Published online Nov 26, 2021. doi: 10.4252/wjsc.v13.i11.1797
Peer-review started: July 19, 2021
First decision: August 15, 2021
Revised: August 28, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 26, 2021
Human spermatogonial stem cells (SSCs) are the basis of spermatogenesis. However, little is known about the developmental regulatory mechanisms of SSC due to sample origin and species differences.
To investigates the mechanisms involved in the proliferation of human SSC.
The expression of mitogen-activated protein kinase kinase 7 (MKK7) in human testis was identified using immunohistochemistry and western blotting (WB). MKK7 was knocked down using small interfering RNA, and cell proliferation and apoptosis were detected by WB, EdU, cell counting kit-8 and fluorescence-activated cell sorting. After bioinformatic analysis, the interaction of MKK7 with c-Jun N-terminal kinases ( JNKs ) was verified by protein co-immunoprecipitation and WB. The phosphorylation of JNKs was inhibited by SP600125, and the phenotypic changes were detected by WB, cell counting kit-8 and fluorescence-activated cell sorting.
MKK7 is mainly expressed in human SSCs, and MKK7 knockdown inhibits SSC proliferation and promotes their apoptosis. MKK7 mediated the phosphorylation of JNKs, and after inhibiting the phosphorylation of JNKs, the phenotypic changes of the cells were similar to those after MKK7 downregulation. The expression of MKK7 was significantly downregulated in patients with abnormal spermatogenesis, suggesting that abnormal MKK7 may be associated with spermatogenesis impairment.
MKK7 regulates the proliferation and apoptosis of human SSC by mediating the phosphorylation of JNKs.
Core Tip: This study demonstrated that mitogen-activated protein kinase kinase 7 regulates the proliferation and apoptosis of human spermatogonial stem cells by mediating the phosphorylation of c-Jun N-terminal kinases in vitro. In addition, we found that mitogen-activated protein kinase kinase 7 was significantly downregulated in patients with reproductive disorders, suggesting that mitogen-activated protein kinase kinase 7 may be associated with human spermatogenesis.
- Citation: Huang ZH, Huang C, Ji XR, Zhou WJ, Luo XF, Liu Q, Tang YL, Gong F, Zhu WB. MKK7-mediated phosphorylation of JNKs regulates the proliferation and apoptosis of human spermatogonial stem cells. World J Stem Cells 2021; 13(11): 1797-1812
- URL: https://www.wjgnet.com/1948-0210/full/v13/i11/1797.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i11.1797
Infertility is an important global health problem that affects approximately 15% of couples of childbearing age[1]. For about 50% of such couples, male factors are a contributing cause[2]. There are many possible types of male subfertility, including idiopathic, acquired and congenital[3]. Although most male infertility problems can be solved through assisted reproductive technology, there are still some patients who are unable to have genetic offspring, especially those with non-obstructive azoospermia (NOA)[4]. Thus, it is necessary to find other effective treatments, such as spermatogonial stem cell (SSC) transplantation and in vitro spermatogenesis.
SSCs are present on the basement membrane of the seminiferous tubules of the testis, and these cells self-renew and differentiate for life-long spermatogenesis[5]. Intricate molecular and cellular interactions form the niche for SSC development[6]. SSC self-renewal is promoted by the secretion of glial cell line-derived neurotrophic factor (GDNF) from the niche[7]. In vitro, GDNF and fibroblast growth factor 2 (FGF2) were observed to function synergistically to promote the growth of undifferentiated spermatogonia[8]. However, the function of basic FGF in SSC maintenance in vivo remains poorly defined[9]. Common molecules are activated by GDNF and FGF2 through Src family kinases, resulting in Ras activation[10]. Glucocorticoid-induced leucine zipper protein antagonizes Ras and stimulates the protein kinase B and MAPK/ERK kinase pathways[11]. When active protein kinase B or mitogen-activated protein kinase kinase 1 (a downstream molecule of the MAPK/ERK kinase pathway) were overexpressed, SSCs transfected with protein kinase B or mitogen-activated protein kinase kinase 1 could proliferate only in the presence of FGF2 or GDNF[12,13], respectively. Maintenance of the self-renewing state requires these pathways to upregulate certain genes, including ETS variant transcription factor 5, LIM homeobox 1, BCL6B transcription repressor and Nanos C2HC-type zinc finger 2[14].
Although studies have revealed a number of regulators of SSC self-renewal in mice, the regulatory mechanisms of SSCs are significantly different in humans and rodents[15]; these results cannot be repeated in human SSCs. In mouse, stemness occurs within the single spermatogonia; by contrast, the chains and pairs of spermatogonia are believed to represent committed progenitors[16,17]. In humans, Adark spermatogonia are regarded as the actual stem cells and slowly produce Apale spermatogonia, which have enough self-renewal capacity to maintain the stem cell pool[18-20]. Moreover, in mouse, the seminiferous epithelium cycle usually comprises 12 stages; however, only 6 stages have been distinguished in humans[21,22]. Notably, mouse and human SSCs have some common biomarkers; however, others differ considerably. For example, octamer-binding protein 4, a marker of mouse SSCs, is absent in humans[23,24]. Likewise, GDNF family receptor alpha 1 (GFRA1) positive spermatogonia are rare in mouse seminiferous tubules but abundant in humans[15].
Mitogen-activated protein kinase kinase 7 (MKK7) is an activator of c-Jun N-terminal kinase (JNK). MKK7 directly activates JNK1 and JNK2 (stressactivated protein kinases) via phosphorylation[25-27]. JNK signaling has an important function in self-renewal, apoptosis and cell proliferation and was reported to be associated with reactive oxygen species-mediated mouse SSC self-renewal[28]. In the present study, we found that MKK7 was mainly expressed in human SSCs [GFRA1+/proliferating cell nuclear antigen (PCNA)+/KIT proto-oncogene, receptor tyrosine kinase (KIT)-] with proliferative activity. Deletion of MKK7 promoted human SSC apoptosis and impaired their proliferation. We further confirmed that MKK7 regulates SSC proliferation by inducing JNK1/JNK2/JNK3 phosphorylation. Additionally, MKK7 levels were confirmed to be decreased in patients with NOA compared with those in patients with obstructive azoospermia with normal spermatogenesis, particularly in patients with spermatogonial or spermatocyte maturation arrest. Our results reveal the molecular mechanisms responsible for human SSC proliferation and apoptosis and provide the basis for further research into the etiology, molecular diagnosis and therapy for male infertility.
The Ethics Committee of the Reproductive & Genetic Hospital of CITICXiangya, Basic Medical Science School, Central South University approved and supervised the present study (approval No.: LL-SC-2020-028). The samples used in the experiments were provided by donors who supplied informed consent. All testis samples were derived from patients undergoing microdissection testicular sperm extraction, and patients with spermatogenic failure because of known hereditary factors, such as Klinefelter syndrome and Y chromosome microdeletions, were excluded. We collected a total of 16 testicular biopsies weighing 30-50 mg and classified them according to the results of hematoxylin-eosin staining, including normal, spermatogenic failure and Sertoli cell only syndrome. Testis tissue samples were rinsed thrice in Dulbecco’s modified Eagle’s medium (DMEM) comprising 10 mL/L streptomycin and penicillin and then fixed with 40 g/L paraformaldehyde or stored in liquid nitrogen.
The method described by Hou et al[29] was used to establish a human SSC line, which was a gift from He ZP, Hunan Normal University, Changsha. Human SSCs were cultured in DMEM/F12 (Gibco, Grand Island, NY, United States) containing 100 mL/L fetal bovine serum (Gibco) and 100 unit/mL streptomycin and penicillin (Invitrogen, Carlsbad, CA, United States) at 34 °C in a 50 mL/L CO2 incubator. Every 4 d, the cells were passaged using 0.53 mmol/L EDTA with 0.5 g/L trypsin (Invitrogen).
RNAiso Plus reagent (Takara, Kusatsu, Japan) was used to extract total RNA from cells following the supplier’s protocol. Total RNA quality and concentration were measured using a Nanodrop instrument (Thermo Scientific, Waltham, MA, United States). The total RNA was converted to cDNA via reverse transcription PCR using a First Strand cDNA Synthesis Kit (Thermo Scientific) and following a previously described method[30]. The cDNA was then subjected to quantitative real-time PCR (qPCR) using SYBR Premix Ex Taq II (Takara) and comprising the following reaction conditions: 95 °C for 5 min; followed by 32 cycles of denaturation at 95 °C for 30 s, annealing at 52-60 °C for 45 s (see Supplementary Table 1 for the specific annealing temperatures) and elonga
The human SSCs were rinsed thrice using cold phosphate buffer saline (PBS) (Gibco) and subjected to fixation for 15 min in 40 g/L paraformaldehyde. After a further three rinses with PBS, the cells were permeabilized for 10 min using 2.5 mL/L Triton X-100 (Sigma, St. Louis, MO, United States). The cells were blocked for 1 h at room temperature in 5% bovine serum albumin before being incubated with primary antibodies overnight at 4 °C. Supplementary Table 2 provides the detailed information regarding the antibodies used. After extensive washes with PBS, Alexa Fluor 488-conjugated immunoglobulin (Ig) G or Alexa Fluor 594-conjugated IgG were used as the secondary antibodies, and the nuclei were stained using 4,6-diamidino-2-phenylindole. Images were captured using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Xylene was used to deparaffinize testis sections, which were then rehydrated using a graded series of ethanol concentrations. Antigens were then retrieved by heating the samples at 98 °C for 18 min in 0.01 mol/L sodium citrate buffer. For immunohistochemistry but not for immunofluorescence, endogenous peroxidase activity was blocked using 30 mL/L H2O2 (Zsbio, Beijing, China). The sections were then subjected to permeabilization for 15 min using 2.5 mL/L Triton X-100 (Sigma). The sections were blocked at room temperature for 1 h using 50 mL/L bovine serum albumin for 1 h and then incubated with primary antibodies overnight at 4 °C. Following extensive washing using PBS for immunohistochemistry, the sections were incubated at room temperature for 1 h with horseradish peroxidaseconjugated secondary antibodies, and then chromogen detection was carried out using a 3,3’-diaminobenzidine chromogen kit (Dako, Glostrup, Denmark). The sections were finally stained with hematoxylin. For immunofluorescence, the sections were incubated at room temperature for 1 h with Alexa Fluorconjugated secondary antibody, and the nuclei were stained with 4,6-diamidino-2-phenylindole. The images were captured under a Zeiss microscope.
Testis samples and human SSCs were ground and lysed on ice for 30 min using radioimmunoprecipitation assay lysis buffer (Thermo Scientific) and then subjected to centrifugation at 12000 g to produce clear lysates. A BCA kit (Thermo Scientific) was then used to determine the protein concentration in the lysates. Primary antibodies or control rabbit IgG were added to cell lysates and incubated at 4 °C overnight. The next day the supernatants were added with Protein G magnetic beads and incubated at 4 °C for 2 h. The samples were washed three times with washing buffer, the beads were pelleted magnetically, and then resuspended and boiled at 95 °C for 5 min. For each sample, 30 micrograms of total protein extracts were subjected to SDS-PAGE (Bio-Rad), and western blotting was performed following a previously published protocol[30]. Supplementary Table 2 details the antibodies used and their dilution ratios. Chemiluminescence (Bio-Rad) was used to visualize the intensities of the immunoreactive protein bands.
RiboBio (Guangzhou, China) synthesized the small interfering RNA (siRNA) sequences targeting human MKK7 mRNA, which are listed in Supplementary Table 3. Scrambled siRNAs were used as negative controls. Lipofectamine 3000 (Life Technologies, Carlsbad, CA, United States) was used to transfect the control-siRNA or MKK7-siRNAs (both 100 nmol/L) into human SSCs, following the manufacturer’s instructions. At 48 h after transfection, the cells were harvested to evaluate the changes in the expression levels of genes and proteins.
After siRNA transfection, human SSC proliferation was detected using a cell counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan) following the manufacturer’s protocol. Briefly, 100 mL/L CCK-8 reagent replaced the cell culture medium, and the cells were incubated for 3 h. A microplate reader (Thermo Scientific) was then used to measure the absorbance at 450 nm.
Human SSCs were seeded at 5000 cells/well in a 96-well plate; each well contained DMEM/F12 medium containing 50 μmol/L EdU (RiboBio). The cells were cultured for 12 h, washed using DMEM, and subjected to fixation using 40 g/L paraformaldehyde. Cell neutralization was achieved using 2 mg/mL glycine, followed by permeabilization for 10 min using 5 mL/L Triton X-100 at room temperature. Apollo staining reaction buffer was then used to reveal the EdU staining. Cell nuclei were stained using Hoechst 33342. A fluorescence microscope (Zeiss) was used to capture images, and the positive rate of EdU staining was calculated by counting at least 500 cells.
Human SSCs were transfected with siRNAs for 48 h and then assessed for their apoptotic percentage. Cells were digested with trypsin, washed twice using cold PBS, and collected by centrifugation. Cells (at least 106) were resuspended in Annexin V Binding Buffer (BD Biosciences, Franklin Lakes, NJ, United States) following the manufacturer’s instructions. Then, 5 μL of Annexin Vconjugated Allophycocyanin and 10 μL of propidium iodide solution were added to the cells and incubated at room temperature for 15 min in the dark. The cells were then subjected to C6 flow cytometry analysis (BD Biosciences).
An In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) was used to determine the percentage of apoptosis among human SSCs, following the manufa
All the statistical review of this study was performed by a biomedical statistician. GraphPad Prism 8.0 (GraphPad software, La Jolla, CA, United States) was used for the descriptive and statistical analyses. All experiments were performed at least in triplicate, and all data are presented as the mean ± SD. A t-test was used to calculate the statistical difference between two groups, with P < 0.05 being considered as statistically significant.
