Published online Sep 16, 2023. doi: 10.4253/wjge.v15.i9.553
Peer-review started: April 5, 2023
First decision: June 1, 2023
Revised: June 15, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: September 16, 2023
Esophageal replacement (ER) with gastric pull-up (GPU) or jejunal interposition (JI) used to be the standard treatment for long-gap esophageal atresia (LGEA). Changes of the ER grafts on a macro- and microscopic level however, are un
To evaluate long-term clinical symptoms and anatomical and mucosal changes in adolescents and adults after ER for LGEA.
A cohort study was conducted including all LGEA patients ≥ 16 years who had undergone GPU or JI between 1985-2003 at two tertiary referral centers in the Netherlands. Patients underwent clinical assessment, contrast study and endoscopy with biopsy. Data was collected prospectively. Group differences between JI and GPU patients, and associations between different outcome measures were assessed using the Fisher’s exact test for bivariate variables and the Mann-Whitney U-test for continuous variables. Differences with a P-value < 0.05 were considered statistically significant.
Nine GPU patients and eleven JI patients were included. Median age at follow-up was 21.5 years and 24.4 years, respectively. Reflux was reported in six GPU patients (67%) vs four JI patients (36%) (P = 0.37). Dysphagia symptoms were reported in 64% of JI patients, compared to 22% of GPU patients (P = 0.09). Contrast studies showed dilatation of the jejunal graft in six patients (55%) and graft lengthening in four of these six patients. Endoscopy revealed columnar-lined esophagus in three GPU patients (33%) and intestinal metaplasia was histologically confirmed in two patients (22%). No association was found between reflux symptoms and macroscopic anomalies or intestinal metaplasia. Three GPU patients (33%) experienced severe feeding problems vs none in the JI group. The median body mass index of JI patients was 20.9 kg/m2vs 19.5 kg/m2 in GPU patients (P = 0.08).
The majority of GPU patients had reflux and intestinal metaplasia in 22%. The majority of JI patients had dys
Core Tip: Long-gap esophageal atresia (LGEA) remains a surgical challenge. Preservation of the native esophagus in LGEA is the treatment of choice. Previously however, almost all LGEA patients underwent esophageal replacement (ER). This study evaluated long-term clinical symptoms and anatomical and mucosal changes in adolescents and adults after ER for LGEA. We found that long-term symptoms and graft alterations were common. The majority of gastric pull-up patients had reflux symptoms with intestinal metaplasia in 22%. The majority of jejunal interposition (JI) patients had dysphagia symptoms and more than half of the JI grafts were dilated.
- Citation: van Tuyll van Serooskerken ES, Gallo G, Weusten BL, Westerhof J, Brosens LA, Zwaveling S, Ruiterkamp J, Hulscher JB, Arets HG, Bittermann AJ, van der Zee DC, Tytgat SH, Lindeboom MY. Graft dilatation and Barrett’s esophagus in adults after gastric pull-up and jejunal interposition for long-gap esophageal atresia. World J Gastrointest Endosc 2023; 15(9): 553-563
- URL: https://www.wjgnet.com/1948-5190/full/v15/i9/553.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i9.553
Long-gap esophageal atresia (LGEA) is present in approximately 10% of all EA[1] and remains a surgical challenge[2,3]. Preservation of the native esophagus in LGEA is the treatment of choice, which can be accomplished by delayed primary anastomosis[4,5] or elongation techniques[6-10] in experienced centers. Previously however, almost all LGEA patients underwent esophageal replacement (ER) with gastric[11], jejunal[12] or colonic[13] conduit.
Since survival rates have improved up to 90% in EA[14], focus has shifted to the investigation and treatment of long-term morbidities and quality of life. Gastrointestinal symptoms, including gastroesophageal reflux (GER) and dysphagia, are frequent in EA[15]. The incidence of (severe) reflux is expected to be even higher in patients after a gastric pull-up (GPU)[16]. This may be explained by mobilization of the stomach into the mediastinum. This results in alteration of the shape of the gastroesophageal junction and consequently the loss of the angle of His, which is one of the anti-reflux barriers. Moreover, the negative intrathoracic pressure and the positive intraluminal pressure in the transposed stomach may increase GER[17]. Micro-aspiration due to GER may contribute to chronic cough and asthma-like symptoms[18,19]. Chronic GER may lead to esophageal mucosal alterations with a four times higher incidence of Barrett’s esophagus compared to healthy controls[20]. Literature on the long-term outcome of ER is scarce[16,21-23]. Studies on long-term endoscopic findings in LGEA patients are lacking. Therefore, this study aims to evaluate the long-term outcome of jejunal interposition (JI) and GPU on clinical symptoms and anatomical and mucosal changes in adolescents and adults after LGEA.
A cohort study was conducted including all LGEA patients ≥ 16 years old who had undergone JI or GPU at the University Medical Center Utrecht (UMCU) and the University Medical Center Groningen between 1985 and 2003. As of 2018, all 17-year-old EA patients are routinely referred to the gastroenterologist for clinical assessment and endoscopic and histologic screening for esophageal mucosal lesions. All adult LGEA patients (> 17 years), that were not yet included in the routine follow-up, were invited for screening. Patients that had ER for LGEA underwent an one-time barium contrast study, to evaluate the anatomy of the graft. Data was collected prospectively. Gastroscopies that were performed after the age of 17 years and within the last four years, were reviewed retrospectively.
