Published online Oct 15, 2018. doi: 10.4251/wjgo.v10.i10.317
Peer-review started: May 30, 2018
First decision: July 3, 2018
Revised: July 13, 2018
Accepted: August 12, 2018
Article in press: August 13, 2018
Published online: October 15, 2018
Pancreatic cancer is a lethal malignancy, whose precursor lesions are pancreatic intraepithelial neoplasm, intraductal papillary mucinous neoplasm, intraductal tubulopapillary neoplasm, and mucinous cystic neoplasm. To better understand the biology of pancreatic cancer, it is fundamental to know its precursors and to study the mechanisms of carcinogenesis. Each of these precursors displays peculiar histological features, as well as specific molecular alterations. Starting from such pre-invasive lesions, this review aims at summarizing the most important aspects of carcinogenesis of pancreatic cancer, with a specific focus on the recent advances and the future perspectives of the research on this lethal tumor type.
Core tip: Pancreatic intraepithelial neoplasm, intraductal papillary mucinous neoplasm, intraductal tubulopapillary neoplasm, and mucinous cystic neoplasm are precursor lesions of invasive pancreatic cancer. Each of these precursors displays peculiar histological and molecular features, which have been summarized in this review along with the most important aspects of pancreatic carcinogenesis. The most recent advances and the future perspectives of the research on this topic have also been highlighted.
- Citation: Riva G, Pea A, Pilati C, Fiadone G, Lawlor RT, Scarpa A, Luchini C. Histo-molecular oncogenesis of pancreatic cancer: From precancerous lesions to invasive ductal adenocarcinoma. World J Gastrointest Oncol 2018; 10(10): 317-327
- URL: https://www.wjgnet.com/1948-5204/full/v10/i10/317.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i10.317
Precursor lesions of pancreatic ductal adenocarcinoma (PDAC) are non-invasive lesions, which can progress to infiltrating carcinoma[1]. Following the 2010 World Health Organization classification, different consensus conferences and international meetings have provided the basis for the current view of the definition and classification of pancreatic precursor lesions[2-4].
One major issue in classification is in regards to the grading of dysplasia of pre-invasive lesions, which should be restricted to the two categories of “low-grade” and “high-grade” with the elimination of the poorly reproducible intermediate entity of “moderate dysplasia”[5]. Furthermore, small intraductal papillary mucinous lesions (PanIN) ranging from 0.5 to 1.0 cm would be better defined as “incipient intraductal papillary mucinous neoplasm (IPMN)”[5].
Three PDAC precursors have been recognized: PanIN, IPMN, and mucinous cystic neoplasm (MCN). In addition, intratubular papillary neoplasms (ITPNs) display the features of pre-invasive lesion. Each of these entities has distinct clinical, histological, and molecular profiles. Through a multi-step carcinogenesis, with the accumulation of cellular and molecular alterations, each of these precursors may lead to the development of invasive ductal adenocarcinoma.
PanIN represents the most common PDAC precursor, affecting both men and women equally. Its incidence increases directly with age[5]. The strict correlation with PDAC is suggested first of all by the fact that these lesions can be found in more than 80% of pancreas with invasive carcinoma[1,6], and by a reported multifocality in patients with PDAC familial history[7,8]. Usually, due to their small size (by definition < 0.5 cm), these lesions are classically found incidentally during histological examination and are not associated with clinical symptoms or specific signs. From the radiological point of view, PanINs are more often associated with acinar atrophy and/or fibrosis, but this correlation is not specific[9,10].
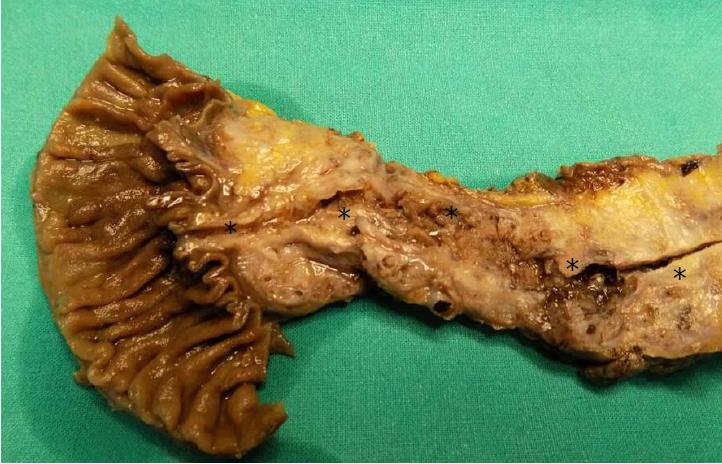
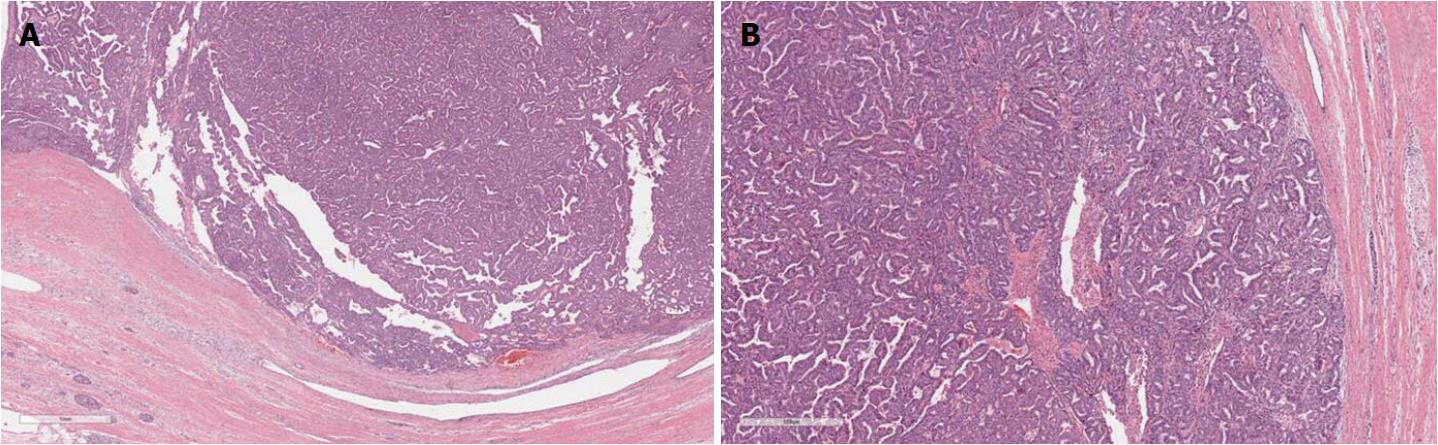
IPMNs are grossly visible lesions with intraductal growth, papillary architecture, and mucous producing cells. The first definition of IPMNs was reported in 1994[11]. The median age of IPMN patients ranges from 60 to 66 years. They are more common in men than in women (ratio: 3/1.3 in Europe, 3/2.1 in United States, and 3/1.8 in Asia) and arise most frequently in the proximal pancreatic head and the “uncinatus” process[12]. Although rare, IPMN involving more than a pancreatic segment or even the entire pancreas have been described (Figure 1)[12]. It is estimated that IPMNs may require up to six years to become invasive, although such estimation may be affected by multiple biases, including the specific IPMN histotype[13-15]. Similarly to PanINs, IPMNs are found more frequently in patients with PDAC familial history, thus highlighting the importance of a genetic predisposition to carcinogenesis of IPMN patients. In fact, such precursors have been found also in the context of multi-organ syndromes such as Peutz-Jeghers, familial adenomatous polyposis, Lynch, and McCune-Albright syndromes[16-18]. Moreover, patients with IPMNs have an increased risk of developing other extrapancreatic cancers[19].
One of the most important distinctions between IPMNs and PanINs is the possibility that IPMNs may be detected with imaging techniques. Patients with IPMNs should be followed-up according to specific protocols based on radiological examination[20]. The typical intraductal growth of IPMNs leads to cystic dilation of the pancreatic tree ducts[21]. Based on their location, IPMNs may be classified from the topographic point of view in: (1) main duct IPMNs where Wirsung’s duct is involved; (2) branch duct IPMNs in the case of the involvement of secondary ducts; or (3) mixed IPMNs in the case of contemporaneous involvement of the main and the branch ducts[1,20,21]. Although this distinction cannot always be confirmed by histopathological examination due to some branch duct IPMNs displaying some degree of Wirsung’s duct involvement[22], this topographic definition has an important clinical impact. Indeed, main duct IPMNs are more often associated with PDAC development and patients with this type of lesion must follow stringent surveillance protocols[20]. IPMN-associated carcinomas usually display a better prognosis than conventional PDACs[1].
MCNs are typically reported in perimenopausal women, with few cases described in men[4]. They usually arise in the distal part of the pancreas (body and/or tail), and by definition do not communicate with the pancreatic ductal tree[1]. It has been hypothesized that the females may be predisposed to MCNs due to embryogenesis or by a carcinogenetic process stimulated by female hormones[23,24]. This theory is also corroborated by their histological aspect because under the mucinous, non-papillary epithelium is a classic ovarian-like stroma[1]. The mean age of patients with MCNs is about 44 years. The mean age of patients with MCNs with an associated adenocarcinoma is about 55 years[20]. This observation is in line with the status of MCN as a PDAC precursor lesion. The association with PDAC is present in up to one third of MCNs[25,26]. In contrast to PanINs, but similar to IPMNs, MCNs can be detected by imaging. MCN patients must undergo strict follow-up or pancreatic resection because there is a non-negligible risk of PDAC development[21].
ITPNs are rare intraductal neoplasms of the pancreas composed of mucinous cells displaying a tubule-papillary architecture[1,5]. The incidence is similar in women and men[1]. These lesions are more commonly located in the head of the pancreas[1], and their symptoms are unspecific, including undefined abdominal pain and vomiting. Notably, about 40% of ITPNs harbor an associated invasive carcinoma[21]. PDACs arising in association with ITPNs usually have a better prognosis than that of conventional PDACs with a 5-year survival rate of more than 30%[21].
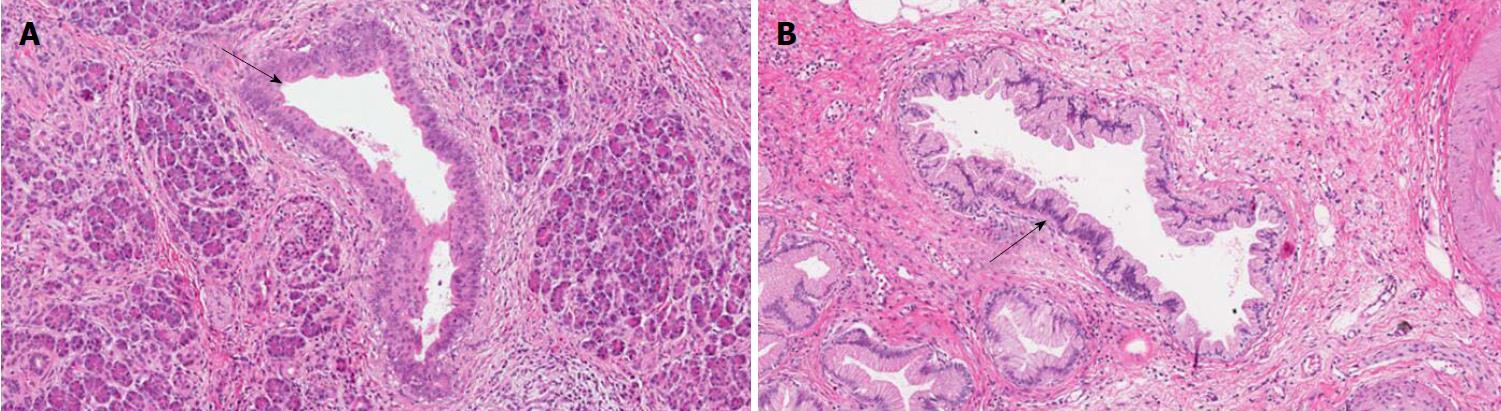
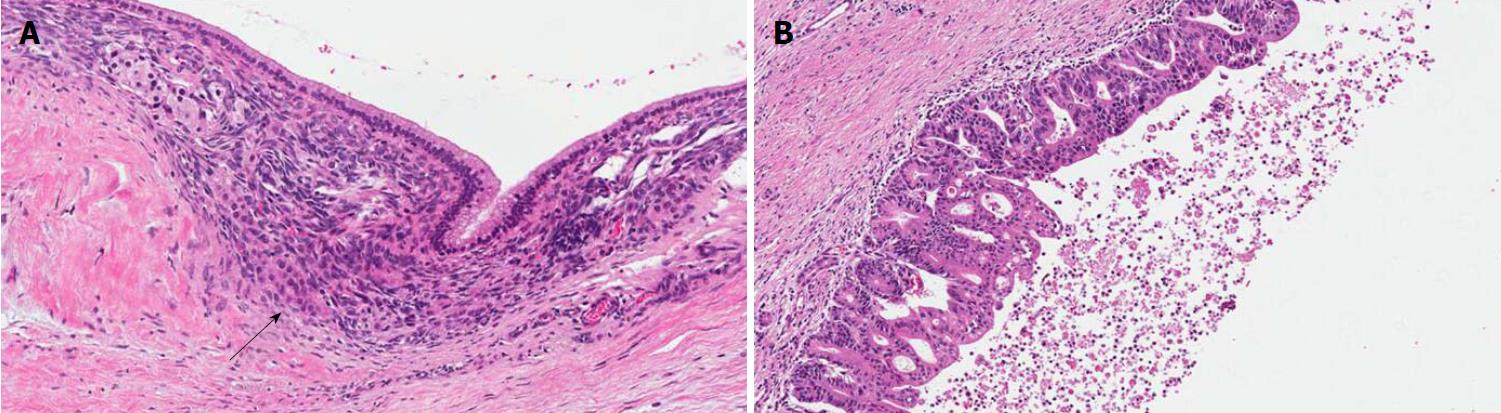
PanINs are non-infiltrating microscopic intraductal lesions with a diameter < 0.5 cm[1,3]. From the histological point of view, they are composed of cuboid to columnar mucinous cells with varying degrees of dysplasia, reflecting the different degrees of cytologic and/or architectural atypia[1,3]. In the vast majority of cases, PanINs show gastric/foveolar differentiation[21]. Hruban et al[4] classified PanINs into a three-tiered scale, based on the degree of dysplasia. In this scheme, PanIN-1 shows low-grade dysplasia, PanIN-2 shows intermediate dysplasia and PanIN-3 shows high-grade dysplasia characterized by marked cell atypia, presence of mitotic figures, loss of polarity, and complex architecture. To improve inter-observer agreement and in order to report only the most important histological information, a recent consensus suggested a new classification system, distinguishing low-grade PanINs, which includes the previously called PanIN-1 and PanIN-2, and high-grade PanINs that includes PanIN-3 (Figure 2)[5]. In high-grade PanINs, cribriform structures, atypical mitosis, tufting of epithelial cells in the lumen, and even necrosis may be present, but in case these features are concomitant with a PDAC, the most important differential diagnosis of high-grade PanINs is with non-dysplastic ducts, which have been colonized by PDAC cells[27]. Notably, high-grade PanINs have been reported almost exclusively in association with an infiltrating PDAC[1,21]. However, a recent report pointed out that high-grade dysplasia PanINs may be found without concomitant infiltrating PDAC, and, when they involve the main duct, they may cause stenosis with extensive upstream duct dilation[28]. At the immunohistochemical level, PanINs show an increased expression of mucin 1 and mucin 5AC (MUC1 and MUC5AC) and a decreased expression of mucin 6 (MUC6)[29-32].
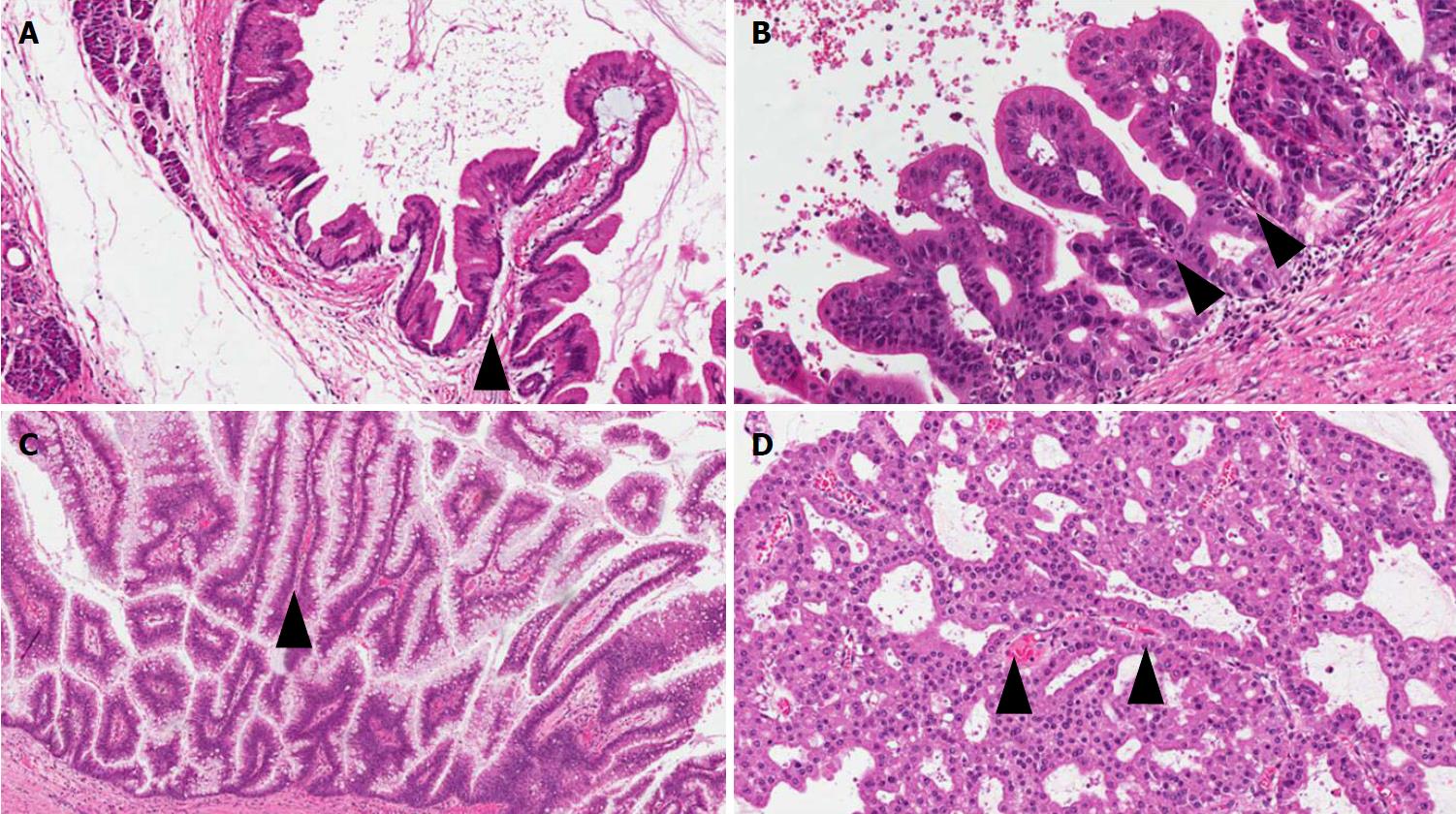
IPMNs are non-infiltrating neoplasms > 1.0 cm with intraductal growth composed of mucinous cells with a papillary architecture[1,5]. Lesions with such features but with a size > 0.5 cm but < 1.0 cm should be classified as “incipient IPMN”[5]. Similarly to PanINs, a recent consensus suggested grading IPMNs into low-grade and high-grade and to avoid the intermediate-grade dysplasia, which should be included into the low-grade category[5]. IPMNs can be classified not only basing on topography (main duct, branch duct, or mixed type), but also from the histological point of view, based on the histotype of the predominant epithelium, which also influences their biological behavior: Gastric, pancreatobiliary, intestinal, and oncocytic[1,33-41] (Figure 3).
Gastric-type IPMNs usually do not involve the Wirsung’s duct. They are composed of cells with the features of the gastric foveolar epithelium. There is a single layer of mucinous cells with polarized nuclei located at the basis of the cells. Usually this epithelium is associated with low-grade dysplasia. It can show a mixture of papillary, pseudopapillary, and flat structures[1]. When high-grade dysplasia is present in a gastric-type IPMN, with complex structure and atypical cells, the lesion becomes histologically very similar to a pancreatobiliary-type IPMN[33]. Questions are still open if these aspects represent different degrees of gastric-epithelial dysplasia, or could represent intratumor heterogeneity of IPMNs with low-grade gastric epithelium and high-grade pancreatobiliary epithelium coexisting in the same lesion[33-36].
Pancreatobiliary IPMNs usually involve the Wirsung’s duct. They are composed of irregular cells usually with enlarged nuclei and prominent nucleoli. Typically, the dysplasia in this type of lesion is high-grade. Among the different IPMN subtypes, they have the highest risk to progress into PDAC[21,33-41]. Indeed, a recent meta-analysis including 14 studies for a total of 1617 patients, showed that pancreatobiliary IPMNs are associated with the most aggressive behavior, while gastric IPMNs display the lowest risk of cancer progression[37].
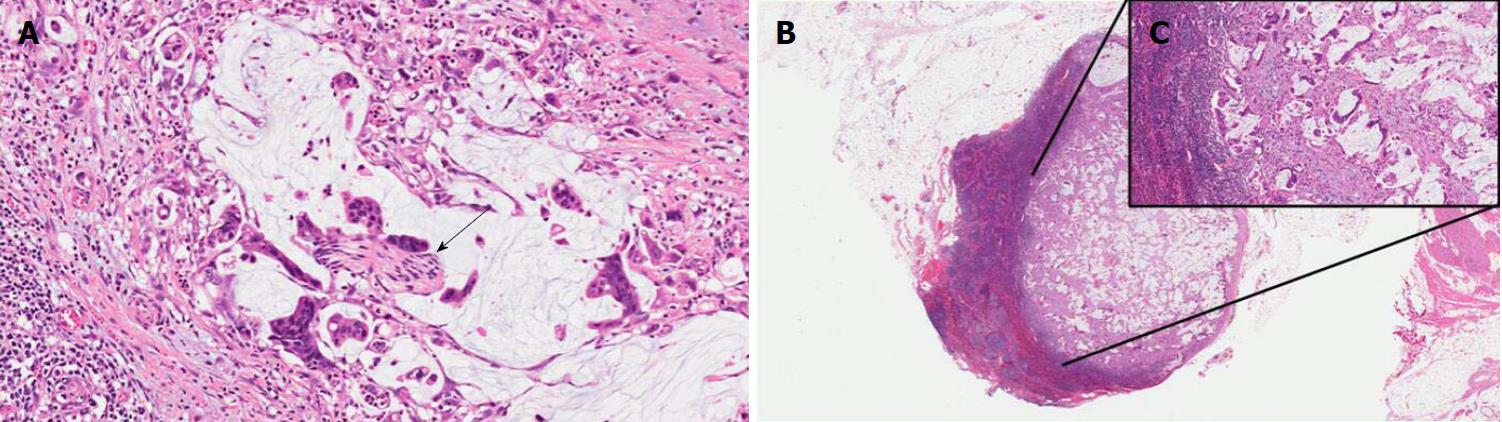
Intestinal IPMNs usually involve Wirsung’s duct. They are histologically similar to villous adenoma of the large bowel. Their most evident morphological feature is represented by the presence of goblet cells, the papillae are long and sometimes branching, and the nuclei of the cells are hyperchromatic, elongated, and show different degrees of pseudostratification[1,21]. Although their risk is lower than pancreaticobiliary type, intestinal IPMNs can progress into invasive adenocarcinoma as well. Interestingly, the latter is not usually represented by a conventional adenocarcinoma but by a colloid carcinoma (Figure 4), which displays a better prognosis than conventional PDAC[1].
Oncocytic IPMNs are rare lesions, which may involve both main and branch ducts, or even the entire pancreatic ductal tree. They are composed of cells with a typical eosinophilic and granular cytoplasm due to the abundance of accumulated mitochondria[32]. Not only is the cytological appearance peculiar, but so is the architecture. Oncocytic IPMN form arborizing papillae, lined by one to five layers of cuboidal cells. A specific feature is represented by punched-out spaces in the epithelium[1,21,32].
The best strategy for pathologists to classify IPMN histotypes is coupling morphology with immunohistochemistry, particularly in the case of high-grade dysplasia. Immunohistochemistry based on mucin staining appears of great importance in this setting (Table 1)[1,33-41]. However, even with this integrated approach about 25% of cases are difficult to classify, mainly due to the presence of phenotypic heterogeneity or dedifferentiated areas[42].
MCNs are composed of columnar cells with abundant mucin located in the luminal part of the cells. The dysplasia of MCNs should be classified with a two-tiered scale (MCNs with low-grade including the previously called intermediate dysplasia, vs MCNs with high-grade dysplasia), following the recommendation of the latest consensus conference[5]. MCNs with low-grade dysplasia show mild cell atypia and lack of architectural complexity. MCNs with high-grade dysplasia are composed of atypical cells often with enlarged nuclei and multi-layer stratification (Figure 5). The diagnostic clues for the diagnosis of MCNs are represented by the lack of communication with the pancreatic ductal tree (always present in IPMNs), and the presence of an ovarian-like stroma located under the mucinous epithelium (Figure 5)[1,5,43]. These stromal cells usually exhibit immunostaining for progesterone and estrogen receptors as well as for alfa-inhibin[44,45]. In the case of an associated invasive adenocarcinoma, the latter is usually represented by a conventional PDAC[21].
ITPNs are composed of relatively uniform and cuboidal cells, without a significant amount of mucin, arranged in densely packed tubules and back-to-back glands, with a typical intraductal, tubulopapillary growth (Figure 6)[1]. In this type of lesion, extracellular mucin production may be lacking or very focal with less common cyst formation as a direct consequence. An intestinal-type necrosis may also be present[46]. ITPNs are typically negative for MUC5AC, while MUC6 is often strongly positive (Table 1)[1,46]. Because of their potential progression to invasiveness as well as for their non-negligible association with PDAC, ITPNs are also considered a PDAC precursor lesion[1,21].
The study of the molecular landscape of PDAC precursor lesions has generated a growing body of knowledge, very useful not only to the comprehension of its oncogenesis but also to plan future strategies for their early detection. From the molecular point of view, KRAS, TP53, CDKN2A, and SMAD4 represent the four major driver genes of PDAC[1,27], and it is of great interest the timing in which its precursors acquire alterations in such genes during their specific carcinogenesis. The most important aspect in this process, which is common to each precursor, is that a KRAS mutation is a fundamental and early event.
The generally accepted definition of PanIN as a true precursor lesion of PDAC has been necessarily confirmed through their molecular characterization. Seminal research on this topic has showed that there is molecular evidence of the progression of PanIN towards PDAC. Early lesions (low-grade PanINs) display KRAS somatic mutations, and high-grade PanINs harbor CDKN2A, TP53, and SMAD4 mutations[47-53]. In PanIN carcinogenesis, TP53 and SMAD4 inactivation appear as the latest events[53].
A recent whole-exome sequencing study on IPMNs has showed an average of 26 mutated genes per case[54]. The most frequently mutated genes in IPMNs are GNAS and KRAS, which are altered in up to 60% and to 80% of cases, respectively[54,55]. Notably, recent studies pointed out that the carcinogenesis of IPMNs may follow two distinct pathways: The first, linked to GNAS mutations, are intestinal IPMNs progressing to colloid adenocarcinomas, and the second, linked to KRAS mutations, are typical of pancreatobiliary IPMNs and leads to conventional PDAC[56-59]. Another frequently mutated gene in IPMNs is RNF43, an E3 ubiquitin-protein ligase, which functions as a negative regulator of the Wnt-signaling pathway[54,60]. Lastly, BRAF, TP53, and SMAD4 mutations can be found in IPMN with high-grade dysplasia. TP53 and SMAD4 mutations, similar to high-grade PanINs, are the latest molecular events in IPMN carcinogenesis[60].
A recent whole-exome sequencing study of MCNs revealed an average of 16 somatic mutations per case[54], and compared to IPMNs there was a lower percentage of cases with loss of heterozygosity events, a molecular feature associated to poor prognosis[54,61]. The fewer number of mutations and chromosomal alterations in MCNs could explain the lower frequency of progression to PDAC of this type of precursor when compared with IPMNs. In MCNs, somatic mutations involving the four classic PDAC driver genes (KRAS, CDKN2A, TP53, and SMAD4) and RNF43 have also been reported[54].
This type of lesion has a peculiar molecular profile. Particularly, mutations involving KRAS, NRAS, and GNAS are very rare[60,62,63]. At the same time, PIK3CA mutations and AKT alterations (and consequently the involvement of the druggable mTOR pathway) are seen in 21% to 27% of ITPN cases[62,63].
Recent molecular advances in pancreatic carcinogenesis have given new interesting insights into the biological behavior of PDAC. The study of the genetic landscape of its precursor lesions has highlighted important implications for the early detection and for the clinical management of patients with pre-invasive and invasive pancreatic tumors.
The most recent advances on the genetics of PanINs come from the study by Hosoda et al[64] of a series of isolated PanINs, i.e. those occurring in the absence of a concomitant PDAC. Whole-exome or targeted sequencing of 23 isolated high-grade PanINs found that KRAS mutations were present in the vast majority of lesions (> 90%), and CDKN2A and RFN43 mutations were relatively frequent (about 20%-25% of cases), but other genes previously considered important in high-grade PanINs, i.e., TP53, SMAD4, GNAS, ARID1A, PIK3CA, and TGFBR2 were very rarely mutated or not altered[64]. In the same study, 16 adjacent low-grade PanINs were sequenced showing very frequent KRAS mutations (> 90% of cases) and lack of mutations in TP53, CDKN2A, and SMAD4 tumor suppressor genes[64]. The main conclusion of this paper was that mutations of TP53 and SMAD4 are events mainly associated with invasive PDAC and not with PanIN precursor lesions. Also the inactivation of chromatin remodeler genes, such as ARID1A tumor suppressor gene, previously thought to be important in PDAC and other invasive malignancies[65-68], appeared to be associated with infiltrating cancers rather than precursor lesions in the pancreas. The refinement of our knowledge on the morphological and molecular alterations of PanINs should be taken into account by future researchers in order to improve the possibilities of PDAC early detection.
The clinical management of IPMNs has changed in the last decade. The most recent guidelines indicate the need of combining clinical and radiological information in order to define the best therapeutic choice. Particular features, whose presence has different implications, have been distinguished in IPMN patients and indicated as “high-risk stigmata” and “worrisome features”[69]. The “high-risk stigmata” are represented by: (1) obstructive jaundice in a patient with cystic lesion of the head of the pancreas; (2) enhancing mural nodule > 5 mm; and (3) main pancreatic duct > 10 mm. The “worrisome features” comprise of: (a) clinical pancreatitis; (b) cyst > 3 cm, (c) enhancing mural nodule < 5 mm; (d) thickened/enhancing cyst walls; (e) main duct size 5–9 mm; (f) abrupt change in caliber of pancreatic duct with distal pancreatic atrophy; (g) lymphadenopathy; (h) increased serum level of CA19-9; and (i) cyst growth rate > 5 mm/2 years[69]. Thus, patients with IPMN should be followed-up with a stringent protocol, which integrates imaging (endoscopic ultrasonography, computed tomography, and magnetic resonance) and clinical data, on the basis of their importance and their specific risk of progression to invasive cancer. From the molecular point of view, the recent advances in this field have provided interesting information from the genetic analysis of cyst fluids[70]. Future protocols should integrate clinical-radiological information with molecular data, to obtain for each patient an integrated estimation of the risk of PDAC development. This approach, however, should also take into account the issue of field-effect carcinogenesis of PDAC. Indeed, IPMNs and PDACs are not necessarily genetically related as recently reported by Felsenstein et al[71], who demonstrated that about 20% of coexisting IPMNs and PDACs are molecularly unrelated, indicating the possibility of PDAC development independent from a coexisting IPMN. The main implication of this research regards the strategy of surveillance of patients with IPMN[72]. Also, the local recurrence of IPMN or PDAC after pancreatic resection for a IPMN may be genetically unrelated, highlighting the existence of a field-effect carcinogenesis of PDAC[73].
The clinical management of patients with MCNs and ITPNs should take into account recent molecular knowledge. MCNs and ITPNs represent true PDAC precursor lesions, thus an integrated approach with clinical-radiological information and molecular data should be implemented in order to define stringent protocols for the surveillance of low-risk subjects as well as precise parameters indicating the need of pancreatic resection in high-risk patients.
Another recent fascinating advance in pancreatic carcinogenesis are the putative cells of origin of this tumor type. Indeed, recent evidence from engineered mice-models suggests that PanIN development seems to be the result of the transdifferentiation of acinar cells, while IPMNs seem to arise from the progenitor niche of the pancreatic ductal epithelium[74-76]. These new concepts have totally changed the previous convictions, which indicated the differentiated ductal cells as the progenitor of PDAC. Understanding these two different pathways of PDAC carcinogenesis, one starting from acinar epithelium and one from ductal epithelium, could also partly explain the different biological behaviors of PanINs and IPMNs and their progression into an overt PDAC[74-76].
The histopathological and molecular features of PDAC precursor lesions have been summarized in Table 2 to provide a complete vision on this important topic. They represent a fundamental issue for the comprehension of PDAC carcinogenesis and its biological behavior. Only an integrated approach coupling histopathology and molecular analysis may guarantee a decisive step for the early detection of PDAC and to design more effective therapeutic strategies.
| Precursor lesions | Main histopathological features | Molecular hallmarks |
| PanIN | Non-infiltrating lesions involving pancreatic ducts and < 0.5 cm, composed of cuboid to columnar mucinous cells, with two degrees of dysplasia: (1) Low-grade PanINs include the previously called PanIN-1 and PanIN-2; and (2) high-grade PanINs include PanIN-3 | KRAS somatic mutations are early molecular events (Low-grade PanINs); CDKN2A, TP53, and SMAD4 mutations are late molecular events (High-grade PanINs) |
| IPMN | Non-infiltrating intraductal neoplasms > 1.0 cm composed of mucinous cells with papillary architecture. IPMNs have two degrees of dysplasia: (1) Low-grade IPMNs; and (2) High-grade IPMNs. IPMNs can be classified based on topography (main duct, branch duct or mixed) and also on histology (gastric, pancreaticobiliary, intestinal, or oncocytic type, see Table 1) | GNAS and KRAS are altered in up to 60% and to 80% of IPMNs, respectively There are two possible carcinogenetic processes: (1) GNAS mutations cause progression to colloid carcinomas; and (2) KRAS mutations lead to conventional PDAC. Other frequently mutated genes in IPMNs are RNF43, BRAF, TP53, and SMAD4 |
| MCN | Composed of columnar cells with abundant mucin located in the upper part. There are two degrees of dysplasia: (1) Low-grade MCN; and (2) High-grade MCN. The histopathologic clues for MCN diagnosis are the lack of communication with the pancreatic ductal tree and the presence of an ovarian-like stroma under the mucinous epithelium | There are fewer mutations and chromosomal alterations in MCNs compared with other precursors, and this could explain the lower frequency of progression of MCN to PDAC. Frequently altered genes are KRAS, CDKN2A, TP53, SMAD4, and RNF43 |
| ITPN | Composed of uniform cuboidal cells without a significant amount of mucin, arranged in densely packed tubules and back-to-back glands with a typical intraductal, tubulopapillary growth | PIK3CA mutations and AKT alterations are frequently seen in ITPNs. Mutations involving KRAS, NRAS, and GNAS are very rare in ITPNs |
Manuscript source: Invited Manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Guo JC, Yang F, Guo XZ, Mukaida N S- Editor: Dou Y L- Editor: Filipodia E- Editor: Tan WW
| 1. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Lyon: IARC Press 2010; . [Cited in This Article: ] |
| 2. | Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 788] [Cited by in F6Publishing: 702] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 3. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 454] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 758] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 496] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 6. | Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Brune K, Abe T, Canto M, O’Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [PubMed] [Cited in This Article: ] |
| 8. | Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737-7743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Takaori K, Matsusue S, Fujikawa T, Kobashi Y, Ito T, Matsuo Y, Oishi H, Takeda H. Carcinoma in situ of the pancreas associated with localized fibrosis: a clue to early detection of neoplastic lesions arising from pancreatic ducts. Pancreas. 1998;17:102-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Morohoshi T, Kanda M, Asanuma K, Klöppel G. Intraductal papillary neoplasms of the pancreas. A clinicopathologic study of six patients. Cancer. 1989;64:1329-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 12. | Ingkakul T, Warshaw AL, Fernández-Del Castillo C. Epidemiology of intraductal papillary mucinous neoplasms of the pancreas: sex differences between 3 geographic regions. Pancreas. 2011;40:779-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Ingkakul T, Sadakari Y, Ienaga J, Satoh N, Takahata S, Tanaka M. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg. 2010;251:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 334] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 15. | Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-685; discussion 685-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 529] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 16. | Sparr JA, Bandipalliam P, Redston MS, Syngal S. Intraductal papillary mucinous neoplasm of the pancreas with loss of mismatch repair in a patient with Lynch syndrome. Am J Surg Pathol. 2009;33:309-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Su GH, Hruban RH, Bansal RK, Bova GS, Tang DJ, Shekher MC, Westerman AM, Entius MM, Goggins M, Yeo CJ. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835-1840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 270] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Gaujoux S, Chanson P, Bertherat J, Sauvanet A, Ruszniewski P. Hepato-pancreato-biliary lesions are present in both Carney complex and McCune Albright syndrome: comments on P. Salpea and C. Stratakis. Mol Cell Endocrinol. 2014;382:344-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Panic N, Macchini F, Solito S, Boccia S, Leoncini E, Larghi A, Berretti D, Pevere S, Vadala S, Marino M. Prevalence of Extrapancreatic Malignancies Among Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas. 2018;47:721-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Tanaka M. Intraductal Papillary Mucinous Neoplasm of the Pancreas as the Main Focus for Early Detection of Pancreatic Adenocarcinoma. Pancreas. 2018;47:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Noë M, Brosens LA. Pathology of Pancreatic Cancer Precursor Lesions. Surg Pathol Clin. 2016;9:561-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Fritz S, Klauss M, Bergmann F, Strobel O, Schneider L, Werner J, Hackert T, Büchler MW. Pancreatic main-duct involvement in branch-duct IPMNs: an underestimated risk. Ann Surg. 2014;260:848-55; discussion 855-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 521] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Ridder GJ, Maschek H, Flemming P, Nashan B, Klempnauer J. Ovarian-like stroma in an invasive mucinous cystadenocarcinoma of the pancreas positive for inhibin. A hint concerning its possible histogenesis. Virchows Arch. 1998;432:451-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Baker ML, Seeley ES, Pai R, Suriawinata AA, Mino-Kenudson M, Zamboni G, Klöppel G, Longnecker DS. Invasive mucinous cystic neoplasms of the pancreas. Exp Mol Pathol. 2012;93:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Luchini C, Capelli P, Scarpa A. Pancreatic Ductal Adenocarcinoma and Its Variants. Surg Pathol Clin. 2016;9:547-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Yokode M, Akita M, Fujikura K, Kim MJ, Morinaga Y, Yoshikawa S, Terada T, Matsukiyo H, Tajiri T, Abe-Suzuki S. High-grade PanIN presenting with localised stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology. 2018;73:247-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Moriya T, Kimura W, Semba S, Sakurai F, Hirai I, Ma J, Fuse A, Maeda K, Yamakawa M. Biological similarities and differences between pancreatic intraepithelial neoplasias and intraductal papillary mucinous neoplasms. Int J Gastrointest Cancer. 2005;35:111-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, Coban I, Adsay NV. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 34. | Mino-Kenudson M, Fernández-del Castillo C, Baba Y, Valsangkar NP, Liss AS, Hsu M, Correa-Gallego C, Ingkakul T, Perez Johnston R, Turner BG. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 35. | Distler M, Kersting S, Niedergethmann M, Aust DE, Franz M, Rückert F, Ehehalt F, Pilarsky C, Post S, Saeger HD. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013;258:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Kim J, Jang KT, Mo Park S, Lim SW, Kim JH, Lee KH, Lee JK, Heo JS, Choi SH, Choi DW. Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol. 2011;32:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Koh YX, Zheng HL, Chok AY, Tan CS, Wyone W, Lim TK, Tan DM, Goh BK. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157:496-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Nakata K, Ohuchida K, Aishima S, Sadakari Y, Kayashima T, Miyasaka Y, Nagai E, Mizumoto K, Tanaka M, Tsuneyoshi M. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas. 2011;40:581-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Sadakari Y, Ohuchida K, Nakata K, Ohtsuka T, Aishima S, Takahata S, Nakamura M, Mizumoto K, Tanaka M. Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery. 2010;147:812-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Kobayashi M, Fujinaga Y, Ota H. Reappraisal of the Immunophenotype of Pancreatic Intraductal Papillary Mucinous Neoplasms (IPMNs)-Gastric Pyloric and Small Intestinal Immunophenotype Expression in Gastric and Intestinal Type IPMNs-. Acta Histochem Cytochem. 2014;47:45-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Schaberg KB, DiMaio MA, Longacre TA. Intraductal Papillary Mucinous Neoplasms Often Contain Epithelium From Multiple Subtypes and/or Are Unclassifiable. Am J Surg Pathol. 2016;40:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1714] [Cited by in F6Publishing: 1540] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 44. | Shiono S, Suda K, Nobukawa B, Arakawa A, Yamasaki S, Sasahara N, Hosokawa Y, Suzuki F. Pancreatic, hepatic, splenic, and mesenteric mucinous cystic neoplasms (MCN) are lumped together as extra ovarian MCN. Pathol Int. 2006;56:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Izumo A, Yamaguchi K, Eguchi T, Nishiyama K, Yamamoto H, Yonemasu H, Yao T, Tanaka M, Tsuneyoshi M. Mucinous cystic tumor of the pancreas: immunohistochemical assessment of “ovarian-type stroma”. Oncol Rep. 2003;10:515-525. [PubMed] [Cited in This Article: ] |
| 46. | Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 47. | Klimstra DS, Longnecker DS. K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol. 1994;145:1547-1550. [PubMed] [Cited in This Article: ] |
| 48. | Lemoine NR, Jain S, Hughes CM, Staddon SL, Maillet B, Hall PA, Klöppel G. Ki-ras oncogene activation in preinvasive pancreatic cancer. Gastroenterology. 1992;102:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 161] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Cooper CL, O’Toole SA, Kench JG. Classification, morphology and molecular pathology of premalignant lesions of the pancreas. Pathology. 2013;45:286-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | DiGiuseppe JA, Hruban RH, Offerhaus GJ, Clement MJ, van den Berg FM, Cameron JL, van Mansfeld AD. Detection of K-ras mutations in mucinous pancreatic duct hyperplasia from a patient with a family history of pancreatic carcinoma. Am J Pathol. 1994;144:889-895. [PubMed] [Cited in This Article: ] |
| 51. | Delpu Y, Hanoun N, Lulka H, Sicard F, Selves J, Buscail L, Torrisani J, Cordelier P. Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr Genomics. 2011;12:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140-2143. [PubMed] [Cited in This Article: ] |
| 53. | Brosens LA, Hackeng WM, Offerhaus GJ, Hruban RH, Wood LD. Pancreatic adenocarcinoma pathology: changing “landscape”. J Gastrointest Oncol. 2015;6:358-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 31] [Reference Citation Analysis (0)] |
| 54. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 457] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 55. | Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 565] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 56. | Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, LaFemina J, Jarnagin WR, Berger MF, Klimstra D. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J Am Coll Surg. 2015;220:845-854.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 57. | Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, Yatabe Y. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch. 2015;466:665-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Molin MD, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802-3808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 59. | Yamada M, Sekine S, Ogawa R, Taniguchi H, Kushima R, Tsuda H, Kanai Y. Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol. 2012;228:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 61. | Southern JF, Warshaw AL, Lewandrowski KB. DNA ploidy analysis of mucinous cystic tumors of the pancreas. Correlation of aneuploidy with malignancy and poor prognosis. Cancer. 1996;77:58-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 62. | Yamaguchi H, Kuboki Y, Hatori T, Yamamoto M, Shiratori K, Kawamura S, Kobayashi M, Shimizu M, Ban S, Koyama I. Somatic mutations in PIK3CA and activation of AKT in intraductal tubulopapillary neoplasms of the pancreas. Am J Surg Pathol. 2011;35:1812-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Yamaguchi H, Kuboki Y, Hatori T, Yamamoto M, Shimizu K, Shiratori K, Shibata N, Shimizu M, Furukawa T. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J Pathol. 2013;231:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Hosoda W, Chianchiano P, Griffin JF, Pittman ME, Brosens LA, Noë M, Yu J, Shindo K, Suenaga M, Rezaee N. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol. 2017;242:16-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 65. | Luchini C, Nottegar A. The Roles of Chromatin Remodeling Genes in Pancreatic-Biliary Malignancies. Crit Rev Oncog. 2017;22:471-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 1771] [Article Influence: 196.8] [Reference Citation Analysis (1)] |
| 67. | Luchini C, Veronese N, Solmi M, Cho H, Kim JH, Chou A, Gill AJ, Faraj SF, Chaux A, Netto GJ. Prognostic role and implications of mutation status of tumor suppressor gene ARID1A in cancer: a systematic review and meta-analysis. Oncotarget. 2015;6:39088-39097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 68. | Luchini C, Veronese N, Yachida S, Cheng L, Nottegar A, Stubbs B, Solmi M, Capelli P, Pea A, Barbareschi M. Different prognostic roles of tumor suppressor gene BAP1 in cancer: A systematic review with meta-analysis. Genes Chromosomes Cancer. 2016;55:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 971] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 70. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 71. | Felsenstein M, Noë M, Masica DL, Hosoda W, Chianchiano P, Fischer CG, Lionheart G, Brosens LAA, Pea A, Yu J. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018;67:1652-1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 72. | Scarpa A, Real FX, Luchini C. Genetic unrelatedness of co-occurring pancreatic adenocarcinomas and IPMNs challenges current views of clinical management. Gut. 2018;67:1561-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Pea A, Yu J, Rezaee N, Luchini C, He J, Dal Molin M, Griffin JF, Fedor H, Fesharakizadeh S, Salvia R. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann Surg. 2017;266:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 74. | Yamaguchi J, Yokoyama Y, Kokuryo T, Ebata T, Nagino M. Cells of origin of pancreatic neoplasms. Surg Today. 2018;48:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Yamaguchi J, Mino-Kenudson M, Liss AS, Chowdhury S, Wang TC, Fernández-Del Castillo C, Lillemoe KD, Warshaw AL, Thayer SP. Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice. Gastroenterology. 2016;151:1232-1244.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Sánchez-Arévalo Lobo VJ, Fernández LC, Carrillo-de-Santa-Pau E, Richart L, Cobo I, Cendrowski J, Moreno U, Del Pozo N, Megías D, Bréant B. c-Myc downregulation is required for preacinar to acinar maturation and pancreatic homeostasis. Gut. 2018;67:707-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |