Published online Jan 26, 2019. doi: 10.4252/wjsc.v11.i1.44
Peer-review started: October 29, 2018
First decision: November 29, 2018
Revised: December 6, 2018
Accepted: January 5, 2019
Article in press: January 6, 2019
Published online: January 26, 2019
Pluripotent stem cell-derived cardiomyocytes (CMs) have become one of the most attractive cellular resources for cell-based therapy to rescue damaged cardiac tissue.
We investigated the regenerative potential of mouse embryonic stem cell (ESC)-derived platelet-derived growth factor receptor-α (PDGFRα)+ cardiac lineage-committed cells (CLCs), which have a proliferative capacity but are in a morphologically and functionally immature state compared with differentiated CMs.
We induced mouse ESCs into PDGFRα+ CLCs and αMHC+ CMs using a combination of the small molecule cyclosporin A, the rho-associated coiled-coil kinase inhibitor Y27632, the antioxidant Trolox, and the ALK5 inhibitor EW7197. We implanted PDGFRα+ CLCs and differentiated αMHC+ CMs into a myocardial infarction (MI) murine model and performed functional analysis using transthoracic echocardiography (TTE) and histologic analysis.
Compared with the untreated MI hearts, the anterior and septal regional wall motion and systolic functional parameters were notably and similarly improved in the MI hearts implanted with PDGFRα+ CLCs and αMHC+ CMs based on TTE. In histologic analysis, the untreated MI hearts contained a thinner ventricular wall than did the controls, while the ventricular walls of MI hearts implanted with PDGFRα+ CLCs and αMHC+ CMs were similarly thicker compared with that of the untreated MI hearts. Furthermore, implanted PDGFRα+ CLCs aligned and integrated with host CMs and were mostly differentiated into α-actinin+ CMs, and they did not convert into CD31+ endothelial cells or αSMA+ mural cells.
PDGFRα+ CLCs from mouse ESCs exhibiting proliferative capacity showed a regenerative effect in infarcted myocardium. Therefore, mouse ESC-derived PDGFRα+ CLCs may represent a potential cellular resource for cardiac regeneration.
Core tip: We demonstrated that mouse embryonic stem cell-derived platelet-derived growth factor receptor-α+ cardiac lineage-committed cells have proliferative capacity but are in a morphologically and functionally immature state compared with differentiated cardiomyocytes; these cells exerted a regenerative effect on infarcted myocardium.
- Citation: Hong SP, Song S, Lee S, Jo H, Kim HK, Han J, Park JH, Cho SW. Regenerative potential of mouse embryonic stem cell-derived PDGFRα+ cardiac lineage committed cells in infarcted myocardium. World J Stem Cells 2019; 11(1): 44-54
- URL: https://www.wjgnet.com/1948-0210/full/v11/i1/44.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i1.44
Myocardial infarction (MI) and heart failure are the most common causes of death in patients with cardiovascular disease[1]. Despite remarkable advances in therapeutic strategies for heart failure, such as novel drugs, ventricular assist device implantation, and heart transplantation, the burden of the disease remains high. Cardiac regeneration using stem cell therapy is an attractive therapeutic strategy to rescue damaged cardiac tissue[2]. Among stem cell populations, pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), exhibit a higher efficacy in cardiomyocyte induction and expansion rate compared with adult stem cells[2]. Indeed, previous large numbers of reports demonstrated functional improvement of damaged myocardium in murine, rodent, and porcine MI models that received PSC-derived cardiomyocytes (CMs)[2-4].
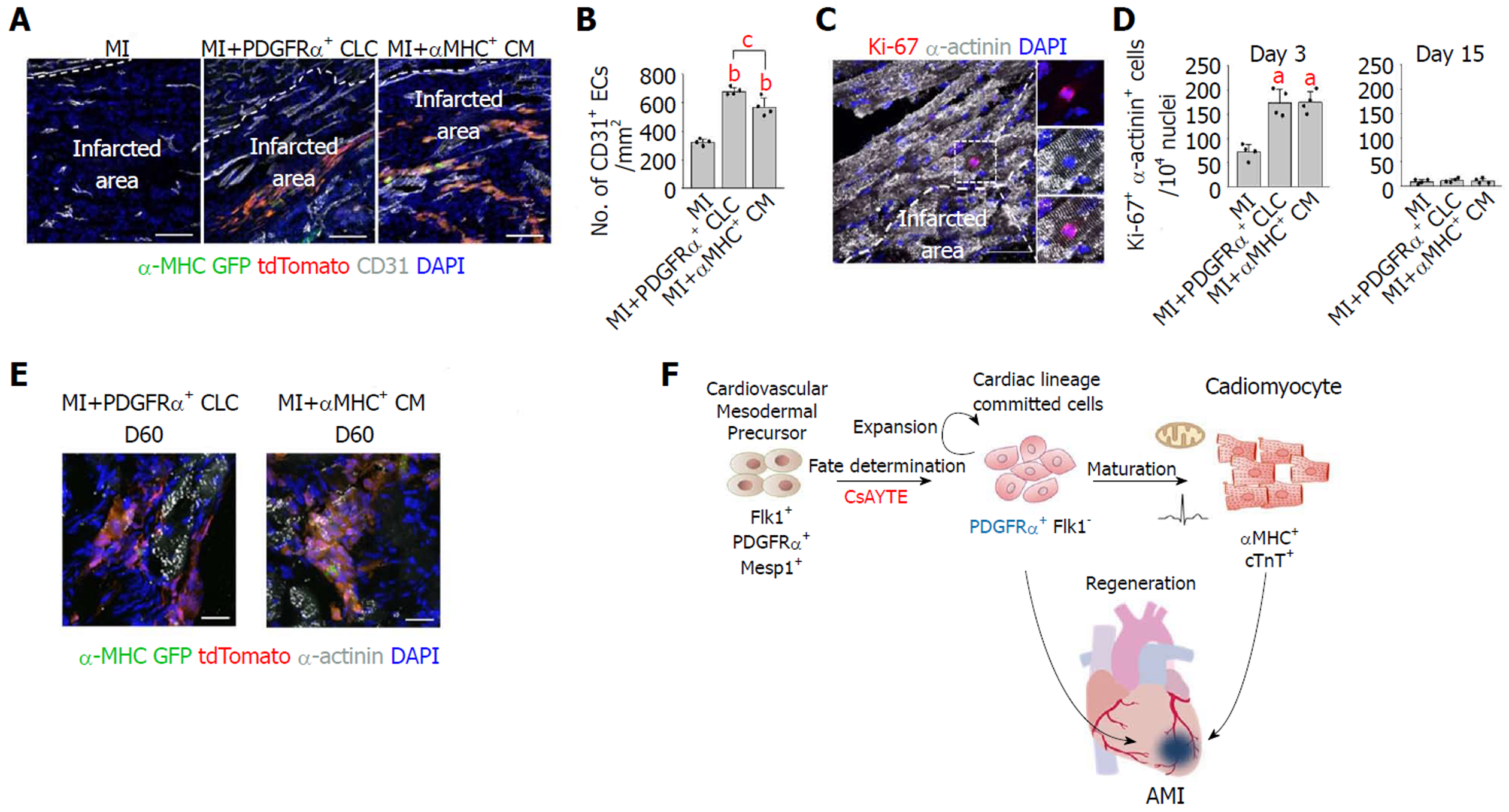
However, the proliferative capacity of PSC-derived CMs is decreased after beating and terminal differentiation[5]. Furthermore, there is no definite surface marker of differentiated PSC-derived CMs to facilitate purification[6]. Recently, several studies have been conducted to identify a novel marker for cardiac progenitor or cardiac lineage-committed cells (CLCs), which are intermediate-stage cells between mesodermal cells and differentiated CMs with proliferative capacity[7-10]. Our group previously established a novel class of cells from PSCs-platelet-derived growth factor receptor-α (PDGFRα)+ CLCs-induced using a combination of four specific modulators: the mitochondrial permeability transition pore inhibitor cyclosporin A (CsA), the ROCK inhibitor Y27632, the antioxidant Trolox, and the activin A receptor type II-like kinase (ALK5) inhibitor EW7197 (collectively referred to here as CsAYTE)[11]. This novel population of actively proliferating cells is cardiac lineage-committed but in a morphologically and functionally immature state compared with differentiated CMs[11]. In the present study, we investigated the regenerative potential of mouse ESC-derived PDGFRα+ CLCs in a murine MI model and compared their efficacy with differentiated CMs.
EMG7 mouse ESCs, which have an αMHC promoter-driven enhanced GFP gene, E14Tg2a ESCs, and OP9 cells were generated as described previously[12-14] and transferred to KAIST.
Lentiviruses were generated by transfecting FUtdTW (Addgene plasmid 22478)[15] with pMD2.G (Addgene plasmid 12259), pMDLg/pRRE (Addgene plasmid 12251) and pRSV-Rev (Addgene plasmid 12253)[16] in 293T cells using jetPEI (Polypus-transfection). Supernatants were collected 48 h after transfection, filtered through a 0.45 μm filter, and concentrated by Lenti-X concentrator (Clontech). Viral particles were resuspended in ESC medium with 4 mg/mL polybrene. EMG7 mouse ESCs were incubated in this medium for 24 h. Selection of ESCs was performed by FACS sorting.
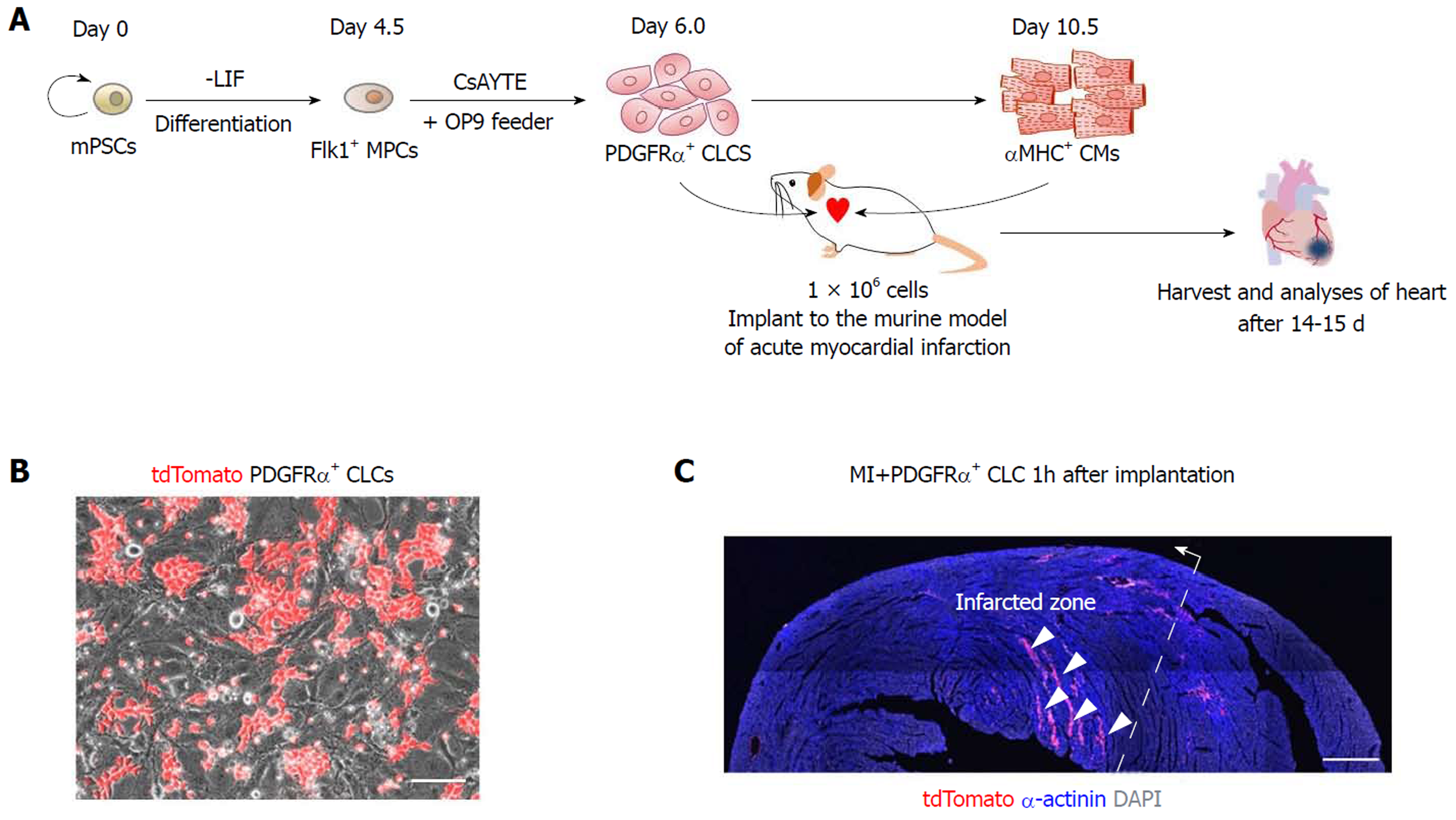
For the induction of Flk1+ mesodermal precursor cells (MPCs), ESCs were cultured without leukemia inhibitory factor (LIF, Millipore) and plated on a 0.1% gelatin-coated dish at a cell density between 1 × 103 and 1.5 × 103 cells cm2 in the differentiation medium, which is αMEM (Invitrogen) containing 10% fetal bovine serum (FBS, Welgene), 0.1 mmol/L of 2-mercaptoethanol (Invitrogen), 2 mmol/L of L-glutamine (Invitrogen) and 50 U/mL of penicillin-streptomycin (Invitrogen). Medium was changed every other day for 4.5 d. At day 4.5, differentiated ESCs were harvested with 0.25% trypsin-EDTA (Invitrogen), and antigen retrieval was performed in the differentiation medium for 30 min in an incubator. Then, cells were washed using 2% FBS in phosphate buffered saline (PBS) and incubated with biotinconjugated anti–mouse Flk1 antibody (clone AVAS12a1, eBioscience) and anti-streptavidin MicroBeads (Miltenyi Biotec). Flk1+ MPCs were sorted by AutoMACS Pro Separator (Miltenyi Biotec). For induction of CLCs, sorted Flk1+ MPCs were plated onto the mitomycin C (AG Scientific)-treated confluent OP9 cells at a density of 5-10 × 103 cells cm2 in the medium containing 3 μg/mL of CsA, 10 μmol/L of Y27632, 400 μmol/L of Trolox, and 1 μg/mL of EW7197 (CsAYTE)[11,17]. The medium was refreshed every other day. Live images of differentiation process of CLCs and CMs were obtained using Axiovert 200M microscope (Carl Zeiss) equipped with AxioCam MRm (Carl Zeiss). Phase contrast images including beating CMs were obtained using an Infinity X digital camera and DpxView LE software (DeltaPix).
The cells were harvested with 0.25% trypsin-EDTA or dissociation buffer (Invitrogen). To analyze live cells, antigen retrieval was performed in the differentiation medium for 30 min in an incubator and the cells were incubated for 20 min with the following antibodies: Allophycocyanin-conjugated anti–mouse PDGFRα (eBioscience, 17-1401, clone APA5, 1:100) and phycoerythrin/Cy7-conjugated anti–mouse Flk1 (BioLegend, 136414, clone AVAS12a1, 1:50). In live cell analysis and sorting, dead cells were excluded using 4,6-diamidino-2-phenylindole (DAPI, Sigma, D8417, 1:1000), and OP9 cells were excluded from Flk1+ MPC by gating in flow cytometry. The differentiated CMs were sorted using αMHC-GFP. Analyses and sorting were performed by FACS Aria II (Beckton Dickinson). Data were analyzed using FlowJo Version 7.5.4 software (TreeStar).
Twenty eight male 9-wk-old BALB/c nude mice were kept in the specific pathogen free before the experiment under a 12:12 h light/dark cycle with lights on at 8:00 AM. They were deprived of food for 18 h but permitted water ad libitum before surgery. Animal care and experimental procedures were performed to conform the NIH guidelines (Guide for the care and use of laboratory animals) and approved by the Animal Care Committee of KAIST (KA2013-40).
All mice were anesthetized through an intraperitoneal injection of a combination of anesthetics (80 mg/kg ketamine, 12 mg/kg xylazine) before any procedures. After intubation, the mice were ventilated with room air (SomnoSuiteTM, Kent scientific). MI was induced by exposing the heart by left thoracotomy and permanently ligating the proximal portion of left anterior descending coronary artery with an 8-0 prolene thread under respiratory support. After ligating the proximal portion of left anterior descending coronary artery, infarction of the anterior wall of left ventricle was confirmed in each mouse by the presence of a pale anterior wall and myocardial hypokinesis. Immediately after ligation of coronary artery and the confirmation of infarction, 100 μL PBS containing 1 x 106 PDGFRα+ CLCs or αMHC+ CMs were intramyocardially injected with a 31-gauge (0.25 mm) insulin syringe into the 3 different sites along the borderline of the infarcted area.
Transthoracic echocardiography (TTE) studies were performed (VIVID 7 dimension system, General Electric-Vingmed Ultrasound) 15 d after MI surgery and cell implantation. Images were obtained using an i13L transducer (5.3-14.0 MHz, GE Healthcare) with high temporal and spatial resolution. Two-dimensionally targeted M-mode parameters were measured at a level of papillary muscle in parasternal short axis view during 6 consecutive cardiac beats. All measurements were performed in a blind fashion according to the guidelines of American Society for Echocardiography.
Before sacrifice, mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). For hematoxylin and eosin (H and E) staining, samples were fixed overnight with 4% paraformaldehyde and embedded in paraffin after tissue processing. For immunofluorescence staining, samples were fixed in 4% paraformaldehyde, dehydrated in 20% sucrose solution overnight, and embedded in tissue freezing medium (Leica). Samples were blocked with 5% goat (or donkey) serum in 0.01% Trition X-100 in PBS and then incubated overnight at 4 °C with the following primary antibodies: Mouse anti-α-actinin monoclonal antibody (Sigma Aldrich, A7811, clone EA-53, 1:100) or rabbit anti-α-actinin polyclonal antibody (Abcam, ab68167, clone EP2529Y, 1:100), rabbit anti-Ki-67 polyclonal antibody (Abcam, ab15580, 1:200), mouse anti-α-SMA monoclonal antibody (Sigma Aldrich, A2547, clone 1A4, 1:500), hamster anti-CD31 monoclonal antibody (Millipore, MAB1398Z, clone 2H8, 1:400), and rabbit anti-GFP polyclonal antibody (Millipore, AB3080, 1:200). After several washes, the samples were incubated for 2 h at RT with the following secondary antibodies: Cy5-conjugated anti-mouse IgG (Jackson ImmunoResearch, 715-175-150, 1:1000) and Cy3-, Cy5-, FITC-conjugated anti-rabbit IgG antibodies (Jackson ImmunoResearch, 711-165-152, 711-175-152, 711-095-152, 1:1000). Then the samples were mounted with fluorescent mounting medium (DAKO) and immunofluorescent images were acquired using a Zeiss LSM780 confocal microscope (Carl Zeiss). To calculate capillary density, number of CD31+ endothelial cells was counted per random 0.5 mm2 area in the infarcted myocardium at 15 d after cell implantation. To analyze the regenerative effects of host myocardium, number of Ki-67+/α-actinin+ CMs was counted per 104 nuclei in the peri-infarcted area, ranged within 200 μm from infarcted region, at 3 and 15 d after cell implantation. Images were analyzed using ImageJ software (http://imagej.nih.gov/ij/, 1.47V, NIH, United States).
Values are presented as mean ± SD. For continuous data, statistical significance was determined with the Mann-Whitney U test between 2 groups and the Kruskal-Wallis test followed by Tukey’s honest significant difference test with ranks or multiple-group comparison. Statistical analysis was performed with SAS 9.4 (SAS Institute Inc). Statistical significance was set at P < 0.05 or 0.01.
To investigate the regenerative potential of PDGFRα+ CLCs and αMHC+ CMs, cells were sorted, and approximately 1 × 106 of each were implanted into the left ventricular myocardium after inducing acute MI. The results of the recipient groups were compared with those of MI hearts without implantation. Analyses were performed at 2 wk after implantation of cells (Figure 1A). To trace the implanted cells in the infarcted heart, we induced PDGFRα+ CLCs from ESCs expressing tdTomato fluorescence (Figure 1B). As shown in Figure 1C, the implanted cells were mainly distributed along several myocardial cavities 1 h after implantation.
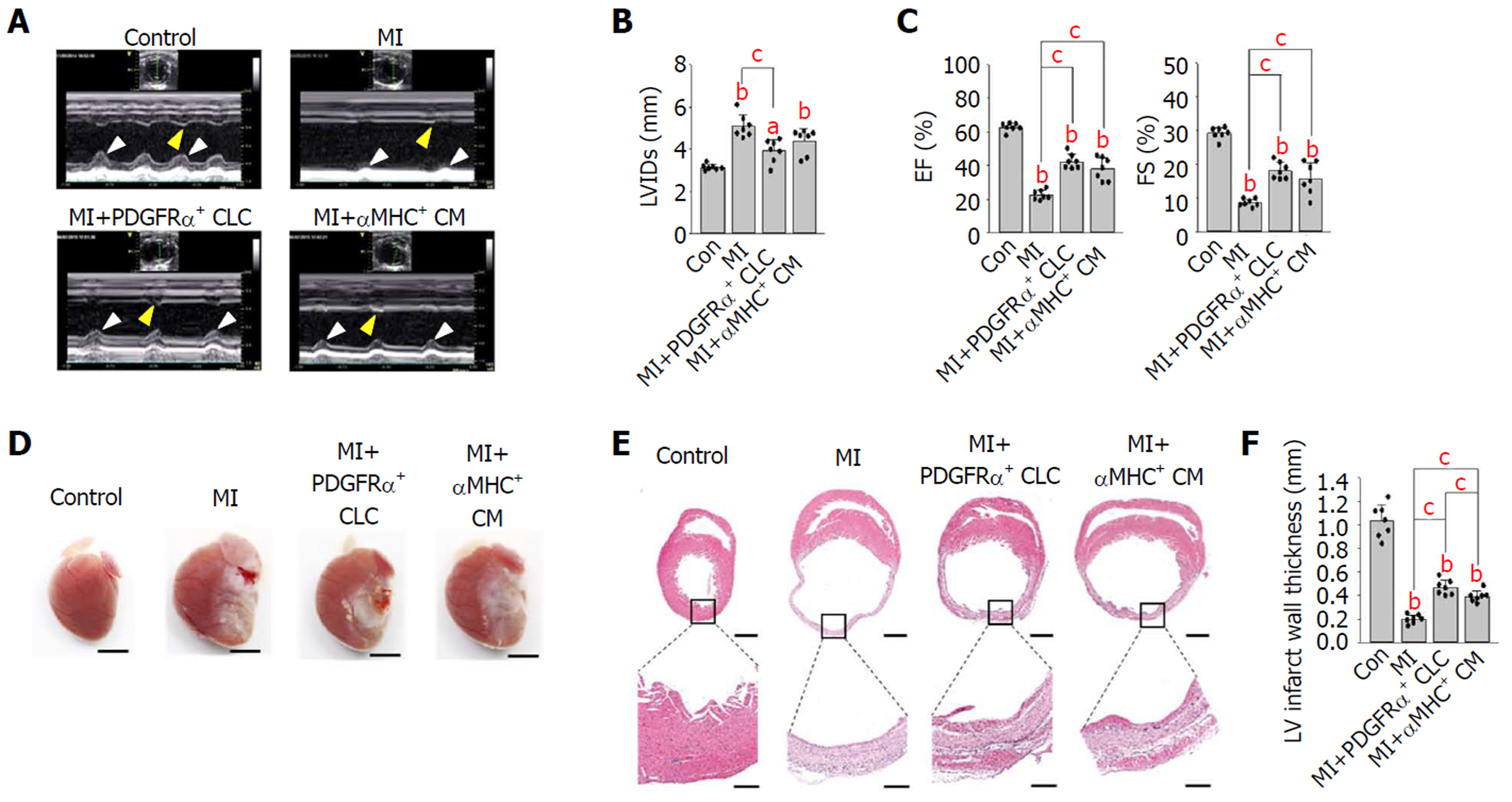
First, to evaluate the functional recovery of infarcted hearts after cell implantation, we performed TTE 14 d after implantation. Compared with that in untreated MI hearts, the anterior and septal regional wall motion was notably and similarly improved (see arrowheads in Figure 2A) in the MI hearts implanted with PDGFRα+ CLCs (hereafter designated as MI+PDGFRα+ CLCs) and αMHC+ CMs (designated as MI+αMHC+ CMs). Moreover, the left ventricular internal dimension during systole of both MI+PDGFRα+ CLCs and MI+αMHC+ CMs was approximately 12%–23% less compared with that of untreated MI hearts (Figure 2B). Both MI+PDGFRα+ CLCs and MI+αMHC+ CMs also showed significant and similar improvements in systolic functional parameters, which included an ejection fraction increased by 20.0/15.6% and fractional shortening increased by 9.5/7.2%, respectively, compared with those of untreated MI hearts (Figure 2C). All TTE parameters are summarized in Table 1. These findings indicate that the implantation of PDGFRα+ CLCs and αMHC+ CMs had similar beneficial effects in the functional recovery of acutely infarcted hearts. Next, to confirm whether the implanted cells were properly engrafted to the infarcted myocardium, we performed histologic analyses at 15 d after implantation. Overall, the gross sizes of MI+PDGFRα+ CLCs and MI+αMHC+ CMs were smaller than that of untreated MI hearts (Figure 2D). Hematoxylin and eosin staining showed that untreated MI hearts had a thinner ventricular wall (0.19 mm) than did controls, while the ventricular walls of MI+PDGFRα+ CLCs and MI+αMHC+ CMs were similarly thicker (0.47 mm and 0.39 mm, respectively) compared with that of untreated MI hearts (Figures 2E and F).
| Group | LVIDd | LVIDs | IVSd | IVSs | LVPWd | LVPWs | LVEDV | LVESV | LVSV | LVEF | FS |
| (mm) | (mm) | (mm) | (mm) | (mm) | (mm) | (mL) | (mL) | (mL) | (%) | (%) | |
| Control | 4.46 ± 0.23 | 3.14 ± 0.14 | 0.76 ± 0.07 | 1.08 ± 0.05 | 0.76 ± 0.10 | 1.11 ± 0.07 | 0.22 ± 0.04 | 0.08 ± 0.01 | 0.14 ± 0.03 | 62.7 ± 2.52 | 29.2 ± 1.72 |
| (n = 7) | |||||||||||
| MI | 5.57 ± 0.60 | 5.09 ± 0.54 | 0.67 ± 0.04 | 0.71 ± 0.07 | 0.74 ± 0.09 | 1.03 ± 0.17 | 0.42 ± 0.14 | 0.32 ± 0.10 | 0.10 ± 0.03 | 22.5 ± 2.91 | 8.61 ± 1.12 |
| (n = 7) | |||||||||||
| MI+PDGFRα+ CLCs | 4.96 ± 0.38 | 3.92b ± 0.54 | 0.68 ± 0.10 | 0.87 ± 0.27 | 0.68 ± 0.06 | 0.99 ± 0.13 | 0.21b ± 0.14 | 0.12b ± 0.08 | 0.09 ± 0.06 | 42.4b ± 4.38 | 18.1b ± 2.54 |
| (n = 7) | |||||||||||
| MI+αMHC+ CMs | 5.15 ± 0.56 | 4.38 ± 0.60 | 0.65 ± 0.04 | 0.69 ± 0.04 | 0.74 ± 0.07 | 1.08 ± 0.12 | 0.28a ± 0.14 | 0.18b ± 0.10 | 0.10 ± 0.05 | 38.1b ± 6.86 | 15.8b ± 4.79 |
| (n = 7) |
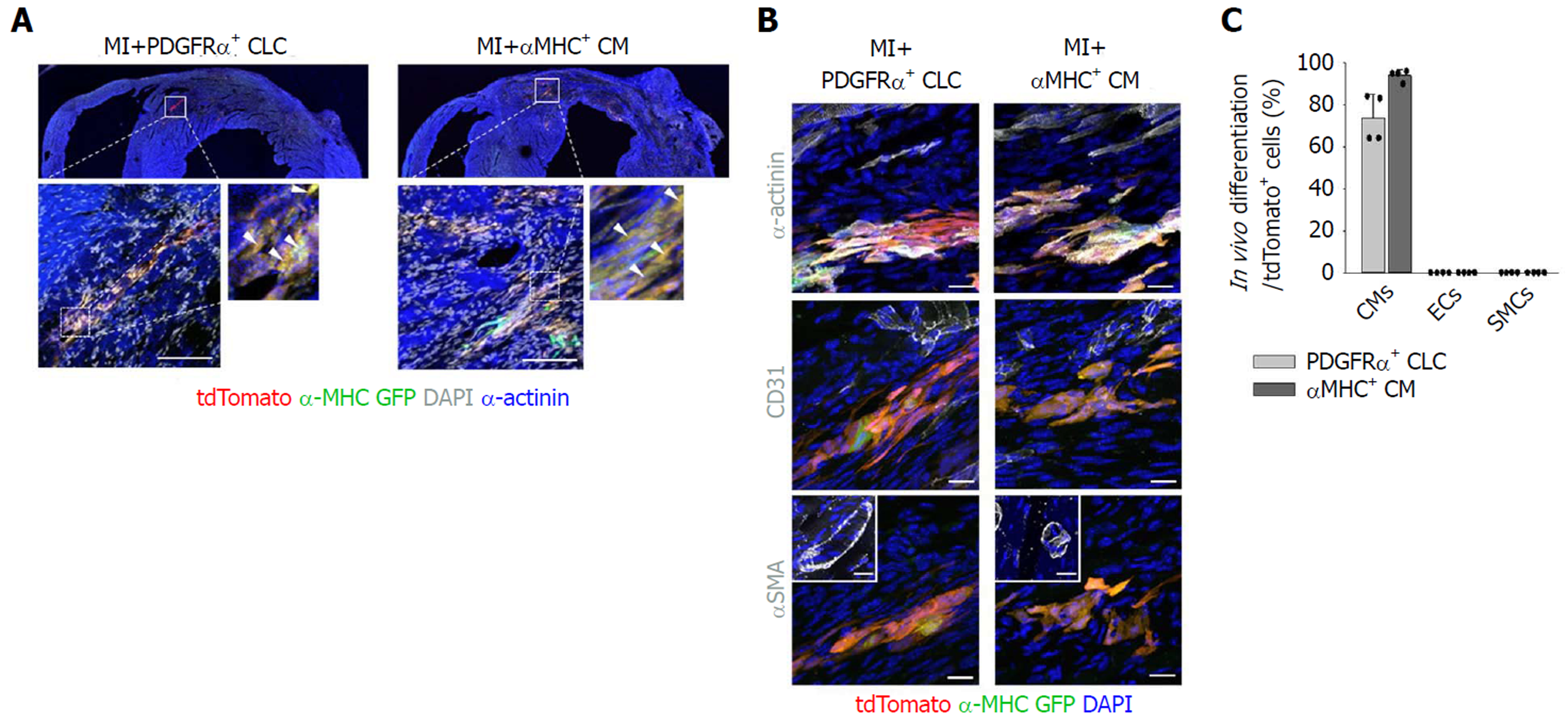
Importantly, implanted PDGFRα+ CLCs and αMHC+ CMs were visible as tdTomato+/α-MHC-GFP+ cells aligned and integrated with host CMs (Figure 3A). Implanted PDGFRα+ CLCs and αMHC+ CMs were mostly differentiated into α-actinin+ CMs, and they did not convert into CD31+ endothelial cells or αSMA+ mural cells (Figures 3B and C). Moreover, CD31+ blood vessels in the infracted area increased by 2.1- and 1.8-fold in MI+PDGFRα+ CLCs and MI+αMHC+ CMs at day 15 after implantation (Figures 4A and B), while the numbers of Ki-67+ CMs also transiently increased equally by 2.4-fold at day 3; no such increases were detected at day 15 in both groups (Figures 4C and D). Thus, in addition to integration of implanted MI+PDGFRα+ CLCs and MI+αMHC+ CMs into the host myocardium, paracrine effects of MI+PDGFRα+ CLCs and MI+αMHC+ CMs appeared to be involved in the functional recovery of acutely infarcted hearts. Both types of implanted cells persisted up to 60 d after implantation (Figure 4E), which was the longest observation period in this study.
In the present study, we demonstrated the regenerative potential of mouse ESC-derived PDGFRα+ CLCs in a murine MI model. Implantation of PDGFRα+ CLCs and αMHC+ CMs equally improved the contractile function and structure in the infarcted heart. Notably, implanted PDGFRα+ CLCs were well integrated with host CMs and mostly differentiated into CMs.
Various transcription factors and cell-surface markers of cardiac progenitors or CLCs have been identified in previous studies[6]. Our group developed PDGFRα+ CLCs induced by CsAYTE, which significantly enhanced the commitment of mesodermal cells to CLCs; in addition, the PDGFRα+ CLCs can spontaneously further differentiate into CMs without additional manipulation or stimulation under in vitro conditions[11]. However, there are few studies regarding the engraftment and regenerative potential of cardiac progenitors or CLCs compared with differentiated CMs after implantation under in vivo pathologic conditions[18,19]. Takeda et al[7] recently found that human iPSC-derived CM-fated progenitors from a subpopulation of kinase insert domain receptor (KDR)+ and PDGFRα+ cells express CD82[7]. Consistent with our findings, purified CD82+ cells gave rise to CMs under both in vitro and in vivo conditions[7]. Interestingly, CD82+ cells showed considerably greater engraftment than differentiated vascular cell adhesion molecule 1 (VCAM1)+ CMs after transplantation to the subrenal space[7]. These data indicated that the proliferative capacity of CLCs is higher than that of differentiated CMs under in vivo conditions. Furthermore, CD82+ cells primarily differentiated into CMs within infarcted hearts at approximately 95% efficiency; nevertheless, there were no data related to functional and structural recovery in the infarcted hearts[7]. The LIM-homeodomain transcription factor ISL1 is the most well-known marker of cardiac progenitors, and recent studies demonstrated that ISL1+ cardiac progenitors also exhibit regenerative potential in the infarcted heart[20,21]. Another developed strategy, direct reprogramming, was used to generate proliferative induced cardiac progenitors from fibroblasts with cardiac-specific transcription factors (Mesp1, Tbx5, Gata4, Nkx2.5, and Baf60c), and these reprogrammed cells were revealed to have regenerative potential in MI[22,23]. Collectively, the previous and current data provide compelling evidence that cardiac progenitors or CLCs are potential cellular resources for cardiac regeneration.
However, our data failed to demonstrate the superior regenerative effect of proliferative PDGFRα+ CLCs compared with differentiated αMHC+ CMs after implantation, consistent with a previous report[19]. Although PDGFRα+ CLCs exhibit more proliferative capacity than differentiated αMHC+ CMs, their expansion might be restricted owing to the limited space of the myocardium, especially in a small mouse model. Further experiments using large animal models, such as swine or non-human primates, might be necessary to confirm the regenerative effect of CLCs. In addition, the pathologic microenvironment of damaged heart might affect the proliferation and survival of implanted cells. Indeed, the previous and current data demonstrated that the engraftment of implanted CLCs and CMs was gradually decreased with time. Despite suboptimal engraftment and eventual death of the implanted cells in infarcted myocardium, the regenerative effect of implanted cells might result from differential paracrine effects[24]. Recent data demonstrated the significant upregulation of promigratory, proangiogenic, and antiapoptotic gene expression in the infarcted myocardium of groups treated with CMs compared with groups treated with PSCs and the controls[24]. Our data also revealed enhanced angiogenesis after implantation of PDGFRα+ CLCs and αMHC+ CMs. Therefore, the previous and current data suggested that not only direct integration but also the paracrine effect of implanted CLCs and CMs contributes to cardiac regeneration[24]. Further studies are needed to better understand the therapeutic mechanisms following transplantation of CLCs and to enhance engraftment.
Proper electromechanical integration of PSC-derived CMs into host myocardium is crucial for preventing fatal arrhythmia after transplantation[1]. In a recent study, Chong et al[25] reported remuscularization of infarcted myocardium after injection of human ESC-derived CMs into non-human primate models of MI[26]. These grafts formed electromechanical junctions with the host myocardium and beat in synchrony, but ventricular arrhythmias were noted after transplantation[25]. Another recent study showed that monkey iPSC-derived CMs improved cardiac contractile function after transplantation into infarcted monkey hearts; nonetheless, the incidence of ventricular tachycardia was transiently but significantly increased[27]. In our study, we could not evaluate the occurrence of ventricular arrhythmia because of the technical difficulties associated with the mouse model. Therefore, further studies using large animal models might be necessary to confirm the arrhythmogenic effect of proliferating CLCs compared with differentiated CMs after transplantation into infarcted heart.
In conclusion, PDGFRα+ CLCs served as the potential donor population for cardiac regeneration, and our findings provide conceptual and technical advances in stem cell therapy for cardiac regeneration.
Pluripotent stem cell (PSC)-derived cardiomyocytes (CMs) have become one of the most attractive cellular resources for cell-based therapy to rescue damaged cardiac tissue.
The proliferative capacity of PSC-derived CMs is decreased after beating and terminal differentiation. Furthermore, there is no definite surface marker of differentiated PSC-derived CMs to facilitate purification.
We investigated the regenerative potential of mouse embryonic stem cell-derived PDGFRα+ cardiac lineage-committed cells (CLCs) in a murine myocardial infarction (MI) model and compared their efficacy with differentiated CMs.
We implanted platelet-derived growth factor receptor-α (PDGFRα)+ CLCs and differentiated αMHC+ CMs into a MI murine model and performed functional analysis using transthoracic echocardiography (TTE) and histologic analysis.
Compared with the untreated MI hearts, the anterior and septal regional wall motion and systolic functional parameters were notably and similarly improved in the MI hearts implanted with PDGFRα+ CLCs and αMHC+ CMs based on TTE. In histologic analysis, the untreated MI hearts contained a thinner ventricular wall than did the controls, while the ventricular walls of MI hearts implanted with PDGFRα+ CLCs and αMHC+ CMs were similarly thicker compared with that of the untreated MI hearts. Furthermore, implanted PDGFRα+ CLCs aligned and integrated with host CMs and were mostly differentiated into α-actinin+ CMs, and they did not convert into CD31+ endothelial cells or αSMA+ mural cells.
PDGFRα+ CLCs from mouse ESCs exhibiting proliferative capacity showed a regenerative effect in infarcted myocardium. Therefore, mouse ESC-derived PDGFRα+ CLCs may represent a potential cellular resource for cardiac regeneration.
PDGFRα+ CLCs served as the potential donor population for cardiac regeneration, and our findings provide conceptual and technical advances in stem cell therapy for cardiac regeneration.
We thank Professor Gou Young Koh for technical advice and valuable discussions. We also thank Su Jin Seo and Sam Mi Yoo for their technical assistance.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Labusca L, Pixley JS, Wakao H S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 895] [Cited by in F6Publishing: 930] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 3. | Chow A, Stuckey DJ, Kidher E, Rocco M, Jabbour RJ, Mansfield CA, Darzi A, Harding SE, Stevens MM, Athanasiou T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Reports. 2017;9:1415-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation. 2018;137:1712-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 5. | Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31:829-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Skelton RJP, Kamp TJ, Elliott DA, Ardehali R. Biomarkers of Human Pluripotent Stem Cell-Derived Cardiac Lineages. Trends Mol Med. 2017;23:651-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Takeda M, Kanki Y, Masumoto H, Funakoshi S, Hatani T, Fukushima H, Izumi-Taguchi A, Matsui Y, Shimamura T, Yoshida Y, Yamashita JK. Identification of Cardiomyocyte-Fated Progenitors from Human-Induced Pluripotent Stem Cells Marked with CD82. Cell Rep. 2018;22:546-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Ishida H, Saba R, Kokkinopoulos I, Hashimoto M, Yamaguchi O, Nowotschin S, Shiraishi M, Ruchaya P, Miller D, Harmer S, Poliandri A, Kogaki S, Sakata Y, Dunkel L, Tinker A, Hadjantonakis AK, Sawa Y, Sasaki H, Ozono K, Suzuki K, Yashiro K. GFRA2 Identifies Cardiac Progenitors and Mediates Cardiomyocyte Differentiation in a RET-Independent Signaling Pathway. Cell Rep. 2016;16:1026-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Yoon C, Song H, Yin T, Bausch-Fluck D, Frei AP, Kattman S, Dubois N, Witty AD, Hewel JA, Guo H, Emili A, Wollscheid B, Keller G, Zandstra PW. FZD4 Marks Lateral Plate Mesoderm and Signals with NORRIN to Increase Cardiomyocyte Induction from Pluripotent Stem Cell-Derived Cardiac Progenitors. Stem Cell Reports. 2018;10:87-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Nelson DO, Lalit PA, Biermann M, Markandeya YS, Capes DL, Addesso L, Patel G, Han T, John MC, Powers PA, Downs KM, Kamp TJ, Lyons GE. Irx4 Marks a Multipotent, Ventricular-Specific Progenitor Cell. Stem Cells. 2016;34:2875-2888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Hong SP, Song S, Cho SW, Lee S, Koh BI, Bae H, Kim KH, Park JS, Do HS, Im I, Heo HJ, Ko TH, Park JH, Youm JB, Kim SJ, Kim I, Han J, Han YM, Koh GY. Generation of PDGFRα+ Cardioblasts from Pluripotent Stem Cells. Sci Rep. 2017;7:41840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Hirai H, Ogawa M, Suzuki N, Yamamoto M, Breier G, Mazda O, Imanishi J, Nishikawa S. Hemogenic and nonhemogenic endothelium can be distinguished by the activity of fetal liver kinase (Flk)-1 promoter/enhancer during mouse embryogenesis. Blood. 2003;101:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Yamashita JK, Takano M, Hiraoka-Kanie M, Shimazu C, Peishi Y, Yanagi K, Nakano A, Inoue E, Kita F, Nishikawa S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005;19:1534-1536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Kodama H, Nose M, Niida S, Nishikawa S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Rompani SB, Cepko CL. Retinal progenitor cells can produce restricted subsets of horizontal cells. Proc Natl Acad Sci USA. 2008;105:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463-8471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Cho SW, Park JS, Heo HJ, Park SW, Song S, Kim I, Han YM, Yamashita JK, Youm JB, Han J, Koh GY. Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells. J Am Heart Assoc. 2014;3:e000693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Chen L, Diaz AD, Benham A, Xu X, Wijaya CS, Fa'ak F, Luo W, Soibam B, Azares A, Yu W, Lyu Q, Stewart MD, Gunaratne P, Cooney A, McConnell BK, Schwartz RJ. Mesp1 Marked Cardiac Progenitor Cells Repair Infarcted Mouse Hearts. Sci Rep. 2016;6:31457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Fernandes S, Chong JJH, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, Murry CE. Comparison of Human Embryonic Stem Cell-Derived Cardiomyocytes, Cardiovascular Progenitors, and Bone Marrow Mononuclear Cells for Cardiac Repair. Stem Cell Reports. 2015;5:753-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Ghazizadeh Z, Fattahi F, Mirzaei M, Bayersaikhan D, Lee J, Chae S, Hwang D, Byun K, Tabar MS, Taleahmad S, Mirshahvaladi S, Shabani P, Fonoudi H, Haynes PA, Baharvand H, Aghdami N, Evans T, Lee B, Salekdeh GH. Prospective Isolation of ISL1+ Cardiac Progenitors from Human ESCs for Myocardial Infarction Therapy. Stem Cell Reports. 2018;10:848-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Bartulos O, Zhuang ZW, Huang Y, Mikush N, Suh C, Bregasi A, Wang L, Chang W, Krause DS, Young LH, Pober JS, Qyang Y. ISL1 cardiovascular progenitor cells for cardiac repair after myocardial infarction. JCI Insight. 2016;1:pii: e80920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Cao N, Huang Y, Spencer CI, Fu JD, Yu C, Liu K, Nie B, Xu T, Li K, Xu S, Bruneau BG, Srivastava D, Ding S. Expandable Cardiovascular Progenitor Cells Reprogrammed from Fibroblasts. Cell Stem Cell. 2016;18:368-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Lalit PA, Salick MR, Nelson DO, Squirrell JM, Shafer CM, Patel NG, Saeed I, Schmuck EG, Markandeya YS, Wong R, Lea MR, Eliceiri KW, Hacker TA, Crone WC, Kyba M, Garry DJ, Stewart R, Thomson JA, Downs KM, Lyons GE, Kamp TJ. Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors. Cell Stem Cell. 2016;18:354-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, Yang PC. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ Res. 2017;121:e22-e36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 948] [Cited by in F6Publishing: 967] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 26. | Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36:597-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 385] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 27. | Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 515] [Article Influence: 64.4] [Reference Citation Analysis (0)] |