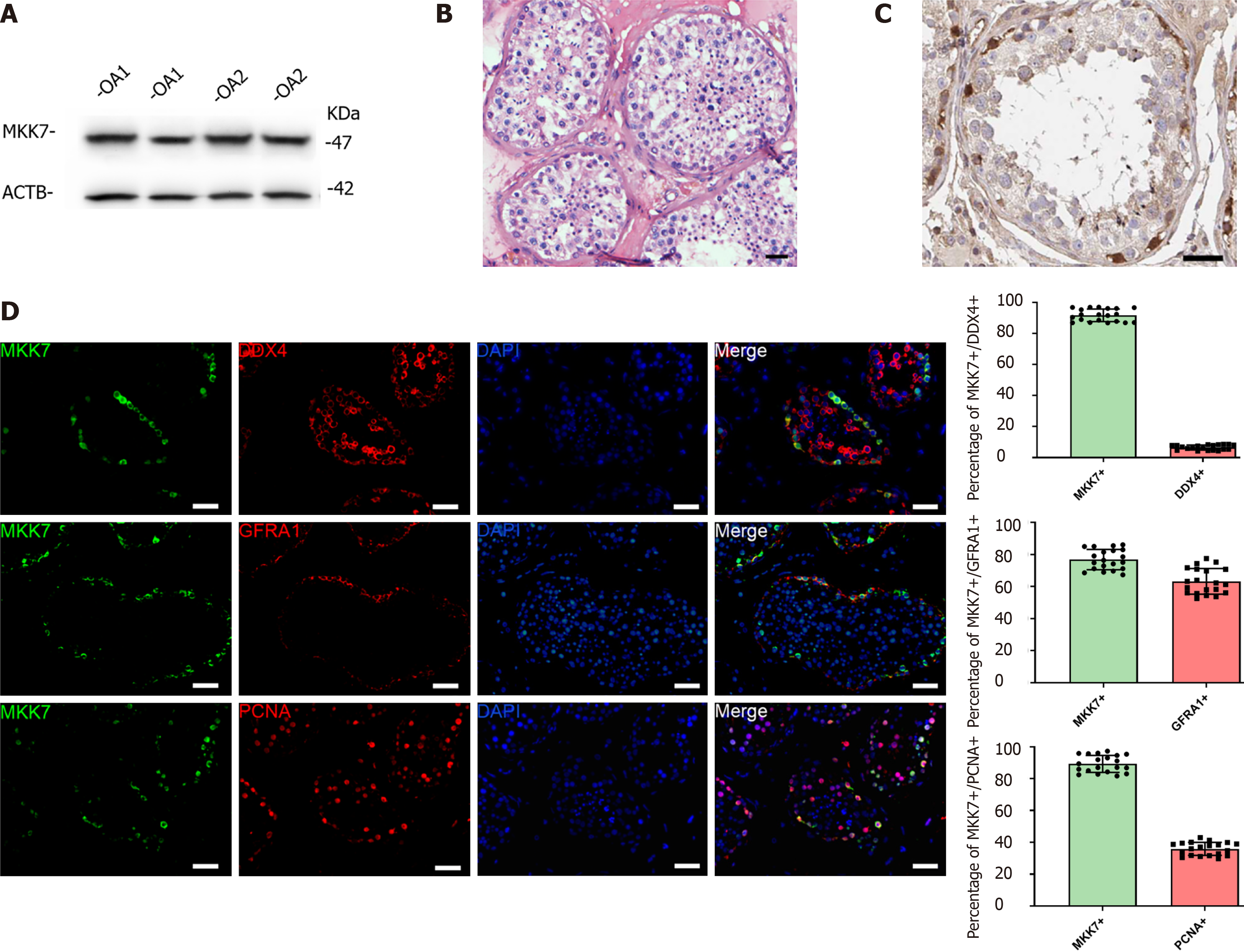
To explore MKK7 function, we first examined its expression in the adult human testis. The MKK7 protein level was measured in testis samples from patients with obstructive azoospermia with normal spermatogenesis using western blotting (Figure 1A). We further analyzed the localization of MKK7 in testicular tissue. MKK7 was mainly found in the cytoplasm of cells close to the seminiferous tubule basement membrane (Figure 1B), indicating that it might be expressed in SSCs. The results of double immunofluorescence staining showed that most MKK7-expressing cells were DEAD-box helicase 4-positive germ cells, and about 76% of MKK7 positive germ cells expressed GFRA1. Interestingly, about 90% of MKK7-expressing cells were PCNA positive (Figure 1C). Taken together, the results suggested that MKK7 was mainly expressed in SSCs and might be involved in cell proliferation.
We then used a human SSC cell line to explore the role of MKK7 in SSC proliferation. First, quantitative real time PCR was used to confirm the identity of the human SSC line. The results indicated that the cells expressed many markers of human SSCs, including GFRA1, Thy-1 cell surface antigen, ubiquitin C-terminal hydrolase L1 and DEAD-box helicase 4, while a hallmark of human Sertoli cells (encoding SRY-box transcription factor 9) was not detected (Supplementary Figure 1A). The results of immunofluorescence also showed that almost all cells expressed SSC markers, such as GFRA1, ubiquitin C-terminal hydrolase L1 and Thy-1 cell surface antigen (Supplementary Figure 1B). These results suggested that the SSC line had similar properties to primary SSCs.
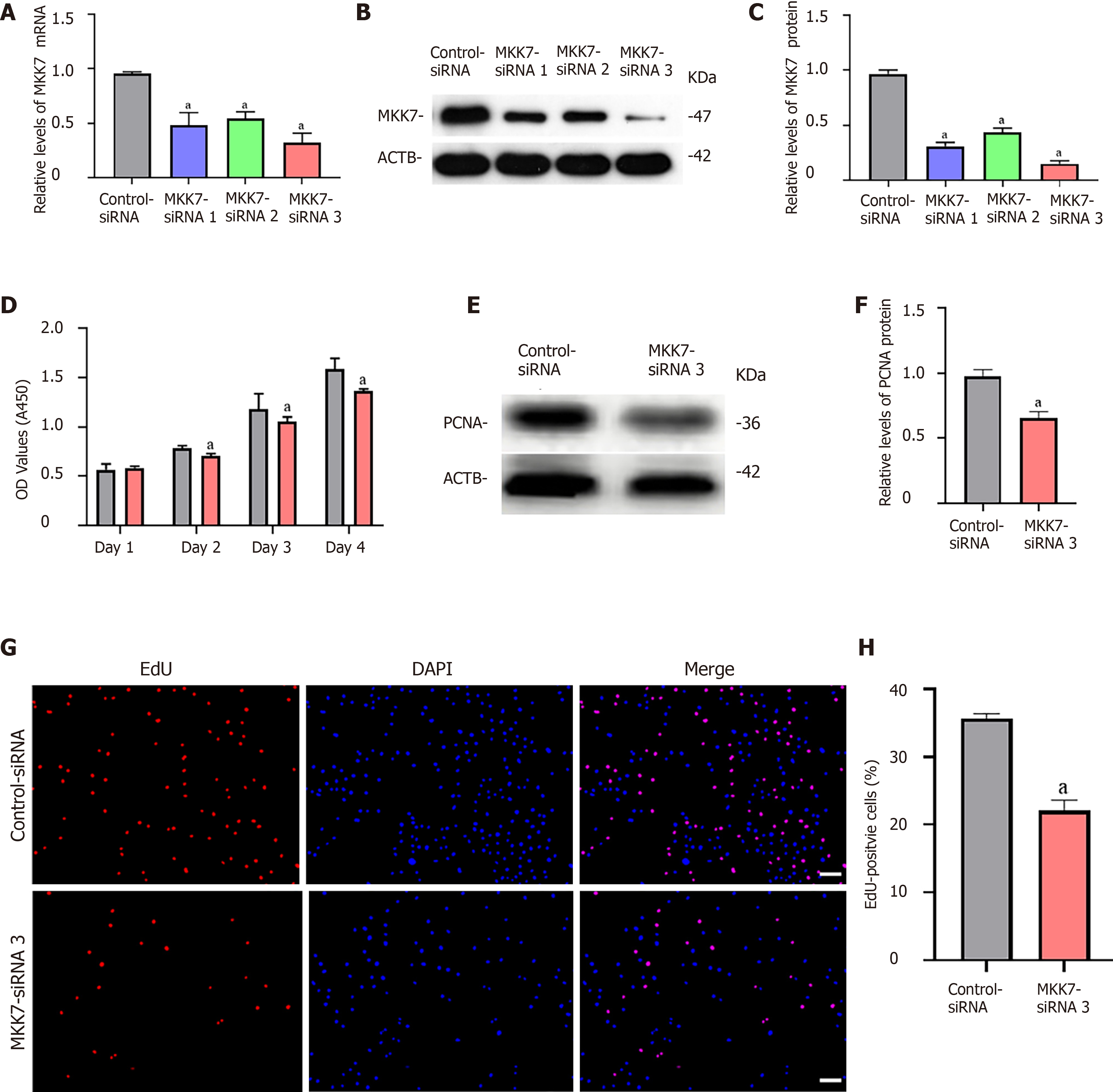
To explore the function of MKK7 in SSCs, siRNA-triggered knockdown of MKK7 was performed in the human SSC line. We examined the knockdown efficiency using quantitative real time PCR (Figure 2A) and western blotting (Figure 2B and C), which showed that MKK7 expression was attenuated by MKK7-siRNA1, MKK7-siRNA2 and MKK7-siRNA3. MKK7-siRNA3 showed the best knockdown effect. We then investigated the proliferative ability of MKK7siRNA3-transfected SSCs using the CCK-8 assay (Figure 2D). We observed that MKK7 knockdown repressed SSC proliferation at 2 d to 5 d after transduction. We also found that the level of PCNA (a cell proliferation hallmark) was significantly reduced (Figure 2E and F). Likewise, at 48 h after transfection, we examined cellular DNA synthesis using an EdU assay. The proportion of EdU-positive cells was decreased compared with that in the control siRNA transfected cells (35.73% ± 0.64% vs 22.05% ± 1.58%, P < 0.05) (Figure 2G and H).
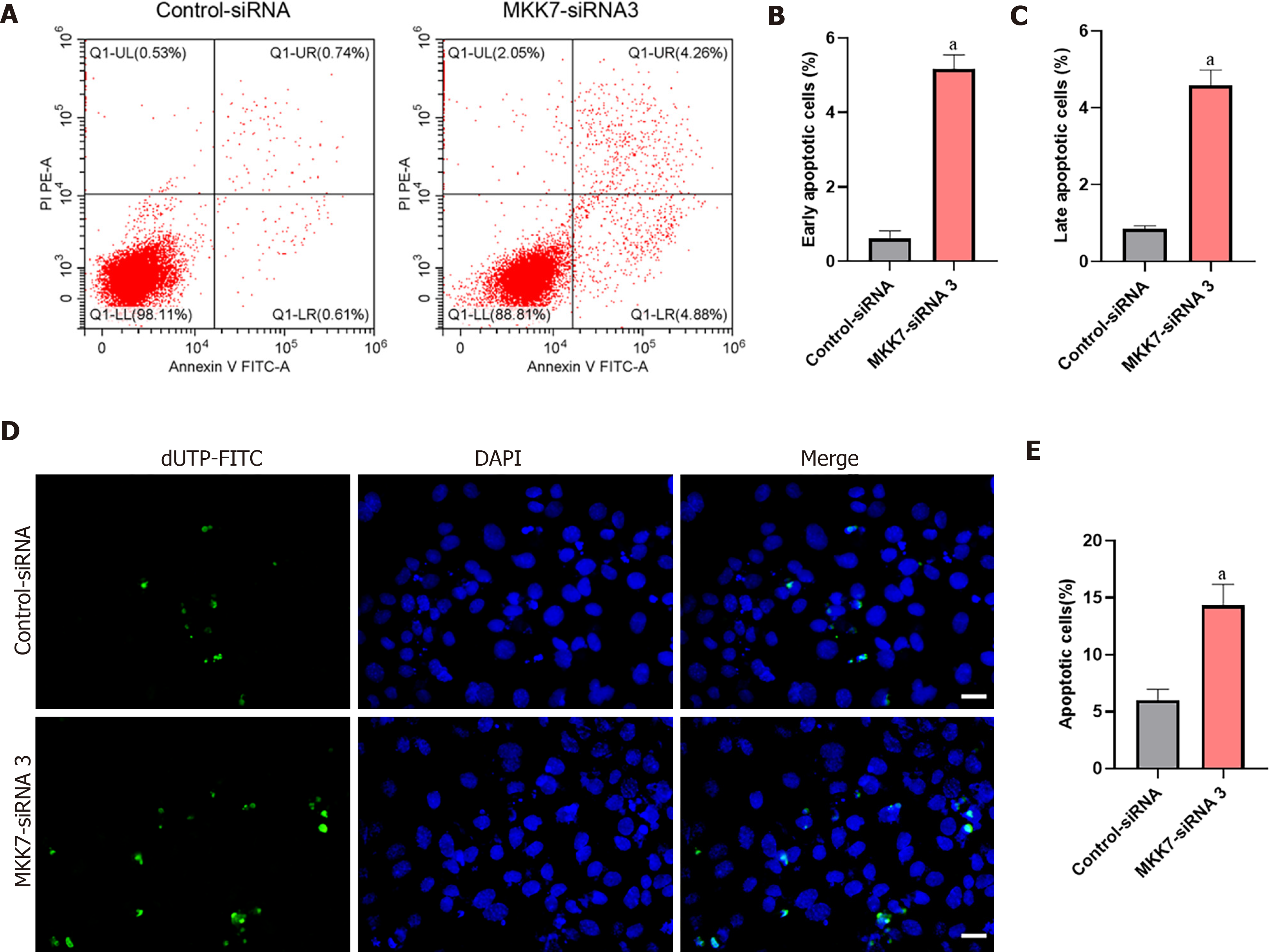
Next, Annexin V/propidium iodide staining and flow cytometry were used to further examine cell apoptosis. MKK7 silencing increased both early and late apoptosis of the human SSC line compared to that in the cells transfected with the controlsiRNA (early apoptosis: 5.17% ± 0.37% vs 0.62% ± 0.19%, P < 0.05; late apoptosis: 4.58% ± 0.40% vs 0.84% ± 0.09%, P < 0.05) (Figure 3A-C). Similarly, the results of terminal deoxynucleotidyl transferase nick-end-labeling (TUNEL) assay displayed that the proportion of TUNEL positive cells increased after MKK7 silencing compared with that in the control siRNAtransfected cells (14.34% ± 1.83% vs 6.01% ± 0.95%, P < 0.05) (Figure 3D and E). Taken together, the results suggested that MKK7 promotes DNA synthesis and cell proliferation, whereas MKK7 knockdown causes apoptosis.
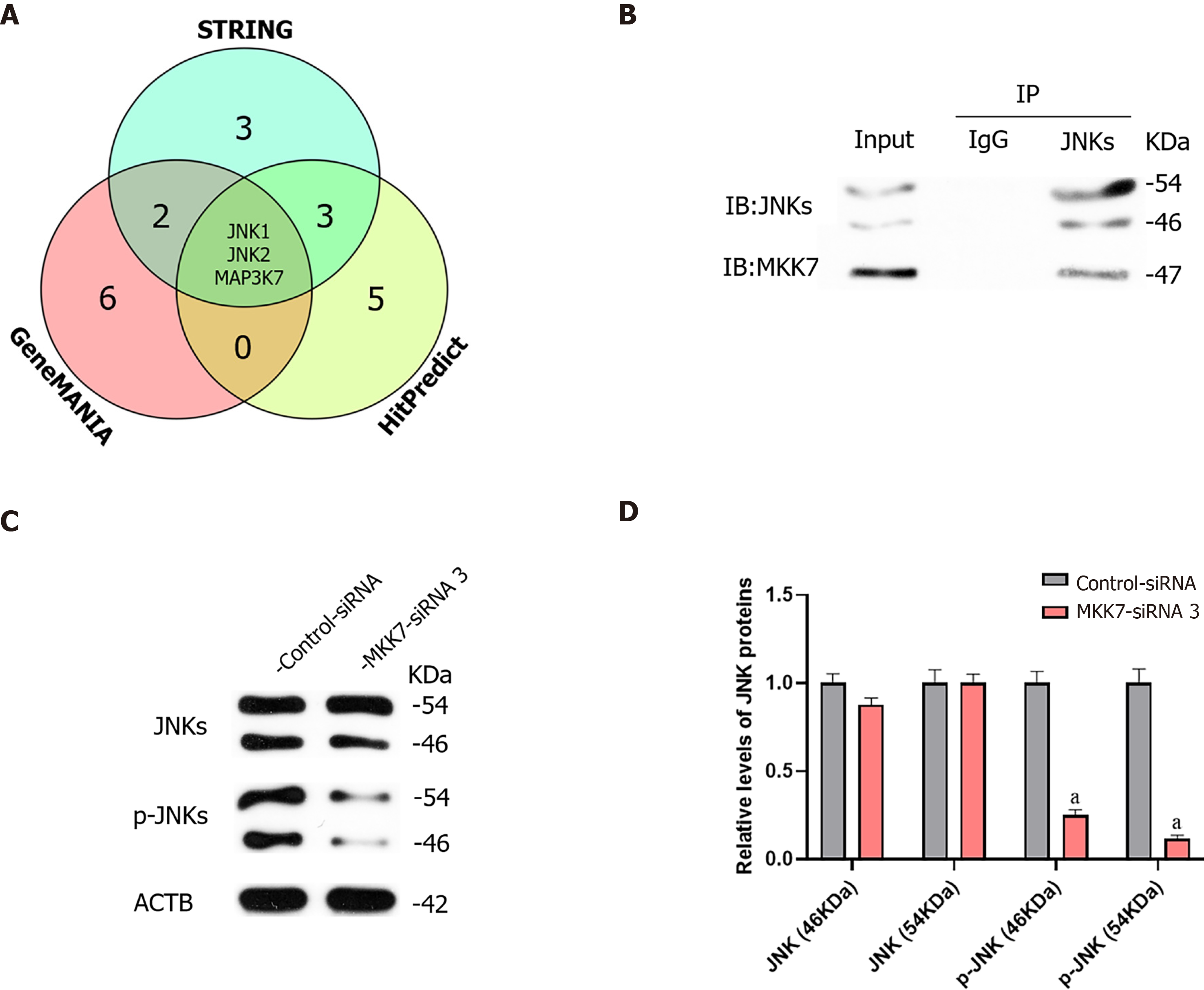
We further investigated the targets of MKK7. We predicted the targets of MKK7 using bioinformatic analysis, (GeneMANIA, HitPredict and String). Comprehensive prediction using the three databases identified JNK1, JNK2 and mitogen-activated protein kinase kinase kinase 7 as MKK7interacting proteins (Figure 4A). Mitogen-activated protein kinase kinase kinase 7 was reported as an upstream activator of the MKK/JNK signal transduction pathway, acting via phosphorylation of MKK7[32], and MKK7 was reported to activate JNKs by phosphorylation[26]; therefore, JNKs might be candidate downstream targets of MKK7 in SSCs. To examine whether there was a physical interaction between MKK7 and JNKs, an immunoprecipitation assay followed by western blotting was carried out (Figure 4B), which showed that MKK7 could bind directly to JNKs in the human SSC line. By contrast, the control IgG did not pull down JNKs. We further detected the levels of JNKs and phosphorylated JNKs in human SSCs transfected with MKK7-siRNA3 (Figure 4C). We found that the levels of phosphorylated JNKs were reduced significantly compared with those in cells transfected with the control siRNA; however, the total level of JNKs did not change significantly. This result indicated that MKK7 could bind directly to JNKs to promote their phosphorylation.
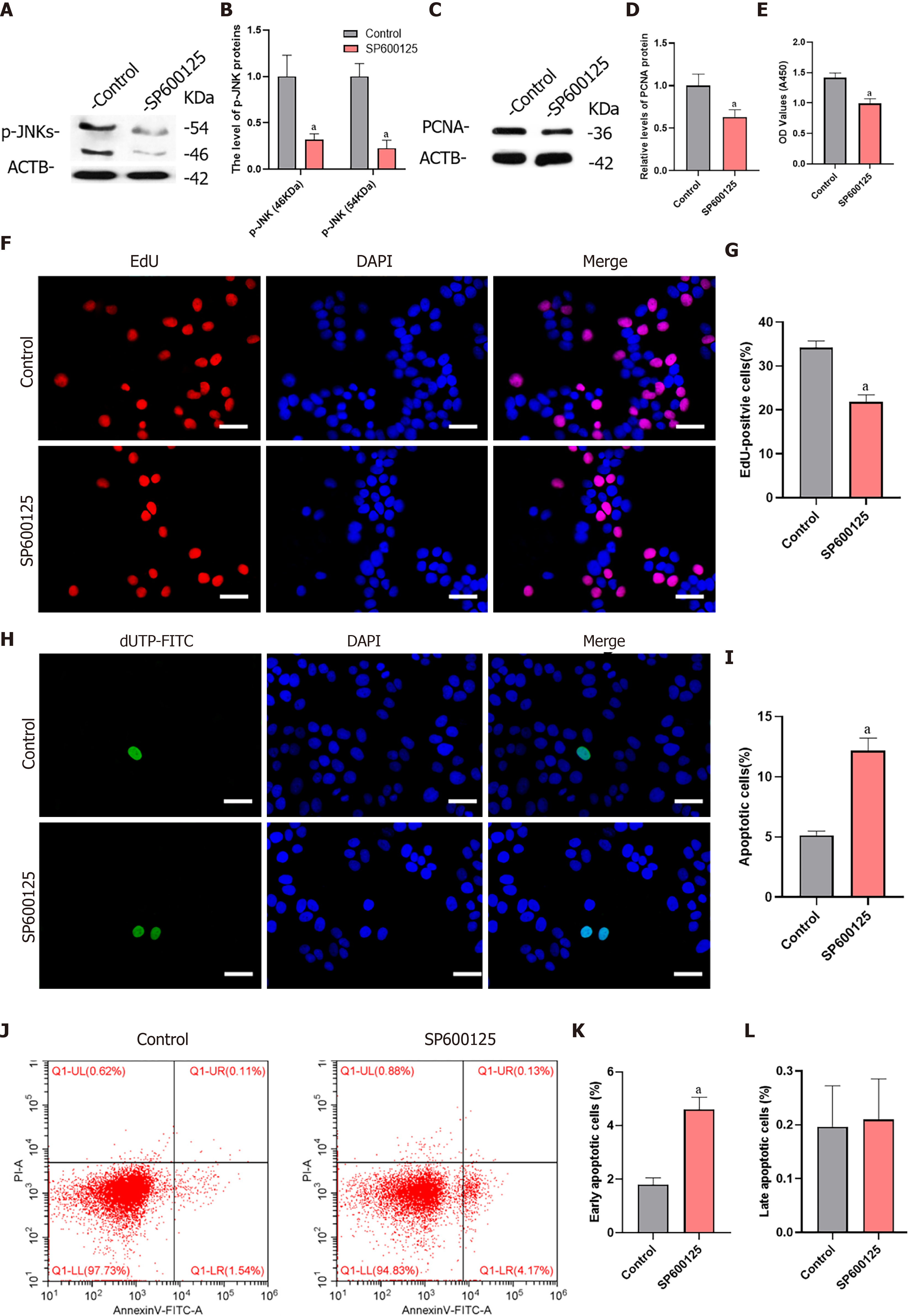
To verify whether MKK7 affects SSC proliferation through phosphorylation of JNKs, we blocked the function of JNKs using SP600125, a selective inhibitor of JNK phosphorylation. We detected the level of phosphorylated JNKs after SP600125 treatment for 24 h, which showed that the levels of phosphorylated JNKs were notably reduced compared with those in the untreated control group (Figure 5A and B). In addition, PCNA protein levels decreased significantly (Figure 5C and D). Then, we examined the proliferation of human SSCs using the CCK-8 assay. SP600125 treatment significantly inhibited cell proliferation compared with that in the control group (Figure 5E). In addition, the proportion of EdU-positive cells decreased after culture with SP600125 for 24 h (34.17% ± 1.56% vs 21.84% ± 1.62%, P < 0.05) (Figure 5F and G). We further analyzed the apoptosis of human SSCs using the TUNEL assay, which showed that TUNEL positive cells increased significantly (5.13% ± 0.34% vs 12.18% ± 1.03%, P < 0.05) after SP600125 treatment (Figure 5H and I). Likewise, SP600125 treatment caused an increase in early apoptosis but not late apoptosis of SSCs according to Annexin V/propidium iodide staining and flow cytometry [1.80% ± 0.25% (control) vs 4.61% ± 0.45% (SP600125), P < 0.05] (Figure 5J-L). Taken together, the results indicated that SP600125-mediated inhibition of JNK phosphorylation in SSCs resulted in impaired cell proliferation and increased cell apoptosis.
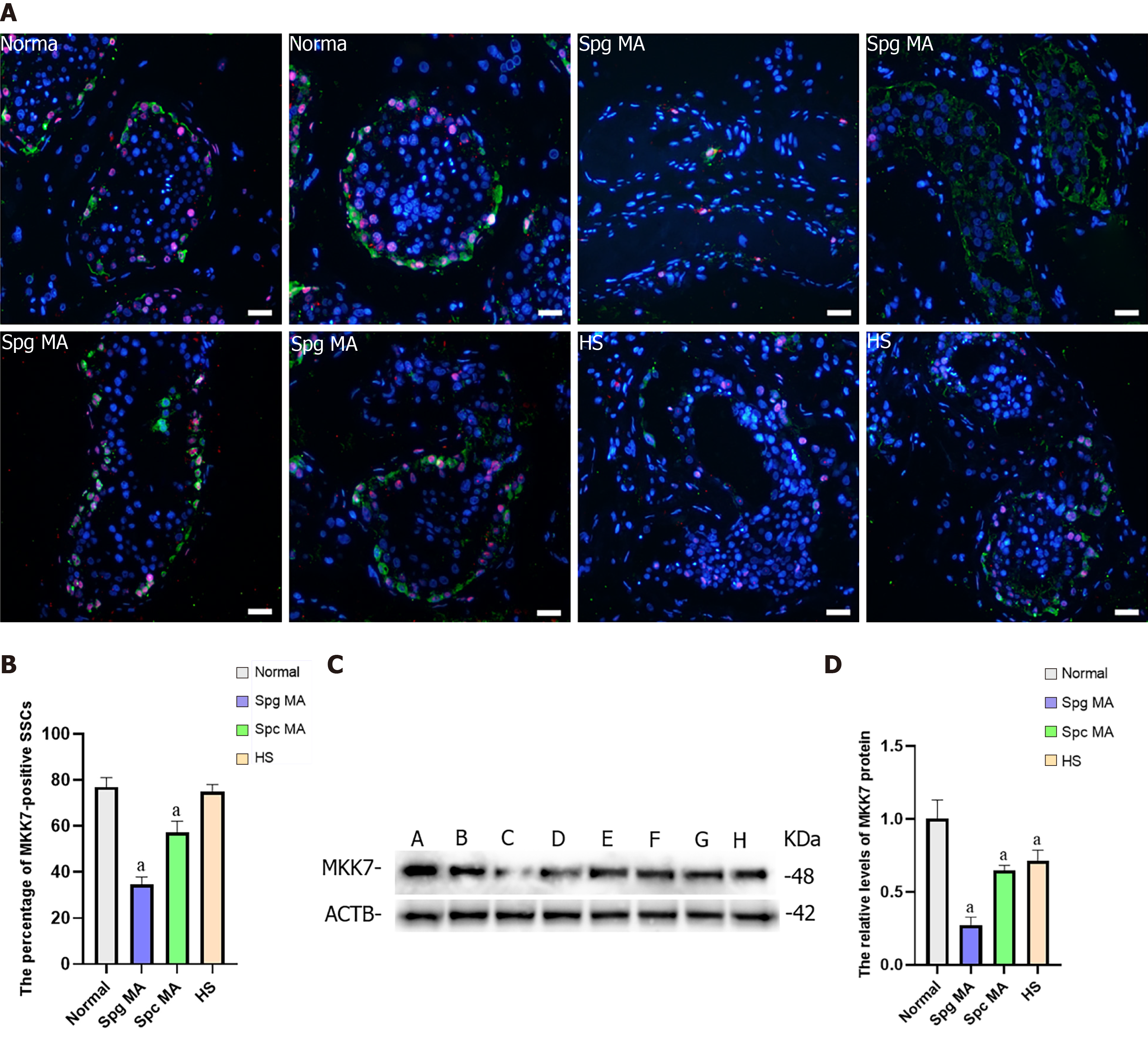
NOA is a serious male infertility disease in clinical practice. According to the results of testicular pathological tissue, NOA can be divided into spermatogonia maturation arrest, spermatocyte maturation arrest, spermatid maturation arrest, hypospermatogenesis and Sertoli cell only syndrome. To investigate whether MKK7 is associated with impaired spermatogenesis in adults, we examined MKK7 expression patterns in eight testes. According to the results of hematoxylin-eosin staining, we confirmed the spermatogenesis status of the testis (Supplementary Figure 2A-H). We examined the positive proportion and localization changes of MKK7 (red) in SSCs using double immunohistochemistry with ubiquitin C-terminal hydrolase L1 (green). The results indicated that the percentage of MKK7-expressing SSC was significantly reduced in spermatogonia maturation arrest and spermatocyte maturation arrest samples compared to samples with normal spermatogenesis (Figure 6A and B), and the fluorescence intensity appeared to decrease in hypospermatogenesis samples, but there were no translocations of MKK7 protein observed in NOA samples. We further detected the relative levels of MKK7 protein by using western blots. The results displayed that the expression of MKK7 was significantly downregulated in all samples with impaired spermatogenesis compared to the normal group (Figure 6C and D). Those results implied that the aberrant expression of MKK7 was correlated with spermatogenesis disorder, especially the maturation of spermatogonia and spermatocytes.
Human SSCs are the origin of spermatogenesis[33]; however, research on human SSCs are hampered because of limited sources and scarce numbers[34,35]. Much is known about the mechanism of SSC regulation in rodents, and spermatogenesis has been reconstructed through SSC transplantation[36]. However, these results are not entirely applicable to primates because many biological processes of rodent SSCs are different from those of primates[15,37]. For example, the spermatogenic epithelial cycle in mice can usually be divided into 12 stages; however, humans generally have 6 stages[21,22]. Furthermore, octamer-binding protein 4, a biomarker of mouse SSCs, is not expressed in human SSCs[23,24]. Therefore, research on the mechanisms of human SSCs are hindered by these discrepancies. Human primary SSCs cannot be cultured in vitro for a long time because of the weak proliferation of human SSC in vitro, and the culture system of mice SSCs is not suitable for human SSC. In addition, the proliferation of human SSCs in vitro is weak[34]. To establish the human SSC line, the human SV40 Large T antigen was overexpressed in human primary GPR125-positive undifferentiated spermatogonia[29,38]. These cells possess similar biological properties to human SSCs and possess an unlimited proliferative capacity, which assisted us in studying the molecular mechanisms of SSCs.
MKK7 is involved in the phosphorylation of JNKs and activates their downstream pathways[39]. However, the promotion of SSC proliferation by MKK7 was unexpected, given that its inhibition often enhances self-renewal division of other stem cells. In mouse embryonic stem cells, activation of c-Jun NH2-terminal kinase by CdCl2- or HgCl2 requires MKK7[40], and it is also involved in the process of embryonic stem cell differentiation into cardiomyocytes[41]. This suggested that MKK7 regulation in SSCs is distinct from that in other stem cells. In our study, we found that MKK7 was primarily expressed in self-renewing SSCs (GFRA1+/PCNA+), which was consistent with the findings of single cell sequencing, in which MKK7 was mainly expressed in SSCs[42]. Therefore, we believe it may be associated with the proliferation or apoptosis of SSCs. We validated the functions of MKK7 in a human SSC cell line using siRNA, and the results showed that MKK7 deficiency inhibited proliferation and promoted apoptosis of SSCs. MKK7 has been reported to be involved in the apoptotic process in neural cells[43] and participates in the proliferation of hepatocytes and cancer cells[44,45]. The functional diversity of MKK7 in various cells might reflect different downstream targets. Our work provides more evidence for the functions of MKK7; however, the specific downstream effectors of MKK7 remain to be explored.
Previous reports and bioinformatic prediction suggested that MKK7 is involved in the phosphorylation modification of JNKs[39] and blocking JNKs using small molecule inhibitors similarly inhibited the proliferation of mouse SSCs[28]. The development of SSCs is regulated by many key growth factors, such as GDNF[46], FGF[12], epidermal growth factor[47] and LIF interleukin 6 family cytokine[8]. Other intracellular regulatory molecules, including promyelocytic leukemia zinc finger[48], Nanos C2HC-type zinc finger 2[49] and Spalt like transcription factor 4[50], have also been demonstrated to be involved in SSC fate determination. Although the phosphorylation of JNK1 and JNK2 were reported to increase after treatment with GDNF and FGF2 in mouse SSCs[28], whether MKK7 is involved in the signal transduction of these important regulatory factors or if other regulatory pathways participate in this process remains to be further investigated. It is also reported that cell division cycle 5 like[51] and TNF receptor associated factor 6[52] have direct interactions with MKK7, which suggests that MKK7 might also affect cell proliferation through other molecules. To explore this question, further investigations are needed, for example, protein co-immunoprecipitation combined with mass spectrometry and transcriptome sequencing.
Although the pathogenesis of NOA is largely unclear, our results displayed that MKK7 levels were reduced in the testis of patients with NOA compared with those in patients with obstructive azoospermia, especially in testis with spermatocyte maturation arrest and spermatogonia maturation arrest. A reduction was also observed in the proportion of MKK7-positive SSCs. Exploration of mutations in the MKK7 gene in patients with NOA and the construction of point mutations or knockout mouse models should be used to confirm the functions of MKK7. In addition, the current study was carried out in a human SSC line, and the proliferation function of MKK7 was only tested in SSCs. Thus, we are unsure whether MKK7 is involved in the differentiation of progenitor cells, which also needs further studies in mouse models.
In conclusion, we revealed that MKK7 is expressed mainly in human SSCs and inhibits the apoptosis and enhances the proliferation of SSCs via JNK phosphorylation. Although some questions remain, our study offers new clues regarding the pathways and genes involved in the determination of SSC fate in humans. The activation of MKK7 or JNK1 using small molecules might contribute to human SSC self-renewal in vitro and might identify molecular targets to diagnose and treat male infertility.
MKK7 regulates the proliferation and apoptosis of human SSC by mediating the phosphorylation of JNKs. Abnormal expression of MKK7 may impair human spermatogenesis.
Human spermatogonial stem cells (SSCs) are the basis of spermatogenesis. However, little is known about the developmental regulatory mechanisms of SSC due to sample origin and species differences.
To investigates the mechanisms involved in the proliferation of human SSCs.
To investigate the functions and mechanisms of mitogen-activated protein kinase kinase 7 (MKK7) during proliferation and apoptosis in human SSCs.
The expression of MKK7 in human testis was identified using immunohistochemistry and western blotting (WB). MKK7 was knocked down using small interfering RNA, and cell proliferation and apoptosis were detected by WB, EdU, cell counting kit-8 and fluorescence-activated cell sorting. After bioinformatic analysis, the interaction of MKK7 with c-Jun N-terminal kinases (JNKs) was verified by protein co-immunoprecipitation and WB. The phosphorylation of JNKs was inhibited by SP600125, and the phenotypic changes were detected by WB, cell counting kit-8 and fluorescence-activated cell sorting.
MKK7 is mainly expressed in human SSCs, and MKK7 knockdown inhibits SSC proliferation and promotes their apoptosis. MKK7 mediated the phosphorylation of JNKs, and after inhibiting the phosphorylation of JNKs, the phenotypic changes of the cells were similar to those after MKK7 downregulation. The expression of MKK7 was significantly downregulated in patients with abnormal spermatogenesis, suggesting that abnormal MKK7 may be associated with spermatogenesis impairment.
MKK7 regulates the proliferation and apoptosis of human SSC by mediating the phosphorylation of JNKs. Abnormal expression of MKK7 may impair human spermatogenesis.
This study intended to reveal the role and regulatory mechanism of MKK7 in the regulation of SSC development and spermatogenesis in humans, which can provide a scientific basis for the etiological interpretation and molecular diagnosis of male infertility. It also provided new molecular targets for the clinical treatment of male infertility and the development of contraceptives.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prasetyo EP S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yu HG
| 1. | Cardona Barberán A, Boel A, Vanden Meerschaut F, Stoop D, Heindryckx B. Diagnosis and Treatment of Male Infertility-Related Fertilization Failure. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 564] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 3. | Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R. Male infertility. Lancet. 2021;397:319-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 391] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 4. | Corona G, Minhas S, Giwercman A, Bettocchi C, Dinkelman-Smit M, Dohle G, Fusco F, Kadioglou A, Kliesch S, Kopa Z, Krausz C, Pelliccione F, Pizzocaro A, Rassweiler J, Verze P, Vignozzi L, Weidner W, Maggi M, Sofikitis N. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:733-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 561] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 6. | de Rooij DG. The nature and dynamics of spermatogonial stem cells. Development. 2017;144:3022-3030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 7. | Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1003] [Cited by in F6Publishing: 947] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 8. | Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 731] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 9. | Takashima S, Kanatsu-Shinohara M, Tanaka T, Morimoto H, Inoue K, Ogonuki N, Jijiwa M, Takahashi M, Ogura A, Shinohara T. Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports. 2015;4:489-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, Toyokuni S, Shinohara T. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842-25851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139:1734-1743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Burgess AM. The effect of calcitonin on the prechordal mesoderm, neural plate and neural crest of Xenopus embryos. J Anat. 1985;140 ( Pt 1):49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Di Persio S, Saracino R, Fera S, Muciaccia B, Esposito V, Boitani C, Berloco BP, Nudo F, Spadetta G, Stefanini M, de Rooij DG, Vicini E. Spermatogonial kinetics in humans. Development. 2017;144:3430-3439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776-798. [PubMed] [Cited in This Article: ] |
| 17. | Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011;85:347-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Clermont Y. Spermatogenesis in man. A study of the spermatogonial population. Fertil Steril. 1966;17:705-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 63] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | de Rooij DG. Spermatogonial stem cell renewal in the mouse. I. Normal situation. Cell Tissue Kinet. 1973;6:281-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Simorangkir DR, Marshall GR, Plant TM. A re-examination of proliferation and differentiation of type A spermatogonia in the adult rhesus monkey (Macaca mulatta). Hum Reprod. 2009;24:1596-1604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Johnson L. A new approach to study the architectural arrangement of spermatogenic stages revealed little evidence of a partial wave along the length of human seminiferous tubules. J Androl. 1994;15:435-441. [PubMed] [Cited in This Article: ] |
| 22. | Muciaccia B, Boitani C, Berloco BP, Nudo F, Spadetta G, Stefanini M, de Rooij DG, Vicini E. Novel stage classification of human spermatogenesis based on acrosome development. Biol Reprod. 2013;89:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Baumrind D. Parental control and parental love. Children. 1965;12:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 24. | Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Wu Z, Wu J, Jacinto E, Karin M. Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol Cell Biol. 1997;17:7407-7416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Foltz IN, Gerl RE, Wieler JS, Luckach M, Salmon RA, Schrader JW. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344-9351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Huang YA, Zhou B, Wernig M, Südhof TC. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell. 2017;168:427-441.e21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 28. | Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12:774-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Hou J, Niu M, Liu L, Zhu Z, Wang X, Sun M, Yuan Q, Yang S, Zeng W, Liu Y, Li Z, He Z. Establishment and Characterization of Human Germline Stem Cell Line with Unlimited Proliferation Potentials and no Tumor Formation. Sci Rep. 2015;5:16922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Zhou D, Wang X, Liu Z, Huang Z, Nie H, Zhu W, Tan Y, Fan L. The expression characteristics of FBXW7 in human testis suggest its function is different from that in mice. Tissue Cell. 2020;62:101315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117419] [Cited by in F6Publishing: 122676] [Article Influence: 5333.7] [Reference Citation Analysis (0)] |
| 32. | Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141-8144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 273] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Guo Y, Hai Y, Gong Y, Li Z, He Z. Characterization, isolation, and culture of mouse and human spermatogonial stem cells. J Cell Physiol. 2014;229:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Medrano JV, Rombaut C, Simon C, Pellicer A, Goossens E. Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil Steril. 2016;106:1539-1549.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 401] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303-11307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 811] [Cited by in F6Publishing: 751] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 37. | Mäkelä JA, Hobbs RM. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction. 2019;158:R169-R187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 38. | Chen W, Cui Y, Liu B, Li C, Du L, Tang R, Qin L, Jiang Y, Li J, Yu X, He Q, He Z. Hsa-miR-1908-3p Mediates the Self-Renewal and Apoptosis of Human Spermatogonial Stem Cells via Targeting KLF2. Mol Ther Nucleic Acids. 2020;20:788-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Yao Z, Diener K, Wang XS, Zukowski M, Matsumoto G, Zhou G, Mo R, Sasaki T, Nishina H, Hui CC, Tan TH, Woodgett JP, Penninger JM. Activation of stress-activated protein kinases/c-Jun N-terminal protein kinases (SAPKs/JNKs) by a novel mitogen-activated protein kinase kinase. J Biol Chem. 1997;272:32378-32383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Matsuoka M, Igisu H, Nakagawa K, Katada T, Nishina H. Requirement of MKK4 and MKK7 for CdCl2- or HgCl2-induced activation of c-Jun NH2-terminal kinase in mouse embryonic stem cells. Toxicol Lett. 2004;152:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Wang J, Chen L, Ko CI, Zhang L, Puga A, Xia Y. Distinct signaling properties of mitogen-activated protein kinase kinases 4 (MKK4) and 7 (MKK7) in embryonic stem cell (ESC) differentiation. J Biol Chem. 2012;287:2787-2797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Hermann BP, Cheng K, Singh A, Roa-De La Cruz L, Mutoji KN, Chen IC, Gildersleeve H, Lehle JD, Mayo M, Westernströer B, Law NC, Oatley MJ, Velte EK, Niedenberger BA, Fritze D, Silber S, Geyer CB, Oatley JM, McCarrey JR. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018;25:1650-1667.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 43. | Syc-Mazurek SB, Rausch RL, Fernandes KA, Wilson MP, Libby RT. Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death. Cell Death Dis. 2018;9:1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Wada T, Stepniak E, Hui L, Leibbrandt A, Katada T, Nishina H, Wagner EF, Penninger JM. Antagonistic control of cell fates by JNK and p38-MAPK signaling. Cell Death Differ. 2008;15:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Park JG, Aziz N, Cho JY. MKK7, the essential regulator of JNK signaling involved in cancer cell survival: a newly emerging anticancer therapeutic target. Ther Adv Med Oncol. 2019;11:1758835919875574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 47. | Fu H, Zhang W, Yuan Q, Niu M, Zhou F, Qiu Q, Mao G, Wang H, Wen L, Sun M, Li Z, He Z. PAK1 Promotes the Proliferation and Inhibits Apoptosis of Human Spermatogonial Stem Cells via PDK1/KDR/ZNF367 and ERK1/2 and AKT Pathways. Mol Ther Nucleic Acids. 2018;12:769-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell. 2010;142:468-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 49. | Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 50. | . Insulin, glucose, and carbohydrate nutrition in hyperlipoproteinemias. Nutr Rev. 1970;28:204-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Llères D, Denegri M, Biggiogera M, Ajuh P, Lamond AI. Direct interaction between hnRNP-M and CDC5L/PLRG1 proteins affects alternative splice site choice. EMBO Rep. 2010;11:445-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Venkatesan B, Valente AJ, Prabhu SD, Shanmugam P, Delafontaine P, Chandrasekar B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 2010;49:655-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |