Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1134
Peer-review started: December 29, 2023
First decision: January 20, 2024
Revised: January 26, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: April 15, 2024
Pancreatic cancer (PC) is characterized by its extremely aggressive nature and ranks 14th in the number of new cancer cases worldwide. However, due to its complexity, it ranks 7th in the list of the most lethal cancers worldwide. The pathogenesis of PC involves several complex processes, including familial genetic factors associated with risk factors such as obesity, diabetes mellitus, chronic pancreatitis, and smoking. Mutations in genes such as KRAS, TP53, and SMAD4 are linked to the appearance of malignant cells that generate pancreatic lesions and, consequently, cancer. In this context, some therapies are used for PC, one of which is immunotherapy, which is extremely promising in various other types of cancer but has shown little response in the treatment of PC due to various resistance mechanisms that contribute to a drop in immunotherapy efficiency. It is therefore clear that the tumor microenvironment (TME) has a huge impact on the resistance process, since cellular and non-cellular elements create an immunosuppressive environment, characterized by a dense desmoplastic stroma with cancer-associated fibroblasts, pancreatic stellate cells, extracellular matrix, and immuno
Core Tip: This study aims to analyze the main mechanisms of resistance to pancreatic cancer immunotherapy and the respective methods of manipulating these processes. Thus, this review provides a compilation of the main mechanisms of resistance to immunotherapy linked to the tumor microenvironment, genetic factors and those linked to T-cell immunosuppression. Finally, this study provides an insight into new avenues that can be followed to manipulate the factors linked to resistance, providing a more efficient treatment and a reduction in lethality.
- Citation: Silva LGO, Lemos FFB, Luz MS, Rocha Pinheiro SL, Calmon MDS, Correa Santos GL, Rocha GR, de Melo FF. New avenues for the treatment of immunotherapy-resistant pancreatic cancer. World J Gastrointest Oncol 2024; 16(4): 1134-1153
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1134.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1134
Pancreatic cancer (PC) is an extremely complex disease and represents a major challenge for oncology. Characterized by its highly aggressive nature, PC ranks 14th among cancers with the highest number of new cases worldwide, with around 495773 cases reported in 2020 and an overall 5-year survival rate of 11%[1,2]. Moreover, PC also garners attention due to its high lethality and aggressiveness, accounting for 466003 new deaths and securing the 7th position on the list of the most lethal types of cancer in 2020[1]. Additionally, the high level of complexity involved in managing PC stems from its late diagnosis and potent metastatic capability, which compromises treatment and prognosis[3].
The pathogenesis of PC involves a combination of factors related to life history and genetic alterations, ultimately leading to an individual's susceptibility[4]. The primary risk factors for developing PC include a family history of the disease, chronic pancreatitis, genetic disorders, smoking, and poor dietary habits[4-6]. Regarding genetic alterations, cancer can stem from mutations in tumor suppressor genes and oncogenes, such as KRAS, CDKN2A, TP53, SMAD4, and BRCA1/2[7].
The treatment of PC relies on surgery, chemotherapy, radiotherapy, and immunotherapy[8]. However, this process is extremely complex due to the elevated rates of metastases that impede surgery in the majority of patients, the intricate nature of the surgical approach due to anatomical challenges, and the mechanisms of resistance to chemotherapy and immunotherapy present in the tumor microenvironment (TME) of PC[9,10].
It is important to note that immunotherapy has emerged as a crucial treatment in recent years for various types of cancer[11]. Nevertheless, resistance to these methods in PC underscores the necessity of comprehending these mechanisms to develop efficient strategies for addressing this new challenge. Therefore, this study aims to report the main mechanisms in PC that lead to resistance to immunotherapy and the new ways to overcome this obstacle.
The development of PC is notably linked to the extensive plasticity of acinar and ductal cells in pancreatic tissue[12]. In physiological situations, these cells already possess a wide capacity for cellular metaplasia (transdifferentiation) for regenerative purposes[12]. This potential for identity reprogramming extends beyond regenerative processes, becoming a favorable factor for pancreatic carcinogenesis[12]. Besides genetic familiar factors, non-hereditary risk events are associated with an increased risk of PC, including obesity[13], chronic pancreatitis[14], cigarette smoking[15], and diabetes mellitus, especially new-onset diabetes mellitus after the 5th decade of life[16].
The onset of PC occurs through the malignant evolution of non-invasive precursor pancreatic lesions, which, according to a prospective epidemiological study, become significantly more common, larger, and more numerous with aging[17-19]. However, the presence of non-invasive precursor pancreatic lesions does not necessarily imply future malignancy; the risk varies according to the level of identified dysplasia[20]. A 2015 international consensus recommends stratifying pancreatic lesions into two levels: "low-grade" for lesions with mild to moderate dysplasia and "high-grade," reserved for lesions with severe dysplasia ("carcinoma in situ" type), exhibiting significantly increased potential for progression to invasive carcinoma[21]. Morphologically, there are three primarily forms of noninvasive precursor lesions, including pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm (MCN)[17,21].
PanIN are noncystic proliferative lesions located in pancreatic ducts up to 5 mm, characterized by the replacement of normal cuboid/columnar epithelial tissue by flat/papillary epithelial cells with varying levels of cytological and architectural atypia[22]. Low-grade PanINs (traditionally described as PanIN 1A, PanIN 1B, and PanIN 2) exhibit mild-to-moderate cytological atypia[23]. In contrast, high-grade PanINs (or PanIN 3) are predominantly papillary, featuring loss of polarity, irregular stratification, severe cytological atypia, and eventual intraluminal necrosis[24].
In addition, some alterations in oncogenes or tumor suppressor genes underlying PC are identified early in PanIN. The endogenous expression of the KRASG12D mutation, associated with telomere shortening, represents the first events in low-grade PanINs, driving all stages of PanIN progression to pancreatic adenocarcinoma (PDAC)[25-27]. The frequency of the KRASG12D mutation in PanINs increases according to the degree of dysplasia, becoming more frequent in high-grade PanINs, and being present in > 90% of PDAC cases[24,28,29].
Conversely, mutations in tumor suppressors TP53, which plays a role in cellular damage repair and apoptosis, and SMAD4, whose protein is a transcription factor for growth inhibition and apoptosis-related genes, are widely described in established PDAC[30-33]. However, these mutations appear almost exclusively in advanced neoplastic stages (high-grade PanINs)[34], being rarely found in isolated PanIN lesions, i.e., without invasive PC[35-37].
IPMN are macroscopic mucin (MUC)-producing cystic neoplasms that communicate with the pancreatic ductal system[38]. Similar to PanINs, IPMNs are predominantly composed of columnar cells with a papillary configuration, exhibiting varying degrees of cytological atypia[38]. However, they substantially differ in diameter, generally being larger than 1.0 cm in IPMNs[38]. IPMNs can be classified based on the duct involved: Main-duct or branch-duct type; or the predominant cell type: Pancreatobiliary, intestinal, or gastric types[24]. Main-duct IPMNs are most frequently associated with high-grade dysplasia, generally consisting of pancreatobiliary- and intestinal-cell types, which are associated, respectively, with an increased risk of tubular adenocarcinomas and colloid carcinomas, according to recent meta-analysis[39-41].
An IPMN lesion may be accompanied by an invasive carcinoma in two ways[42]. The first scenario involves the IPMN serving as a direct precursor to the existing carcinoma, commonly main duct IPMNs accompanied by colloid carcinomas[42]. The second possibility is the coexistence of an IPMN alongside an independently established carcinoma, with branch-duct IPMNs being more likely in this context[42]. Main risk factors for IPMN progression to PDAC include main pancreatic duct dilation, a size of 3 cm, and the presence of associated solid components[43,44].
Like PanINs, KRAS mutations are consistently reported in IPMNs, tipically manifesting in early stages of dysplasia (low-grade)[45]. Mutations in TP53 and SMAD4 tend to develop in more advanced stages of neoplasia[46,47]. SMAD4 mutation is strongly associated with neoplastic capacity[47]. GNAS mutations are frequent and highly specific to IPMNs, potentially aiding in their differentiaton from other cystic lesions[48].
Also in this context, MCN is the least common precursor of PC, and are almost exclusive to women aged 40-50 years[24]. Similarly to IPMNs, MCNs are composed of MUC-producing columnar epithelial cells, but differ in the presence of a subepithelial ovarian-type stroma, a pathognomonic finding of MCN[48,49]. Generally located in the body and tail of the pancreas (with less than 10% in the pancreatic head), MCNs are rarely multifocal[25,50]. In comparison to IPMNs, MCNs have a lower risk of evolving into invasive carcinoma[51]. Predictive factors for malignancy include cyst diameter and the presence of mural nodules[51]. While MCNs typically exhibit slow growth, high exposure to sex hormones during pregnancy can trigger rapid enlargement[52]. Histological types of invasive carcinoma frequently associated with MCNs include tubular adenocarcinomas, mucinous non-cystic (colloid) carcinomas, undifferentiated carcinomas, undifferentiated carcinomas with osteoclast-like giant cells, adenosquamous carcinomas, and sarcomas[25]. While there is a lack of studies focusing on the genetic bases of MCNs, mutations in KRAS, TP53, SMAD4, and CDKN2A/p16 have been verified[53].
Finally, it is important to note that the TME plays a fundamental role in the establishment and persistence of pancreatic neoplasia. Histologically, the TME of PDAC is characterized by a dense stroma composed of cellular and acellular components, initiating development from the early stages of neoplastic precursors[54]. The cellular component of the stroma forms a network that includes: Myeloid cells (macrophages, neutrophils, regulatory cells, cytotoxic cells), cancer-associated fibroblasts (CAFs), neurons, and endothelial cells[54]. Interaction between these agents co-stimulates the production of molecules such as growth factors, matricellular proteins, tissue inhibitors of metalloproteinases, and cytokines[54]. Such structural changes are intimately related to tumor maintenance and progression, altering vascular density and tissue perfusion[29].
The management of PC is contingent upon the disease stage. Consequently, the application of surgical interventions is reserved for individuals presenting with resectable tumors devoid of distant metastases, possibly in conjunction with adjuvant chemotherapy[17]. Within this framework, surgery is undertaken with the objective of achieving complete tumor resection, thereby fostering a more favorable prognosis for the patient. However, pancreatic tumor excisions constitute anatomically intricate procedures, frequently culminating in incomplete resection[55]. Moreover, the pancreatoduodenectomy, a commonly employed procedure, is associated with a morbidity rate of up to 45%[55]. This is compounded by the circumstance that a considerable proportion of PC diagnoses occur at an advanced stage, characterized by metastasis, rendering surgery unviable. This elucidates the intricacies associated with performing surgical interventions on pancreatic tumors[56].
Chemotherapeutic interventions for PC encompass three distinct regimens: Neoadjuvant, adjuvant, or first-line strategies[55]. The neoadjuvant approach is employed preemptively, preceding surgical resection, with the aim of diminishing tumor size. Conversely, the adjuvant regimen is administered post-surgical resection, while patients with metastatic PC receive first-line chemotherapy[55,57]. Noteworthy chemotherapy protocols for PC include gemcitabine, nab-paclitaxel, and folinic acid, 5-fluorouracil, irinotecan and oxaliplatin[57]. However, the anticipated efficacy of chemotherapy in treating PC has not been fully realized, as the intricate oncological landscape of PC is characterized by pronounced chemoresistance[58]. Within this context, gemcitabine emerges as the chemotherapy agent exhibiting the highest degree of chemoresistance to date. This phenomenon can be attributed to various factors inherent in PC, such as components of the TME, the release of inflammatory enzymes, altered signaling pathways involving cells like fibroblasts and pancreatic stellate cells (PSCs), and genetic alterations, including microRNA (miRNA)[58].
Radiotherapy has been incorporated into neoadjuvant, adjuvant, and first-line treatment regimens for patients with metastatic and advanced PC[59]. While chemoradiotherapy in neoadjuvant and adjuvant settings has demonstrated a marginal increase in patient survival, the majority of diagnoses occur at an advanced disease stage. Consequently, the use of chemotherapy and radiotherapy as first-line treatments becomes imperative[59,60]. Nevertheless, the application of radiotherapy in the treatment of patients with metastatic PC yields conflicting data and falls short of anticipated effectiveness. This underscores the necessity for novel clinical studies dedicated to scrutinizing the role and efficacy of radiotherapy in addressing the complexities of this disease[60].
Immunotherapy stands as a groundbreaking frontier in the realm of cancer treatment. The concept of leveraging the body's own immune system to target cancerous cells has brought about a profound shift in the overall survival (OS) rates for several types of cancer[61-63]. Moreover, it distinguishes itself by presenting fewer side effects in comparison to conventional approaches, such as chemotherapy[64].
In the context of PC, however, the use of immunotherapy, particularly immune checkpoint inhibitors (ICIs), as a standalone treatment in unselected patients has not demonstrated the same level of success observed in other tumor types[65]. From this perspective, anti-CTLA-4 drugs are already a reality in immunotherapy treatment, and drugs of this class, such as Ipilimumab, have received approval in both the United States and Europe[66]. However, both Ipilimumab and Tremelimumab (anti-CTLA-4) proved unsuccessful in clinical trials focused on treating PC[67,68]. It is also important to mention the anti-programmed cell death (PD)-1/PD-L1 drugs, with Nivolumab and Pembrolizumab being the primary representatives; however, these drugs have not demonstrated significant success in studies targeting PC, largely due to the complexity of this cancer model[66,69,70].
In another scenario, the investigation of vaccines in PC therapy has also become a subject of study in the eager pursuit of an efficient treatment to combat resistance to this complex cancer[71]. Thus, various vaccine models already exist in the scientific world and are currently undergoing testing for PC, with the primary ones being GVAX (cell-based) and vaccination with Listeria monocytogenes[71]. However, these methods have also demonstrated limitations in the ongoing analyses[71].
Finally, it is important to highlight Adoptive Cell Transfer as an immunotherapy model that has also been employed in treating PC[72]. The method is based on CAR T cells produced from T cells extracted from an individual and genetically altered to enhance their efficiency against cancer when reintroduced into the patient[73]. Despite this, clinical studies targeting various aspects of adoptive cell therapy (ACT) for PC, such as mesothelin and epidermal growth factor receptor, have demonstrated limited responses and minimal impact on patient survival[74,75].
Therefore, it is clear that understanding the mechanisms in the PC TME that induce resistance to immunotherapy treatment is crucial for developing new techniques to overcome the complexity imposed by this oncological model.
The TME of PC is characterized by a complex network of cellular and non-cellular elements that create a highly immunosuppressive environment. It is composed of a dense desmoplastic stroma, comprising CAFs, PSCs, extracellular matrix (ECM) and immune suppressive cells[76].
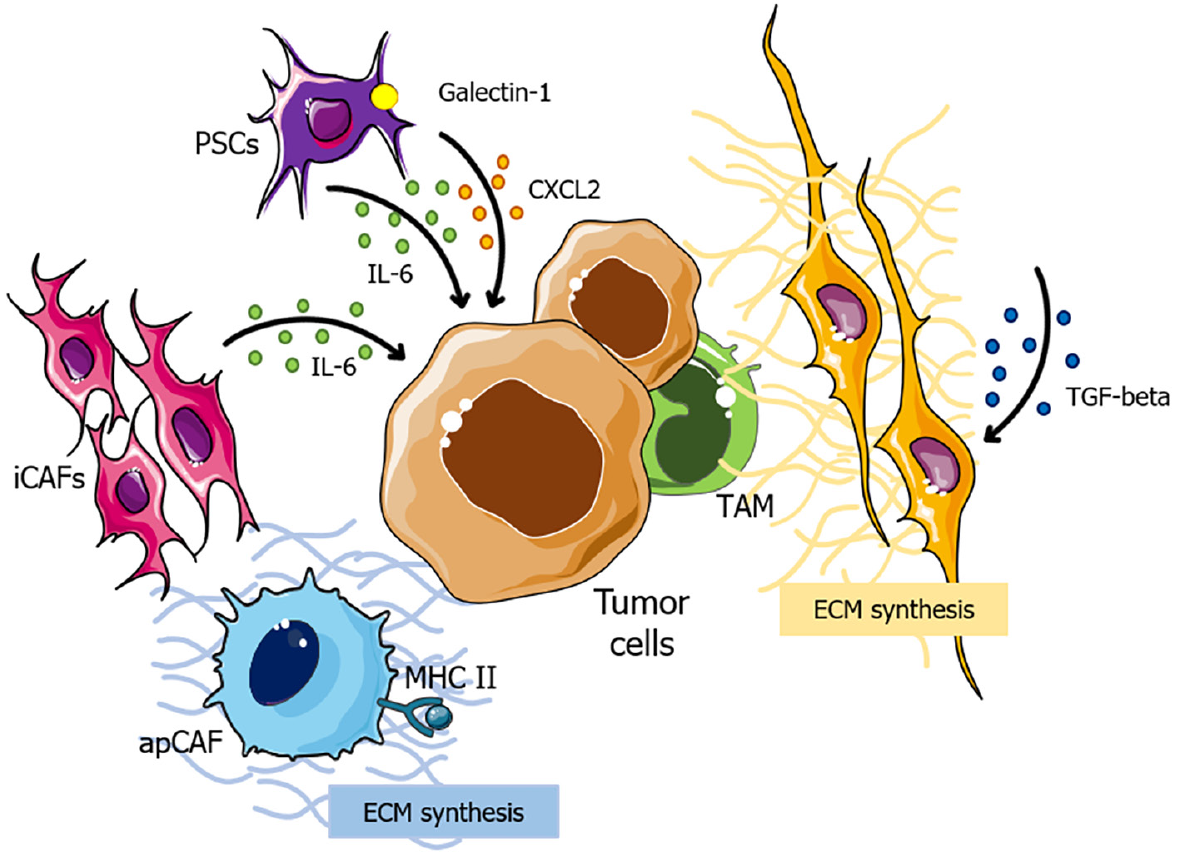
Initially, the notable capacity of CAFs to generate and remodel the ECM plays a pivotal role in immunotherapy resistance in PCs. These cells comprise distinct subtypes, each exerting specific influences on the TME. Firstly, α-smooth muscle actin, + myofibroblasts (myCAFs), which are transforming growth factor (TGF) signaling-dependent, contribute to the synthesis of ECM components[77,78]. On the other hand, inflammatory CAFs, exhibit elevated expression of interleukin (IL)-6, which is related to cancer progression[78,79], while major histocompatibility complex class II (MHC class II) + CAFs are able to present antigens but lack costimulatory molecules, potentially leading to deactivation of CD4+ T cells and further immune suppression in the TME (Figure 1)[80,81].
In the context of immunotherapy resistance, myCAFs emerge as a key player in this process, as they lead to the development of a dense, fibrotic stroma around the tumor, which provides a physical barrier that impedes the infiltration of immune cells and, consequently, impairs the effectiveness of immunotherapeutic agents[77,82]. Also, evidence suggests that secreted phosphoprotein 1 (SPP1) derived from CAFs in hepatocellular carcinomas apparently increases resistance to tyrosine kinase inhibitors (TKIs) through the induction of epithelial-to-mesenchymal transition[83,84]. However, as the direct correlation between SPP1 and TKI resistance remains unexplored in PC, further research is required to clarify this issue.
Additionally, the combination of a dense stroma and limited vascularization induces severe hypoxia within the TME, triggering the stabilization of hypoxia-inducible factors 1 and 2 (HIF2)[82]. CAF-specific deletion of HIF2 is associated to increased survival in PC, by reducing the intratumoral recruitment of M2 macrophages; and therapeutic HIF2 inhibition leads to increased response to immune checkpoint blockade[85]. These findings highlight the critical role of hypoxia in shaping the pancreatic TME and influencing immunotherapy resistance.
Finally, PSCs also seem to play a role in desmoplasia, as they become activated in response to signals from PC cells and contribute to the formation of the fibrotic stroma[82]. PSCs are associated with the secretion of immunosuppressive molecules, including C-X-C chemokine ligand (CXCL) 2, IL-6 and galectin-1, sustaining immunosuppression within the TME[86]. These dynamic interactions between PSCs and PC cells sustain underlying mechanisms that promote immunotherapy resistance (Figure 1).
Therefore, therapeutic agents that target the aforementioned components could be a potential next step in overcoming immunotherapy resistance in PCs.
Evidence also suggests that genetic/epigenetic factors may play a role in immunotherapy resistance in PC, even though current literature provides minimal information on the subject. Some studies indicate that mutations in the p53 gene are associated with alterations in the innate immune response, which may underlie tumorigenesis and promote immunotherapeutic resistance in PDACs[87]. In this regard, the Trp53R172H mutation in PC cells has been identified as a promoter of neutrophil accumulation, potentially contributing to resistance against immunotherapy[88].
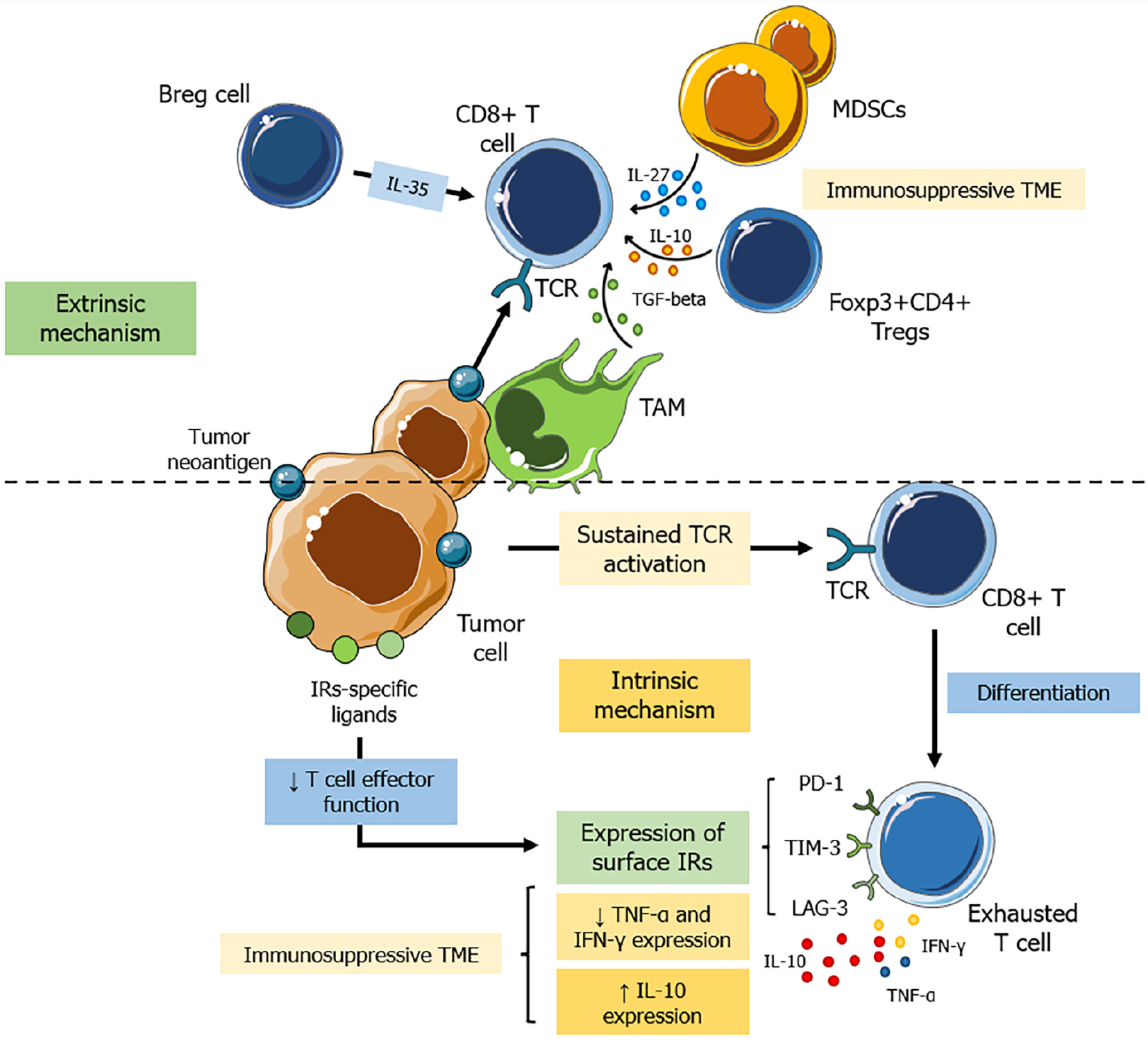
Pancreatic ductal adenocarcinoma is typically associated with a low mutation burden, resulting in a paucity of neoantigens and a scarcity of tumor-infiltrating effector T cells[89,90]. This gives rise to an "immunologically cold" TME, which is characterized by a dearth of tumor antigen-specific CD8+ T cells and a limited expression of activation markers such as interferon-gamma (IFN-γ) and granzyme B[91-95]. In turn, CD4+ helper T cells are more abundant within the TME compared to CD8+ T cells, displaying diverse immunological effects, including both anti- and pro-tumor activities across various phenotypes such as effector CD4+ T helper (Th) 1, Th2, Th17, FoxP3+ regulatory Ts (Tregs), and γδ T cells[96]. Nevertheless, the evaluation of antigen-specific CD4+ T cells remains elusive in both animal and human PDAC models[97]. Moreover, These observations imply a deficit or impediment in adaptive T cell immunity, recognized as the primary factor contributing to the concerning resistance against immune checkpoint blockade therapies[81].
Accordingly, PDAC appears to utilize two primary strategies to circumvent anti-tumor immune responses: (1) intrinsic T-cell receptor (TCR)-mediated exhaustion; and (2) extrinsic TME-driven immunosuppression[81,98]. Indeed, CD8+ T cells targeting tumor-specific antigens can trigger cell death. Nonetheless, in cases where the tumor persists and these cells face continual antigen exposure, sustained TCR activation leads to their differentiation into exhausted T (Tex) cells[99-101]. Tex cells express cell surface inhibitory receptors such as PD-1, MUC-3/T-cell immunoglobulin, and T-cell activation gene[94,102-105]. Upon interaction with their specific ligands expressed on cells within the TME, Tex cells undergo a progressive decline in effector function, differentiation state, and proliferative capacity[81]. Notably, these cells not only experience a reduction in functionality, such as decreased tumor necrosis factor (TNF)-α and IFN-γ expression, but also demonstrate a gradual increase in IL-10 expression within the TME[106]. These alterations thus contribute to the establishment of a local immunosuppressive milieu (Figure 2).
On the other hand, extrinsic factors encompass elements in the TME that hinder T cell function[107]. The oncogenic activation of KRAS in pancreatic cells initiates PanIN at the outset of cancer development, which triggers an immunosuppressive milieu orchestrated by tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and Treg and Breg cells, compounded by inhibitory cytokines and metabolic limitations[108-110].
In this scenario, macrophages represent the predominant leukocyte population identified within PDAC[111]. Studies have unveiled the origin of TAMs in PDAC from two primary sources: (1) Bone marrow-derived inflammatory monocytes; and (2) embryonic-derived tissue-resident macrophages[112,113]. These cells typically exhibit an immunosuppressive phenotype, which is characterized by the expression of immune checkpoints, inhibitory ligands, and the secretion of immunoregulatory cytokines such as IL-10[114-116].
Concurrently, the recruitment of bone marrow-derived myeloid cells toward PDAC involves a complex process orchestrated by granulocyte-macrophage (GM) colony-stimulating factor (CSF), granulocyte-CSF (G-CSF), IL-3, vascular endothelial growth factor, and the orchestrated interplay between the CXCL12/C-X-C chemokine receptor 4 (CXCR4) or C-C chemokine ligand 2/C-C chemokine receptor 2 signaling cascades[111,117,118]. Ultimately, tumor cell production of GM-CSF and G-CSF and tumor cell production of IL-1β fosters the proliferation of immature myeloid cells and drives their acquisition of a suppressive phenotype (MDSCs)[119-121].
Additionally, stromal-associated fibroblasts are known to produce CXCL13, which serves as a recruitment signal for IL-35-producing regulatory B cells within the TME[122]. This phenomenon further exacerbates PDAC immune evasion by harnessing IL-35-mediated inhibition, effectively suppressing T cell proliferation[123].
Collectively, TAMs, MDSCs, and Bregs exhibit a robust capacity to suppress the proliferation in both CD4+ and CD8+ T cells[124]. They also generate elevated levels of immunosuppressive cytokines, such as IL-10, IL-27, and TGF-β[106,122,124]. This coordinated activity facilitates the recruitment of regulatory Foxp3+CD4+ Tregs, which may also impede the antitumor immunity of CD8+ T cells at local intratumoral sites[125,126]. In summary, these factors collectively reduce T cell infiltration, impair their function, or promote their exhaustion within the TME, contributing to resistance against immunotherapy interventions (Figure 2).
Over the past decade, there has been significant interest in the pancreatic TME, particularly in its capacity to influence therapy response. The focus has shifted towards recognizing the TME as a key factor and obstacle affecting the effectiveness of immunotherapy in pancreatic ductal adenocarcinoma[127].
Numerous promising therapies targeting various mechanisms are currently undergoing preclinical and clinical development. These approaches encompass novel strategies to enhance T-cell responses, modify myeloid and stromal compartments, and attract new immune cells to the TME of PDAC[128].
From this perspective, targeting the tumor stroma holds potential advantages in the treatment of PC. The matricellular protein Secreted Protein Acidic and Rich in Cysteine (SPARC), produced by CAFs, has the ability to bind albumin[127]. This led to the hypothesis that SPARC could enhance the accumulation of nab-paclitaxel within the PC microenvironment, thereby augmenting its anti-tumor efficacy[129]. In a phase III study combining albumin-bound paclitaxel (nab-paclitaxel) with gemcitabine, the results indicated an increased intracellular concentration of gemcitabine, possibly attributed to the disruption of the tumor stroma and the reduction of CAFs[129].
Despite the promising outcomes mentioned earlier, efforts to target the tumor stroma have yielded contradictory consequences. Matrix metalloproteinases, a family of proteolytic enzymes essential for maintaining tissue homeostasis, play a crucial role in cancer invasion when expressed abnormally[130]. However, attempts to target matrix metalloproteinases using marimastat and tanomastat did not show any discernible benefits when combined with gemcitabine[131,132].
Furthermore, follow-up studies have indicated that depleting the stroma may, in fact, promote tumor growth, highlighting the intricate and multifaceted role that stroma plays in tumor biology. This underscores the complexity of the interactions within the TME and suggests that a nuanced approach is needed when considering stroma-targeted therapies in cancer treatment[133].
In preclinical mouse models of PC, the depletion of stroma by inhibiting the Hedgehog cellular signaling pathway has been demonstrated to enhance the delivery of gemcitabine to tumors[134]. This intervention resulted in improved survival and reduced metastasis by increasing the intracellular concentration of gemcitabine. The Hedgehog pathway's involvement in the formation of desmoplasia highlights its role in impairing drug delivery in the context of PC[134]. Despite the conflicting results of tumor response to stroma-depleting therapies, the TME plays a significant role in tumor biology and in modulating the immune recognition of PC.
Another therapeutic avenue under investigation involves targeting hyaluronic acid, which is abundant in PCs and contributes to angiogenesis and chemoresistance[135]. In a phase II study involving untreated, metastatic PC patients, the targeting of hyaluronic acid using pegvorhyaluronidase alfa, a pegylated formulation of recombinant hyaluronidase, in combination with nab-paclitaxel/gemcitabine resulted in a significant improvement in progression-free survival and OS[136].
Still in this scenario of trying to manipulate the microenvironment, it is known that the TME in PC represents a formidable therapeutic challenge when using traditional immunotherapies. Nevertheless, there is a shift towards utilizing combination approaches to reprogram the TME, aiming to unlock the potential benefits of immunotherapy. Early results from these endeavors are showing promise in the pursuit of more effective treatments for PC.
As aforementioned, targeting immunosuppressive cells within the TME enhances the likelihood of efficacy in immunotherapy treatments. One primary target is CSF1 receptor (CSF1-R), located on TAMs. The binding of CSF1 to CSF1-R facilitates TAM proliferation and extended survival, promoting tumor growth, resistance to treatments, and metastasis[137]. Inhibition of CSF1-R results in fewer TAMs, leading to a heightened immune response, increased tumor regression, and improved survival[138].
Looking at another relevant pathway for manipulation, it is notable that pancreatic ductal adenocarcinoma tumors show infiltration of M2 macrophages, which have an immunosuppressive function. This phenotype is characterized by the expression of CD206, CSF-1R, and IL-10, along with reduced expression of MHC class II[81]. The CSF-1 pathway plays a crucial role in the differentiation and survival of M2 macrophages. Inhibiting the CSF-1 pathway has been demonstrated to redirect TAMs toward the M1 phenotype, leading to distinct remodeling of the TME[139-141].
From a molecular perspective, it is important to analyze that CXCL12, a chemokine produced by CAFs, is frequently expressed at elevated levels in the PDAC TME. This creates a network of dense stroma, which, in turn, hinders the migration of immune cells and the recognition of cancer cell antigens. The elevated levels of CXCL12 in the PDAC TME play a role in creating an immunosuppressive environment, thereby diminishing the effectiveness of immune responses directed at cancer cells[142]. In preclinical studies, interrupting the interaction between CXCL12 and its receptor, CXCR4, enhanced the impact of ICIs in models of PDAC[142,143].
Also in this scenario, pancreatic tumor cells, fibroblasts, and other stromal cells release TGF-β, a cytokine that contributes to the creation of an immunosuppressive structure in the TME[144]. Using the small molecule inhibitor galunisertib to target TGF-β, combined with gemcitabine as the initial treatment for PDAC, resulted in only a marginal improvement in median mOS compared to gemcitabine alone and did not achieve statistical significance[145]. Following this, galunisertib was evaluated in conjunction with durvalumab in a cohort of 32 patients with advanced PDAC and demonstrated restricted effectiveness, yielding only one partial response[146]. Novel approaches, such as exploring a bifunctional fusion comprising a monoclonal antibody targeting TGF-β along with other ICIs, are currently being investigated[147].
Therefore, the combination of treatment strategies aimed at stimulating the immune response and overcoming barriers in the TME represent a promising avenue for improving the treatment of patients with PC.
The genetic mutation background of PC is well known, being found in CDKN2A, MLH1, BRCA2, ATM, KRAS and
Thus, the key principles of gene therapy are to induce immune effects that combat tumors with different signaling pathways, delivering genetic material to cells, focusing on the resolution of a disorder[154]. An effective gene therapy regimen is dependent upon the following factors: Efficient delivery of the gene, therapy specifically targeted at the tumor, and careful selection of optimal targets[155].
Against this backdrop, there are many possibilities that surround gene-therapy on PC cells. Gene editing and gene transfer can be utilized as a therapeutic intervention, employing an array of vectors and molecular tools, including interference RNA and genome editing techniques, which have shown promise in bridging preclinical cancer research and clinical trials[156,157].
In addition, various strategies have been applied to eliminate tumor cells based on known genetic alterations. Gene transfer strategies with TP53 have been utilized to treat multiple cancers[158]. However, attempts to restore TP53 expression during tumor growth have yielded disappointing results, indicating the limited efficacy of gene transfer in vitro[158].
Still in the scenario of genetic manipulation, suicide gene therapy is a major topic of discussion, based on the transfer of a suicide gene with a strong neighboring antitumor effect that can compensate the weakness of gene expression within the tumor[155]. The classic suicide gene strategy is the herpes simplex virus thymidine kinase gene (HSV-TK gene)[159]. This therapy is capable of causing toxicity and cell death, through metabolites and inhibition of DNA synthesis[159,160], It also elicits a robust immune response targeting tumor cells by releasing tumor antigens, resulting in a reaction against additional tumor cells by the body[159-161]. HSV-TK delivery via adenovirus and retrovirus have shown great anti-tumor efficiency in pancreatic cells both in vitro and in vivo[160,162].
Other examples of suicide/prodrug gene system that has been also tested, with success, in PC models is the cytochrome P450/isofosfamide system, was developed through in vitro and in vivo proof of concept to conduct phase I and II trials in patients with PC, the treatment has shown significant success in improving survival rates[158,163-165].
Other than that, miRNA is another potent point for therapeutic approach. Several studies have shown that miRNAs play important roles in the development of pancreatic tumors and also in the process of resistance to various therapies, including immunotherapy[166-168]. Loss of miRNA expression may result in significant dysfunctionality and promote carcinogenesis, owing to their crucial role in modulating apoptosis, cell cycle, and differentiation[158].
Thus, the methods for modulating intracellular miRNA levels primarily consist of miRNA replacement therapy and anti-miRNA oligonucleotides. In miRNA replacement therapy, oligonucleotide mimics are used to increase miRNA levels, while in anti-miRNA oligonucleotides, miRNA silencing is induced[169,170]. However, some barriers still exist when thinking in miRNA therapy such as low in vivo stability, improper biodistribution, insufficient cell specificity, disruption and saturation of endogenous RNA machinery, as some examples[171].
In this scenario, several miRNAs that are upregulated or downregulated in PC have demonstrated their contribution to tumor cell growth by targeting specific molecules[172-174]. An elevation in circulating miRNAs, such as miR-21, miR-25, miR-155 and miR-196, demonstrated a strong correlation with chemotherapy resistance among PC patients[175,176]. In this sense, an experimental study showed that targeting the oncogenic miRNA21 could suppress tumor growth in PC in vitro and in vivo[177]. However, no miRNA therapeutics have been tested clinically for PC treatment[178].
From another perspective of genetic manipulation, oncolytic virotherapy is one of the most promising anti-cancer therapies using agents with high antitumor potency and strong oncolytic effect[154,179]. Natural pathogens have either been selected or designed to specifically infect and destroy cancer cells, being engineered in a way that enables the production of cytokines, antigens, or suicide genes[158]. Oncolytic adenoviruses have been considered highly eligible vehicles for delivery of therapeutic genes to treat cancer due to their tumor-restricted replication capabilities[158,180], and because of them being non-pathogenic and with a high selectivity and cytotoxicity to cancer cells[181].
With this in mind, it is important to point out that a known viral vector is the HSV type designed with an efficient blockade of experimental tumor growth, used alone or in combination with gemcitabine[182]. A study with a HSV that showed an promising anti-cancer activity was Myb34.5, which has been assessed preclinically in PC models, being used inducing apoptosis and inhibition of pancreatic tumor growth[183]. Other studies are being conducted, dealing with possible viruses that can be used on a therapeutic concept, such as the oncolytic parvovirus H-1, in some clinical trials[184,185].
Numerous genetic alterations that directly contribute to pancreatic tumorigenesis have been identified or are being actively studied; because of it, novel therapies for PC patients by targeting specific genes are a promising future, associated with a lot of new trials searching novel possibilities[154,156,158]. This approach to personalized medicine can be utilized for patients with PC, providing appropriate treatment that is tailored to their individual needs.
The mechanisms of resistance to immunotherapy present in PC are rooted in an intense interaction between molecules and cells of the TME and cells of the immune system, with this impact being particularly focused on the activity of T cells, leading to a reduction in the tumor activity of these cells[186]. Thus, novel approaches to treating immunotherapy-resistant PC involve manipulating the activity of T cells and immune system cells that directly interact with these anti-tumor cells[187].
One of the primary immunotherapy strategies used in oncology is to block the immune checkpoint, but resistance to existing methods has prompted the need to employ immunomodulators, such as PD1-IL2v[188,189]. PD1-IL2v is a bispecific antibody molecule that binds to PD-1 on CD8+ T cells and incorporates a modified IL-2 molecule in its structure[189]. This modification stimulates the cytotoxic activity of CD8+ T cells without binding to CD25 present on Treg cells, consequently avoiding the activation of these regulatory cells[189]. Thus, the study conducted by Tichet et al[189] in mice was based on the combination of anti-PD-L1 with the immunomodulator PD1-IL2v and achieved promising results, including tumor regression, improved efficiency of anti-tumor T cells, increased infiltration of CD8+ T cells into the TME, modulation of TAMs, and demonstrated a positive response for cancers resistant to immunotherapy[189].
From another perspective, Siglec-15 was detailed by Wang et al[190] in 2019, demonstrating that it can be expressed in cancer cells and TAMs, with increased expression in macrophages leading to the inhibition of anti-tumor T cell proliferation[190]. Consequently, Siglec-15 has become a new target for therapy based on blocking the immune checkpoint, despite the absence of published studies to comprehend all its functions in PC[190,191]. Sun et al[191] describe Siglec-15 as a potential therapeutic target for cancer based on ongoing clinical studies[191]. Therefore, as analyzed by Chen et al[192], Siglec-15-based therapy may offer a promising solution for PC patients resistant to anti-PD-1 treatment[192]. It can be utilized alone or in conjunction with other immune checkpoint blockade techniques, resulting in increased infiltration of CD8+ T cells into the TME[192].
Another growing immunotherapy technique in cancer treatment is ACT, based on the ex vivo manipulation of T cells to expand their anti-tumor activity[193]. After manipulation, these cells are reinserted into the individual to exert more potent anti-tumor effects[193]. Despite the various factors contributing to resistance to immunotherapy in PC, impacting the efficiency of ACT for this type of cancer, the primary obstacle remains the immunosuppressive and challenging-to-access TME, hindering the infiltration of immune cells[72]. In this regard, Rataj et al[194] demonstrated a promising approach to overcoming resistance by developing a fusion protein with the extracellular domain of the PD-1 receptor fused to the intracellular T-cell activation domain of CD28[194]. This fusion protein was implanted into CD4+ T cells using the ACT technique. When these modified cells were introduced into the TME, the PD-1 domain interacted with its ligand PD-L1[194]. However, instead of inducing immunosuppression, the activated CD28 protein coupled to PD-1 resulted in increased antitumor activity of CD4+ T cells through enhanced cytokine secretion and stimulation of the cytotoxic activity of CD8+ T cells[194].
Still from the perspective of manipulating the extrinsic and intrinsic mechanisms associated with T cells, even more recent studies have concentrated on the use of nanomedicines to overcome resistance to immunotherapy[195]. In this context, Jung et al[196] conducted a study in humanized mice employing an siRNA nanoparticle targeting PD-L1 as the therapeutic approach[196]. The study yielded promising results, leading to an increase in CD8+ T cells in the TME as a result of the blockade of PD-L1 induction caused by the nanoparticle absorbed by cells in the TME[196].
When analyzing molecular pathways, it is known that the use of cytokines as immunomodulators is an immunotherapeutic strategy employed in cancer treatment, but it has not yielded promising responses when used alone in PC, primarily due to immune resistance mechanisms[197]. Many therapies utilizing cytokines with immunosuppressive activity are employed as adjuvants to other immunotherapy models[197]. Nonetheless, more targeted studies investigating the action of specific cytokines may lead to new strategies for addressing immunotherapy-resistant PC.
With this in mind, Huang et al[198] conducted a study with the aim of inhibiting the immunosuppressive activity resulting from the action of IFN-γ, a pivotal cytokine in the process of resistance to immunotherapy[198]. According to this analysis, the action of gamma interferon leads to the production of proteins such as indoleamine 2, 3-dioxygenase 1 and CD274, which possess immunosuppressive properties and are included in the category of therapies based on inhibiting the immune checkpoint[198]. The study utilized dinaciclib to block the expression of these proteins induced by IFN-γ in murine models and achieved promising results in curtailing the immunosuppressive activity of IFN-γ, reducing cancer immune invasion, and blocking the expression of immune checkpoint[198].
In a similar vein, Tsukamoto et al[199] demonstrated in a study involving 235 patients that TNF-α overexpression is directly associated with increased PD-L1 expression in TME cells, leading to immunosuppression through the neutralization of cytotoxic T cells[199]. Consequently, anti-TNF-α may also exhibit promising efficacy in addressing resistance to existing immunotherapy in PC by impacting the PD-L1 receptor, which is immunosuppressive and also a target of immune checkpoint blockade[199]. Nevertheless, additional clinical trials aimed at analyzing the impact of anti-TNF-α on PD-L1 expression are still necessary.
The mechanisms of resistance to immunotherapy in the treatment of PC have brought a new challenge for medicine: To identify therapeutic combinations that help overcome resistance, thus increasing the efficiency of immunotherapy and improving the patient's prognosis[200].
From this perspective, Mehla et al[201] utilized a murine monoclonal antibody (mAb-AR20.5), which modulates the TME by binding to MUC1, in conjunction with PolyICLC (a vaccine adjuvant) and anti-PD-L1 in their murine models for the treatment of PC[201]. The combined therapy induced superior anti-tumor activity, leading to the rejection of tumor cells expressing MUC1 and heightened cytotoxic activity of CD8+ T cells[201]. This resulted in immune modulation and promising tumor control for PC through the use of an TME modulator, an immune checkpoint blocker and a vaccine adjuvant[201].
In the same context, it is pertinent to examine the promising aspect of using TME modulators alongside immune checkpoint inhibitors, as demonstrated by Rana et al[202]. They conducted a preclinical study with murine models, employing an inhibitor of the TGF-β receptor, responsible for TME progression, and an immune checkpoint blocker (anti-PD-L1/anti-CTLA-4)[202]. The study resulted in the inhibition of tumor growth, enhanced CD8+ T cell infiltration, and increased the population of M1 macrophages in the TME[202]. Additionally, Cappellesso et al[203] adopted a similar approach by analyzing the single-cell RNA of individuals with PC and identifying solute carrier family 4 member 4 (SLC4A4), primarily responsible for maintaining the acidity of the TME and, consequently, tumor progression[203]. This led to the association of an SLC4A4 inhibitor with ICI, such as anti-PD-1/anti-CTLA-4, in studies with mice, resulting in improved survival and overcoming resistance mechanisms that impact treatment alone[203]. Finally, Datta et al[204] employed mitogen-activated protein kinase/extracellular signal-regulated kinase and signal transducer and activator of transcription 3 inhibitors, crucial components of existing resistance mechanisms in the TME, in conjunction with anti-PD-1 in mice[204]. This approach yielded promising responses in terms of enhanced survival and increased anti-tumor response, driven by the greater recruitment of cytotoxic T cells in the TME[204]. These preclinical studies underscore the significant clinical potential of combining TME modelers with immune checkpoint blockers, opening up new possibilities for innovative therapies in the treatment of resistant PC.
In the context of resistance mediated by immunosuppressive molecules, Zhang et al[205] demonstrated the involvement of IL-17 in the process of triggering the inactivation of CD8+ T cells and shaping the TME[205]. Subsequent to this analysis, a study in murine models was established utilizing a triple combination of anti-IL17/IL17R/PD-1 antibodies[205]. This approach resulted in a reduction in tumor size based on increased sensitization of the TME to the action of ICI, a fact corroborated when replacing anti-PD-1 with anti-CTLA-4 in the combination[205]. Similarly, Nelson et al[206] devised a triple combination involving natural killer T cells, a recombinant oncolytic virus designed to express the cytokine IL-15, and anti-PD-1[206]. The study's triple therapy tested in mice exhibited promising results, including prolonged tumor regression and complete elimination of the tumor in 20% of the mice[206].
Another compelling approach was demonstrated by Saung and Zheng[207] using a cancer vaccine (GVAX) in conjunction with anti-PD-1, anti-CSF-1R, and the chemotherapy drug gemcitabine in murine models[207]. The combination yielded enhanced survival of the mice, along with more efficient infiltration of anti-tumor cells and a reduction in myeloid cells[207]. Table 1 summarizes all the pre-clinical studies reported and their respective findings.
| Methods | Combination | Results | Ref. |
| TME modulator + Vaccine + ICI | mAb-AR20.5 + PolyICLC + anti-PD-L1 | Rejection of tumor cells expressing MUC1 and increased cytotoxic activity of CD8+ T cells | [201] |
| TME modulator + ICI | TGF-β inhibitor + anti-PD-L1/anti-CTLA-4 | Inhibited tumor growth, improved CD8+ T cell infiltration and increased the population of M1 macrophages in the TME | [202] |
| TME modulator + ICI | SLC4A4 inhibitor + anti-PD-1/anti-CTLA-4 | It reduced the acidity of the TME, increased the infiltration of CD8+ T cells and the number of M1 macrophages | [203] |
| TME modulator + ICI | MEK and STAT3 inhibitors + anti-PD-1 | Attenuated the pro-inflammatory CAF myofibroblastic phenotypes expressing IL6/CXCL1 and increased the recruitment of CD8+ T cells | [204] |
| IL-17 signaling blocker + ICI | Anti-IL17+ anti-IL17R + anti-PD-1/anti-CTLA-4 | Favored the activation of CD8+ T cells, achieved a 50% response rate and increased survival | [205] |
| NKT activation + recombinant oncolytic virus + ICI | NKT + VSV-IL-15 + anti-PD-1 | It increased overall tumor regression, survival time, NK/T CD8 cell infiltration and resulted in complete tumor elimination in 20% of the mice | [206] |
| Vaccine + ICI + TME modulator + chemotherapy | GVAX + anti-PD-1 + anti-CSF-1R + gemcitabine | It increased the number of infiltrated CD8+T cells, reduced the infiltration of myeloid cells, myeloid-derived suppressor cells and reduced the number of TAMs | [207] |
The realm of combined immunotherapy to overcome resistant PC has expanded in recent years, and clinical trials are already underway to tackle this significant challenge. Thus, Bockorny et al[208] conducted a phase IIa study to assess the efficacy of the combination of a CXCR4 blocker, BL-8040 (motixafortide), and pembrolizumab (anti-PD-1) for the treatment of 37 patients[208]. They achieved a disease control rate of 34.5% and an increase in OS of 7.5 months, attributed to greater infiltration of anti-tumor CD8+ T cells and a reduction in immunosuppressive cells such as MDSCs and T regs[208]. Similarly, Overman et al[209] used a Bruton's TKI combined with anti-PD-1 in a randomized phase II clinical trial with 40 patients[209]. Although the combination was tolerated and showed limited clinical activity, with a disease control rate of only 21.1%, blood analysis revealed a reduction in MDSCs[209]. Consequently, these clinical trials reveal a relevant potential, paving the way for new studies aimed at manipulating the TME in combination with ICI and other treatment models, seeking improved results for the treatment of patients with PC.
Finally, it is important to analyze the clinical studies carried out with a combination of different PC vaccine models. From this perspective, Le et al[210] employed a combination of the GVAX vaccine and CRS-207 (live attenuated mesothelin-expressing Listeria monocytogenes) in a clinical trial with 90 patients[210]. They observed prolonged survival in these patients treated with this vaccine combination, with little toxicity in the therapeutic process[210]. Similarly, Nair et al[211] used the same immunotherapy combination in a phase IIa clinical trial with 38 patients[211]. They found that patients with higher CD8+ T expression achieved a longer OS under this therapeutic regimen[211]. Table 2 summarizes all the clinical studies reported and their respective findings.
| Combination | Pacients | Phase | Results | Identification | Ref. |
| Motixafortide (CXCR4 blocker) + pembrolizumab (anti-PD-1) | 37 | IIa | The disease control rate was 34.5%, survival rate of 7.5 months, a more efficient infiltration of CD8+ T cells and a reduction in MDSCs | NCT02826486 | |
| Acalabrutinib (BTK inhibitor) + pembrolizumab (anti-PD-1) | 40 | II | The disease control rate was 21.1%, the survival rate was 1.4 months and there was a reduction in MDSCs | NCT02362048 | |
| GVAX + CRS-207 | 90 | II | The disease control rate was 31%, the survival rate was 6.1 months and there was an increase in mesothelin-specific CD8+ T cells | NCT01417000 | |
| GVAX + CRS-207 | 200 | IIb | The survival rate of 175 days (average) and a more efficient infiltration of CD8+ T cells | NCT02004262 |
In summary, PC has evolved into a complex challenge for the medical community, given the intricate resistance to immunotherapy treatments and other applied therapies. Due to its distinctive tumor environment coupled with underlying genetic and immunosuppressive factors, various mechanisms of opposition to immunomodulatory methods manifest, with the primary one associated with immune checkpoint inhibitors. Given this perspective, it is imperative to progress to unexplored stages, elucidating and presenting solutions that enable science to overcome the challenges posed by this demanding oncological model. In this context, emerging approaches aimed at modulating the TME to reduce immunosuppression are proving promising, as are innovative techniques for modulating the immune system. The goal is to enhance the efficacy and infiltration of anti-tumor cells by manipulating the intrinsic and extrinsic systems within the immune system. Based on these considerations, a combination of these techniques is feasible to achieve more auspicious prognoses and results, as evidenced in clinical studies exploring the efficacy of a combination of a CXCR4 blocker and pembrolizumab, yielding promising results, and the combination of two vaccine models, such as GVAX and CRS-207. In this way, a new path is emerging that presents itself as a promising prospect for overcoming the resistance to immunotherapy present in the treatment of PC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chatterjee B, India S-Editor: Liu H L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Jiang S, Fagman JB, Ma Y, Liu J, Vihav C, Engstrom C, Liu B, Chen C. A comprehensive review of pancreatic cancer and its therapeutic challenges. Aging (Albany NY). 2022;14:7635-7649. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Ushio J, Kanno A, Ikeda E, Ando K, Nagai H, Miwata T, Kawasaki Y, Tada Y, Yokoyama K, Numao N, Tamada K, Lefor AK, Yamamoto H. Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors. Diagnostics (Basel). 2021;11. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Korc M, Jeon CY, Edderkaoui M, Pandol SJ, Petrov MS; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2017;31:529-536. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Hayashi A, Hong J, Iacobuzio-Donahue CA. The pancreatic cancer genome revisited. Nat Rev Gastroenterol Hepatol. 2021;18:469-481. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Zhao Z, Liu W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol Cancer Res Treat. 2020;19:1533033820962117. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, Maitra A. Pancreatic cancer: Advances and challenges. Cell. 2023;186:1729-1754. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Sherman MH, Beatty GL. Tumor Microenvironment in Pancreatic Cancer Pathogenesis and Therapeutic Resistance. Annu Rev Pathol. 2023;18:123-148. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Guo Y, Gao F, Ahmed A, Rafiq M, Yu B, Cong H, Shen Y. Immunotherapy: cancer immunotherapy and its combination with nanomaterials and other therapies. J Mater Chem B. 2023;11:8586-8604. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Grimont A, Leach SD, Chandwani R. Uncertain Beginnings: Acinar and Ductal Cell Plasticity in the Development of Pancreatic Cancer. Cell Mol Gastroenterol Hepatol. 2022;13:369-382. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and Pancreatic Cancer: Overview of Epidemiology and Potential Prevention by Weight Loss. Pancreas. 2018;47:158-162. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Walling A, Freelove R. Pancreatitis and Pancreatic Cancer. Prim Care. 2017;44:609-620. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Yang J, Chheda C, Lim A, Hauptschein D, Zayou L, Tang J, Pandol SJ, Edderkaoui M. HDAC4 Mediates Smoking-Induced Pancreatic Cancer Metastasis. Pancreas. 2022;51:190-195. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Roy A, Sahoo J, Kamalanathan S, Naik D, Mohan P, Kalayarasan R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J Gastroenterol. 2021;27:4939-4962. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology. 2022;163:386-402.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Goral V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac J Cancer Prev. 2015;16:5619-5624. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, Völzke H, Mayerle J, Kühn JP. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138-145. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, DeMatteo R, Fong Y, Blumgart LH, Brennan MF. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572-582. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Klöppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T; Baltimore Consensus Meeting. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730-1741. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Ottenhof NA, Milne AN, Morsink FH, Drillenburg P, Ten Kate FJ, Maitra A, Offerhaus GJ. Pancreatic intraepithelial neoplasia and pancreatic tumorigenesis: of mice and men. Arch Pathol Lab Med. 2009;133:375-381. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, Lüttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579-586. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Kim JY, Hong SM. Precursor Lesions of Pancreatic Cancer. Oncol Res Treat. 2018;41:603-610. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Scarlett CJ, Salisbury EL, Biankin AV, Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathology. 2011;43:183-200. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437-450. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Mahadevan KK, McAndrews KM, LeBleu VS, Yang S, Lyu H, Li B, Sockwell AM, Kirtley ML, Morse SJ, Moreno Diaz BA, Kim MP, Feng N, Lopez AM, Guerrero PA, Paradiso F, Sugimoto H, Arian KA, Ying H, Barekatain Y, Sthanam LK, Kelly PJ, Maitra A, Heffernan TP, Kalluri R. KRAS(G12D) inhibition reprograms the microenvironment of early and advanced pancreatic cancer to promote FAS-mediated killing by CD8(+) T cells. Cancer Cell. 2023;41:1606-1620.e8. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Löhr M, Klöppel G, Maisonneuve P, Lowenfels AB, Lüttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17-23. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062-1078. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350-1358. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Rosenfeldt MT, O'Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296-300. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Kojima K, Vickers SM, Adsay NV, Jhala NC, Kim HG, Schoeb TR, Grizzle WE, Klug CA. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res. 2007;67:8121-8130. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002-2006. [PubMed] [Cited in This Article: ] |
| 35. | Yokode M, Akita M, Fujikura K, Kim MJ, Morinaga Y, Yoshikawa S, Terada T, Matsukiyo H, Tajiri T, Abe-Suzuki S, Itoh T, Hong SM, Zen Y. High-grade PanIN presenting with localised stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology. 2018;73:247-258. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Kanda M, Sadakari Y, Borges M, Topazian M, Farrell J, Syngal S, Lee J, Kamel I, Lennon AM, Knight S, Fujiwara S, Hruban RH, Canto MI, Goggins M. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719-30.e5. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Hosoda W, Chianchiano P, Griffin JF, Pittman ME, Brosens LA, Noë M, Yu J, Shindo K, Suenaga M, Rezaee N, Yonescu R, Ning Y, Albores-Saavedra J, Yoshizawa N, Harada K, Yoshizawa A, Hanada K, Yonehara S, Shimizu M, Uehara T, Samra JS, Gill AJ, Wolfgang CL, Goggins MG, Hruban RH, Wood LD. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol. 2017;242:16-23. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Klöppel G, Longnecker DS, Lüttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977-987. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Hackert T, Fritz S, Büchler MW. Main- and Branch-Duct Intraductal Papillary Mucinous Neoplasms: Extent of Surgical Resection. Viszeralmedizin. 2015;31:38-42. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Zhou H, Li X, Wang Y, Wang Z, Zhu J, Chen X. Threshold of main pancreatic duct for malignancy in intraductal papillary mucinous neoplasm at head-neck and body-tail. BMC Gastroenterol. 2022;22:473. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Koh YX, Zheng HL, Chok AY, Tan CS, Wyone W, Lim TK, Tan DM, Goh BK. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157:496-509. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Attiyeh MA, Fernández-Del Castillo C, Al Efishat M, Eaton AA, Gönen M, Batts R, Pergolini I, Rezaee N, Lillemoe KD, Ferrone CR, Mino-Kenudson M, Weiss MJ, Cameron JL, Hruban RH, D'Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, Wolfgang CL, Allen PJ. Development and Validation of a Multi-institutional Preoperative Nomogram for Predicting Grade of Dysplasia in Intraductal Papillary Mucinous Neoplasms (IPMNs) of the Pancreas: A Report from The Pancreatic Surgery Consortium. Ann Surg. 2018;267:157-163. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Lee JH, Kim Y, Choi JW, Kim YS. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus. 2016;5:1172. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Miyasaka Y, Nagai E, Yamaguchi H, Fujii K, Inoue T, Ohuchida K, Yamada T, Mizumoto K, Tanaka M, Tsuneyoshi M. The role of the DNA damage checkpoint pathway in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2007;13:4371-4377. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755-761. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Pittman ME, Rao R, Hruban RH. Classification, Morphology, Molecular Pathogenesis, and Outcome of Premalignant Lesions of the Pancreas. Arch Pathol Lab Med. 2017;141:1606-1614. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Macgregor-Das AM, Iacobuzio-Donahue CA. Molecular pathways in pancreatic carcinogenesis. J Surg Oncol. 2013;107:8-14. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Fernández-del Castillo C. Mucinous cystic neoplasms. J Gastrointest Surg. 2008;12:411-413. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Yamao K, Yanagisawa A, Takahashi K, Kimura W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, Hifumi M, Kobayashi Y, Tobita K, Tanno S, Sugiyama M, Miyasaka Y, Nakagohri T, Yamaguchi T, Hanada K, Abe H, Tada M, Fujita N, Tanaka M. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67-71. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Wiseman JE, Yamamoto M, Nguyen TD, Bonadio J, Imagawa DK. Cystic pancreatic neoplasm in pregnancy: a case report and review of the literature. Arch Surg. 2008;143:84-86. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188-21193. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Hessmann E, Buchholz SM, Demir IE, Singh SK, Gress TM, Ellenrieder V, Neesse A. Microenvironmental Determinants of Pancreatic Cancer. Physiol Rev. 2020;100:1707-1751. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Robatel S, Schenk M. Current Limitations and Novel Perspectives in Pancreatic Cancer Treatment. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Tonini V, Zanni M. Pancreatic cancer in 2021: What you need to know to win. World J Gastroenterol. 2021;27:5851-5889. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP. Chemotherapy for pancreatic cancer. Presse Med. 2019;48:e159-e174. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in Pancreatic Cancer. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Falco M, Masojć B, Sulikowski T. Radiotherapy in Pancreatic Cancer: To Whom, When, and How? Cancers (Basel). 2023;15. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Ejlsmark MW, Schytte T, Bernchou U, Bahij R, Weber B, Mortensen MB, Pfeiffer P. Radiotherapy for Locally Advanced Pancreatic Adenocarcinoma-A Critical Review of Randomised Trials. Curr Oncol. 2023;30:6820-6837. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Cope S, Keeping ST, Goldgrub R, Ayers D, Jansen JP, Penrod JR, Korytowsky B, Juarez-Garcia A, Yuan Y. Indirect comparison of nivolumab ± ipilimumab (CheckMate 032) versus other treatments for recurrent small-cell lung cancer. J Comp Eff Res. 2019;8:733-751. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, Liu M, Yuan Y. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e11936. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol. 2018;24:2137-2151. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A, Karamouzis MV. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat Rev. 2020;86:102016. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, Leone F. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750-1755. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, Chiorean EG, Waterhouse DM, Guiterrez M, Parikh A, Jain R, Carrizosa DR, Soliman HH, Lila T, Reiss DJ, Pierce DW, Bhore R, Banerjee S, Lyons L, Louis CU, Ong TJ, O'Dwyer PJ. Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin Cancer Res. 2020;26:4814-4822. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res. 2020;26:2124-2130. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Huang X, Zhang G, Tang TY, Gao X, Liang TB. Personalized pancreatic cancer therapy: from the perspective of mRNA vaccine. Mil Med Res. 2022;9:53. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Mukherji R, Debnath D, Hartley ML, Noel MS. The Role of Immunotherapy in Pancreatic Cancer. Curr Oncol. 2022;29:6864-6892. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Brown CE, Adusumilli PS. Next frontiers in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16028. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Haas AR, Tanyi JL, O'Hara MH, Gladney WL, Lacey SF, Torigian DA, Soulen MC, Tian L, McGarvey M, Nelson AM, Farabaugh CS, Moon E, Levine BL, Melenhorst JJ, Plesa G, June CH, Albelda SM, Beatty GL. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol Ther. 2019;27:1919-1929. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Liu Y, Guo Y, Wu Z, Feng K, Tong C, Wang Y, Dai H, Shi F, Yang Q, Han W. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: A phase I clinical trial. Cytotherapy. 2020;22:573-580. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Truong LH, Pauklin S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579-596. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Beatty GL, Werba G, Lyssiotis CA, Simeone DM. The biological underpinnings of therapeutic resistance in pancreatic cancer. Genes Dev. 2021;35:940-962. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9:1102-1123. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Schmiechen ZC, Stromnes IM. Mechanisms Governing Immunotherapy Resistance in Pancreatic Ductal Adenocarcinoma. Front Immunol. 2020;11:613815. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Deng D, Patel R, Chiang CY, Hou P. Role of the Tumor Microenvironment in Regulating Pancreatic Cancer Therapy Resistance. Cells. 2022;11. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Argentiero A, Delvecchio A, Fasano R, Andriano A, Caradonna IC, Memeo R, Desantis V. The Complexity of the Tumor Microenvironment in Hepatocellular Carcinoma and Emerging Therapeutic Developments. J Clin Med. 2023;12. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Eun JW, Yoon JH, Ahn HR, Kim S, Kim YB, Lim SB, Park W, Kang TW, Baek GO, Yoon MG, Son JA, Weon JH, Kim SS, Cho HJ, Cheong JY. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun (Lond). 2023;43:455-479. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Garcia Garcia CJ, Huang Y, Fuentes NR, Turner MC, Monberg ME, Lin D, Nguyen ND, Fujimoto TN, Zhao J, Lee JJ, Bernard V, Yu M, Delahoussaye AM, Jimenez Sacarello I, Caggiano EG, Phan JL, Deorukhkar A, Molkentine JM, Saur D, Maitra A, Taniguchi CM. Stromal HIF2 Regulates Immune Suppression in the Pancreatic Cancer Microenvironment. Gastroenterology. 2022;162:2018-2031. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Farran B, Nagaraju GP. The dynamic interactions between the stroma, pancreatic stellate cells and pancreatic tumor development: Novel therapeutic targets. Cytokine Growth Factor Rev. 2019;48:11-23. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Calheiros J, Corbo V, Saraiva L. Overcoming therapeutic resistance in pancreatic cancer: Emerging opportunities by targeting BRCAs and p53. Biochim Biophys Acta Rev Cancer. 2023;1878:188914. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Siolas D, Vucic E, Kurz E, Hajdu C, Bar-Sagi D. Gain-of-function p53(R172H) mutation drives accumulation of neutrophils in pancreatic tumors, promoting resistance to immunotherapy. Cell Rep. 2021;36:109578. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Bailey P, Chang DK, Forget MA, Lucas FA, Alvarez HA, Haymaker C, Chattopadhyay C, Kim SH, Ekmekcioglu S, Grimm EA, Biankin AV, Hwu P, Maitra A, Roszik J. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci Rep. 2016;6:35848. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:569. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Wang L, Geng H, Liu Y, Liu L, Chen Y, Wu F, Liu Z, Ling S, Wang Y, Zhou L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm (2020). 2023;4:e343. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG, Ramsay AG, Kocher HM. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121-1132. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20:3422-3433. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Burrack AL, Spartz EJ, Raynor JF, Wang I, Olson M, Stromnes IM. Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma. Cell Rep. 2019;28:2140-2155.e6. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, Hingorani SR. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2015;28:638-652. [PubMed] [DOI] [Cited in This Article: ] |
| 96. | Stromnes IM, Hulbert A, Pierce RH, Greenberg PD, Hingorani SR. T-cell Localization, Activation, and Clonal Expansion in Human Pancreatic Ductal Adenocarcinoma. Cancer Immunol Res. 2017;5:978-991. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Patterson MT, Burrack AL, Xu Y, Hickok GH, Schmiechen ZC, Becker S, Cruz-Hinojoza E, Schrank PR, Kennedy AE, Firulyova MM, Miller EA, Zaitsev K, Williams JW, Stromnes IM. Tumor-specific CD4 T cells instruct monocyte fate in pancreatic ductal adenocarcinoma. Cell Rep. 2023;42:112732. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | Mundry CS, Eberle KC, Singh PK, Hollingsworth MA, Mehla K. Local and systemic immunosuppression in pancreatic cancer: Targeting the stalwarts in tumor's arsenal. Biochim Biophys Acta Rev Cancer. 2020;1874:188387. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, Schumacher TN, Schwartzberg PL, Sharpe AH, Speiser DE, Wherry EJ, Youngblood BA, Zehn D. Defining 'T cell exhaustion'. Nat Rev Immunol. 2019;19:665-674. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19:775-790. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Stromnes IM, Hulbert A, Rollins MR, Basom RS, Delrow J, Bonson P, Burrack AL, Hingorani SR, Greenberg PD. Insufficiency of compound immune checkpoint blockade to overcome engineered T cell exhaustion in pancreatic cancer. J Immunother Cancer. 2022;10. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Lee J, Ahn E, Kissick HT, Ahmed R. Reinvigorating Exhausted T Cells by Blockade of the PD-1 Pathway. For Immunopathol Dis Therap. 2015;6:7-17. [PubMed] [DOI] [Cited in This Article: ] |
| 103. | Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989-1004. [PubMed] [DOI] [Cited in This Article: ] |
| 104. | Seifert L, Plesca I, Müller L, Sommer U, Heiduk M, von Renesse J, Digomann D, Glück J, Klimova A, Weitz J, Schmitz M, Seifert AM. LAG-3-Expressing Tumor-Infiltrating T Cells Are Associated with Reduced Disease-Free Survival in Pancreatic Cancer. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Bai M, Zheng Y, Liu H, Su B, Zhan Y, He H. CXCR5(+) CD8(+) T cells potently infiltrate pancreatic tumors and present high functionality. Exp Cell Res. 2017;361:39-45. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Burrack AL, Rollins MR, Spartz EJ, Mesojednik TD, Schmiechen ZC, Raynor JF, Wang IX, Kedl RM, Stromnes IM. CD40 Agonist Overcomes T Cell Exhaustion Induced by Chronic Myeloid Cell IL-27 Production in a Pancreatic Cancer Preclinical Model. J Immunol. 2021;206:1372-1384. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Herting CJ, Karpovsky I, Lesinski GB. The tumor microenvironment in pancreatic ductal adenocarcinoma: current perspectives and future directions. Cancer Metastasis Rev. 2021;40:675-689. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Goulart MR, Stasinos K, Fincham REA, Delvecchio FR, Kocher HM. T cells in pancreatic cancer stroma. World J Gastroenterol. 2021;27:7956-7968. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | Orth M, Metzger P, Gerum S, Mayerle J, Schneider G, Belka C, Schnurr M, Lauber K. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol. 2019;14:141. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | de Santiago I, Yau C, Heij L, Middleton MR, Markowetz F, Grabsch HI, Dustin ML, Sivakumar S. Immunophenotypes of pancreatic ductal adenocarcinoma: Meta-analysis of transcriptional subtypes. Int J Cancer. 2019;145:1125-1137. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Stone ML, Beatty GL. Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol Ther. 2019;201:202-213. [PubMed] [DOI] [Cited in This Article: ] |
| 112. | Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404-3415. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, DeNardo DG. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity. 2017;47:323-338.e6. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Diskin B, Adam S, Cassini MF, Sanchez G, Liria M, Aykut B, Buttar C, Li E, Sundberg B, Salas RD, Chen R, Wang J, Kim M, Farooq MS, Nguy S, Fedele C, Tang KH, Chen T, Wang W, Hundeyin M, Rossi JAK, Kurz E, Haq MIU, Karlen J, Kruger E, Sekendiz Z, Wu D, Shadaloey SAA, Baptiste G, Werba G, Selvaraj S, Loomis C, Wong KK, Leinwand J, Miller G. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21:442-454. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Yang X, Wang G, Song Y, Zhuang T, Li Y, Xie Y, Fei X, Zhao Y, Xu D, Hu Y. PD-1(+)CD8(+) T Cells Proximal to PD-L1(+)CD68(+) Macrophages Are Associated with Poor Prognosis in Pancreatic Ductal Adenocarcinoma Patients. Cancers (Basel). 2023;15. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844-854. [PubMed] [DOI] [Cited in This Article: ] |
| 117. | Thyagarajan A, Alshehri MSA, Miller KLR, Sherwin CM, Travers JB, Sahu RP. Myeloid-Derived Suppressor Cells and Pancreatic Cancer: Implications in Novel Therapeutic Approaches. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, André P, Rigot V, Iovanna J, van de Pavert S, Lombardo D, Mas E, Martirosyan A. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Front Immunol. 2019;10:3070. [PubMed] [DOI] [Cited in This Article: ] |
| 119. | Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836-847. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822-835. [PubMed] [DOI] [Cited in This Article: ] |
| 121. | Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020;80:1088-1101. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6:247-255. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Mirlekar B, Michaud D, Lee SJ, Kren NP, Harris C, Greene K, Goldman EC, Gupta GP, Fields RC, Hawkins WG, DeNardo DG, Rashid NU, Yeh JJ, McRee AJ, Vincent BG, Vignali DAA, Pylayeva-Gupta Y. B cell-Derived IL35 Drives STAT3-Dependent CD8(+) T-cell Exclusion in Pancreatic Cancer. Cancer Immunol Res. 2020;8:292-308. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208-220. [PubMed] [DOI] [Cited in This Article: ] |
| 125. | Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e91551. [PubMed] [DOI] [Cited in This Article: ] |
| 126. | Barilla RM, Diskin B, Caso RC, Lee KB, Mohan N, Buttar C, Adam S, Sekendiz Z, Wang J, Salas RD, Cassini MF, Karlen J, Sundberg B, Akbar H, Levchenko D, Gakhal I, Gutierrez J, Wang W, Hundeyin M, Torres-Hernandez A, Leinwand J, Kurz E, Rossi JAK, Mishra A, Liria M, Sanchez G, Panta J, Loke P, Aykut B, Miller G. Specialized dendritic cells induce tumor-promoting IL-10(+)IL-17(+) FoxP3(neg) regulatory CD4(+) T cells in pancreatic carcinoma. Nat Commun. 2019;10:1424. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Bockorny B, Grossman JE, Hidalgo M. Facts and Hopes in Immunotherapy of Pancreatic Cancer. Clin Cancer Res. 2022;28:4606-4617. [PubMed] [DOI] [Cited in This Article: ] |
| 129. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [PubMed] [DOI] [Cited in This Article: ] |
| 130. | Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9-34. [PubMed] [DOI] [Cited in This Article: ] |
| 131. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [PubMed] [DOI] [Cited in This Article: ] |
| 132. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B, Tu D, Ottaway J, Humphrey R, Seymour L; National Cancer Institute of Canada Clinical Trials Group. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. [PubMed] [DOI] [Cited in This Article: ] |
| 133. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [PubMed] [DOI] [Cited in This Article: ] |
| 134. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [PubMed] [DOI] [Cited in This Article: ] |
| 135. | Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244-250. [PubMed] [DOI] [Cited in This Article: ] |
| 136. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [PubMed] [DOI] [Cited in This Article: ] |
| 137. | Patel K, Siraj S, Smith C, Nair M, Vishwanatha JK, Basha R. Pancreatic Cancer: An Emphasis on Current Perspectives in Immunotherapy. Crit Rev Oncog. 2019;24:105-118. [PubMed] [DOI] [Cited in This Article: ] |
| 138. | Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15:8-18. [PubMed] [DOI] [Cited in This Article: ] |
| 139. | Candido JB, Morton JP, Bailey P, Campbell AD, Karim SA, Jamieson T, Lapienyte L, Gopinathan A, Clark W, McGhee EJ, Wang J, Escorcio-Correia M, Zollinger R, Roshani R, Drew L, Rishi L, Arkell R, Evans TRJ, Nixon C, Jodrell DI, Wilkinson RW, Biankin AV, Barry ST, Balkwill FR, Sansom OJ. CSF1R(+) Macrophages Sustain Pancreatic Tumor Growth through T Cell Suppression and Maintenance of Key Gene Programs that Define the Squamous Subtype. Cell Rep. 2018;23:1448-1460. [PubMed] [DOI] [Cited in This Article: ] |
| 140. | Saung MT, Muth S, Ding D, Thomas DL 2nd, Blair AB, Tsujikawa T, Coussens L, Jaffee EM, Zheng L. Targeting myeloid-inflamed tumor with anti-CSF-1R antibody expands CD137+ effector T-cells in the murine model of pancreatic cancer. J Immunother Cancer. 2018;6:118. [PubMed] [DOI] [Cited in This Article: ] |
| 141. | Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057-5069. [PubMed] [DOI] [Cited in This Article: ] |
| 142. | Seo YD, Jiang X, Sullivan KM, Jalikis FG, Smythe KS, Abbasi A, Vignali M, Park JO, Daniel SK, Pollack SM, Kim TS, Yeung R, Crispe IN, Pierce RH, Robins H, Pillarisetty VG. Mobilization of CD8(+) T Cells via CXCR4 Blockade Facilitates PD-1 Checkpoint Therapy in Human Pancreatic Cancer. Clin Cancer Res. 2019;25:3934-3945. [PubMed] [DOI] [Cited in This Article: ] |
| 143. | Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212-20217. [PubMed] [DOI] [Cited in This Article: ] |
| 144. | Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18:9-34. [PubMed] [DOI] [Cited in This Article: ] |
| 145. | Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, Smith C, Estrem ST, Gueorguieva I, Lahn MMF, Blunt A, Benhadji KA, Tabernero J. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119:1208-1214. [PubMed] [DOI] [Cited in This Article: ] |
| 146. | Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, Lamping E, Marté JL, Donahue RN, Grenga I, Cordes L, Christensen O, Mahnke L, Helwig C, Gulley JL. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res. 2018;24:1287-1295. [PubMed] [DOI] [Cited in This Article: ] |
| 147. | Melisi D, Oh DY, Hollebecque A, Calvo E, Varghese A, Borazanci E, Macarulla T, Merz V, Zecchetto C, Zhao Y, Gueorguieva I, Man M, Gandhi L, Estrem ST, Benhadji KA, Lanasa MC, Avsar E, Guba SC, Garcia-Carbonero R. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer. 2021;9. [PubMed] [DOI] [Cited in This Article: ] |
| 148. | Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, Bamlet WR, Chaffee KG, DiCarlo J, Wu Z, Samara R, Kasi PM, McWilliams RR, Petersen GM, Couch FJ. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319:2401-2409. [PubMed] [DOI] [Cited in This Article: ] |
| 149. | di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220-1229. [PubMed] [DOI] [Cited in This Article: ] |
| 150. | Caldas C, Kern SE. K-ras mutation and pancreatic adenocarcinoma. Int J Pancreatol. 1995;18:1-6. [PubMed] [DOI] [Cited in This Article: ] |
| 151. | Hahn SA, Schmiegel WH. Recent discoveries in cancer genetics of exocrine pancreatic neoplasia. Digestion. 1998;59:493-501. [PubMed] [DOI] [Cited in This Article: ] |
| 152. | Delpu Y, Hanoun N, Lulka H, Sicard F, Selves J, Buscail L, Torrisani J, Cordelier P. Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr Genomics. 2011;12:15-24. [PubMed] [DOI] [Cited in This Article: ] |
| 153. | Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945-D950. [PubMed] [DOI] [Cited in This Article: ] |
| 154. | Galanopoulos M, Doukatas A, Gkeros F, Viazis N, Liatsos C. Room for improvement in the treatment of pancreatic cancer: Novel opportunities from gene targeted therapy. World J Gastroenterol. 2021;27:3568-3580. [PubMed] [DOI] [Cited in This Article: ] |
| 155. | Liu SX, Xia ZS, Zhong YQ. Gene therapy in pancreatic cancer. World J Gastroenterol. 2014;20:13343-13368. [PubMed] [DOI] [Cited in This Article: ] |
| 156. | Kamimura K, Yokoo T, Terai S. Gene Therapy for Pancreatic Diseases: Current Status. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] |
| 157. | Vassaux G, Angelova A, Baril P, Midoux P, Rommelaere J, Cordelier P. The Promise of Gene Therapy for Pancreatic Cancer. Hum Gene Ther. 2016;27:127-133. [PubMed] [DOI] [Cited in This Article: ] |
| 158. | Rouanet M, Lebrin M, Gross F, Bournet B, Cordelier P, Buscail L. Gene Therapy for Pancreatic Cancer: Specificity, Issues and Hopes. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] |
| 159. | Fogar P, Greco E, Basso D, Habeler W, Navaglia F, Zambon CF, Tormen D, Gallo N, Cecchetto A, Plebani M, Pedrazzoli S. Suicide gene therapy with HSV-TK in pancreatic cancer has no effect in vivo in a mouse model. Eur J Surg Oncol. 2003;29:721-730. [PubMed] [DOI] [Cited in This Article: ] |
| 160. | Wang J, Lu XX, Chen DZ, Li SF, Zhang LS. Herpes simplex virus thymidine kinase and ganciclovir suicide gene therapy for human pancreatic cancer. World J Gastroenterol. 2004;10:400-403. [PubMed] [DOI] [Cited in This Article: ] |
| 161. | Greco E, Fogar P, Basso D, Stefani AL, Navaglia F, Zambon CF, Mazza S, Gallo N, Piva MG, Scarpa A, Pedrazzoli S, Plebani M. Retrovirus-mediated herpes simplex virus thymidine kinase gene transfer in pancreatic cancer cell lines: an incomplete antitumor effect. Pancreas. 2002;25:e21-e29. [PubMed] [DOI] [Cited in This Article: ] |
| 162. | Mäkinen K, Loimas S, Wahlfors J, Alhava E, Jänne J. Evaluation of herpes simplex thymidine kinase mediated gene therapy in experimental pancreatic cancer. J Gene Med. 2000;2:361-367. [PubMed] [DOI] [Cited in This Article: ] |
| 163. | Carrió M, Visa J, Cascante A, Estivill X, Fillat C. Intratumoral activation of cyclophosphamide by retroviral transfer of the cytochrome P450 2B1 in a pancreatic tumor model. Combination with the HSVtk/GCV system. J Gene Med. 2002;4:141-149. [PubMed] [DOI] [Cited in This Article: ] |
| 164. | Löhr M, Müller P, Karle P, Stange J, Mitzner S, Jesnowski R, Nizze H, Nebe B, Liebe S, Salmons B, Günzburg WH. Targeted chemotherapy by intratumour injection of encapsulated cells engineered to produce CYP2B1, an ifosfamide activating cytochrome P450. Gene Ther. 1998;5:1070-1078. [PubMed] [DOI] [Cited in This Article: ] |
| 165. | Löhr M, Hoffmeyer A, Kröger J, Freund M, Hain J, Holle A, Karle P, Knöfel WT, Liebe S, Müller P, Nizze H, Renner M, Saller RM, Wagner T, Hauenstein K, Günzburg WH, Salmons B. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet. 2001;357:1591-1592. [PubMed] [DOI] [Cited in This Article: ] |
| 166. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [PubMed] [DOI] [Cited in This Article: ] |
| 167. | Li M, Marin-Muller C, Bharadwaj U, Chow KH, Yao Q, Chen C. MicroRNAs: control and loss of control in human physiology and disease. World J Surg. 2009;33:667-684. [PubMed] [DOI] [Cited in This Article: ] |
| 168. | Wei L, Sun J, Wang X, Huang Y, Huang L, Han L, Zheng Y, Xu Y, Zhang N, Yang M. Noncoding RNAs: an emerging modulator of drug resistance in pancreatic cancer. Front Cell Dev Biol. 2023;11:1226639. [PubMed] [DOI] [Cited in This Article: ] |
| 169. | Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199-208. [PubMed] [DOI] [Cited in This Article: ] |
| 170. | Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043-2050. [PubMed] [DOI] [Cited in This Article: ] |
| 171. | Passadouro M, Faneca H. Managing Pancreatic Adenocarcinoma: A Special Focus in MicroRNA Gene Therapy. Int J Mol Sci. 2016;17. [PubMed] [DOI] [Cited in This Article: ] |
| 172. | Fang Y, Yao Q, Chen Z, Xiang J, William FE, Gibbs RA, Chen C. Genetic and molecular alterations in pancreatic cancer: implications for personalized medicine. Med Sci Monit. 2013;19:916-926. [PubMed] [DOI] [Cited in This Article: ] |
| 173. | Ohuchida K, Mizumoto K, Lin C, Yamaguchi H, Ohtsuka T, Sato N, Toma H, Nakamura M, Nagai E, Hashizume M, Tanaka M. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene. Ann Surg Oncol. 2012;19:2394-2402. [PubMed] [DOI] [Cited in This Article: ] |
| 174. | Chen Z, Chen LY, Dai HY, Wang P, Gao S, Wang K. miR-301a promotes pancreatic cancer cell proliferation by directly inhibiting Bim expression. J Cell Biochem. 2012;113:3229-3235. [PubMed] [DOI] [Cited in This Article: ] |
| 175. | Ren C, Chen H, Han C, Wang D, Fu D. Increased plasma microRNA and CD133/CK18-positive cancer cells in the pleural fluid of a pancreatic cancer patient with liver and pleural metastases and correlation with chemoresistance. Oncol Lett. 2012;4:691-694. [PubMed] [DOI] [Cited in This Article: ] |
| 176. | Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila). 2009;2:807-813. [PubMed] [DOI] [Cited in This Article: ] |
| 177. | Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986-994. [PubMed] [DOI] [Cited in This Article: ] |
| 178. | Merhautova J, Demlova R, Slaby O. MicroRNA-Based Therapy in Animal Models of Selected Gastrointestinal Cancers. Front Pharmacol. 2016;7:329. [PubMed] [DOI] [Cited in This Article: ] |
| 179. | Sato-Dahlman M, Wirth K, Yamamoto M. Role of Gene Therapy in Pancreatic Cancer-A Review. Cancers (Basel). 2018;10. [PubMed] [DOI] [Cited in This Article: ] |
| 180. | Lou W, Chen Q, Ma L, Liu J, Yang Z, Shen J, Cui Y, Bian XW, Qian C. Oncolytic adenovirus co-expressing miRNA-34a and IL-24 induces superior antitumor activity in experimental tumor model. J Mol Med (Berl). 2013;91:715-725. [PubMed] [DOI] [Cited in This Article: ] |
| 181. | Haller SD, Monaco ML, Essani K. The Present Status of Immuno-Oncolytic Viruses in the Treatment of Pancreatic Cancer. Viruses. 2020;12. [PubMed] [DOI] [Cited in This Article: ] |
| 182. | Gayral M, Lulka H, Hanoun N, Biollay C, Sèlves J, Vignolle-Vidoni A, Berthommé H, Trempat P, Epstein AL, Buscail L, Béjot JL, Cordelier P. Targeted oncolytic herpes simplex virus type 1 eradicates experimental pancreatic tumors. Hum Gene Ther. 2015;26:104-113. [PubMed] [DOI] [Cited in This Article: ] |
| 183. | Yokoda R, Nagalo BM, Arora M, Egan JB, Bogenberger JM, DeLeon TT, Zhou Y, Ahn DH, Borad MJ. Oncolytic virotherapy in upper gastrointestinal tract cancers. Oncolytic Virother. 2017;7:13-24. [PubMed] [DOI] [Cited in This Article: ] |
| 184. | Réjiba S, Bigand C, Parmentier C, Masmoudi A, Hajri A. Oncosuppressive suicide gene virotherapy "PVH1-yCD/5-FC" for pancreatic peritoneal carcinomatosis treatment: NFκB and Akt/PI3K involvement. PLoS One. 2013;8:e70594. [PubMed] [DOI] [Cited in This Article: ] |
| 185. | Geletneky K, Huesing J, Rommelaere J, Schlehofer JR, Leuchs B, Dahm M, Krebs O, von Knebel Doeberitz M, Huber B, Hajda J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. [PubMed] [DOI] [Cited in This Article: ] |
| 186. | Yu S, Zhang C, Xie KP. Therapeutic resistance of pancreatic cancer: Roadmap to its reversal. Biochim Biophys Acta Rev Cancer. 2021;1875:188461. [PubMed] [DOI] [Cited in This Article: ] |
| 187. | Luo W, Wang J, Chen H, Qiu J, Wang R, Liu Y, Su D, Tao J, Weng G, Ma H, Zhang T. Novel strategies optimize immunotherapy by improving the cytotoxic function of T cells for pancreatic cancer treatment. Cancer Lett. 2023;576:216423. [PubMed] [DOI] [Cited in This Article: ] |
| 188. | Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37:443-455. [PubMed] [DOI] [Cited in This Article: ] |
| 189. | Tichet M, Wullschleger S, Chryplewicz A, Fournier N, Marcone R, Kauzlaric A, Homicsko K, Deak LC, Umaña P, Klein C, Hanahan D. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8(+) T cells and reprogramming macrophages. Immunity. 2023;56:162-179.e6. [PubMed] [DOI] [Cited in This Article: ] |
| 190. | Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, Zhang J, Song C, Zarr M, Zhou X, Han X, Archer KA, O'Neill T, Herbst RS, Boto AN, Sanmamed MF, Langermann S, Rimm DL, Chen L. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25:656-666. [PubMed] [DOI] [Cited in This Article: ] |
| 191. | Sun J, Lu Q, Sanmamed MF, Wang J. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin Cancer Res. 2021;27:680-688. [PubMed] [DOI] [Cited in This Article: ] |
| 192. | Chen X, Mo S, Zhang Y, Ma H, Lu Z, Yu S, Chen J. Analysis of a novel immune checkpoint, Siglec-15, in pancreatic ductal adenocarcinoma. J Pathol Clin Res. 2022;8:268-278. [PubMed] [DOI] [Cited in This Article: ] |
| 193. | Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62-68. [PubMed] [DOI] [Cited in This Article: ] |
| 194. | Rataj F, Kraus FBT, Chaloupka M, Grassmann S, Heise C, Cadilha BL, Duewell P, Endres S, Kobold S. PD1-CD28 Fusion Protein Enables CD4+ T Cell Help for Adoptive T Cell Therapy in Models of Pancreatic Cancer and Non-hodgkin Lymphoma. Front Immunol. 2018;9:1955. [PubMed] [DOI] [Cited in This Article: ] |
| 195. | Elzoghby AO, Ferrone CR, Ferrone S, Nasr ML. Engineered nanomedicines to overcome resistance of pancreatic cancer to immunotherapy. Drug Discov Today. 2023;28:103434. [PubMed] [DOI] [Cited in This Article: ] |
| 196. | Jung JY, Ryu HJ, Lee SH, Kim DY, Kim MJ, Lee EJ, Ryu YM, Kim SY, Kim KP, Choi EY, Ahn HJ, Chang S. siRNA Nanoparticle Targeting PD-L1 Activates Tumor Immunity and Abrogates Pancreatic Cancer Growth in Humanized Preclinical Model. Cells. 2021;10. [PubMed] [DOI] [Cited in This Article: ] |
| 197. | Timmer FEF, Geboers B, Nieuwenhuizen S, Dijkstra M, Schouten EAC, Puijk RS, de Vries JJJ, van den Tol MP, Bruynzeel AME, Streppel MM, Wilmink JW, van der Vliet HJ, Meijerink MR, Scheffer HJ, de Gruijl TD. Pancreatic Cancer and Immunotherapy: A Clinical Overview. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 198. | Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, Peng Q, Liu ZX, Li C, Kroemer G, Lotze M, Zeh H, Kang R, Tang D. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2021;70:890-899. [PubMed] [DOI] [Cited in This Article: ] |
| 199. | Tsukamoto M, Imai K, Ishimoto T, Komohara Y, Yamashita YI, Nakagawa S, Umezaki N, Yamao T, Kitano Y, Miyata T, Arima K, Okabe H, Baba Y, Chikamoto A, Ishiko T, Hirota M, Baba H. PD-L1 expression enhancement by infiltrating macrophage-derived tumor necrosis factor-α leads to poor pancreatic cancer prognosis. Cancer Sci. 2019;110:310-320. [PubMed] [DOI] [Cited in This Article: ] |
| 200. | Bear AS, Vonderheide RH, O'Hara MH. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell. 2020;38:788-802. [PubMed] [DOI] [Cited in This Article: ] |
| 201. | Mehla K, Tremayne J, Grunkemeyer JA, O'Connell KA, Steele MM, Caffrey TC, Zhu X, Yu F, Singh PK, Schultes BC, Madiyalakan R, Nicodemus CF, Hollingsworth MA. Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors. Cancer Immunol Immunother. 2018;67:445-457. [PubMed] [DOI] [Cited in This Article: ] |
| 202. | Rana M, Kansal R, Chaib M, Teng B, Morrrison M, Hayes DN, Stanfill AG, Shibata D, Carson JA, Makowski L, Glazer ES. The pancreatic cancer immune tumor microenvironment is negatively remodeled by gemcitabine while TGF-β receptor plus dual checkpoint inhibition maintains antitumor immune cells. Mol Carcinog. 2022;61:549-557. [PubMed] [DOI] [Cited in This Article: ] |
| 203. | Cappellesso F, Orban MP, Shirgaonkar N, Berardi E, Serneels J, Neveu MA, Di Molfetta D, Piccapane F, Caroppo R, Debellis L, Ostyn T, Joudiou N, Mignion L, Richiardone E, Jordan BF, Gallez B, Corbet C, Roskams T, DasGupta R, Tejpar S, Di Matteo M, Taverna D, Reshkin SJ, Topal B, Virga F, Mazzone M. Targeting the bicarbonate transporter SLC4A4 overcomes immunosuppression and immunotherapy resistance in pancreatic cancer. Nat Cancer. 2022;3:1464-1483. [PubMed] [DOI] [Cited in This Article: ] |
| 204. | Datta J, Dai X, Bianchi A, De Castro Silva I, Mehra S, Garrido VT, Lamichhane P, Singh SP, Zhou Z, Dosch AR, Messaggio F, Ban Y, Umland O, Hosein PJ, Nagathihalli NS, Merchant NB. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts With Mesenchymal Stem Cell-Like Features to Overcome Immunotherapy Resistance in Pancreatic Cancer. Gastroenterology. 2022;163:1593-1612. [PubMed] [DOI] [Cited in This Article: ] |
| 205. | Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S, Horne W, Burks J, Xu H, Hussain P, Wang H, Gupta S, Maitra A, Bailey JM, Moghaddam SJ, Banerjee S, Sahin I, Bhattacharya P, McAllister F. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217. [PubMed] [DOI] [Cited in This Article: ] |
| 206. | Nelson A, Gebremeskel S, Lichty BD, Johnston B. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J Immunother Cancer. 2022;10. [PubMed] [DOI] [Cited in This Article: ] |
| 207. | Saung MT, Zheng L. Adding combination immunotherapy consisting of cancer vaccine, anti-PD-1 and anti-CSF1R antibodies to gemcitabine improves anti-tumor efficacy in murine model of pancreatic ductal adenocarcinoma. Ann Pancreat Cancer. 2019;2. [PubMed] [DOI] [Cited in This Article: ] |
| 208. | Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, Park JO, Ramirez RA, Abad DG, Feliu J, Muñoz A, Ponz-Sarvise M, Peled A, Lustig TM, Bohana-Kashtan O, Shaw SM, Sorani E, Chaney M, Kadosh S, Vainstein Haras A, Von Hoff DD, Hidalgo M. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878-885. [PubMed] [DOI] [Cited in This Article: ] |
| 209. | Overman M, Javle M, Davis RE, Vats P, Kumar-Sinha C, Xiao L, Mettu NB, Parra ER, Benson AB, Lopez CD, Munugalavadla V, Patel P, Tao L, Neelapu S, Maitra A. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J Immunother Cancer. 2020;8. [PubMed] [DOI] [Cited in This Article: ] |
| 210. | Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T Jr, Brockstedt DG, Jaffee EM. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325-1333. [PubMed] [DOI] [Cited in This Article: ] |
| 211. | Nair N, Chen SY, Lemmens E, Chang S, Le DT, Jaffee EM, Murphy A, Whiting C, Müller T, Brockstedt DG. Single-Cell Immune Competency Signatures Associate with Survival in Phase II GVAX and CRS-207 Randomized Studies in Patients with Metastatic Pancreatic Cancer. Cancer Immunol Res. 2020;8:609-617. [PubMed] [DOI] [Cited in This Article: ] |