Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1676
Peer-review started: December 27, 2023
First decision: February 5, 2024
Revised: February 17, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: May 15, 2024
Gastrointestinal (GI) cancer is a malignancy arising in the digestive system and accounts for approximately a third of increasing global cancer-related mortality, especially in the colorectum, esophagus, stomach, and liver. Interleukin-1β (IL-1β) is a leukocytic pyrogen recognized as a tumor progression-related cytokine. IL-1β secretion and maturation in inflammatory responses could be regulated by nu
Core Tip: Interleukin-1β (IL-1β) is a pro-inflammatory cytokine primarily secreted by leukocytes to activate the immune response at the site of infection or inflammation. As tumor-promoting inflammation is one of the cancer hallmarks, IL-1β then plays central roles in tumor-promoting activities in many cancers, including cancers of the gastrointestinal tract. On the other hand, by activating and recruiting immune cells into the tumor microenvironments, IL-1β also has anti-tumor effects depending on the subtypes of immune cells that respond and infiltrate into the tumor site. Whether it could be a promising target for future therapeutic development is then discussed in this article.
- Citation: Khawkhiaw K, Panaampon J, Imemkamon T, Saengboonmee C. Interleukin-1β: Friend or foe for gastrointestinal cancers. World J Gastrointest Oncol 2024; 16(5): 1676-1682
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1676.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1676
Tumor microenvironments are composed of various cell populations, not only the cancer cells themselves but also others, e.g., fibroblasts, endothelial cells, and infiltrated white blood cells[1]. The infiltrated white blood cells or immune cells that reside in the tumors create a biological niche in the so-called tumor immune microenvironment that interacts with the cancer cells via signals between cancer and immune cell communities. This communication between immune and cancer cells could direct the progression or recession of cancers depending on the subtypes of immune cells and the “media” of communication. The proinflammatory cytokine interleukin-1β (IL-1β) has a recognized central inflammatory signaling role in innate immunity in tissues and has been questioned for decades whether it possesses pro- or anti-tumorigenic effects[2]. As a player in innate immunity, IL-1β potentially possesses anti-tumor effects by activating antigen-presenting cells and phagocytes. However, this cytokine can be produced and released by both cancer cells and other cells in tumor microenvironments, suggesting different functions in tumor biology. Thus, IL-1β’s actual roles have been investigated extensively in gastrointestinal (GI) cancers for guidance in developing novel therapeutic methods.
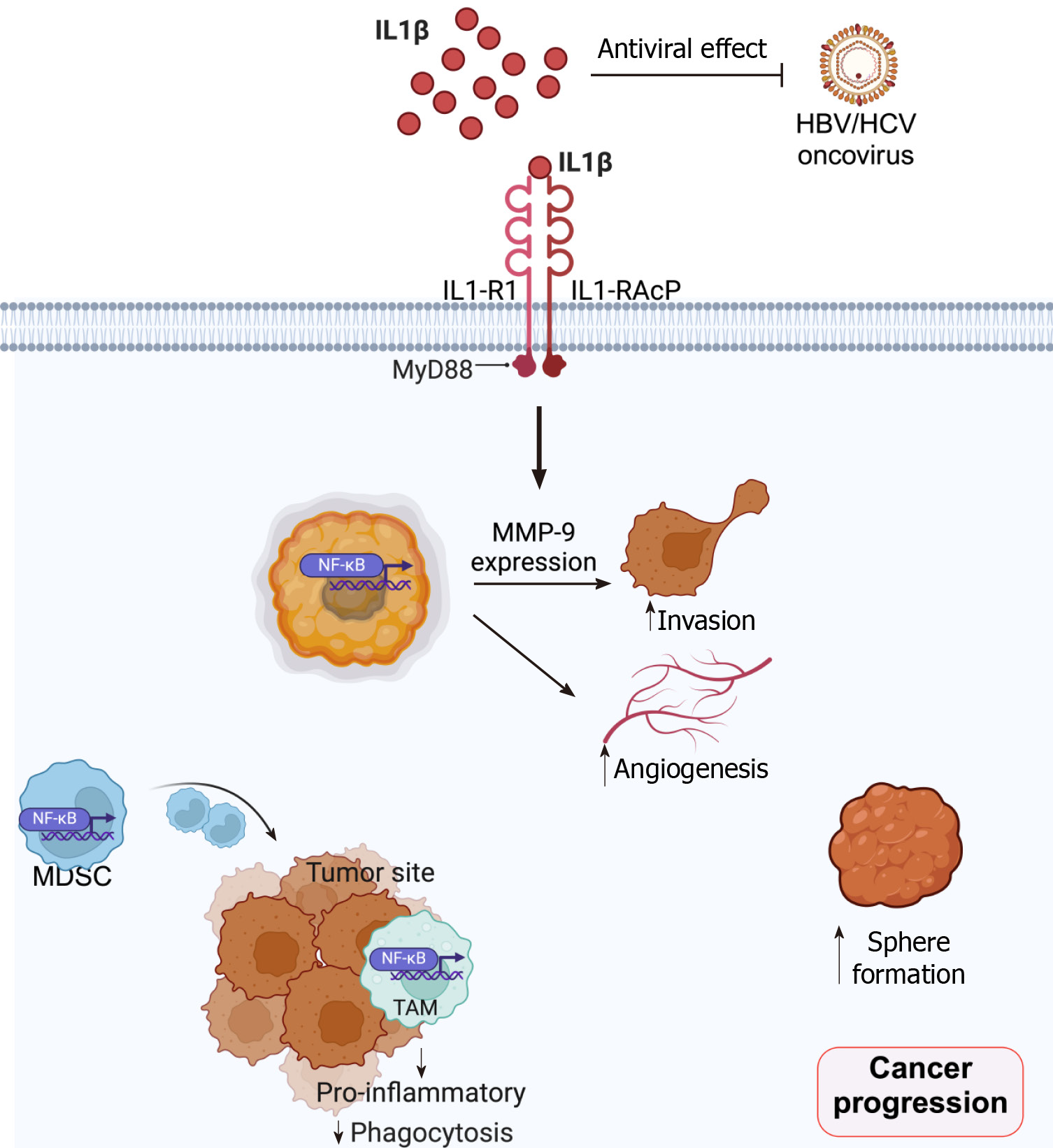
IL-1β, an influential pro-inflammatory cytokine, is synthesized and secreted by various cell types in tumor microenvironments[2], such as white blood cells, cancer-associated fibroblasts (CAFs), and cancer cells. Signaling of IL-1β was conventionally initiated by binding to interleukin-1 receptor (IL-1R) type 1 (IL-1R1), which presents three extra-cellular immunoglobulin binding domains and is associated with the highly homologous IL-1R accessory protein (IL-1RAcP or IL-1R3). These signaling functions can be controlled by several cell-secreted inhibitors, such as IL-1 receptor antagonist (IL-1RA), IL-1 receptor type II (IL-1RII), and other soluble receptors[3]. Upon activation, IL-1R/IL1-RAcP recruits myeloid differentiation primary response 88 (Myd88) through Toll/interleukin-1 receptor/resistance protein domains (Figure 1). Then, MyD88 associates with interleukin 1 receptor-associated kinase (IRAK) 1, IRAK 2, and IRAK4. IRAK4 then phosphorylates IRAK1 and IRAK2 to enable their association with tumor necrosis factor receptor 6, which further recruits and activates the transforming growth factor β-activated kinase 1 (TAK1). TAK1 then activates p38 and c-Jun N-terminal kinase, leading to the activation of the inhibitor nuclear factor-kappaB (NF-κB) kinase complex. This subsequently degrades the inhibitor of NF-κB, rendering NF-κB translocation from the cytosol into the nucleus[2,4]. IL-1β signaling plays various roles in cancer progression and malignancy, such as serving as an inducer of carcinogenesis, angiogenesis, and metastasis (Figure 1)[5]. Careful consideration is, then, needed when it is proposed as the therapeutic target for cancers.
GI cancers include malignancies along the alimentary tracts (esophagus, stomach, small intestine, colon-rectum, and anus) and the digestive accessory organs (liver, pancreas, and biliary tracts). It is the most common cancer group affecting both males and females worldwide. In this article, the roles of IL-1β in different types of GI cancer are reviewed and discussed.
Expression analysis based on a dataset indicated significantly elevated expression levels of IL-1β in esophageal cancer cases compared to normal samples[6]. A positive immunohistochemistry score for IL-1β correlated with a diminished response to neoadjuvant therapy and poorer overall survival among patients with esophageal squamous cell carcinoma (ESCC)[7]. In the context of ESCC, investigations revealed that the overexpression of IL-1RA, conversely, curtailed the proliferation, migration, and lymphangiogenesis of ESCC cells. This effect was attributed to the downregulation of vascular endothelial growth factor-C and matrix metalloproteinase 9 (MMP-9). Downregulation of IL-1RA was also observed in esophageal carcinomas[8]. Furthermore, in vivo assessments demonstrated a significant reduction in the growth rate of ESCC upon IL-1RA overexpression[9]. In another in vivo model, IL-1β transgenic mice exhibited inflammation-driven, age-dependent progression toward malignancy of the esophagus, characterized by squamous epithelial hyperplasia, dysplasia, and finally ESCC. Notably, this inflammation-based progression occurred independently of the gut microbiome[10]. Within the transgenic L2-IL1B mice cohort subjected to a high-fat diet to promote inflammation-based progressive esophageal adenocarcinoma[11], IL-1R antagonist and anti-inflammatory agent administration significantly diminished inflammatory scores and also led to a reduction in metaplasia and dysplasia scores in L2-IL1B mice exposed to a high-fat diet. All these findings emphasize the significant roles of IL-1β as a linking cytokine for chronic inflammation and esophageal cancer in both histological subtypes of squamous cell carcinoma and adenocarcinoma.
IL-1β exerts a multifaceted influence on the development and progression of gastric cancer, primarily through its capacity to inhibit gastric acid secretion, induce epigenetic alterations, facilitate angiogenesis, attract adhesive factors, and release various inflammatory mediators[12]. A meta-analysis revealed an association between IL-1B−511C/T polymorphisms and susceptibility to an intestinal subtype of gastric adenocarcinoma[13]. Within gastric carcinoma cell lines, such as BGC-823, IL-1β was observed to upregulate retinoid X receptors via the activation of NF-κB signaling pathways[14]. Additionally, IL-1β enhanced NF-κB activation, MMP-9 expression, and invasive capabilities of gastric cancer cells[15]. The gastric-specific overexpression of human IL-1β in transgenic mice was demonstrated to be sufficient for the progression of gastric dysplasia to cancer. Moreover, the activation of NF-κB in myeloid-derived suppressor cells (MDSCs) was significantly associated with cancer development. Notably, blockade by IL-1RA significantly impeded histological progression and diminished MDSC recruitment[16]. Examining the tumor microenvironment, the majority of tumor-associated macrophages (TAMs) exhibit expression of proinflammatory cytokine/chemokine genes, including IL1B, CCL2, CCL3, and CCL20. IL-1β and its decoy receptor IL1-R2 are also the most abundant cytokine–receptor pair interaction between TAMs and tumor cells. This suggests a potential mechanism where tumor cells can be inhibited by targeting IL-1β-mediated proinflammatory signaling within the tumor microenvironment[17].
Polymorphic variations within the IL1B gene, concomitant with heightened levels of IL-1β, have been significantly associated with an augmented susceptibility to the development of colon cancer[18]. Conversely, single-nucleotide poly
The examination of allele frequencies pertaining to IL-1B-511 revealed significant elevations among individuals afflicted with hepatitis B virus-associated hepatocellular carcinoma (HCC) compared to healthy controls[31]. Notably, within hepatitis C-infected individuals, the IL-1B-511 genotype T/T emerged as a risk factor for HCC. Polymorphic variations within the IL-1B-511 genetic locus were raised as possible risk factors for HCC development[32]. Mechanistically, miR-4756 inhibition reversed the effects induced by circUBAP2 silencing on the IL-17 and IL-1β levels and HCC cell migration[33]. Orthotopic liver tumor models and RNA-sequencing demonstrated a pivotal role for pulmonary IL-1β in creating a permissive lung pre-metastatic niche via enhancing MMP-9 expression and recruiting myeloid cells, thus promoting pulmonary metastasis of HCC[34]. In contrast, the hepatitis B virus modulated liver macrophage function to favor the establishment and likely maintenance of infection. It impaired the production of IL-1β, playing roles as antiviral cytokines, while promoting that of IL-10 in the microenvironment[35]. An in vitro model revealed that anakinra, an IL-1R1 antagonist, reversed IL-1β-promoted HCC metastasis[36]. IL-1β can promote TAMs and MDSCs infiltration via induction of solute carrier family 7-member 11 overexpression, thus up-regulating programmed death-ligand 1 and colony-stimulating factor 1 through the α-ketoglutarate/hypoxia-inducible factor 1 subunit alpha axis. These result in homeobox protein Hox-C10 overexpression and exacerbate HCC metastasis through the upregulation of phosphoinositide-depen
IL-1β expression in pancreatic tumors was associated with shorter survival of patients with pancreatic ductal adenocarcinoma (PDAC)[39]. Physical proximity with IL-1β+ TAMs was associated with inflammatory reprogramming and acquisition of pathogenic properties by a subset of PDAC cells[40]. Inhibiting IL-1β activity caused TAM reprogramming and antagonized tumor cell inflammation, leading to PDAC control in vivo. Tumor cell-derived IL-1β regulated an immune-modulatory program that supported pancreatic tumorigenesis[41]. IL-1β also served as a key cytokine that activated IRAK4 in pancreatic CAF[42,43]. Targeting IRAK4 or IL-1β thus rendered PDAC less fibrotic and more sensitive to gemcitabine in vivo.
IL-1β expression was significantly upregulated in gallbladder cancer (GBC) cases as compared with non-malignant cholelithiasis controls[44]. The study also showed that levels of IL-6, IL-1β, and IL-23 were significantly higher in GBC patients, whereas transforming growth factor-β was lower compared with healthy individuals[45]. In a north Indian population, the haplotype 1/C of IL1B was found to confer a significantly enhanced risk of GBC in patients with gallstones[46]. Exogenous IL-1β promoted the proliferation of GBC-SD and SGC996 cells in vitro and in vivo and also promoted migration in vitro via TWIST activation[47]. To date, most studies in GBC point toward the roles of IL-1β as a pro-tumorigenic cytokine; however, studies on the roles and clinical significance of IL-1R are still lacking.
A genetic polymorphism study revealed that the IL-1B +3954 C/C genotype was associated with a shorter overall sur
Although IL-1β plays central roles in inflammation and innate immunity that could potentially be a foe for retarding tumorigenesis and tumor cell progression, it is likely to become a friend for cancer cells in most GI cancers. Still, IL-1β possesses anti-tumor effects in some subtypes of cancers, possibly because of the underlying tumor microenvironments. The current understanding of IL-1β’s roles in GI cancer is summarized in Figure 1. Further studies on inhibiting the activations of IL-1β, hence, hold promise for developing a new approach for anti-tumor agents, especially for GI malignancies.
We would like to thank Prof. John F Smith for editing this manuscript via the KKU Publication Clinic, Khon Kaen University, Thailand (PCO-1151).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Medical Council of Thailand, No. 62243; The Medical Association of Thailand under the Patronage of HM the King, No. 28796.
Specialty type: Oncology
Country of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sivaraj N, India S-Editor: Fan JR L-Editor: A P-Editor: Zheng XM
| 1. | Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2019] [Cited by in F6Publishing: 3004] [Article Influence: 500.7] [Reference Citation Analysis (0)] |
| 2. | Rébé C, Ghiringhelli F. Interleukin-1β and Cancer. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 4. | Loiarro M, Ruggiero V, Sette C. Targeting TLR/IL-1R signalling in human diseases. Mediators Inflamm. 2010;2010:674363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Gelfo V, Romaniello D, Mazzeschi M, Sgarzi M, Grilli G, Morselli A, Manzan B, Rihawi K, Lauriola M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Zhao R, Chen X, Ren W, Dai H, Li H, Jia A, Wu Y, Han P, Shao Y. IL-1B rs2853550 polymorphism contributes to esophageal cancer susceptibility in Chinese Han population of Northwest China. Mol Med. 2020;26:57. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 7. | Chen MF, Lu MS, Chen PT, Chen WC, Lin PY, Lee KD. Role of interleukin 1 beta in esophageal squamous cell carcinoma. J Mol Med (Berl). 2012;90:89-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Chen S, Shen Z, Liu Z, Gao L, Han Z, Yu S, Kang M. IL-1RA suppresses esophageal cancer cell growth by blocking IL-1α. J Clin Lab Anal. 2019;33:e22903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Shen Z, Zhang P, Zhang W, Luo F, Xu H, Chen S, Kang M. IL-1RA inhibits esophageal carcinogenesis and lymphangiogenesis via downregulating VEGF-C and MMP9. Funct Integr Genomics. 2023;23:164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 10. | Muthupalani S, Annamalai D, Feng Y, Ganesan SM, Ge Z, Whary MT, Nakagawa H, Rustgi AK, Wang TC, Fox JG. IL-1β transgenic mouse model of inflammation driven esophageal and oral squamous cell carcinoma. Sci Rep. 2023;13:12732. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Baumeister T, Ingermann J, Marcazzan S, Fang HY, Oellinger R, Rad R, Engleitner T, Kleigrewe K, Anand A, Strangmann J, Schmid RM, Wang TC, Quante M. Anti-inflammatory chemoprevention attenuates the phenotype in a mouse model of esophageal adenocarcinoma. Carcinogenesis. 2021;42:1068-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Yin S, Lan C, Pei H, Zhu Z. Expression of interleukin 1β in gastric cancer tissue and its effects on gastric cancer. Onco Targets Ther. 2016;9:31-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Xu J, Yin Z, Cao S, Gao W, Liu L, Yin Y, Liu P, Shu Y. Systematic review and meta-analysis on the association between IL-1B polymorphisms and cancer risk. PLoS One. 2013;8:e63654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Ren HY, Huang GL, Liu WM, Zhang W, Liu Y, Su GQ, Shen DY. IL-1β induced RXRα overexpression through activation of NF-κB signaling in gastric carcinoma. Biomed Pharmacother. 2016;78:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yamanaka N, Morisaki T, Nakashima H, Tasaki A, Kubo M, Kuga H, Nakahara C, Nakamura K, Noshiro H, Yao T, Tsuneyoshi M, Tanaka M, Katano M. Interleukin 1beta enhances invasive ability of gastric carcinoma through nuclear factor-kappaB activation. Clin Cancer Res. 2004;10:1853-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 633] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 17. | Eum HH, Kwon M, Ryu D, Jo A, Chung W, Kim N, Hong Y, Son DS, Kim ST, Lee J, Lee HO, Park WY. Tumor-promoting macrophages prevail in malignant ascites of advanced gastric cancer. Exp Mol Med. 2020;52:1976-1988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1126-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Graziano F, Ruzzo A, Canestrari E, Loupakis F, Santini D, Rulli E, Humar B, Galluccio N, Bisonni R, Floriani I, Maltese P, Falcone A, Tonini G, Catalano V, Fontana A, Giustini L, Masi G, Vincenzi B, Alessandroni P, Magnani M. Variations in the interleukin-1 receptor antagonist gene impact on survival of patients with advanced colorectal cancer. Pharmacogenomics J. 2009;9:78-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Liu L, Zhai Z, Wang D, Ding Y, Chen X, Wang Q, Shu Z, Wu M, Chen L, He X, Fan D, Pan F, Xing M. The association between IL-1 family gene polymorphisms and colorectal cancer: A meta-analysis. Gene. 2021;769:145187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Liu H, Zeng J, Huang W, Xu Q, Ye D, Sun R, Zhang D. Colorectal Cancer Is Associated with a Deficiency of Lipoxin A(4), an Endogenous Anti-inflammatory Mediator. J Cancer. 2019;10:4719-4730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 23. | Koncina E, Nurmik M, Pozdeev VI, Gilson C, Tsenkova M, Begaj R, Stang S, Gaigneaux A, Weindorfer C, Rodriguez F, Schmoetten M, Klein E, Karta J, Atanasova VS, Grzyb K, Ullmann P, Halder R, Hengstschläger M, Graas J, Augendre V, Karapetyan YE, Kerger L, Zuegel N, Skupin A, Haan S, Meiser J, Dolznig H, Letellier E. IL1R1(+) cancer-associated fibroblasts drive tumor development and immunosuppression in colorectal cancer. Nat Commun. 2023;14:4251. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 24. | Hai Ping P, Feng Bo T, Li L, Nan Hui Y, Hong Z. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch Biochem Biophys. 2016;604:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Teimourian S, Moghanloo E. Role of PTEN in neutrophil extracellular trap formation. Mol Immunol. 2015;66:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Eissing M, Ripken L, Schreibelt G, Westdorp H, Ligtenberg M, Netea-Maier R, Netea MG, de Vries IJM, Hoogerbrugge N. PTEN Hamartoma Tumor Syndrome and Immune Dysregulation. Transl Oncol. 2019;12:361-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Wang G, Li Y, Wang P, Liang H, Cui M, Zhu M, Guo L, Su Q, Sun Y, McNutt MA, Yin Y. PTEN regulates RPA1 and protects DNA replication forks. Cell Res. 2015;25:1189-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 553] [Cited by in F6Publishing: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 29. | Bent R, Moll L, Grabbe S, Bros M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 30. | Qian H, Zhang D, Bao C. Two variants of Interleukin-1B gene are associated with the decreased risk, clinical features, and better overall survival of colorectal cancer: a two-center case-control study. Aging (Albany NY). 2018;10:4084-4092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Hirankarn N, Kimkong I, Kummee P, Tangkijvanich P, Poovorawan Y. Interleukin-1beta gene polymorphism associated with hepatocellular carcinoma in hepatitis B virus infection. World J Gastroenterol. 2006;12:776-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Tanaka Y, Furuta T, Suzuki S, Orito E, Yeo AE, Hirashima N, Sugauchi F, Ueda R, Mizokami M. Impact of interleukin-1beta genetic polymorphisms on the development of hepatitis C virus-related hepatocellular carcinoma in Japan. J Infect Dis. 2003;187:1822-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Liu G, Sun J, Yang ZF, Zhou C, Zhou PY, Guan RY, Sun BY, Wang ZT, Zhou J, Fan J, Qiu SJ, Yi Y. Cancer-associated fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. 2021;12:260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 34. | Zhang C, Li Q, Xu Q, Dong W, Li C, Deng B, Gong J, Zhang LZ, Jin J. Pulmonary interleukin 1 beta/serum amyloid A3 axis promotes lung metastasis of hepatocellular carcinoma by facilitating the pre-metastatic niche formation. J Exp Clin Cancer Res. 2023;42:166. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 35. | Faure-Dupuy S, Delphin M, Aillot L, Dimier L, Lebossé F, Fresquet J, Parent R, Matter MS, Rivoire M, Bendriss-Vermare N, Salvetti A, Heide D, Flores L, Klumpp K, Lam A, Zoulim F, Heikenwälder M, Durantel D, Lucifora J. Hepatitis B virus-induced modulation of liver macrophage function promotes hepatocyte infection. J Hepatol. 2019;71:1086-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 36. | He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, Wang Y, Liu D, Xie M, Ji X, Sun M, Tian D, Xia L. IL-1β-Induced Elevation of Solute Carrier Family 7 Member 11 Promotes Hepatocellular Carcinoma Metastasis Through Up-regulating Programmed Death Ligand 1 and Colony-Stimulating Factor 1. Hepatology. 2021;74:3174-3193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 37. | Dang Y, Chen J, Feng W, Qiao C, Han W, Nie Y, Wu K, Fan D, Xia L. Interleukin 1β-mediated HOXC10 Overexpression Promotes Hepatocellular Carcinoma Metastasis by Upregulating PDPK1 and VASP. Theranostics. 2020;10:3833-3848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Su B, Luo T, Zhu J, Fu J, Zhao X, Chen L, Zhang H, Ren Y, Yu L, Yang X, Wu M, Feng G, Li S, Chen Y, Wang H. Interleukin-1β/Iinterleukin-1 receptor-associated kinase 1 inflammatory signaling contributes to persistent Gankyrin activation during hepatocarcinogenesis. Hepatology. 2015;61:585-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Takahashi R, Macchini M, Sunagawa M, Jiang Z, Tanaka T, Valenti G, Renz BW, White RA, Hayakawa Y, Westphalen CB, Tailor Y, Iuga AC, Gonda TA, Genkinger J, Olive KP, Wang TC. Interleukin-1β-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte-mediated immune suppression. Gut. 2021;70:330-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Caronni N, La Terza F, Vittoria FM, Barbiera G, Mezzanzanica L, Cuzzola V, Barresi S, Pellegatta M, Canevazzi P, Dunsmore G, Leonardi C, Montaldo E, Lusito E, Dugnani E, Citro A, Ng MSF, Schiavo Lena M, Drago D, Andolfo A, Brugiapaglia S, Scagliotti A, Mortellaro A, Corbo V, Liu Z, Mondino A, Dellabona P, Piemonti L, Taveggia C, Doglioni C, Cappello P, Novelli F, Iannacone M, Ng LG, Ginhoux F, Crippa S, Falconi M, Bonini C, Naldini L, Genua M, Ostuni R. IL-1β(+) macrophages fuel pathogenic inflammation in pancreatic cancer. Nature. 2023;623:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 41. | Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020;80:1088-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 42. | Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, Tuveson DA. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 711] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 43. | Zhang D, Li L, Jiang H, Li Q, Wang-Gillam A, Yu J, Head R, Liu J, Ruzinova MB, Lim KH. Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018;78:1700-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 44. | Chaturmohta A, Dixit R, Narayan G, Gupta P, Prasad SB, Yadav S, Shukla VK. Do Expression Profiles of Cytokines VEGF, TNF- α, IL-1β, IL-6 and IL-8 Correlate with Gallbladder Cancer? J Cancer Sci Clin Oncol. 2015;2:202. [DOI] [Cited in This Article: ] |
| 45. | Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139:869-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 46. | Vishnoi M, Pandey SN, Choudhuri G, Mittal B. IL-1 gene polymorphisms and genetic susceptibility of gallbladder cancer in a north Indian population. Cancer Genet Cytogenet. 2008;186:63-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Guo R, Qin Y, Shi P, Xie J, Chou M, Chen Y. IL-1β promotes proliferation and migration of gallbladder cancer cells via Twist activation. Oncol Lett. 2016;12:4749-4755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Lurje I, Gaisa NT, Dahl E, Knüchel R, Strnad P, Trautwein C, Tacke F, Neumann UP, Czigany Z, Lurje G. Genetic polymorphisms in interleukin-1β (rs1143634) and interleukin-8 (rs4073) are associated with survival after resection of intrahepatic cholangiocarcinoma. Sci Rep. 2023;13:12283. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 49. | Song J, Li Y, Bowlus CL, Yang G, Leung PSC, Gershwin ME. Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis (PSC): a Comprehensive Review. Clin Rev Allergy Immunol. 2020;58:134-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 50. | Kunzmann LK, Schoknecht T, Poch T, Henze L, Stein S, Kriz M, Grewe I, Preti M, Hartl J, Pannicke N, Peiseler M, Sebode M, Zenouzi R, Horvatits T, Böttcher M, Petersen BS, Weiler-Normann C, Hess LU, Ahrenstorf AE, Lunemann S, Martrus G, Fischer L, Li J, Carambia A, Kluwe J, Huber S, Lohse AW, Franke A, Herkel J, Schramm C, Schwinge D. Monocytes as Potential Mediators of Pathogen-Induced T-Helper 17 Differentiation in Patients With Primary Sclerosing Cholangitis (PSC). Hepatology. 2020;72:1310-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |