Published online Aug 16, 2014. doi: 10.4253/wjge.v6.i8.385
Revised: April 8, 2014
Accepted: June 27, 2014
Published online: August 16, 2014
Esophageal lymphoepithelioma-like carcinoma (LELC) is extremely rare. We report the first case of esophageal LELC showing macroscopic reduction. A 67-year-old male presented with dysphagia and, by endoscopic examination, was found to have a significantly raised tumor of 10 mm in diameter in the thoracic esophagus. The biopsied material showed esophageal cancer. We performed endoscopic submucosal dissection. However, the tumor became flattened, similar to a scar, in only 2 mo. Histologically, the carcinoma cells had infiltrated the submucosal layer. Prominent infiltration of T lymphoid cells that stained positive for CD8 was observed around the carcinoma cells. Therefore, this lesion was considered to be an LELC with poorly differentiated squamous cells. Because the margin was positive, an esophagectomy was performed. Carcinoma cells were detected in the neck in one lymph node. The staging was T1N0M1b. However, the patient has been well, without adjuvant therapy or recurrence, for more than 5 years.
Core tip: The first case of esophageal lymphoepithelioma-like carcinoma showing macroscopic reduction is reported. In only 2 mo, the appearance of the esophageal tumor changed from a protruding lesion to a flat scar-like entity. After esophagectomy, one lymph node was diagnosed with metastasis. Prominent infiltration of T lymphoid cells that stained positive for CD8 was observed around the carcinoma cells. Strong expression of human leukocyte antigen-DR was evident in the cell membrane. The immune responses against the main tumor and the denatured carcinoma cells in the metastatic lymph node developed at the same time. Therefore, systemic immune responses against the carcinoma might have been occurring.
- Citation: Uesato M, Kono T, Shiratori T, Akutsu Y, Hoshino I, Murakami K, Horibe D, Maruyama T, Semba Y, Urahama R, Ogura Y, Oide T, Tanizawa T, Matsubara H. Lymphoepithelioma-like esophageal carcinoma with macroscopic reduction. World J Gastrointest Endosc 2014; 6(8): 385-389
- URL: https://www.wjgnet.com/1948-5190/full/v6/i8/385.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i8.385
Lymphoepithelioma-like carcinoma (LELC) is defined as a tumor with histological similarity to undifferentiated nasopharyngeal carcinoma, with lymphoid stroma (lymphoepithelioma) that occurs outside the nasopharynx. Although LELC has been detected in the salivary gland[1], stomach[2], thymic gland[3], breast[4], and lungs[5], it is extremely rare in the esophagus[6]. The prognosis of patients suffering from this type of cancer has been reported to be favorable[7]. However, there have been no reports of a tumor being reduced macroscopically.
We herein describe the treatment of a patient suffering from an esophageal LELC that spontaneously became small macroscopically and also discuss the results of a pathological analysis of the tumor.
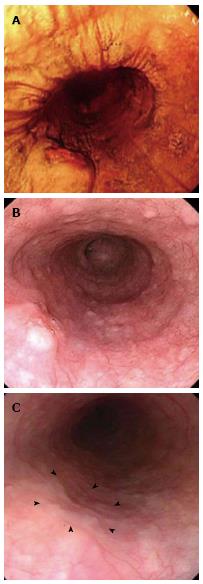
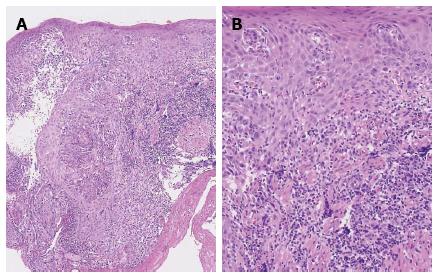
A 67-year-old Japanese male with a seven-month history of intermittent dysphagia underwent an upper endoscopic examination at another clinic in May 2008 (Figure 1A) and was diagnosed as having squamous cell carcinoma of the esophagus, with prominent infiltration of lymphoid cells, based on biopsied material (Figure 2). He was admitted to our hospital for treatment in June 2008. No familial disease and no history of previous gastrointestinal disorders were documented. The patient was a heavy drinker and a non-smoker. A physical examination and laboratory data on admission did not reveal any abnormalities.
A barium esophagogram demonstrated a protruding lesion of 10 mm in diameter on the left and posterior wall of the middle thoracic esophagus. An endoscopic examination in June 2008 revealed a raised tumor with a central dip 33 cm from the upper incisors (Figure 1B). Moreover, the tumor had a smooth surface, similar to a submucosal tumor. Endoscopic ultrasonography in July 2008 demonstrated a well-circumscribed, hypoechoic mass originating in the mucosa, without involvement of the submucosal layer. A computed tomography (CT) scan revealed no metastasis. Therefore, we believed that endoscopic submucosal dissection of the tumor was possible and performed the procedure in July 2008.
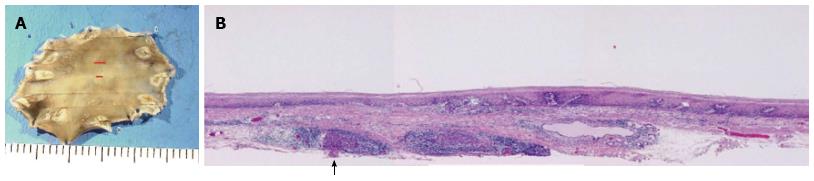
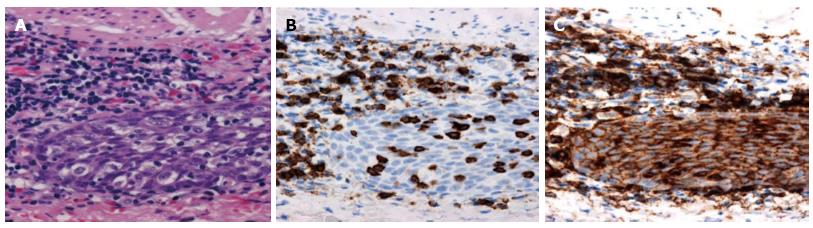
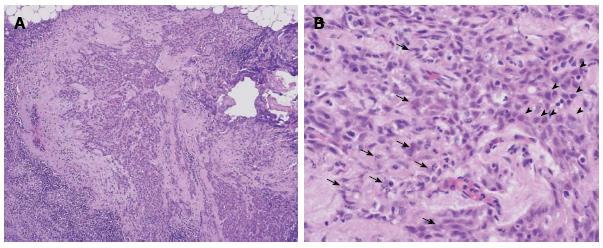
However, the tumor became flattened, similar to a scar (Figure 1C), and there was a superficial smooth tumor measuring 6 mm × 3 mm in the resected mucosa (Figure 3A). Histologically, the large-sized carcinoma cells formed small focal nests and infiltrated into the submucosal layer, 400 μm from the muscularis mucosae (Figure 3B). Prominent infiltration of T lymphoid cells, which stained positive for CD8, was observed between and around the carcinoma cells (Figure 4A and B). Based on these histopathological features, this lesion was considered to be an LELC with poorly differentiated squamous cells. The other pathological findings included human leukocyte antigen-DR (HLA-DR), strongly positive (Figure 4C); p53, strongly positive; Ki67, moderately positive; and Epstein-Barr virus (EBV)-encoded small RNA1 (EBER-1), negative. Because the vertical margin was positive, a subtotal esophagectomy and dissection of the lymph nodes were performed in October 2008. No remnant tumor was found. However, carcinoma cells were detected in the neck area in one of the 53 dissected lymph nodes (Figure 5).
According to the tumor node metastasis (TNM) classification of esophageal cancer, the tumor was diagnosed to be stage IVB (T1, N0, M1b). However, many denatured carcinoma cells were observed in the metastatic lymph node (Figure 5B). The patient’s postoperative course was uneventful, and he was discharged two weeks later. He has been well, without adjuvant therapy or any evidence of recurrence, for more than five years after the surgery. After the diagnosis of the esophageal cancer, he did not smoke or drink alcohol. Furthermore, he did not take any special medicines or examinations.
LELC was first reported by Bégin et al[8] in 1987. LELC is defined as a tumor with histological similarity to undifferentiated nasopharyngeal carcinoma with lymphoid stroma, and this condition has been described in various organs[1-5]. However, an esophageal LELC is extremely rare. According to the PubMed database, there have been only 21 cases, including the present case, reported in the English-language literature to date[6,9-21]. Our report presents the first case of esophageal LELC that was macroscopically reduced in size.
Burke[22] first detected EBV DNA in gastric cancer that histologically resembled nasopharyngeal lymphoepithelioma, and after that, several reports also demonstrated a close relationship between that type of gastric cancer and EBV. However, there does not appear to be a relationship between esophageal cancer and EBV. In the current case, no relationship between the cancer and EBV was revealed by EBER-1 staining. In addition, we investigated 17 of the 21 described patients to determine whether they had an EBV infection. Only three (17.6%) of the 17 described patients were revealed to have an EBV infection. However, Nakasono[20] reported that esophageal LELC may not always be linked with EBV, but that the sensitivity of the detection methods may also be problematic, leading to false-negative findings. Therefore, further investigations are needed to clarify the role of EBV infection in esophageal LELC.
Endoscopically, most cases of LELC have been documented as submucosal tumors covered with normal-appearing esophageal epithelium and with a depression or ulcer in the center of the lesion[6,16,20], as in the present case. However, there has been no previous report of the diagnosis of LELC by endoscopy or biopsy. Because esophageal LELC may be confused with a benign tumor, a biopsy of deeper tissue is required. Moreover, regarding the clinical course, there was a previous report that one year after the first endoscopic examination, the size of the lesion remained unchanged, despite no treatment[20]. However, in our case, the tumor was gradually reduced in size macroscopically and fully disappeared in only 2 mo. It may help the diagnosis of LELC if these endoscopic courses could be followed more closely.
The prominent lymphocytic infiltration in LELC is associated with HLA-DR expression in the deeper carcinoma cells[16]. The T cell infiltration is significantly increased at the sites of HLA-DR expression[23]. In our case, T lymphocytes were found around the lymphoid follicles or within the tumor cell nests, and most of these T cells were positive for CD8, which supports their classification as cytotoxic T lymphocytes.
The role of diffuse infiltrating lymphocytes, consisting of a large number of T lymphocytes and a small number of B lymphocytes, has not yet been clarified in LELC. Two hypotheses have been proposed[13]. In one, the presence of diffuse lymphocytes is explained by the immune response of the host against the carcinoma. In the other, the diffuse lymphocytes are explained in terms of a cell reaction caused by the cytokines produced by the carcinoma cells. In our case, the immune responses against the main tumor and the denatured carcinoma cells in the metastatic lymph node developed at the same time. Therefore, systemic immune responses against the carcinoma might have been occurring. Furthermore, we suppose that the systemic immune response against carcinoma becomes more significant over time and that the tumor becomes smaller when there is increased expression of HLA-DR, as was observed in our case.
Generally, the prognosis of patients suffering from poorly differentiated esophageal squamous cell carcinoma is extremely poor. However, esophageal LELC seems to have a relatively good prognosis[6]. The prognosis is indicated by the survival curves and recurrence rates after treatments. It was fortunate that we could endoscopically follow an LELC lesion that did not receive any treatment for two months because this study helped to demonstrate the natural course of the disease. During this short time, the tumor was gradually reduced in size macroscopically. Histologically, prominent lymphocytic infiltration was revealed in the main tumor and the metastatic lymph node. The patient has remained in good health for the 5 years since the surgery. The immune responses in this case seem to have had nothing to do with his lifestyle. This unique observation was not described in any previous cases.
In conclusion, esophageal LELC has unique clinical and pathological features. In particular, the strength or weakness of the expression of HLA-DR in esophageal LELC may lead to differences in the patient’s clinical course. Furthermore, LELC should be treated differently than other esophageal cancers based on its unique features.
A 67-year-old male with a 7-mo history of intermittent dysphagia.
A protruding lesion of 10 mm in diameter was observed on the left and posterior wall of the middle thoracic esophagus.
Malignant lymphoma, gastrointestinal stromal tumor, endocrine cell tumor.
In only 2 mo, an endoscopic examination revealed that the appearance of the esophageal tumor had changed from a protruding lesion to a flat, scar-like lesion.
This lesion was considered to be an esophageal lymphoepithelioma-like carcinoma with poorly differentiated squamous cells.
After the endoscopic submucosal dissection, a subtotal esophagectomy and dissection of the lymph nodes were performed.
The esophageal lymphoepithelioma-like carcinoma that was macroscopically reduced in size might have occurred due to systemic immune responses against the carcinoma.
This is a very interesting case report. Esophageal cancers are quite common in some parts of the world and this report highlights the fact that not all are associated with a dismal prognosis. The report is well written and adequate references. The photographs are very good and illustrative.
P- Reviewer: Rajeshwari K, Ratnasari N S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Saemundsen AK, Albeck H, Hansen JP, Nielsen NH, Anvret M, Henle W, Henle G, Thomsen KA, Kristensen HK, Klein G. Epstein-Barr virus in nasopharyngeal and salivary gland carcinomas of Greenland Eskimoes. Br J Cancer. 1982;46:721-728. [Cited in This Article: ] |
| 2. | Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38:232-243. [Cited in This Article: ] |
| 3. | Leyvraz S, Henle W, Chahinian AP, Perlmann C, Klein G, Gordon RE, Rosenblum M, Holland JF. Association of Epstein-Barr virus with thymic carcinoma. N Engl J Med. 1985;312:1296-1299. [Cited in This Article: ] |
| 4. | Moore OS, Foote FW. The relatively favorable prognosis of medullary carcinoma of the breast. Cancer. 1949;2:635-642. [Cited in This Article: ] |
| 5. | Butler AE, Colby TV, Weiss L, Lombard C. Lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol. 1989;13:632-639. [Cited in This Article: ] |
| 6. | Sashiyama H, Nozawa A, Kimura M, Nomura E, Tamaru JI, Ninomiya E, Koide Y, Iino M, Ozawa K. Case report: A case of lymphoepithelioma-like carcinoma of the oesophagus and review of the literature. J Gastroenterol Hepatol. 1999;14:534-539. [Cited in This Article: ] |
| 7. | Gaffey MJ, Weiss LM. Association of Epstein-Barr virus with human neoplasia. Pathol Annu. 1992;27 Pt 1:55-74. [Cited in This Article: ] |
| 8. | Bégin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol. 1987;36:280-283. [Cited in This Article: ] |
| 9. | Mori M, Watanabe M, Tanaka S, Mimori K, Kuwano H, Sugimachi K. Epstein-Barr virus-associated carcinomas of the esophagus and stomach. Arch Pathol Lab Med. 1994;118:998-1001. [Cited in This Article: ] |
| 10. | Mori M, Ohno S, Shimono R, Kuwano H, Sugimachi K. Ten-year survivors after surgical treatment and perioperative irradiation for esophageal carcinoma. J Surg Oncol. 1991;47:71-74. [Cited in This Article: ] |
| 11. | Shimizu K, Takiyama W, Mandai K, Tanada M, Kawabuchi Y, Heike Y. Undifferentiated carcinoma with lymphoid infiltration of the esophagus: a case report. Jpn J Clin Oncol. 1999;29:494-497. [Cited in This Article: ] |
| 12. | Parra P, Aguilar J, López-Garrido J, Meléndez B, Merino E, Martinez E, Roldán JP. Primary esophageal lymphoepithelioma. Tumori. 1999;85:519-522. [Cited in This Article: ] |
| 13. | Yamada T, Tatsuzawa Y, Yagi S, Fujioka S, Kitagawa S, Nakagawa M, Minato H, Kurumaya H, Matsunou H. Lymphoepithelioma-like esophageal carcinoma: report of a case. Surg Today. 1999;29:542-544. [Cited in This Article: ] |
| 14. | Takubo K, Lambie NK. Barrett’s adenocarcinoma of the esophagus with lymphoid stroma. J Clin Gastroenterol. 2001;33:141-144. [Cited in This Article: ] |
| 15. | Squillaci S, Martignoni G, Chiodera PL, Vago L, Polonioli S, Capitanio A. [Lymphoepithelioma-like carcinoma of the esophagus: description of a case]. Pathologica. 2001;93:221-225. [Cited in This Article: ] |
| 16. | Chino O, Kijima H, Shimada H, Mizutani K, Nishi T, Tanaka H, Tanaka M, Serizawa A, Tajima T, Makuuchi H. Esophageal squamous cell carcinoma with lymphoid stroma: report of 3 cases with immunohistochemical analyses. Gastrointest Endosc. 2001;54:513-517. [Cited in This Article: ] |
| 17. | Kuwano H, Sumiyoshi K, Sonoda K, Kitamura K, Toh Y, Nakashima H, Sugimachi K. Pathogenesis of esophageal squamous cell carcinoma with lymphoid stroma. Hepatogastroenterology. 2001;48:458-461. [Cited in This Article: ] |
| 18. | Chen PC, Pan CC, Hsu WH, Ka HJ, Yang AH. Epstein-Barr virus-associated lymphoepithelioma-like carcinoma of the esophagus. Hum Pathol. 2003;34:407-411. [Cited in This Article: ] |
| 19. | Angulo-Pernett F, Smythe WR. Primary lymphoepithelioma of the esophagus. Ann Thorac Surg. 2003;76:603-605. [Cited in This Article: ] |
| 20. | Nakasono M, Hirokawa M, Suzuki M, Takizawa H, Okitsu H, Okamura S, Muguruma N, Ito S, Sano T. Lymphoepithelioma-like carcinoma of the esophagus: report of a case with non-progressive behavior. J Gastroenterol Hepatol. 2007;22:2344-2347. [Cited in This Article: ] |
| 21. | Valbuena JR, Retamal Y, Bernal C, Eizuru Y, Corvalan A. Epstein-Barr virus-associated primary lymphoepitheliomalike carcinoma of the esophagus. Diagn Mol Pathol. 2007;16:27-31. [Cited in This Article: ] |
| 22. | Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377-380. [Cited in This Article: ] |