All ERs had been performed by experienced pediatric surgeons. The GPU was performed as previously described by Spitz et al[11,24]. In short, after mobilization of the stomach and a pyloromyotomy transhiatal posterior mediastinal tunnel is created and the stomach is transposed into the thorax through the esophageal hiatus. Thereafter, the proximal esophagus and the apex of the stomach are anastomosed in the neck. JI was performed as described in these studies[12,25,26]. The pedicle graft is created: The jejunum is transected close to Treitz ligament and at the level of the third mesenteric artery branch. The uppermost part of the graft is tunneled into the right chest, behind the stomach and through the posterior part of the hiatus. Thereafter, two anastomosis are performed, one between the proximal esophagus and the jejunal graft and another between the distal esophagus and the jejunal graft.
Baseline characteristics, including gender, age, type of EA and associated anomalies were obtained from the electronic medical records.
Gastro-intestinal symptoms: Gastrointestinal symptom assessment (e.g., reflux, dysphagia) was derived from the routine outpatient follow-up at the Gastroenterology Department.
Contrast study: Upper gastrointestinal barium contrast studies were analyzed by an experienced radiologist and pediatric surgeon for the following parameters: Anastomotic stenosis, stasis of contrast, reflux, graft-dilatation and graft-lengthening (resulting in a siphon shaped graft) of the JI and the position of the stomach in GPU patients.
Upper endoscopy and histology: Upper endoscopy was performed by a gastroenterologist to assess the esophagus, the anastomotic site(s), the grafts, the gastroesophageal junction and the stomach. Reflux esophagitis and intestinal metaplasia were scored according to the Los Angeles (LA) classification[27] and Prague criteria[28]. Barrett’s esophagus was defined as columnar lined esophagus on endoscopy in combination with intestinal metaplasia on histology. In patients with JI, biopsies were taken from both the distal and proximal esophagus. Jejunal grafts were evaluated on proximal or distal stenosis, (distal) dilatation of the graft and on macroscopic lesions. Biopsies of the jejunal graft were taken if mucosal abnormalities were present. The GPU was evaluated on anastomotic stenosis, macroscopic lesions and altered anatomy. In patients with GPU, biopsies were taken just proximal to the anastomosis. In case of macroscopic abnormalities of the GPU, biopsies were taken. Endoscopies were reviewed by an experienced gastroenterologist and a pediatric surgeon. Biopsies were evaluated for inflammation, eosinophilia and metaplasia by the Pathology Department by an expert gastrointestinal pathologist.
This study was part of a larger cohort study on the long-term outcome in LGEA patients. The study protocol was submitted to the UMCU Ethics Committee (METC 18-458/C). According to the Medical Research Involving Human Subject Act, no ethical approval was required.
Continuous skewed variables were presented as median and range, categorical data were presented as frequencies and percentage. Group differences between JI and GPU patients, and associations between different outcome measures were assessed using the Fisher’s exact test for bivariate variables and the Mann-Whitney U-test for continuous variables. Differences with a P value < 0.05 were considered statistically significant. The analyses were performed using SPSS for Windows, version 25.0 (IBM Corp., Armonk, NY).
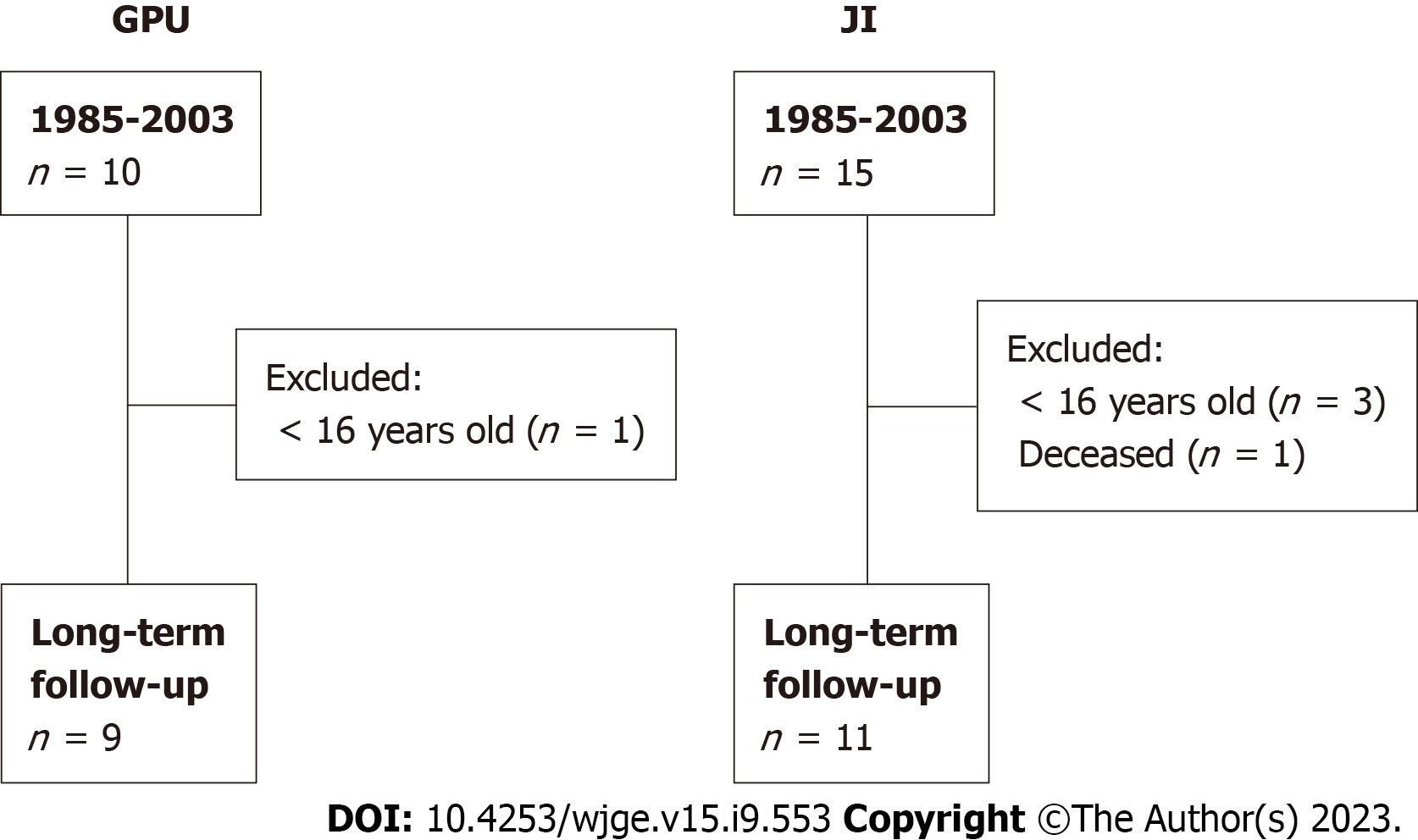
Between 1985 and 2003, a total of 24 patients underwent ER for LGEA (Figure 1). One JI patient was deceased at the age of 10 years due to massive aspiration. After following the exclusion criteria, twenty patients were included in this study. Nine patients underwent GPU and eleven underwent JI. Median age at follow-up was 21.5 years (range 20.2-34.1) for GPU patients and 24.4 years (range 16.1-31.2) for JI patients. Five JI patients (46%) and all GPU patients were male (P = 0.01). Associated anomalies (e.g., cardiac, renal, musculoskeletal anomalies) were more present in GPU patients than in JI patients (100% vs 55%, P = 0.04). In both groups severe mental retardation and Down syndrome were present in one patient. Patient characteristics are shown in Table 1. Preoperative gastrostomy was present in all JI patients and in eight (89%) GPU patients. Anastomotic strictures requiring dilatation had developed in eight JI patients (73%) and five GPU patients (55%). Fundoplication was required in one JI patient at the age of 2 years (Table 2).
| Value | GPU (n = 9) | JI (n = 11) | P value |
| Male | 9 (100%) | 5 (46%) | 0.01a |
| Gestational age (wk) | 33.9 (29-39) | 34.7 (32.3-41.3) | 0.15 |
| Premature | 7 (78%) | 8 (73%) | 1.0 |
| Birthweight (grams) | 1680 (1030-3040) | 2010 (1115-3755) | 0.49 |
| Gross type EA | 0.63 | ||
| A | 5 (56%) | 4 (36%) | |
| B | 3 (33%) | 6 (55%) | |
| C | 1 (11%) | 1 (9%) | |
| Associated anomalies1 | 9 (100%) | 6 (55%) | 0.04a |
| Down syndrome | 1 (11%) | 1 (9%) | |
| Anorectal malformations | 1 (11%) | 1 (9%) | |
| Duodenal atresia | 1 (11%) | 1 (9%) | |
| Musculoskeletal | 4 (44%) | 3 (27%) | |
| Cardiac | 1 (11%) | 2 (18%) | |
| Renal anomaly | 4 (44%) | 1 (9%) | |
| Palatoschisis | 2 (22%) | 0 |
| Variable | GPU (n = 9) | JI (n = 11) | P value |
| Age at surgery (d) | 128 (1-323) | 67 (41-149) | 0.21 |
| Gastrostomy | 8 (89%) | 11 (100%) | 0.45 |
| Fundoplication | 0 | 1 (9.1%) | 1.0 |
| Stenosis1 | 5 (56%) | 8 (73%) | 0.64 |
| Dilatations total (n) | 3 (1-4) | 3 (1-15) | 0.76 |
| Dilatations within 1st yr (n) | 1 (1-3) | 2.5 (0-15) | 0.26 |
Reflux complaints were reported in six of the nine GPU patients (67%) and in four out of 11 JI patients (36%) (P = 0.37). Dysphagia symptoms were scored in seven JI patients (64%) vs two GPU patients (22%) (P = 0.09). Three GPU patients (33%) experienced severe feeding problems. Due to swallowing disabilities, one patient was still fully dependent on jejunostomy feeding, with minimal attempts of liquid oral feeds. Another patient required additional jejunostomy feeding until the age of 21 years, but has recently reached a full oral diet. One patient required additional drink nutrition to achieve a full oral diet. In the JI group, no severe feeding problems were observed.
The median body mass index (BMI) of JI patients was 20.9 kg/m2 (range 17.9-27.6) vs 19.5 kg/m2 (range 17.5-21.6) in GPU patients (P = 0.08). Two JI patients (18%) were underweight (BMI < 18.5 kg/m2) and one patient was overweight (BMI > 25 kg/m2) (Table 3). Three GPU patients (33%) were underweight, none of the patients were overweight.
| Variable | GPU (n = 9) | JI (n = 11) | P value |
| Age at follow-up (median, yr) | 21.5 (20.2-34.1) | 24.4 (16.1-31.2) | 0.85 |
| GER complaints | 6 (67%) | 4 (36%) | 0.37 |
| Dysphagia | 2 (22%) | 7 (64%) | 0.09 |
| FOIS | |||
| Total oral diet with no restrictions | 5 | 5 | |
| Specific food limitations | 1 | 2 | |
| Multiple consistencies, requiring special preparation | 1 | 0 | |
| Tube-dependent | 1 | 0 | |
| Missing | 1 | 4 | |
| PPI use | 4 (44%) | 3 (27%) | 0.38 |
| BMI (kg/m2) | 19.5 (17.5-21.6) | 20.9 (17.9-27.6) | 0.08 |
GPU: Barium contrast studies were performed in five of the nine GPU patients (56%). In one patient, the stomach was completely transposed into the thorax. This patient showed some lengthening of the distal esophagus and stasis of liquids in the distal esophagus. Another patient, with Down syndrome, also showed stasis of contrast in the esophagus. No reflux was observed in these patients.
Four out of nine GPU patients did not undergo a contrast study; three patients did not consent because they did not experience major gastro-intestinal complaints. One patient with mental retardation was unable to perform a contrast study due to severe swallowing difficulties.
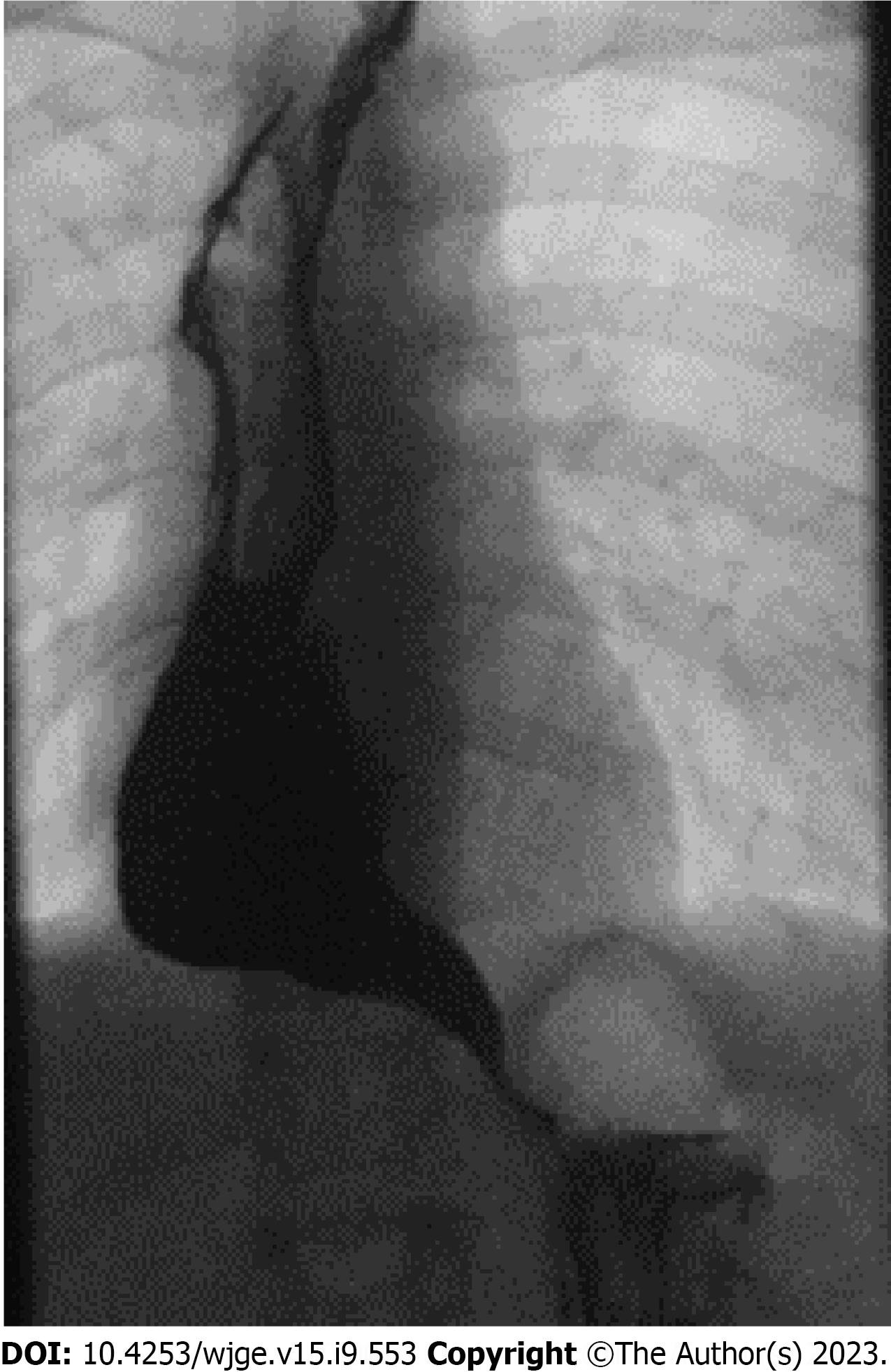
JI: Barium contrast studies were performed in all 11 JI patients. Ten patients (91%) showed stasis of contrast in the ER graft. None of the patients had a proximal or distal stenosis. The jejunal graft was dilated in six (55%) patients. In two of these patients, graft dilatation was severe. In four of these six patients, mild to moderate lengthening of the distal part of the jejunal graft was observed (Figure 2).
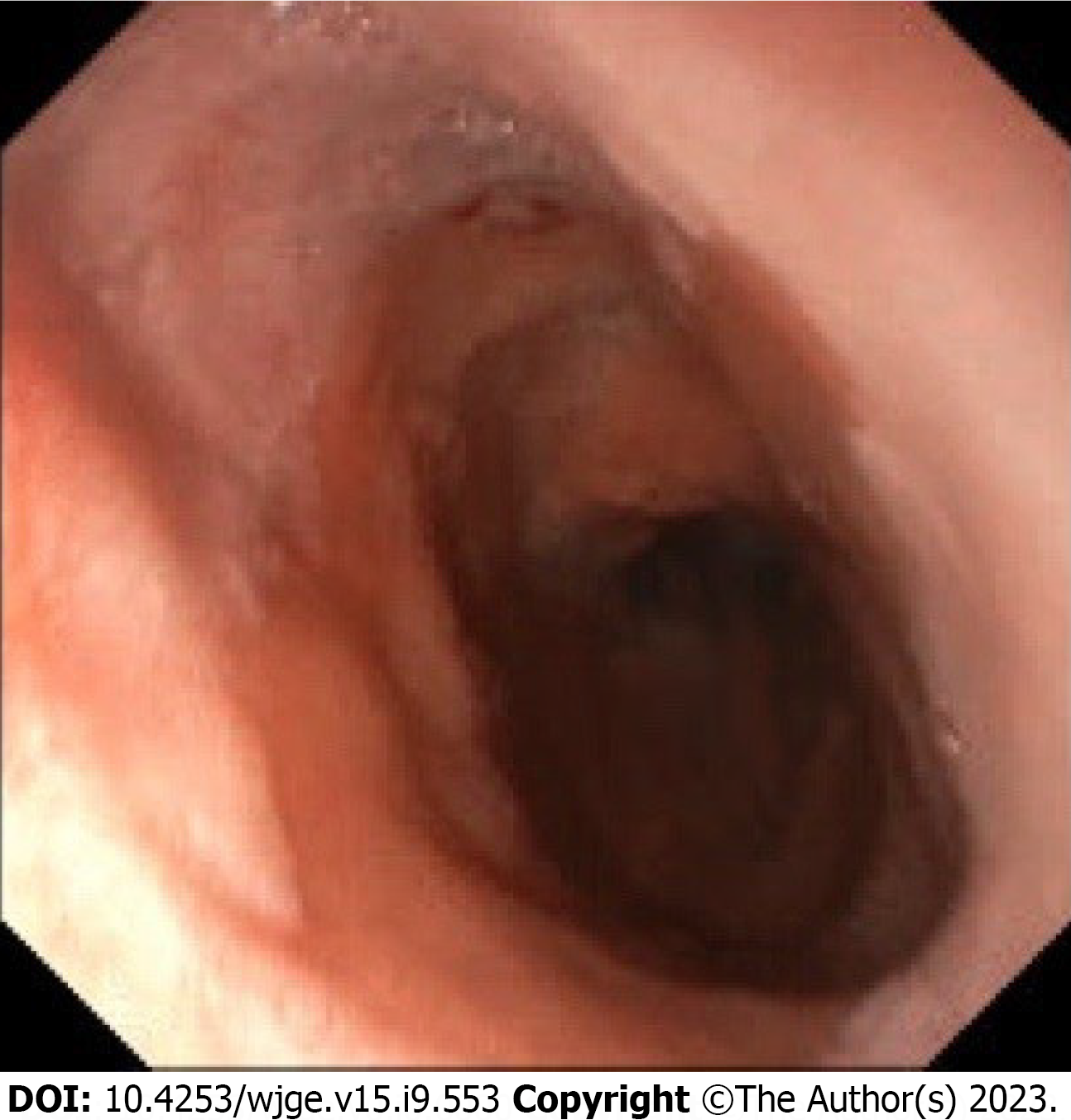
GPU: All GPU patients (n = 9) had undergone gastroscopy. The median distance from the incisors to the anastomosis was 19 cm (range 17-24). Macroscopic anomalies of the native esophagus were seen in five patients (56%); three patients showed columnar lined esophagus (33%) (C0M2, C0M2, C1M2) (Figure 3). One patient had an erosion at the distal part of the esophagus and another patient, who was jejunostomy dependent due to severe swallowing difficulties, had a pinpoint stenosis of the anastomosis (Table 4).
| Variable | GPU (n = 9) | JI (n = 11) | P value |
| Barium contrast results | n = 5 | n = 11 | |
| Stasis | 3 (60%) | 10 (91%) | 0.14 |
| Stricture | 0 | 0 | - |
| Dilated JI graft | N/A | N/A | |
| Mild | 4 (36%) | ||
| Severe | 2 (18%) | ||
| Lengthening of JI graft | N/A | 4 (36%) | N/A |
| Endoscopy results | n = 9 | n = 11 | |
| Length proximal esophagus (cm) | 20 (17-24) | 21 (18-25) | - |
| Length jejunal graft (cm) | N/A | 15 (12-22) | - |
| Length distal esophagus (cm) | N/A | 4.5 (0-8) | - |
| Macroscopic anomalies | 5 (56%) | 5 (45%) | 1.0 |
| Macroscopic esophagitis1 | |||
| Grade A | 0 | 1 (9%) | - |
| Grade B | 0 | 1 (9%) | - |
| Columnar-lined esophagus | 3 (33%) | 0 | 0.07 |
| Histology results | n = 8 | n = 7 | |
| Normal mucosa | 1 (13%) | 3 (43%) | 0.58 |
| Inflammation | 2 (25%) | 1 (14%) | 1 |
| Intestinal metaplasia | 2 (25%) | 0 | 0.47 |
| Other | 3 (38%) | 3 (43%) | 1.0 |
JI: All JI patients (n = 11) had undergone upper endoscopy. The median distance from the incisors to the proximal anastomosis was 21 cm (range 18-25), the median length of the jejunal graft was 15 cm (range 12-22) and the median length of the distal esophagus was 4.5 cm (range 0-8). In none of the patients a proximal or distal anastomotic stenosis was present. Macroscopic anomalies were seen in five patients (45%): Two patients showed macroscopic esophagitis of the distal esophagus according to the LA classification (grade A, n = 1; grade B, n = 1), one patient had fields of squamous epithelium in the proximal part of the jejunal graft, one patient showed elevation of normal mucosa in the distal esophagus and a neurological impaired patient had stasis of food and an ulcer at the distal part of the jejunal graft. None of the JI patients showed columnar-lined esophagus (Table 4).
GPU: In three patients with macroscopic columnar-lined esophagus, biopsies of the native distal esophagus showed intestinal metaplasia in two patients (22%), both with Prague classification C0M2 (2 men; median age 21.6 years). In two patients, biopsies of the distal esophagus showed chronic inflammation. Biopsies in another two patients showed hyperplastic squamous epithelium without dysplasia. In one patient, histopathology revealed that biopsies of cardia and corpus were obtained. Histopathology showed no signs of dysplasia in any of the patients. In one patient without macroscopic anomalies, no biopsies specimens were taken (Table 4).
JI: In three patients, histology of the native distal esophagus showed normal esophageal mucosa. In one patient, biopsy of the native distal esophagus showed a single glandular tube with signs of intestinal metaplasia. A target biopsy of a small mucosal elevation of the distal esophagus in another patient showed mild reactive changes of the mucosa. In two patients, biopsies of the stomach were obtained. Biopsies in one patient showed no abnormalities. In the other patient without macroscopic anomalies, biopsy of the stomach showed lymphoid infiltration, further investigation excluded lymphoma. None of the biopsies showed signs of esophageal dysplasia. In four patients without suspected macroscopic anomalies (36%), no biopsies specimens were taken (Table 4).
Columnar-lined esophagus of the native esophagus occurred more often in the GPU group compared to the JI-group (3 vs 0 patients, P = 0.07). No associations were found in GPU patients between reflux symptoms and macroscopic mucosal abnormalities during upper endoscopy or with intestinal metaplasia. Both patients that had confirmed intestinal metaplasia, reported reflux symptoms and were treated with proton-pump inhibitors (PPIs). No association was found between intestinal metaplasia and GER symptoms. No association was found between BMI and reflux.
Of the six patients with a dilated JI-graft, five (83%) reported dysphagia complaints. Of the four patients with lengthening of the JI-graft, three (75%) reported dysphagia symptoms. However, there was no statistically significant association between dilatation or lengthening and dysphagia.
This is the first study to evaluate very long-term changes in ER grafts for LGEA by contrast study and endoscopy, showing intestinal metaplasia in 22% of GPU patients and graft dilatation in JI patients. Furthermore, this study evaluates gastrointestinal symptoms during a long-term follow-up.
We found that the majority of GPU patients had reflux symptoms, which is in line with the outcome of the study of Hannon et al[21]. In our study, reflux symptoms were assessed at the outpatient clinic by a gastroenterologist. EA patients might consider reflux symptoms as normal after prolonged periods of reflux. Symptom-related questions asked by a specialist may identify patients with reflux symptoms who would otherwise consider themselves free of symptoms[29]. This can explain the high incidence of reflux found in this study.
This study showed that reflux symptoms occurred less in JI patients compared to GPU patients. This difference may be explained by the fact that several physiological anti-reflux mechanisms are altered in GPU patients, such as the intrathoracic position of the stomach with a negative intrathoracic pressure and loss of the His angle[17]. In the JI patient group, the distal esophagus remained intact with an intra-abdominal position in all but one patient. Although peristalsis of the graft is not as efficient as a native esophagus, the other antireflux barriers are preserved.
Postoperative dysphagia was present in the majority of JI patients. Their nutritional status, however, was good on the long term and all JI patients had a full oral intake. This is in contrast to previous studies[30,31], with only 33%-57% of JI patients tolerating a complete oral intake. This difference may be explained by the occurrence of severe postoperative complications in both studies, including graft loss.
In our study, GPU patients reported less dysphagia symptoms compared to JI patients. Our GPU group also reported less dysphagia symptoms than the GPU group of Hannon et al[21], although this difference is relatively small. Lower BMI has been described in GPU patients compared to primary repair EA patients[21]. This is in line with our findings, in which one third of the GPU patients were underweight and needed nutritional supplements. One might speculate that reflux negatively influences the achievement of an adequate caloric intake and consequent lower BMI[32,33]. However, in our study, an association between reflux and BMI could not be found.
Our study showed that the majority of patients had a dilated JI graft. Although almost all of these patients reported dysphagia complaints, an association between the dilatation and dysphagia was not statistically significant. The dilatation of the jejunal graft may be explained by the slower motility of the jejunal graft compared to the faster motility of the esophagus. Stasis of food due to dysmotility of the jejunal graft and the distal esophageal remnant may result in dilatation of the graft and may cause dysphagia symptoms in these patients. Lengthening of the JI graft may also contribute to dysmotility and therefore dysphagia due to the siphon shape. Previously, JI graft dilatation has only been described by Saeki et al[22]. In his study on JI for LGEA (mean age 10 years) dilatation of a graft was observed in one patient. This was due to a stenosis of the distal anastomosis. In our study, lengthening of the jejunal graft was seen in 36% of JI patients, which is in line with previous studies[22,23].
Upper endoscopy showed columnar-lined esophagus in one third of the GPU patients and in none of the JI patients in our study. Histology reported intestinal metaplasia in 22% of GPU patients and in none of the JI patient. These findings are in contrast to the only other published study using endoscopy in adults after LGEA by Vergouwe et al[20]. The latter showed no signs of Barrett’s esophagus in LGEA patients with ER. However, they showed an incidence of 6.6% Barrett’s esophagus in their total cohort of 151 adult EA patients. Vergouwe et al[20] also showed two patients with esophageal cancer. Esophageal cancer after primary repair of EA at the site of the anastomosis in a patient with severe reflux has also been described[34]. In our study, no patients were found with esophageal cancer.
Our findings reveal that the macroscopic and microscopic tissue changes seen in the GPU grafts were not significantly associated with reflux symptoms. This may be explained by the fact that many patients were treated with PPIs. Also, metaplasia of the esophageal mucosa can protect against acid reflux and therefore prevent symptoms of discomfort. Furthermore, one can expect that EA patients may get used to reflux symptoms, although this is not evidence based. Reflux symptoms can thus not be used as a reliable detector for the presence of intestinal metaplasia. Since GPU is the most frequently performed ER procedure for LGEA and intestinal metaplasia or Barrett’s esophagus may occur more frequently in this subset of patients, further follow-up of GPU in the long-term may clarify this concern. Barrett’s esophagus in the normal population increases steeply from young adulthood until the 6th decade of life. Since our cohort consists of young patients, the prevalence of Barrett’s esophagus will become more clear after long term follow-up.
Due to the rarity of LGEA, data are scarce. This inevitably limits our study and therefore, interpretations must be made with caution. Furthermore, treatment for LGEA is being corrected by using the thoracoscopic traction technique in our center. In our opinion, this is now the treatment of choice for LGEA, but only in experienced centers. Alternatively, if experience in this challenging procedure is not available, a GPU can be performed.
Other limitations in this study include the retrospective design of the study and the missing histology in five JI patients and one GPU patient. Although the macroscopic aspects during endoscopy seemed normal in these patients, histological evidence would be preferred. Also, contrast studies were missing in four GPU patients. Furthermore, review of contrast studies is not standardized and therefore subjective. However, all contrast studies were analyzed by an experienced radiologist and pediatric surgeon to minimize bias.
This study shows that ER grafts show significant macroscopic and microscopic abnormalities after long-term follow-up. Dilatation of the graft and dysphagia symptoms were present in the majority of JI patients. GPU patients may have an increased risk of intestinal metaplasia. Therefore, increased awareness and endoscopic follow-up during adulthood is suggested for LGEA patients after ER. Especially since GPU has been and still is the most frequently used treatment for LGEA.
Previously, esophageal replacement (ER) with gastric pull-up (GPU) or jejunal interposition (JI) used to be the standard treatment for long-gap esophageal atresia (LGEA). Gastrointestinal symptoms are common in EA patients and may occur even more frequently after ER, due to a change of the anatomy.
Long-term macroscopic and microscopic graft changes are currently unknown and may be clinically relevant in patients with LGEA.
This study aims to evaluate clinical symptoms and macroscopic and microscopic graft changes in adolescence and adulthood.
A cohort study including all LGEA patients ≥ 16 years who had undergone ER between 1985-2003 at two tertiary centers in the Netherlands was conducted. Clinical symptoms, contrast studies and endoscopies were collected prospectively.
Nine GPU patients and eleven JI patients were included in this study, with a median age of 21.5 years and 24.4 years respectively. Six of nine GPU patients (67%) reported reflux complaints and 64% of JI patients reported dysphagia symptoms. Dilatation of the jejunal graft was observed in 55%. Three GPU patients had columnar-lined epithelium and in two of these patients intestinal metaplasia was histologically confirmed.
Long-term follow-up revealed significant macroscopic and microscopic graft changes after ER. Furthermore, this study revealed long-term clinical symptoms after both GPU and JI. GPU patients may have an increased risk on intestinal metaplasia. Dilatation of the graft and dysphagia symptoms were present in the majority of JI patients. Follow-up during adulthood after ER for LGEA is therefore suggested.
This study highlights the importance of implementing an endoscopic follow-up program after ER for LGEA, particularly after GPU. Further investigations with larger patient cohorts are necessary to validate these findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qvist N, Denmark; Tharavej C, Thailand S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sfeir R, Michaud L, Salleron J, Gottrand F. Epidemiology of esophageal atresia. Dis Esophagus. 2013;26:354-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Holland AJ, Ron O, Pierro A, Drake D, Curry JI, Kiely EM, Spitz L. Surgical outcomes of esophageal atresia without fistula for 24 years at a single institution. J Pediatr Surg. 2009;44:1928-1932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Ron O, De Coppi P, Pierro A. The surgical approach to esophageal atresia repair and the management of long-gap atresia: results of a survey. Semin Pediatr Surg. 2009;18:44-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Puri P, Ninan GK, Blake NS, Fitzgerald RJ, Guiney EJ, O'Donnell B. Delayed primary anastomosis for esophageal atresia: 18 months' to 11 years' follow-up. J Pediatr Surg. 1992;27:1127-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Puri P, Khurana S. Delayed primary esophageal anastomosis for pure esophageal atresia. Semin Pediatr Surg. 1998;7:126-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | van der Zee DC, Vieirra-Travassos D, Kramer WL, Tytgat SH. Thoracoscopic elongation of the esophagus in long gap esophageal atresia. J Pediatr Surg. 2007;42:1785-1788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | van der Zee DC, Gallo G, Tytgat SH. Thoracoscopic traction technique in long gap esophageal atresia: entering a new era. Surg Endosc. 2015;29:3324-3330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Bogusz B, Patkowski D, Gerus S, Rasiewicz M, Górecki W. Staged Thoracoscopic Repair of Long-Gap Esophageal Atresia Without Temporary Gastrostomy. J Laparoendosc Adv Surg Tech A. 2018;28:1510-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Foker JE, Linden BC, Boyle EM Jr, Marquardt C. Development of a true primary repair for the full spectrum of esophageal atresia. Ann Surg. 1997;226:533-41; discussion 541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 161] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Kimura K, Soper RT. Multistaged extrathoracic esophageal elongation for long gap esophageal atresia. J Pediatr Surg. 1994;29:566-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Spitz L. Gastric transposition for esophageal substitution in children. J Pediatr Surg. 1992;27:252-7; discussion 257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Bax NM, van der Zee DC. Jejunal pedicle grafts for reconstruction of the esophagus in children. J Pediatr Surg. 2007;42:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Sherman CD Jr, Waterston D. Oesophageal reconstruction in children using intrathoracic colon. Arch Dis Child. 1957;32:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sulkowski JP, Cooper JN, Lopez JJ, Jadcherla Y, Cuenot A, Mattei P, Deans KJ, Minneci PC. Morbidity and mortality in patients with esophageal atresia. Surgery. 2014;156:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Connor MJ, Springford LR, Kapetanakis VV, Giuliani S. Esophageal atresia and transitional care--step 1: a systematic review and meta-analysis of the literature to define the prevalence of chronic long-term problems. Am J Surg. 2015;209:747-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Gallo G, Zwaveling S, Van der Zee DC, Bax KN, de Langen ZJ, Hulscher JB. A two-center comparative study of gastric pull-up and jejunal interposition for long gap esophageal atresia. J Pediatr Surg. 2015;50:535-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Orlando RC. Overview of the mechanisms of gastroesophageal reflux. Am J Med. 2001;111 Suppl 8A:174S-177S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Gallo G, Vrijlandt EJLE, Arets HGM, Koppelman GH, Van der Zee DC, Hulscher JBF, Zwaveling S. Respiratory function after esophageal replacement in children. J Pediatr Surg. 2017;52:1736-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Schachter LM, Dixon J, Pierce RJ, O'Brien P. Severe gastroesophageal reflux is associated with reduced carbon monoxide diffusing capacity. Chest. 2003;123:1932-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Vergouwe FWT, IJsselstijn H, Biermann K, Erler NS, Wijnen RMH, Bruno MJ, Spaander MCW. High Prevalence of Barrett's Esophagus and Esophageal Squamous Cell Carcinoma After Repair of Esophageal Atresia. Clin Gastroenterol Hepatol. 2018;16:513-521.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Hannon E, Eaton S, Curry JI, Kiely EM, Spitz L, De Coppi P. Outcomes in adulthood of gastric transposition for complex and long gap esophageal atresia. J Pediatr Surg. 2020;55:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Saeki M, Tsuchida Y, Ogata T, Nakano M, Akiyama H. Long-term results of jejunal replacement of the esophagus. J Pediatr Surg. 1988;23:483-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Steves Ring W, Varco RL, L'Heureux PR, Foker JE. Esophageal replacement with jejunum in children: An 18 to 33 year follow-up. J Thorac Cardiov Sur. 1982;83:918-927. [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 101] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Spitz L. Oesophageal atresia treatment: a 21st-century perspective. J Pediatr Gastroenterol Nutr. 2011;52 Suppl 1:S12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Bax NMA, Rövekamp MH, Pull ter Gunne AJ, van der Zee DC. Early one-stage orthotopic jejunal pedicle-graft interposition in long-gap esophageal atresia. Pediatr Surg Int. 1994;9:483-485. [DOI] [Cited in This Article: ] |
| 26. | Bax KM. Jejunum for bridging long-gap esophageal atresia. Semin Pediatr Surg. 2009;18:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1518] [Cited by in F6Publishing: 1520] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 28. | Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 731] [Cited by in F6Publishing: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 29. | Ijsselstijn H, van Beelen NW, Wijnen RM. Esophageal atresia: long-term morbidities in adolescence and adulthood. Dis Esophagus. 2013;26:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Koivusalo A, Suominen J, Salminen J, Pakarinen M. Indications, Surgical Complications, and Long-Term Outcomes in Pediatric Esophageal Reconstructions with Pedicled Jejunal Interposition Graft. Eur J Pediatr Surg. 2020;30:111-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Cauchi JA, Buick RG, Gornall P, Simms MH, Parikh DH. Oesophageal substitution with free and pedicled jejunum: short- and long-term outcomes. Pediatr Surg Int. 2007;23:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, Gupta S, Langendam M, Staiano A, Thapar N, Tipnis N, Tabbers M. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 33. | Baird DC, Harker DJ, Karmes AS. Diagnosis and Treatment of Gastroesophageal Reflux in Infants and Children. Am Fam Physician. 2015;92:705-714. [PubMed] [Cited in This Article: ] |
| 34. | Pultrum BB, Bijleveld CM, de Langen ZJ, Plukker JT. Development of an adenocarcinoma of the esophagus 22 years after primary repair of a congenital atresia. J Pediatr Surg. 2005;40:e1-e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |