Published online Dec 16, 2021. doi: 10.4253/wjge.v13.i12.593
Peer-review started: March 12, 2021
First decision: October 17, 2021
Revised: October 19, 2021
Accepted: November 26, 2021
Article in press: November 26, 2021
Published online: December 16, 2021
Composite intestinal adenoma-microcarcinoid (CIAM) is a rare intestinal lesion consisting of conventional adenoma and small, well differentiated carcinoid [microcarcinoid (MC)] at its base. The incidence of CIAM is 3.8% in surgically resected colorectal polyps. While its pathogenesis is unknown, studies support the role of Wnt/β-catenin pathway in the tumorigenesis of CIAM. CIAMs have been primarily reported in the colon wherein they present as polyps with well-defined margins, similar to conventional adenomatous polyps. MC is usually found in adenomatous polyps with high-risk features such as large size, villous architecture, or high grade dysplasia. Histologically, the MC component is often multifocal and spans 3.9 to 5.8 millimeters in size. MC is usually confined within the mucosa but occasional CIAM cases with MC extending to the submucosa have been reported. MC of CIAM demonstrates bland cytology and inconspicuous proliferative activity. The lesional cells are positive for synaptophysin and 60% to 100% of cases show nuclear β-catenin positivity. MC poses a diagnostic challenge with its morphologic and immunohistochemical resemblance to both benign and malignant lesions, including squamous morules/metaplasia, adenocarcinoma, squamous cell carcinoma, sporadic neuroendocrine tumor and goblet cell adenocarcinoma. CIAM is an indolent lesion with a favorable outcome. Complete removal by polypectomy is considered curative. Awareness and recognition of this rare entity will help arrive at correct diagnosis and improve patient care. Currently, CIAM is not recognized as a subtype of mixed neuroendocrine-non-neuroendocrine neoplasm by WHO.
Core Tip: Composite intestinal adenoma-microcarcinoid (CIAM) is a rare intestinal lesion consisting of adenoma and well differentiated microcarcinoid components. While it is a form of mixed neoplasm with both neuroendocrine and non-neuroendocrine elements, CIAM is currently not recognized as a distinct subtype of mixed neoplasm by WHO. It is found incidentally during the pathologic examination of adenomatous polyps. Altered Wnt/β-catenin pathway appears to play a role in its pathogenesis. Other benign and malignant lesions need to be distinguished from CIAM given differing therapeutic implications. CIAM is an indolent disease with a favorable outcome.
- Citation: Fu ZY, Kmeid M, Aldyab M, Lagana SM, Lee H. Composite intestinal adenoma-microcarcinoid: An update and literature review. World J Gastrointest Endosc 2021; 13(12): 593-606
- URL: https://www.wjgnet.com/1948-5190/full/v13/i12/593.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i12.593
Composite intestinal adenoma-microcarcinoid (CIAM) is a rare intestinal lesion consisting of conventional adenoma and associated microscopic well-differentiated neuroendocrine cell clusters [microcarcinoid (MC)] at its base. The adenoma component presents as a typical polyp, which is removed either endoscopically or surgically[1-3]. The MC component does not form grossly evident nodules or masses[1,3] and is typically located at the base of the polyp, usually within the mucosa. Occasional cases of CIAM with the MC component extending into the submucosa have been reported[1,4,5]. As MC occupies only a minute area and forms small nests or clusters microscopically, the overall architecture of the polyp is preserved[2,3].
CIAM was first described by Moyana et al[6] in 1988. In this report, the authors described two adenomas co-existing with carcinoids: One was in the center of a dome-shaped polyp, and the other was at the base of a sessile villous adenoma. The authors also noticed a transition zone between the two components. It is unclear how much of the lesion was composed of carcinoid component in their report. However, based on the illustrations provided in the report, the carcinoid components do not appear subtle[6]. Since its first description, CIAM have been sporadically documented as case reports or small case series[2,5,7,8].
Although CIAM is a rare entity, endocrine cell “differentiation” is not uncommon in colorectal adenomas, wherein the cells of neuroendocrine phenotype are considered to originate from the endoderm[9,10]. For example, argyrophil cells have been reported in 59% to 85% of adenomatous polyps[10,11]. In Iwashita’s study, argyrophil cells and argentaffin cells were found in 76.4% and 60.4% of 212 colorectal adenomas, respectively. These cells were usually located in the lower third portion of the adenomatous glands[9]. In 8% to 10% of these cases, the density of the neuroendocrine cells may be higher than usual[9,10]. Therefore, it is not surprising that endocrine cell neoplasia may arise within adenomas and that it localizes preferentially at the base of the adenoma[2].
CIAM is distinct from mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN). MiNEN is an umbrella term referring to a neoplasm with both neuroendocrine and non-neuroendocrine components[4,12]. It is required that each component constitutes at minimum 30% of the neoplasm to qualify for MiNEN[12-14]. The terms “low grade” MiNEN and mixed adenoma well-differentiated neuroendocrine tumor (MANET) have been interchangeably used in the literature for a subset of CIAM meeting the required criterion of 30% for each component[4,12]. However, not all CIAMs described in the literature are necessarily low grade MiNEN. Moreover, recent WHO did not officially endorse a composite tumor consisting of an adenoma (a precursor of invasive adenocarcinoma) and well-differentiated neuroendocrine tumor as a subtype of MiNEN in the gastrointestinal tract and hepatopancreatobiliary organs[14].
Although this rare entity is not recognized by the current WHO classification, its recognition will allow for more efficient pathological diagnosis and more detailed clinicopathologic studies, thus leading to better patient care. CIAM may be under-recognized given its rarity and occasional morphologic subtlety. Moreover, it can resemble other benign and malignant lesions and can be mis-diagnosed. Its prognosis is vastly different from that of malignant composite tumors with expansile growth. We summarize the current state of knowledge on CIAM and provide an overview on its pathogenesis, microscopic features, differential diagnosis, as well as prognosis and treatment options. The differences in terminologies–CIAM, collision tumor and MiNEN–are also briefly discussed.
CIAM is identified in middle-aged to elderly patients, with a reported mean age of 60 years[1-4]. Slight male predilection has been reported[1,4,15], while another study found no gender predilection[3]. It is unknown whether there is a demographic divergence between CIAM and typical adenomatous polyps.
Recently we reported that the incidence of CIAM is 3.8% in surgically resected colorectal polyps. Our cohort consisted of consecutive, surgically resected 158 colorectal polyps from one tertiary care center over a span of 16 years[1]. Its incidence in endoscopically removed polyps is unknown.
To date, the largest series of colorectal CIAM has been reported by Kim et al[3] in South Korea, consisting of 24 cases. In their series, the polyps were excised endoscopically (91.7%) or surgically (8.3%) over a span of 7 years[3]. In the United States, the largest series of intestinal (to include 4 cases in the duodenum) CIAM was reported by Estrella et al[15] in a Cancer Center, consisting of 25 cases over a span of nearly 18 years[15]. However, the incidence of CIAM was not reported in these studies.
Colorectal MC is likely exceedingly rare and no minimum size criterion is currently available. MC has been observed in patients with chronic colitis, such as diversion colitis[16] and inflammatory bowel disease (IBD), especially in ulcerative colitis[17-21]. Likewise, Weyant et al[22] described a case of colonic MC and diffuse neuroendocrine cell hyperplasia following long-term cystoplasty[22]. These associations suggest that MC may represent an exaggerated proliferative response of gut mucosa to chronic inflammation.
On the other hand, it is largely unknown whether these patients with inflammatory conditions actually have a higher incidence of CIAM. Most reported CIAMs are sporadic, and it appears to be a much rarer condition than solitary MC[3]. Sigel and Goldblum[17] described a well differentiated neuroendocrine tumor adjacent to high grade glandular dysplasia in the setting of IBD. The authors postulated that the neuroendocrine tumor might have originated from multipotential dysplastic cells in the adjacent mucosa[17]. Alternatively, the MC component may reflect a metaplastic phenomenon related to chronic injury of the overlying adenomatous component[7].
Genetic predisposition may account for some cases of CIAM. Carcinoids at the base of duodenal adenomas have been reported in association with familial adenomatous polyposis (FAP)[15,23]. These observations support a role of the adenomatous polyposis coli (APC)/β-catenin pathway in the pathogenesis of CIAM (to be discussed below), although the risk of CIAM is probably explained by the risk of adenoma in this cohort.
The mechanism for the development of MC component in CIAM is not well understood. Earlier, authors postulated that CIAM represents a form of collision tumor wherein the two components arise from two different clones and they coincidentally occur adjacent to one another[8]. However, evolving knowledge regarding the multipotent stem cells in the gut and their role in tumorigenesis has shed light on the possible histogenesis of tumors with different histologic components such as CIAM. Indeed, in vitro studies of the ileal epithelial cells (IEC-18) of rat have shown that these cells can transform into differing cell types with one type showing neuroendocrine-like morphology and expressing serotonin receptor gene, and the other with adenoma-like mRNA transcription and protein expression[24].
Likewise, a morphologic “transition zone” has been observed in several studies of CIAM[2,4,6,25]. In Pulitzer et al[2]’s study, the MC appeared to arise directly from the basal epithelium of adenomatous crypts, penetrating the basement membrane and infiltrating the lamina propria[2]. La Rosa et al[4] also observed numerous cells with both morphologic and immunohistochemical neuroendocrine differentiation along the base of the adenomatous glands. In addition, these cells demonstrated the same mutational and microsatellite instability profile as the adenomatous components, further supporting the hypothesis that these two components most likely represent divergent differentiation of a common precursor[4]. Interestingly, unlike conventional adenomas without MC, no KRAS mutation was identified in either component of CIAM. These findings suggested that the adenoma component of CIAM may develop through an alternative KRAS-independent pathway[4].
The finding of CIAM in FAP patients suggests the involvement of the Wnt/β-catenin pathway in the tumorigenesis of CIAM, as expected based on the canonical pathway by which normal mucosa becomes adenomatous. The MC components of CIAMs frequently display strong and diffuse nuclear β-catenin reactivity by immunohistochemistry[1,2,7,15]. In Estrella et al[15] study, the level of nuclear β-catenin expression was higher in the MC component of CIAM when compared with either the sporadic neuroendocrine tumors without associated adenoma, or neuroendocrine carcinomas associated with adenoma. Moreover, there was no difference in the level of β-catenin expression between CIAM patients with and without FAP[15].
This plausible hypothesis, though, requires confirmation by additional molecular studies as neither the presence nor absence of nuclear β-catenin expression by immunohistochemistry appears to be a true reflection of an activated Wnt signaling pathway[15,26-29]. For example, Su et al[29] found that carcinoid tumors can show nuclear β-catenin immunohistochemical staining without mutations in the β-catenin and APC genes[29].
In summary, CIAM appears to represent a true composite tumor with a common origin for the MC and adenoma components, and is not a collision tumor. Further molecular studies are needed to better understand the mechanisms driving its tumorigenesis.
CIAMs have been reported in the stomach, duodenum, ileum, colon, and rectum[4]. They are predominantly found in the colon, usually in the cecum and right colon[1-3]. They present as polyps with well-defined margins, similar to conventional adenomatous polyps. The reported mean size of the polyps is 2.4 cm[3]. As the MC component is microscopic, it is incidentally found during the pathologic examination of otherwise typical adenomatous polyps.
To the best of our knowledge, no definite clinical symptoms related to the MC component of CIAM have been established, however, one case report of rectal “collision tumor” consisting of adenoma and carcinoid tumor presented with carcinoid syndrome (elevated serum serotonin and chromogranin A, elevated urine 5-hydroxyindoleacetic acid level, and moderate tricuspid regurgitation). The patient’s symptoms subsided following the endoscopic removal of the polyp with wide margins[30]. It is unclear whether this case represents a composite tumor (CIAM) or a collision tumor, as the author did not provide detailed histologic examination and classified the lesion as “collision” tumor[30].
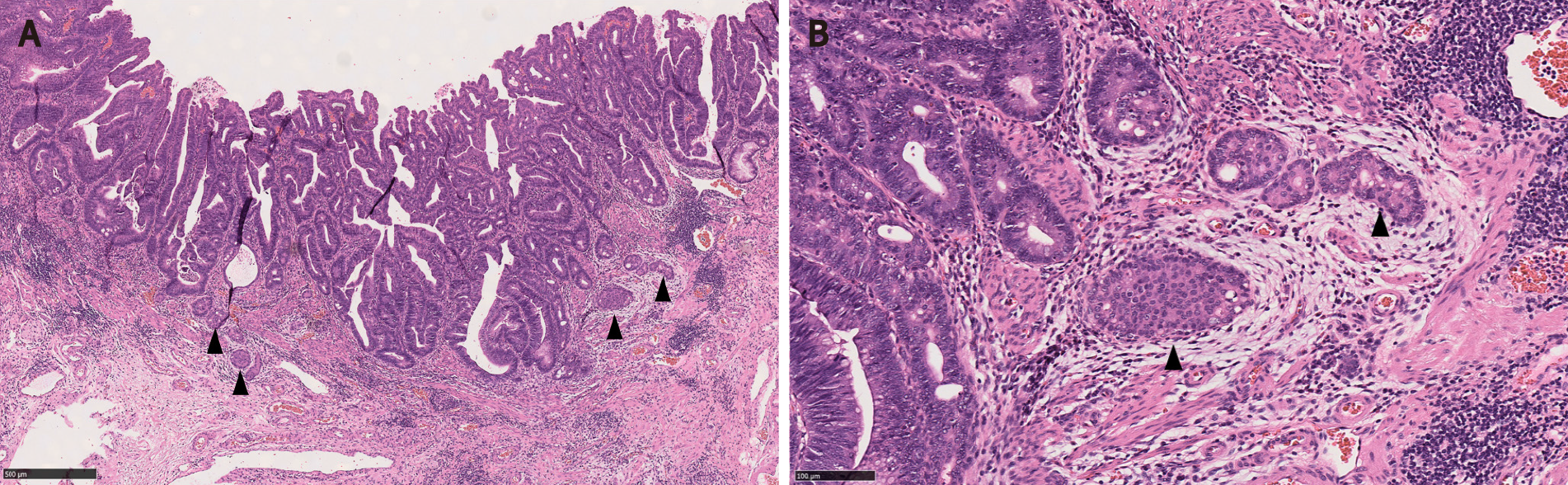
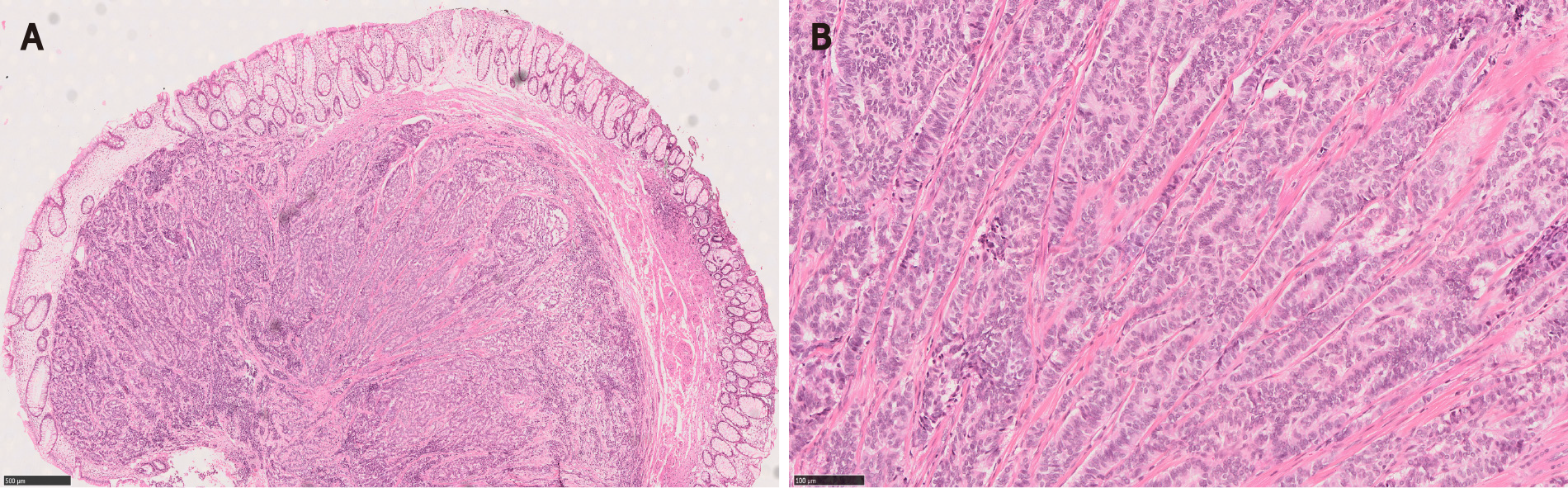
Adenomas with a MC component are usually high-risk adenomas (size ≥ 10 mm, villous components and/or high grade dysplasia)[1,3,5,7,15]. Therefore, the adenomatous components of CIAM tend to be large. For example, the mean size of polyps was 24 mm in Kim et al[3]’s study. In our study, the average size of the polyps was 42 mm (probably because our cohort consisted of surgically removed polyps that were deemed endoscopically unresectable), all of the adenomas showed villous components and 50% had high grade dysplasia (Figure 1). However, no statistically significant differences in terms of polyp size, polyp location (right vs left) or the frequency of associated high grade dysplasia between the adenomas with and without MC were found[1]. In contrast, in Kim et al[3]’s study where most of CIAMs were detected in endoscopically resected polyps, 86% of CIAMs had conventional adenoma with low grade dysplasia[3]. In Salaria et al[7]’s study, high grade glandular dysplasia was seen in 4 (36%) of 11 CIAMs[7].
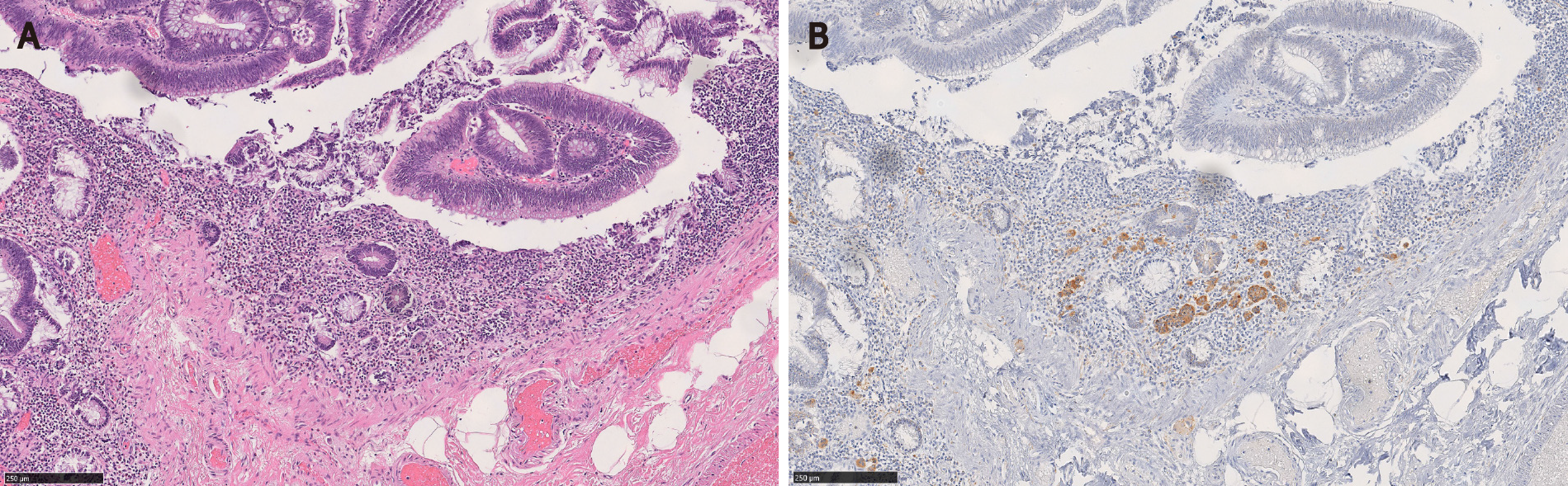
Microscopically, the MC component is found at the base of full-thickness adenomatous glands. The background lamina propria is myxoinflammatory with sometimes conspicuous eosinophils. The MC components are oftentimes connected to the overlying glandular components[3]. These small nests, irregular cords or clusters of neuroendocrine cells are sparsely distributed and do not form grossly evident nodules or masses (Figure 1). Occasional acinar structures may be seen[1-3,7].
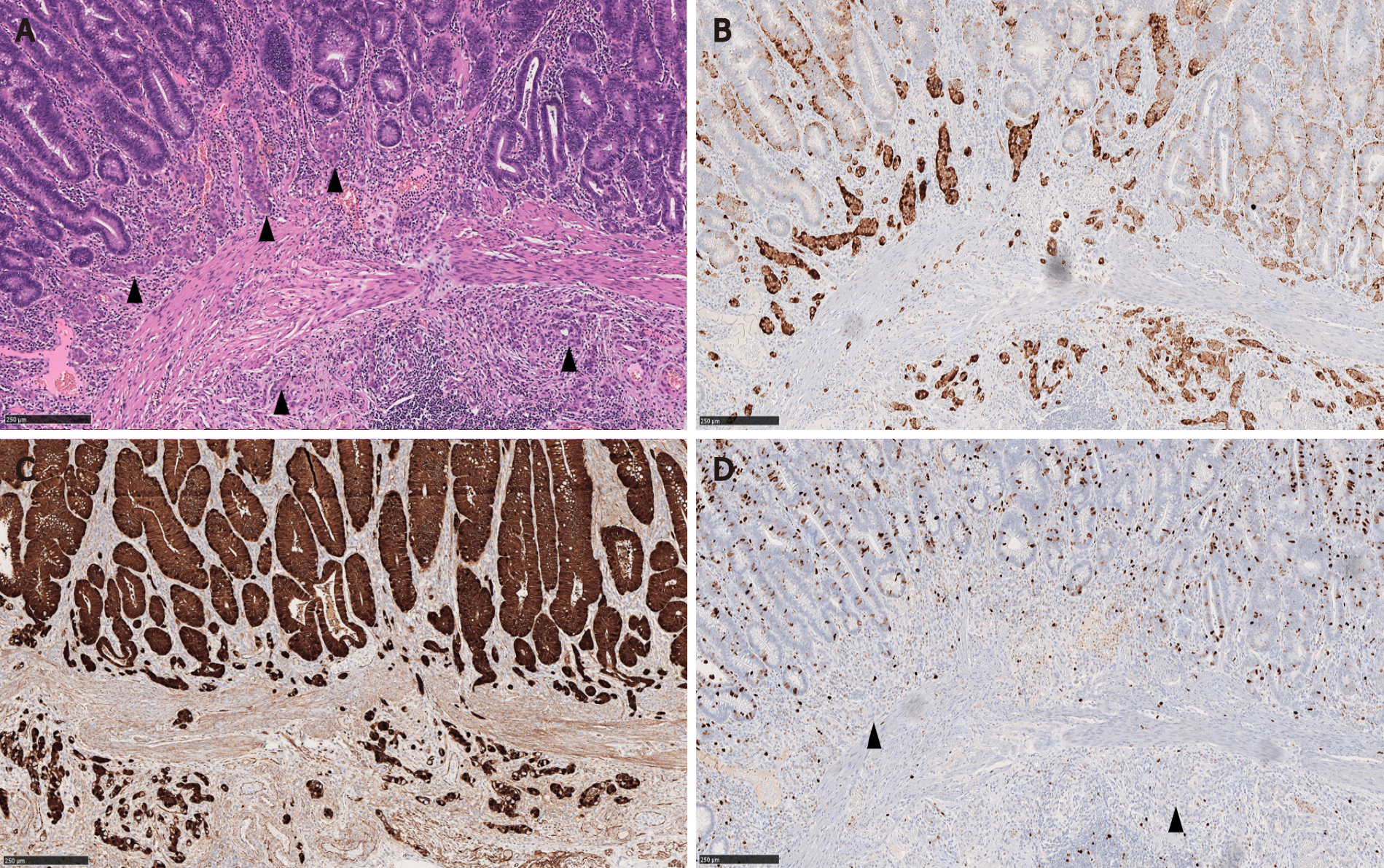
In Salaria et al[7]’s study, the MC component extended over an average length of 3.9 mm. Also 64% (7/11) of the MCs were multifocal[7]. In Kim et al[3]’s and La Rosa et al[4]’s studies, the mean size of the MC components was 4.7 mm and 3.2 mm, respectively[3,4]. In our study, MCs were distributed over a mean area of 5.8 mm and were multifocal in 83% of the cases. In a majority of CIAMs, the MC components are confined within the mucosa, though extension into the submucosa can be seen[1,4,15] (Figure 2).
Cytologically, the neuroendocrine cells constituting MC are bland and monotonous (Figure 1). The cells show scant to abundant granular or eosinophilic cytoplasm and round central nuclei with salt and pepper-pattern chromatin. They are devoid of nuclear atypia, hyperchromasia, nuclear pleomorphism, conspicuous mitotic activity, and apoptosis. In other words, they are typical well-differentiated neuroendocrine cells.
By immunohistochemistry, the MC components are positive for synaptophysin (Figure 2B), supporting their neuroendocrine differentiation[1,3,15]. Chromograinin-A and CD56 show variable staining[4,5]. Variable immunolabeling with squamous markers such as p63 and CK5/6 can be seen[1,7]. They are well-differentiated with a low Ki-67 proliferation index (usually < 1%-2%) (Figure 2D), although sometimes the total number of neuroendocrine cells in MC may be insufficient (< 500 cells in total) for reliable Ki-67 index measurement[1,3,7]. The MC component shows nuclear β-catenin positivity (Figure 2C) in 60% to 100% of the cases, suggesting the role of Wnt/β-catenin pathway in the CIAM tumorigenesis[1,7,15].
La Rosa et al[4] carried out mutational analysis for KRAS, BRAF, PIK3CA and microsatellite instability analysis on 6 CIAMs. No mutations were identified, and all cases were microsatellite stable in both adenoma and MC components[4].
MCs in CIAM may pose diagnostic challenge and may lead to misdiagnosis or overdiagnosis. MC can resemble squamous morules/metaplasia, invasive adenocarcinoma, squamous cell carcinoma (SCC), sporadic neuroendocrine tumor, and goblet cell adenocarcinoma (GCA). Awareness and recognition of this entity is crucial for accurate diagnosis and patient care.
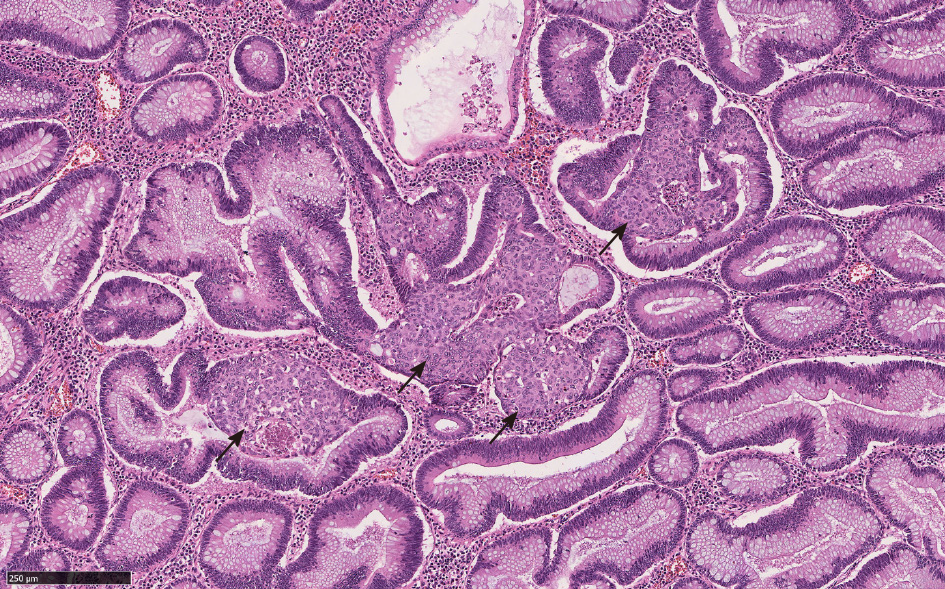
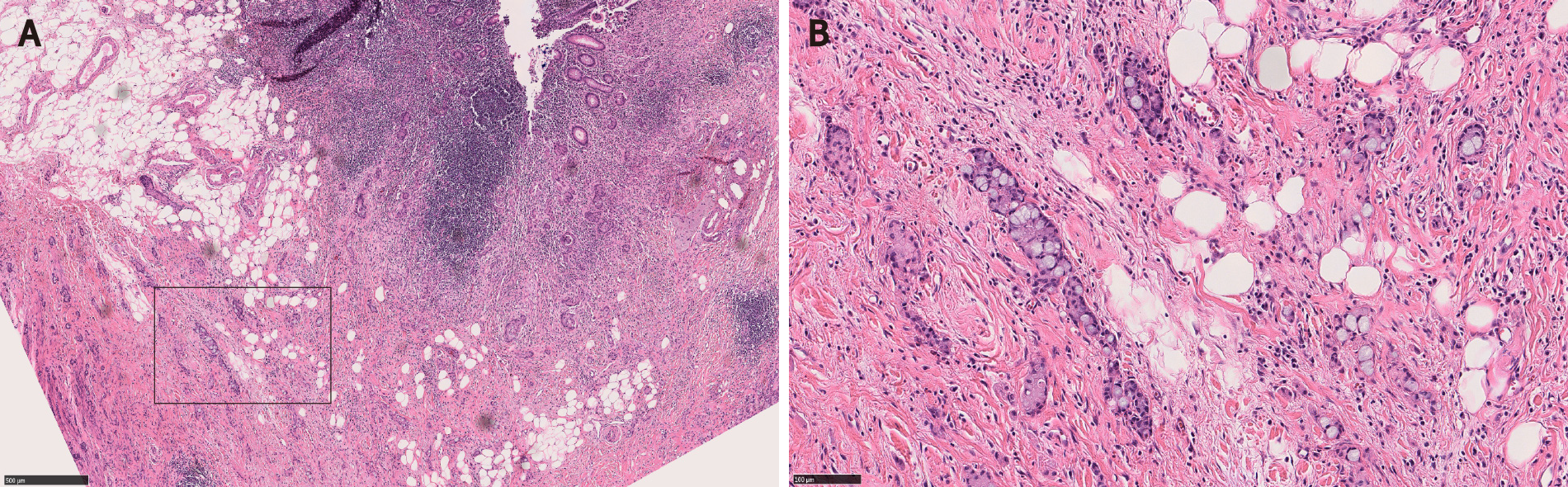
Squamous morules/metaplasia is an incidental histologic lesion that can be seen in colorectal adenomas[13,31]. The reported incidence of squamous morules in colonic adenoma is about 0.4%[11,32,33]. In our study, the incidence of squamous morules was 5.1% in surgically resected large colonic polyps[1].
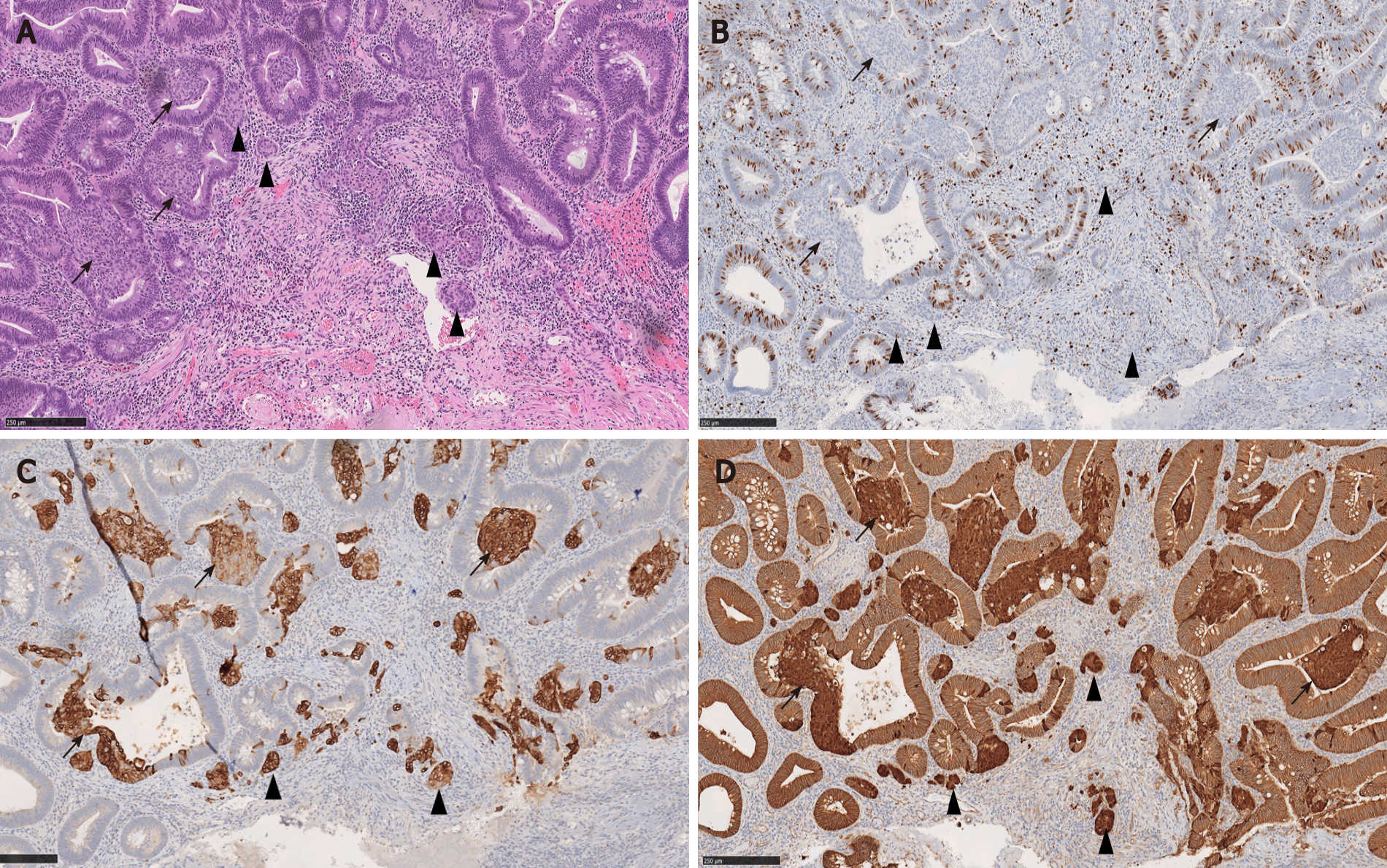
Microscopically, squamous morules are characterized by a proliferation of immature squamoid or spindled cells forming nests and nodules without definitive keratinization or intercellular bridges[11,13,32]. Usually the nests protrude into the lumen of adenomatous glands (Figure 3), or may be identified at the base of the polyps especially in the cases of torsion and prolapse[1,13,32]. Immunohistochemically, squamous morules are positive for pan cytokeratin, CK5/6, cyclin D1 and β-catenin (nuclear staining)[1,13,34,35] (Figure 4) and show variable staining for p63[15,32]. Focal synaptophysin and chromogranin positivity can be seen[32].
There can be significant histomorphologic overlap between the MC component of CIAM and squamous morules. Both can present as solid nests around the bottom of adenomatous glands or myxoinflammatory stroma[1,32]. Indeed, in Kim et al[3]'s study, 6 CIAM cases were initially diagnosed as adenoma with squamous morules/metaplasia[3]. In Pulitzer et al[2]'s study, one CIAM was originally interpreted as adenoma with focal squamous metaplasia owing to the presence of abundant eosinophilic cytoplasm in MC[2]. In Salaria et al[7]'s study, MC was initially interpreted as squamous morules in 5 of 10 CIAMs[7].
In addition, there is immunophenotypic resemblance between the MC component of CIAM and squamous morules. Squamous morules may show focal positivity for neuroendocrine markers such as synaptophysin and chromogranin[32]. Conversely, the MC components of CIAM are variably immunoreactive with p63 and/or CK5/6 (Figure 4), suggesting squamous differentiation. In Salaria et al[7]’s study, 2 of 6 MC were focally positive for p63, and 5 of 6 MC were positive for CK5/6[7].
Given the morphologic and immunohistochemical overlap between squamous morules and the MC component of CIAM, we hypothesized that these two entities may be related. Interestingly, 33.3% (2 of 6) of CIAM showed concurrent squamous morule (Figure 4), compared to 4.0% (6 of 152) of adenomas without MC in our cohort, suggesting shared pathogenesis between the two (P < 0.05)[1]. Similarly, Estrella et al[15] reported that 4 (16%) of 25 CIAMs had squamous metaplasia in the adjacent adenomatous component[15].
Nevertheless, given that squamous morules/metaplasia is benign and the MC of CIAM is likely indolent, misdiagnosing MC as squamous morules/metaplasia may not have a significant clinical impact. In fact, it may be nearly impossible to distinguish these two in some cases.
As stated above, 16 to 33% of CIAMs can co-exist with squamous morules/metaplasia[1,15]. Moreover, MC components can demonstrate squamous differentiation with variable p63 and/or CK5/6 immunoreactivity (Figure 4) in a myxoinflammatory background mimicking desmoplasia. Therefore, SCC is considered a differential consideration for MC component of CIAM.
Primary colorectal SCC is a rare malignancy with an incidence of 0.1%-0.25%[36]. To date, less than 100 cases of colorectal SCC have been reported in the literature[37].
Usually, SCC of colon presents late in the disease course and shows an aggressive behavior with early metastasis and poor overall survival[38,39]. Thus, it is important not to overdiagnose the MC of CIAM as SCC. It will be helpful to be aware that MC can show immunohistochemical squamous differentiation to avoid this misinterpretation.
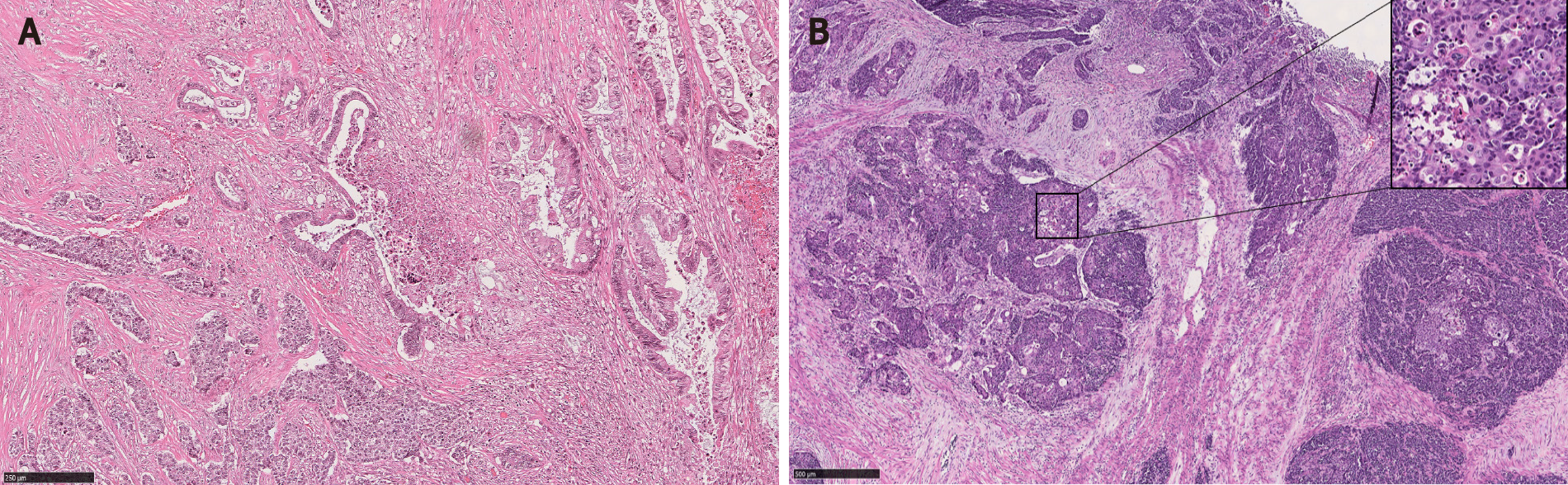
MC components of CIAM may be misdiagnosed as invasive adenocarcinoma or tumor budding. Possible and reasonable explanations for this are: First, MC may show infiltrative or single-cell patterns at the polyp base, mimicking invasive disease[2] (Figure 5). Second, the background myxoinflammatory lamina propria associated with MC may resemble the edema and fibroblastic proliferation of desmoplasia that is usually associated with invasive disease[5,7]. Third, MC is commonly found at the base of full-thickness adenomatous mucosa frequently with high grade glandular dysplasia[1,5]. In fact, one of the CIAM cases reported by Lin et al[5] had been initially misinterpreted as adenocarcinoma[5].
Awareness of this entity and the recognition of bland cytoarchitecture and negligible mitotic activity of MC will be helpful to avoid misclassification[2]. Confirming neuroendocrine differentiation can be a useful diagnostic tool in challenging cases[7] (Figure 5).
CIAMs and sporadic neuroendocrine tumors are treated differently. The MC components in CIAMs are usually situated at the polyp base in the mucosa, therefore complete polypectomy may suffice to remove the MC component with negative margin. On the other hand, the usual epicenter of sporadic neuroendocrine tumors is the submucosa. Therefore, additional surgery may be required to achieve complete resection with negative margin when the initial endoscopic biopsy shows sporadic neuroendocrine tumor[3].
For example, sporadic rectal neuroendocrine tumors are relatively common and oftentimes present as nodules or polyps on endoscopy[14,40-43]. They are usually small (over 50% of the cases < 1.0 cm in diameter), low grade, and located in the mucosa or submucosa[14] (Figure 6). Moreover, 79% to 84% of rectal neuroendocrine tumors are L-cell type that is known to be associated with rather indolent biologic behavior[44,45]. Therefore, rectal neuroendocrine tumors have an excellent overall prognosis especially after an endoscopic resection[41,42,45]. However, tumor stage and grade are still important prognosticators[41,43,46]. Large tumor size [(≥ 1.0 cm), high grade (WHO grade 2 to 3)], and the presence of muscular and lymphovascular invasion are often associated with metastatic disease, requiring aggressive treatment[43].
Nevertheless, MCs of CIAMs may also invade the submucosa[1,4,5,15]. Thus, to ensure complete removal of the MC component, further surgery may still be required following polypectomy[47]. Therefore, from a management standpoint, the tumor size and depth appear to be more relevant than their classifications.
Few studies have explored the biological differences between the MC components in CIAMs and sporadic intestinal carcinoid tumors without associated adenomatous components. Estrella et al[15] observed significantly higher β catenin expression score in CIAMs compared with sporadic neuroendocrine tumors, suggesting that CIAM may develop via a distinct pathway from the latter (i.e., the adenoma pathway). In this study the overall 3- and 5-year survival of CIAM patients was significantly lower than those with sporadic NET[15]. This likely is due to the co-existing adenoma in CIAM as no CIAM patients died of neuroendocrine tumor in this study.
GCA, previously known as goblet cell carcinoid, adenocarcinoid, crypt cell carcinoma and microglandular carcinoma, is a subtype of appendiceal neoplasm. GCA is a mixed tumor with both glandular and neuroendocrine elements, and contains goblet cells (Figure 7). The tumor nests stain positively for neuroendocrine markers and mucin[14]. Despite its mixed phenotype, GCA is officially recognized as a subtype of adenocarcinoma in the current WHO given its aggressive biologic behavior that is akin to adenocarcinoma[14,48]. GCA may co-exist with adjacent cecal adenoma[49]. Therefore, it is possible that cecal adenoma with underlying GCA may be interpreted as CIAM. Indeed, based on the provided illustrations, some authors raised a possibility that one of Lin et al[5]’s CIAM cases with lymph node metastasis may represent GCA with overlying adenoma[3,50]. GCA is an aggressive tumor and often presents with metastatic disease[51-53]. Further surgical management and chemotherapy are commonly required[53].
Composite tumor, such as CIAM, is considered pathogenetically distinct from collision tumor. MiNEN is a broader category than CIAM.
Lewin[54] first proposed to separate composite tumor and collision tumor when neoplastic endocrine cells and nonendocrine epithelial cells are admixed. In a composite tumor, glandular and neuroendocrine components are intermingled, and both components may share common origin. Whereas in a collision tumor, the two elements “collide” but are pathogenetically independent of each other. One of the two elements may represent a metastasis from another primary site[14,54].
Recently, Schizas et al[55] carried out a literature review on collision tumors of the digestive system. In this review, the authors defined collision tumors as those consisting of two or more independent neoplasms without intermingling (thus without transition zone). In colon, adenocarcinoma was the main component of collision tumors, found in 78.6% of the cases, followed by carcinoid, seen in 35.7%[55]. Collision tumors are often high grade with early metastasis and a shorter survival[56-58].
Traditionally, collision tumors have been believed to represent “double primaries” though a few studies challenged this concept[56,58,59]. For example, Minaya-Bravo et al[58] reported a case of colonic collision tumor consisting of adenocarcinoma and large cell neuroendocrine carcinoma without identifiable transition zone. Three years later, the tumor metastasized to the retroperitoneum. Interestingly, both components metastasized, suggesting that both components of this collision tumor may have originated from the same clone[58]. Similarly, Pecorella et al[56] reported a cecal collision tumor consisting of adenocarcinoma and high grade well-differentiated neuroendocrine tumor (reported Ki67 proliferation index was 36%). There was focal positivity for CEA in the neuroendocrine tumor component without clear transition zone between the two components. The authors concluded that some mixed tumors cannot be precisely classified.
MiNEN is a recently introduced umbrella terminology referring to a neoplasm demonstrating a mixture of neuroendocrine and non-neuroendocrine components[4,12,14]. The terms “low grade” MiNEN and MANET have been proposed to describe mixed tumors with adenomatous components and well-differentiated neuroendocrine tumors (to include WHO grades 1 to 3)[4,12]. However, neither low grade MiNEN nor MANET has been officially recognized as a subtype of MiNEN in the current WHO[14]. In fact, in the gastrointestinal tract and hepatopancreatobiliary organs, WHO limits the use of the MiNEN term only to the mixed tumors with malignant non-neuroendocrine components[14] (Figure 8).
Even if low grade MiNEN (MANET) were to be recognized by WHO, there are differences between CIAM and low grade MiNEN. In MiNEN, each component should represent at least 30% of the total volume of the neoplasm. Therefore, some CIAMs with minor MC components would not meet the 30% cutoff criterion for low grade MiNEN. As many studies on CIAM did not specify the amount of MC components relative to the tumor volume, it is difficult to assess how many of the reported CIAM cases had MC components that occupied over 30% of the total tumor volume[2,5,7]. In our study, all 6 CIAM cases had minor MC components constituting much less than 30% of the tumor volume[1]. In addition, most of the MC components in CIAM are low grade with a negligible ki67 proliferation index, whereas low grade MiNEN can have grade 2 and 3 levels of proliferation in the neuroendocrine components[4]. Typical MiNEN with malignant non-neuroendocrine component mixed with neuroendocrine carcinoma is an aggressive neoplasm with a median overall survival of 13.2 mo. The ki67 proliferation index of the neuroendocrine component may drive the prognosis of these tumors[60].
CIAM is an indolent disease with a favorable outcome. One study found that after mean follow-up of 6 (range 0.5 to 27) years, none of the patients had recurrence of CIAM or metastasis after endoscopic or surgical treatment[4,15]. In our study, after mean follow-up of 53 mo, all patients were free of CIAM. In addition, all the lymph nodes retrieved during the surgical resection were devoid of adenocarcinoma or neuroendocrine tumor. Our two patients with MC components extending into the submucosa were followed for 14 and 15 mo, respectively. There was no evidence of recurrence or metastasis of neuroendocrine tumor at the end of the follow-up[1]. In La Rosa et al[4]’s study, one CIAM case had MC in the submucosa. The patient was followed for 12 years without evidence of disease[4]. No tumor-related death has been reported in the literature.
The size of MC component appears to have no bearing on the outcome[3]. This is likely due to the fact that the MC component tends to be small, and is usually confined in the mucosa. Likewise, the lesional cells constituting MC are bland with low proliferative activity.
Given its indolent course, complete removal of both adenoma and MC by polypectomy is considered curative[4]. Additional radical surgeries should be reserved for cases with adverse histologic features such as deep submucosal extension or increased proliferative activity of the MC component[3].
CIAM is a rare intestinal lesion consisting of a conventional adenoma and a well differentiated MC component at its base. CIAM is considered to represent a true composite tumor wherein both adenoma and MC appear to share a common origin and develop via the Wnt/β-catenin pathway. MC in CIAM poses diagnostic challenges with its morphologic resemblance to other benign and malignant lesions. CIAM is an indolent lesion with a favorable outcome. Complete removal of both adenoma and MC by polypectomy is considered curative. Raising awareness of this rare entity will lead to correct diagnosis and appropriate management.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yeniova A S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Fu Z, Saade R, Koo BH, Jennings TA, Lee H. Incidence of composite intestinal adenoma-microcarcinoid in 158 surgically resected polyps and its association with squamous morule. Ann Diagn Pathol. 2019;42:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Pulitzer M, Xu R, Suriawinata AA, Waye JD, Harpaz N. Microcarcinoids in large intestinal adenomas. Am J Surg Pathol. 2006;30:1531-1536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Kim MJ, Lee EJ, Kim DS, Lee DH, Youk EG, Kim HJ. Composite intestinal adenoma-microcarcinoid in the colon and rectum: a case series and historical review. Diagn Pathol. 2017;12:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | La Rosa S, Uccella S, Molinari F, Savio A, Mete O, Vanoli A, Maragliano R, Frattini M, Mazzucchelli L, Sessa F, Bongiovanni M. Mixed Adenoma Well-differentiated Neuroendocrine Tumor (MANET) of the Digestive System: An Indolent Subtype of Mixed Neuroendocrine-NonNeuroendocrine Neoplasm (MiNEN). Am J Surg Pathol. 2018;42:1503-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Lin J, Goldblum JR, Bennett AE, Bronner MP, Liu X. Composite intestinal adenoma-microcarcinoid. Am J Surg Pathol. 2012;36:292-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Moyana TN, Qizilbash AH, Murphy F. Composite glandular-carcinoid tumors of the colon and rectum. Report of two cases. Am J Surg Pathol. 1988;12:607-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Salaria SN, Abu Alfa AK, Alsaigh NY, Montgomery E, Arnold CA. Composite intestinal adenoma-microcarcinoid clues to diagnosing an under-recognised mimic of invasive adenocarcinoma. J Clin Pathol. 2013;66:302-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Lyda MH, Fenoglio-Preiser CM. Adenoma-carcinoid tumors of the colon. Arch Pathol Lab Med. 1998;122:262-265. [PubMed] [Cited in This Article: ] |
| 9. | Iwashita A, Watanabe H, Enjoji M. Argyrophil and argentaffin cells in adenomas of the colon and rectum. Fukuoka Igaku Zasshi. 1989;80:114-124. [PubMed] [Cited in This Article: ] |
| 10. | Van den Ingh HF, Van den Broek LJ, Verhofstad AA, Bosman FT. Neuroendocrine cells in colorectal adenomas. J Pathol. 1986;148:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Bansal M, Fenoglio CM, Robboy SJ, King DW. Are metaplasias in colorectal adenomas truly metaplasias? Am J Pathol. 1984;115:253-265. [PubMed] [Cited in This Article: ] |
| 12. | de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology. 2017;105:412-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Dabir PD, van der Post RS, Nagtegaal ID. Incidental morphological findings in colorectal adenomas. Histopathology. 2021;78:348-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | WHO Classification of Tumours Editorial Board Digestive system tumours. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumours series, 5th ed.; vol 1). [cited 10 March 2021]. Available from: http://publications.oarc.fr/579. [Cited in This Article: ] |
| 15. | Estrella JS, Taggart MW, Rashid A, Abraham SC. Low-grade neuroendocrine tumors arising in intestinal adenomas: evidence for alterations in the adenomatous polyposis coli/β-catenin pathway. Hum Pathol. 2014;45:2051-2058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Griffiths AP, Dixon MF. Microcarcinoids and diversion colitis in a colon defunctioned for 18 years. Report of a case. Dis Colon Rectum. 1992;35:685-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Sigel JE, Goldblum JR. Neuroendocrine neoplasms arising in inflammatory bowel disease: a report of 14 cases. Mod Pathol. 1998;11:537-542. [PubMed] [Cited in This Article: ] |
| 18. | Matsumoto T, Jo Y, Mibu R, Hirahashi M, Yao T, Iida M. Multiple microcarcinoids in a patient with long standing ulcerative colitis. J Clin Pathol. 2003;56:963-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Nascimbeni R, Villanacci V, Di Fabio F, Gavazzi E, Fellegara G, Rindi G. Solitary microcarcinoid of the rectal stump in ulcerative colitis. Neuroendocrinology. 2005;81:400-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Etienne D, Ofosu A, Ona MA, Reddy M. Microcarcinoid and Ulcerative Colitis: Case Report and Literature Review. Cureus. 2020;12:e8803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Stewart CJ, Matsumoto T, Jo Y, Mibu R, Hirahashi M, Yao T, Iida M. Multifocal microcarcinoid tumours in ulcerative colitis. J Clin Pathol. 2005;58:111-2; author reply 1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Weyant GW, Karamchandani DM, Rassaei N. Colorectal microcarcinoids in association with long-term exposure to urinary content: a case report and review of the literature. Case Rep Pathol. 2015;2015:806310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | July LV, Northcott KA, Yoshida EM, Carr DM, Owen DA. Coexisting carcinoid tumors in familial adenomatous polyposis-associated upper intestinal adenomas. Am J Gastroenterol. 1999;94:1091-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Gordon PV, Paxton JB, Fox NS. A methodology for distinguishing divergent cell fates within a common progenitor population: adenoma- and neuroendocrine-like cells are confounders of rat ileal epithelial cell (IEC-18) culture. BMC Cell Biol. 2005;6:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Bazerbachi F, Kermanshahi TR, Monteiro C. Early precursor of mixed endocrine-exocrine tumors of the gastrointestinal tract: histologic and molecular correlations. Ochsner J. 2015;15:97-101. [PubMed] [Cited in This Article: ] |
| 26. | Fodde R, Tomlinson I. Nuclear beta-catenin expression and Wnt signalling: in defence of the dogma. J Pathol. 2010;221:239-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Brabletz T, Jung A, Hermann K, Günther K, Hohenberger W, Kirchner T. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract. 1998;194:701-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 603] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 29. | Su MC, Wang CC, Chen CC, Hu RH, Wang TH, Kao HL, Jeng YM, Yuan RH. Nuclear translocation of beta-catenin protein but absence of beta-catenin and APC mutation in gastrointestinal carcinoid tumor. Ann Surg Oncol. 2006;13:1604-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Hui CK. Collision adenoma-carcinoid tumour of the colon complicated by carcinoid syndrome. Singapore Med J. 2012;53:e195-e197. [PubMed] [Cited in This Article: ] |
| 31. | Chen KT. Colonic adenomatous polyp with focal squamous metaplasia. Hum Pathol. 1981;12:848-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Lee HE, Chandan VS, Lee CT, Wu TT. Squamoid morules in the pseudoinvasive foci of colonic polyp morphologically mimic invasive carcinoma. Hum Pathol. 2017;68:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Nirodi NS, Orr KW. Adenomatous polyp of the sigmoid colon with focal squamous metaplasia. J R Coll Surg Edinb. 1986;31:379-381. [Cited in This Article: ] |
| 34. | Pantanowitz L. Colonic adenoma with squamous metaplasia. Int J Surg Pathol. 2009;17:340-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Mochizuki K, Kondo T, Oishi N, Tahara I, Inoue T, Kasai K, Nakazawa T, Katoh R. Squamous morula formation in colorectal adenoma: Immunohistochemical and molecular analyses. Pathol Res Pract. 2015;211:797-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Vyas N, Ahmad S, Bhuiyan K, Catalano C, Alkhawam H, Sogomonian R, Nguyen J, Walfish A, Aron J. Primary squamous cell carcinoma of the rectum: a case report and literature review. J Community Hosp Intern Med Perspect. 2016;6:31708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Elkbuli A, Dowd B, McKenney M, Boneva D. Mixed neuroendocrine and squamous cell carcinoma of the colon: A case report and literature review. Int J Surg Case Rep. 2019;60:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Fahim F, Al-Salamah SM, Alam MK, Al-Akeely MH. Squamous cell carcinoma of colon and rectum. Saudi Med J. 2006;27:874-877. [PubMed] [Cited in This Article: ] |
| 39. | Shi JX, Sun Y, Gao P, Song YX, Sun JX, Chen XW, Yu DH, Lv XE, Zhou X, Wang ZN. Basic characteristics and therapy regimens for colorectal squamous cell carcinoma. Transla Cancer Resea. 2018;7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Rakici H, Akdogan RA, Yurdakul C, Canturk N. A case of rectal neuroendocrine tumor presenting as polyp. Int J Surg Case Rep. 2015;8C:59-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Rajca BP, Wagh MS. Dilemmas in Endoscopic Management of Rectal Neuroendocrine Tumors: A Case-Based Discussion. Gastroenterol Res Pract. 2015;2015:539861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Cha JH, Jung DH, Kim JH, Youn YH, Park H, Park JJ, Um YJ, Park SJ, Cheon JH, Kim TI, Kim WH, Lee HJ. Long-term outcomes according to additional treatments after endoscopic resection for rectal small neuroendocrine tumors. Sci Rep. 2019;9:4911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Fine C, Roquin G, Terrebonne E, Lecomte T, Coriat R, Do Cao C, de Mestier L, Coffin E, Cadiot G, Nicolli P, Lepiliez V, Hautefeuille V, Ramos J, Girot P, Dominguez S, Céphise FV, Forestier J, Hervieu V, Pioche M, Walter T. Endoscopic management of 345 small rectal neuroendocrine tumours: A national study from the French group of endocrine tumours (GTE). United European Gastroenterol J. 2019;7:1102-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Gastrointestinal Pathology Study Group of Korean Society of Pathologists; Sohn JH, Cho MY, Park Y, Kim H, Kim WH, Kim JM, Jung ES, Kim KM, Lee JH, Chan HK, Park DY, Joo M, Kim S, Moon WS, Kang MS, Jin SY, Kang YK, Yoon SO, Han H, Choi E. Prognostic Significance of Defining L-Cell Type on the Biologic Behavior of Rectal Neuroendocrine Tumors in Relation with Pathological Parameters. Cancer Res Treat. 2015;47:813-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Fu Z, Zuo C, Sheehan CE, Patil DT, Lin J, Yang Z, Lee H. Novel Finding of Paired Box 5 (PAX5) Cytoplasmic Staining in Well-differentiated Rectal Neuroendocrine Tumors (Carcinoids) and Its Diagnostic and Potentially Prognostic Utility. Appl Immunohistochem Mol Morphol. 2019;27:454-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Ito M, Hirano Y, Isii T, Kondo H, Wang L, Asari M, Obara N, Yamaguchi S. Grade 3 well-differentiated neuroendocrine tumor of the rectum: a case report. Surg Case Rep. 2020;6:130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Kuang-I F, Sano Y, Kon H, Ikematsu H, Kaji Y, Fujimori T. A composite adenoma and carcinoid tumor in a single rectal polyp. Endoscopy. 2006;38 Suppl 2:E62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Yozu M, Johncilla ME, Srivastava A, Ryan DP, Cusack JC, Doyle L, Setia N, Yang M, Lauwers GY, Odze RD, Misdraji J. Histologic and Outcome Study Supports Reclassifying Appendiceal Goblet Cell Carcinoids as Goblet Cell Adenocarcinomas, and Grading and Staging Similarly to Colonic Adenocarcinomas. Am J Surg Pathol. 2018;42:898-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Özemir İA, Baysal H, Zemheri E, Bilgiç Ç, Yiğitbaşı R, Alimoğlu O. Goblet cell carcinoid of the appendix accompanied by adenomatous polyp with high-grade dysplasia at the cecum. Turk J Surg. 2018;34:234-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Tamura H, Ando H, Doi R, Adachi S. Combined Intestinal Adenoma/Microcarcinoids. Case Rep Gastroenterol. 2019;13:410-417. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Clift AK, Kornasiewicz O, Drymousis P, Faiz O, Wasan HS, Kinross JM, Cecil T, Frilling A. Goblet cell carcinomas of the appendix: rare but aggressive neoplasms with challenging management. Endocr Connect. 2018;7:268-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Hennessy MM, Ivanovski I. Appendiceal adenocarcinoma-Two unique cases of adenocarcinoma ex-goblet cell carcinoid. Clin Case Rep. 2019;7:806-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Gilmore G, Jensen K, Saligram S, Sachdev TP, Arekapudi SR. Goblet cell carcinoid of the appendix - diagnostic challenges and treatment updates: a case report and review of the literature. J Med Case Rep. 2018;12:275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11 Suppl 1:71-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 168] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Schizas D, Katsaros I, Michalinos A, Damaskos C, Garmpis N, Ntomi V, Agrogiannis G, Stergiopoulos S, Tsaroucha AK. Collision Tumors of the Gastrointestinal Tract: A Systematic Review of the Literature. Anticancer Res. 2018;38:6047-6057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Pecorella I, Memeo L, Ciardi A, Rotterdam H. An unusual case of colonic mixed adenoendocrine carcinoma: collision vs composite tumor. A case report and review of the literature. Ann Diagn Pathol. 2007;11:285-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Eze O, Harari S, Cho M, Neto AG. Colonic polyp presenting as a tubulovillous adenoma and harbinger of high-grade neuroendocrine carcinoma: a unique presentation. Case Rep Clin Path. 2015;. [DOI] [Cited in This Article: ] |
| 58. | Minaya-Bravo AM, Garcia Mahillo JC, Mendoza Moreno F, Noguelares Fraguas F, Granell J. Large cell neuroendocrine - Adenocarcinona mixed tumour of colon: Collision tumour with peculiar behaviour. What do we know about these tumours? Ann Med Surg (Lond). 2015;4:399-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Li Y, Yau A, Schaeffer D, Magliocco A, Gui X, Urbanski S, Waghray R, Owen D, Gao ZH. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am J Surg Pathol. 2011;35:413-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, Vanoli A, Tagliabue G, Pisa E, Messerini L, Centonze G, Inzani F, Scarpa A, Papotti M, Volante M, Sessa F, Fazio N, Pruneri G, Rindi G, Solcia E, La Rosa S, Capella C. Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer. 2018;25:583-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |