Abstract
Aims: To compare stent strut coverage using optical coherence tomography (OCT) at three-month follow-up between a PLGA-polymer with electro-grafting base layer sirolimus-eluting stent (SES) (BuMA) and a PLA-polymer SES (EXCEL).
Methods and results: This prospective, single-centre, non-inferiority randomised BuMA-OCT trial enrolled patients with de novo coronary artery lesions, treated with either the BuMA or the EXCEL stent. The study primary endpoint was OCT-evaluated stent strut coverage at three months. Secondary endpoints were neointimal thickness of stent struts, and incomplete stent apposition evaluated with OCT. A total of 80 patients were randomly assigned to receive the BuMA (n=40) or the EXCEL (n=40) stent. In OCT follow-up (achieved in 86.3% of cases: BuMA, n=33; EXCEL, n=36), the percentage of stent strut coverage was significantly higher in the BuMA vs. the EXCEL group (strut level: 94.2% vs. 90.0%, p<0.01; pnon-inferiority <0.0001; psuperiority <0.0001), while the proportion of malapposed struts (strut level: 1.28% vs. 1.80%, p=0.51) and the mean neointimal thickness (strut level: 0.07±0.03 mm vs. 0.06±0.02 mm, p=0.31) were similar. Rates of myocardial infarction (periprocedural non-Q-wave, 7.5% vs. 7.5%, p=1.00) and target lesion failure (7.5% vs. 7.5%, p=1.00) were similar between groups, with no cardiac death or stent thrombosis.
Conclusions: In the BuMA-OCT randomised trial, the novel BuMA PLGA-polymer with electro-grafting base layer SES was superior to the EXCEL PLA-polymer SES in the primary endpoint of stent strut coverage at three-month follow-up.
Introduction
Metallic drug-eluting stents (DES) have become widely used in patients with coronary artery disease, significantly reducing restenosis compared with bare metal stents (BMS)1-3. However, delayed vascular healing and impaired endothelialisation, potentially caused by polymer-related inflammation, hypersensitivity, and toxicity in first-generation DES, increase the risk of late and very late stent thrombosis (ST)4-6. Biodegradable polymer DES may address the problem both by controlling drug release for neointimal hyperplasia inhibition and by subsequent polymer degradation. A recent study performed by Kim and colleagues using optical coherence tomography (OCT) found that the biodegradable-polymer biolimus-eluting stent (BES) BioMatrix™ (Biosensors Interventional, Singapore) had a non-significantly higher percentage of incomplete strut coverage compared with a durable-polymer sirolimus-eluting stent (SES) at three months, but a greater reduction in the percentage of uncovered struts was observed at 12 months7. The EXCEL SES (JW Medical Systems, Weihai, China), a DES with similar features to the BioMatrix BES, has been extensively used in China8; however, data on earlier vascular response following its implantation are limited.
The BuMA stent (SINOMED, Beijing, China) is a novel biodegradable poly-lactic-co-glycolic acid (PLGA) polymer SES, with an electro-grafting (eG) base layer added between the polymer and the stainless steel stent strut9. The eG layer secures adhesion of the biodegradable PLGA coating through interdigitation, and the PLGA coating ensures 100% drug release within 30 days. Initial investigation showed a high strut coverage rate in both the BuMA and the XIENCE V® (Abbott Vascular, Santa Clara, CA, USA) stents at three-month follow-up10. Earlier vascular healing after drug-eluting stent implantation may reduce the incidence of ST at follow-up and potentially shorten dual antiplatelet therapy duration. Therefore, we performed this randomised trial to compare OCT-evaluated vascular response at three-month follow-up between the BuMA and the EXCEL stents, both eluting sirolimus from different biodegradable polymer carriers.
Methods
STUDY DESIGN AND PATIENT POPULATION
The BuMA trial is a prospective, single-centre, randomised, noninferiority study comparing the BuMA versus the EXCEL stent in terms of OCT-evaluated stent strut coverage at three-month follow-up. The protocol was approved by the institutional review board at FuWai Hospital (Beijing, China), and all patients had to provide written informed consent.
From January through April 2013, patients were eligible for enrolment in the trial if they were over 18 years of age, had evidence of myocardial ischaemia without raised cardiac markers, and had a planned intervention of de novo coronary artery lesions, in different epicardial vessels. Lesions were required to have a visually estimated diameter stenosis of ≥50% and <100%, and reference vessel diameter between 2.5 mm and 4.0 mm. Major exclusion criteria were: acute myocardial infarction within one week; left main coronary artery lesion; true bifurcation with side branch diameter ≥2.25 mm; total occlusion lesion; left ventricular ejection fraction <30%; renal insufficiency; in-stent restenosis lesion; and any lesions with severely tortuous, calcified or angulated coronary anatomy (ClinicalTrials.gov Identifier: NCT01752582).
RANDOMISATION AND STUDY PROCEDURE
Patients meeting the study criteria were randomly assigned to receive either the BuMA or the EXCEL stent in a 1:1 ratio. Randomisation was performed centrally after diagnostic coronary angiography and before percutaneous coronary intervention (PCI) by use of a sealed envelope. All patients received a loading dose of 300 mg clopidogrel and 100 mg aspirin at least 12 hrs before PCI. All PCI procedures were performed according to current standard techniques. Post-PCI treatment included six months of dual antiplatelet therapy (DAPT) with 100 mg aspirin and 75 mg clopidogrel daily.
DEVICES USED
The BuMA stent is an SES that is fully coated with two different layers - the electro-grafting base layer (poly [n-butyl methacrylate] coating) and the biodegradable PLGA drug carrier (Figure 1A)9. The stent platform is made of laser-cut 316L stainless steel with a strut thickness of 100 μm. The base layer is electro-grafted onto the stent surface with a thickness of 100 to 200 nm. The biodegradable polymer coating sits on top of the base layer and consists of a sprayed PLGA drug carrier with 6-8 μg sirolimus per mm to both sides of the struts. The degradation period of the PLGA polymer is around 10 weeks with sirolimus being completely released in a controlled manner for 30 days in vivo.
The EXCEL stent is an SES that is coated with a biodegradable poly-lactic acid (PLA) polymer (Figure 1B)8. The stent platform is made of laser-cut 316L stainless steel with a strut thickness of 120 μm. The coating is mixed with sirolimus and applied onto the abluminal surface of the stent with a thickness of 10-15 μm. Total sirolimus dosage is 13-14 μg/mm according to the stent length. Animal studies showed that the polymer coating has a complete degradation cycle of six to nine months.

Figure 1. Devices. A) The BuMA stent is a sirolimus-eluting stent, fully coated with two different functional coating layers: the eGTM base layer and the PLGA polymer drug carrier. B) The EXCEL stent is a sirolimus-eluting stent, abluminally coated with PLA polymer drug carrier. Scanning electron microscopy images of the device platforms are presented in the lower right corner of the two panels (original magnification ×150). eGTM: electro-grafting; PLA: poly-lactic acid; PLGA: poly-lactic-co-glycolic acid
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
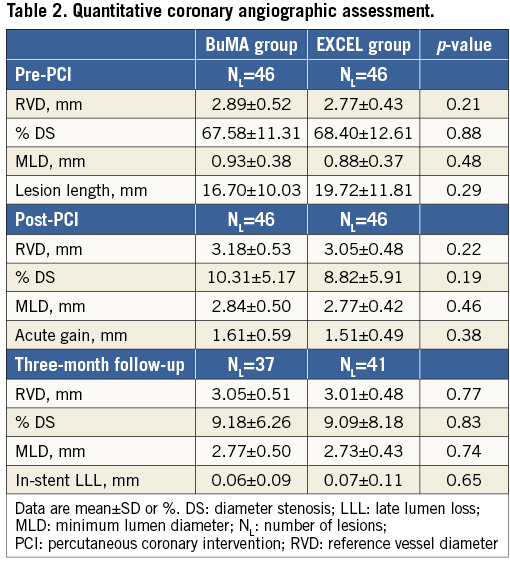
Quantitative coronary angiography (QCA) analysis was performed by two independent operators from an angiographic core laboratory (FuWai Hospital, Beijing, China), using the QAngio XA analysis software (Medis Medical Imaging Systems Inc., Leiden, The Netherlands). Standard QCA methodology was used including analysis of stent and peri-stent segments defined as spanning 5 mm proximal and distal to the stent edge. Binary restenosis was defined in every segment (proximal, distal, and in-stent) as >50% diameter stenosis at follow-up. Late lumen loss (LLL) was defined as the difference between follow-up and post-procedure minimal lumen diameter.
OPTICAL COHERENCE TOMOGRAPHY ASSESSMENT
OCT acquisitions were performed using the C7XR Fourier-Domain system (St. Jude Medical, St. Paul, MN, USA). After confirming the correct position of the OCT catheter, 100-200 μg nitrates were administered. The targeted artery was then cleared of blood by automatic injection of contrast at a flow rate of 3-4 ml/sec and a pressure of 300-600 psi according to the diameter of the artery and quality of the OCT images. An injection of 10-12 mL or 12-15 mL of contrast for the right and left coronary arteries, respectively, was sufficient to achieve a good blood clearing effect. Automated pullback was performed at a speed of 20 mm/sec with a frame rate of 100 frames/sec. Frequency-domain OCT images were calibrated adjusting the Z-offset. This critical step was performed before and after image acquisition to obtain accurate measurements.
All OCT frames were digitally stored and cross-sectional OCT images of stented segments were analysed at 0.4/0.6 mm alternative intervals by an independent core laboratory (FuWai Hospital, Beijing, China), using a validated software (QIvus version 2.2; Medis Medical Imaging Systems Inc.). Two independent analysts blinded to stent-type allocation and clinical information of the patients completed the measurements. The workload was almost evenly split between the two analysts and all measurements were reviewed by another analyst, with discrepancies resolved by consensus with the participation of a third referee. OCT parameters were calculated and defined as follows11-13. Neointimal thickness was measured as the perpendicular distance between the endoluminal surfaces of the neointima to the stent strut. The absence of definite neointima over the stent strut was defined as an uncovered stent strut. The distance between the inner surface of the strut reflection and the vessel wall was measured by extending the contours of the walls on the outside of the strut shadow. Stent strut malapposition was defined as struts with detachment from the vessel wall >138 μm for BuMA and >155 μm for EXCEL (stent strut thickness+coating thickness+OCT resolution limit of 20 μm). The incomplete strut apposition (ISA) area was defined as the fraction of the lumen area that lies outside the stent in each cross-section with ISA. Stent struts located at the ostium of a side branch were excluded from analysis. Thrombus was defined as an irregular mass ≥300 µm2 protruding into the lumen or as an intraluminal mass with signal-free shadowing unconnected to the luminal surface. The percentage of cross-section with a rate of uncovered to total struts (RUTTS) score >30% was analysed in all eligible cross-sections6.
STUDY ENDPOINTS AND CLINICAL FOLLOW-UP
The primary endpoint was the rate of stent strut coverage evaluated with OCT at three months. Secondary endpoints included neointimal thickness of stent struts, ISA evaluated with OCT, and angiographic in-stent LLL at follow-up.
Clinical follow-up visits were conducted at three months (including angiography/OCT investigation), six months, and one and two years post PCI. Target lesion failure was defined as the composite of cardiac death, target vessel myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (TLR). MI was adjudicated according to the third universal definition of MI14. Stent thrombosis (definite and probable) was defined according to the Academic Research Consortium classification. All clinical events were adjudicated by an independent clinical events committee.
Device success was defined as attainment of <30% residual stenosis of the target lesion by visual assessment; lesion success was defined as attainment of <50% residual stenosis, TIMI 3 flow, and no residual dissection or thrombosis of the target lesion using any percutaneous method; and clinical success was defined as attainment of lesion success of the target lesion and no in-hospital major adverse cardiac event.
Statistical analysis
Sample size calculation in this study was based on the predefined hypothesis that the BuMA stent would show a non-inferiority increase in strut coverage relative to that of the EXCEL stent at three-month follow-up OCT. If non-inferiority was met, a superiority test would be conducted. Assuming a neointimal coverage rate for both BuMA and EXCEL stents of 94%, a non-inferior margin of 1.2%, and a one-sided α of 0.025, 19,510 stent struts would have at least 94% power to detect non-inferiority. Based on previous OCT analysis experiences, it was assumed that 1.3 stents would be implanted in each patient with a 20 mm length for each stent. Each stent would be scanned by OCT at 0.5 mm intervals, and 6.7 struts would be detected in each cross-section. Therefore, 56 patients would be required. Estimating a rate of 20% loss to follow-up, the final sample size should be greater than 70.
Continuous variables are presented, if normally distributed, as mean ± standard deviation and compared with the Student’s t-test, and otherwise as median with first and third quartiles and compared using a non-parametric Wilcoxon rank-sum test. Categorical variables are presented as counts and percentages, and compared with chi-square statistics or Fisher’s exact test. Considering the correlation of lesion characteristics within patients and their variation between patients in the BuMA and EXCEL groups, a multilevel GLIMMIX model with random-effects model was used15,16. A p-value of <0.05 was considered a statistically significant level. Statistical analysis was performed with SAS software (SAS version 9.1.3; SAS Institute, Cary, NC, USA).
Results
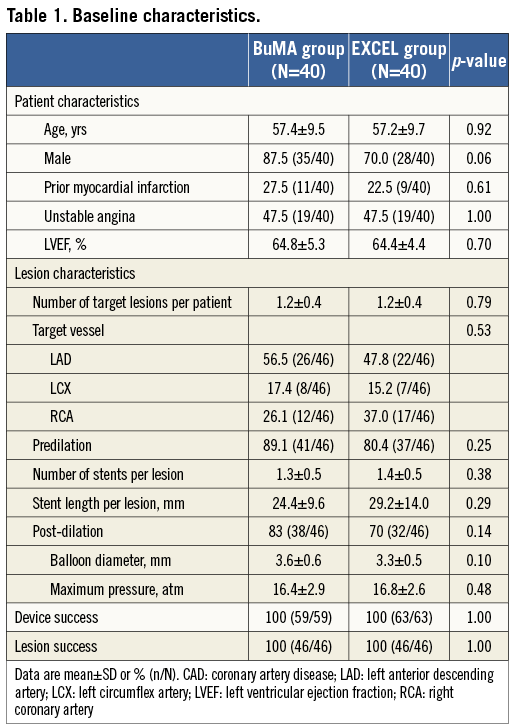
PATIENT CHARACTERISTICS
A flow chart summarising patient enrolment and scheduled follow-up is shown in Figure 2. Briefly, a total of 80 patients were randomly assigned to receive either the BuMA (n=40) or the EXCEL (n=40) stent. Baseline characteristics between the two groups were comparable (Table 1). The mean age was 57 years, 63% of patients were male, 25% had a previous history of MI, and approximately 50% presented with unstable angina. The number of target lesions per patient was 1.2, and the lesions most frequently addressed were in the left anterior descending and right coronary arteries (52% and 32%, respectively).
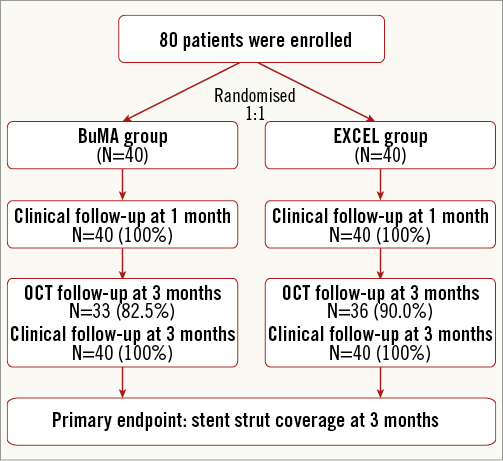
Figure 2. Flow chart of the BuMA-OCT randomised trial.
ANGIOGRAPHIC RESULTS
Baseline and three-month follow-up angiographic results are summarised in Table 2. Before and after the procedure, both groups had similar mean reference vessel diameters, % diameter stenosis, and minimal lumen diameters. At three-month follow-up, in-stent LLL was comparable between the BuMA and EXCEL groups (0.06±0.09 mm vs. 0.07±0.11 mm, p=0.65).
OCT RESULTS
Three-month angiographic follow-up and OCT acquisitions were performed in 86.3% of patients (BuMA: n=33, EXCEL: n=36). Eleven patients (BuMA: n=7, EXCEL: n=4) refused to undergo follow-up angiography and OCT examination. Out of six patients with target vessel failure (BuMA: 3, EXCEL: 3), four underwent OCT examination and were included in the analysis (BuMA: 2, EXCEL: 2), while two patients refused invasive follow-up (BuMA: 1, EXCEL: 1).
Table 3 compares OCT analysis results between groups. Representative cross-section images with covered, uncovered, and malapposed stent struts at three months after BuMA or EXCEL stent implantation are shown in Figure 3. The percentage of covered struts was significantly higher in the BuMA vs. the EXCEL group (strut level: 94.2% vs. 90.0%, p<0.01; difference and 95% confidence interval: 4.2% [3.7%-4.8%]; pnon-inferiority <0.0001, psuperiority <0.0001; Figure 4A), while the proportion of malapposed struts (strut level: 1.28% vs. 1.80%, p=0.51; Figure 4B) and the mean neointimal thickness of stent struts (strut level: 0.07±0.03 mm vs. 0.06±0.02 mm, p=0.31) were not significantly different between the BuMA and EXCEL groups.
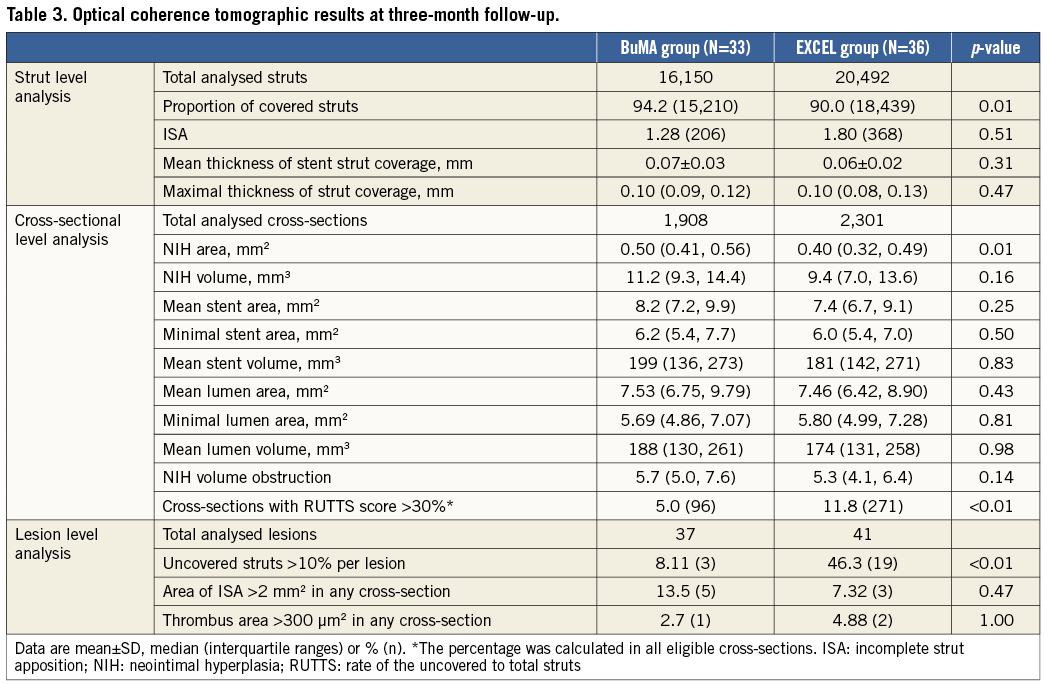
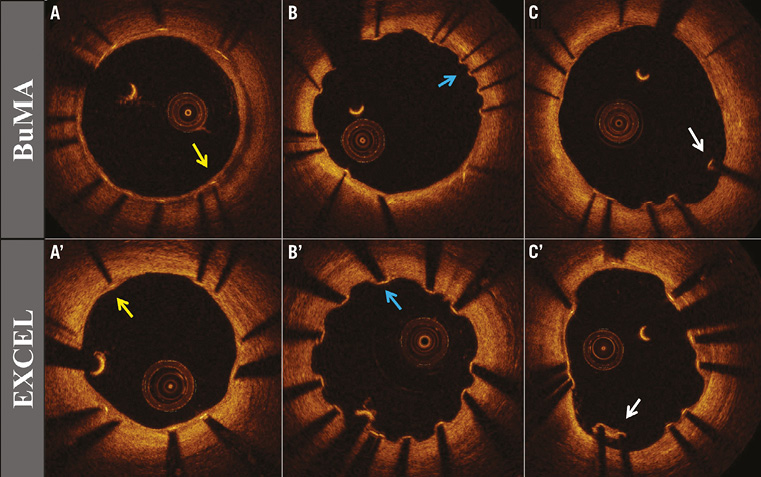
Figure 3. Optical coherence tomographic images. Representative optical coherence tomographic images from the BuMA and EXCEL groups at three-month follow-up: covered struts (A, A’, yellow arrow); uncovered struts (B, B’, blue arrow); and malapposed struts (C, C’, white arrow).
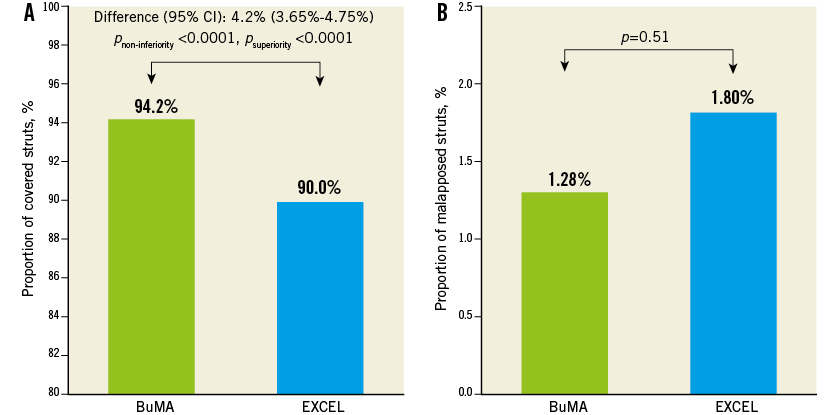
Figure 4. Strut level analysis. In strut level analysis, the proportion of covered struts was significantly higher in the BuMA vs. the EXCEL group (A), while the proportion of malapposed struts was significantly lower in the BuMA vs. the EXCEL group (B).
At the cross-sectional level, mean percentage of NIH volume obstruction (5.7% vs. 5.3%, p=0.14) was comparable between the BuMA and EXCEL stent groups, while mean neointimal hyperplasia (NIH) area was significantly higher in the BuMA than in the EXCEL group (0.50 vs. 0.40 mm2 , p=0.01) and the percentage of cross-sections with RUTTS score >30% was significantly lower in the BuMA than in the EXCEL group (5.0% vs. 11.8%, p<0.01) (Table 3).
In lesion level analysis, the proportion of uncovered struts >10% per lesion was significantly lower in the BuMA than in the EXCEL group (8.11% vs. 46.3%, p<0.01). An area of ISA >2 mm2 and a thrombus area >300 μm2 in any cross-section were similar between groups (Table 3).
Clinical outcomes
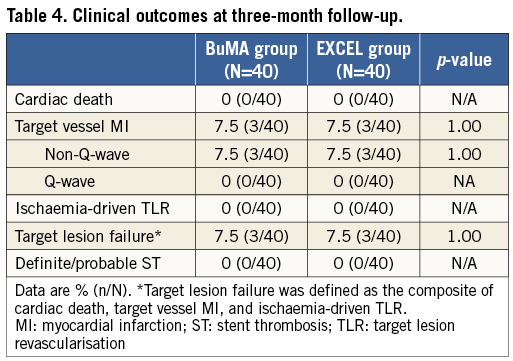
At three months, clinical follow-up was completed in 100% of patients and results are presented in Table 4. There were three periprocedural non-Q-wave MIs which resulted in 7.5% of patients adjudicated with target lesion failure in each group. There was no cardiac death, ST or ischaemia-driven TLR during the follow-up period.
Discussion
To the best of our knowledge, this is the first randomised trial comparing in vivo vascular response, evaluated by OCT at three months, between the BuMA and the EXCEL stents that elute the same antiproliferative drug sirolimus but from different biodegradable polymer coatings. The BuMA stent was shown to be superior to the EXCEL stent in terms of the predefined primary endpoint of strut coverage rate at three-month follow-up. The rate of malapposed struts was similar for the BuMA and the EXCEL groups. The latter analyses were at the strut level using multilevel models. The percentage both of cross-section with RUTTS score >30% and of uncovered struts >10% per lesion was lower following BuMA rather than EXCEL stent implantation.
An autopsy study showed that incomplete endothelialisation due to lack of neointimal coverage and stent strut malapposition at follow-up after DES implantation could be an important underlying cause for ST17. As compared to intravascular ultrasound, intravascular OCT is a useful tool to detect thin neointima following DES implantation and to understand the performance characteristics of stents with new design features18-20. Therefore, the present study utilised OCT for a comparative assessment of early vascular response after implantation of the BuMA and EXCEL stents, both biodegradable polymer-based SES extensively used in China. The EXCEL stent has features similar to those of the BioMatrix BES8. Full conversion of PLLA into lactic acid takes place by six months, and degradation of the entire coating is completed by nine months after implantation. Two small studies, one of which was randomised, reported delayed re-endothelialisation in the BioMatrix stent at short-term (three or 12 months) follow-up7,21. In the randomised study, OCT examination at 12 months showed that BES had a greater reduction of percentage Δ of uncovered struts from three to 12 months7. The BuMA stent is a novel biodegradable PLGA-polymer SES with the novel design feature of an additional electro-grafting (eG) base layer. The degradation period for the PLGA polymer is around 10 weeks9. Therefore, in the setting of similar clinical presentations, procedural and lesion characteristics, and strut thickness, the shorter polymer degradation and drug elution periods for the BuMA stent are expected to translate into better vascular healing than for the EXCEL stent, with both stents sharing a similar ability to inhibit neointimal hyperplasia.
There have been no previous studies using OCT to assess early vascular responses between PLA-coated and novel PLGA-coated biodegradable polymer SES. Therefore, the current randomised trial provides an opportunity to understand better the impact of polymer degradation duration on stent strut coverage at short-term follow-up. Our study evaluated the percentage of cross-sections with RUTTS score >30% and of uncovered struts >10% per lesion in contrast to previous stent studies reporting only the percentage of uncovered struts. All strut-, cross-sectional-, and lesion-level analyses of stent strut coverage at three-month follow-up favoured the BuMA stent over the EXCEL stent. Although stent strut coverage thickness was similar between stents, the NIH area was significantly higher in the BuMA than in the EXCEL stent, which suggests that better vascular healing may be associated with adaptive neointimal hyperplasia at early follow-up. Additionally, the non-significant difference in the rate of malapposed struts might be influenced by differences in clinical, procedural and lesions factors. However, the design of eG technology used in the BuMA stent potentially has the benefit of suppressing corrosion and ion release from metal stent substrates, which could contribute to a lower local inflammation response. Furthermore, the struts are thinner (100 μm vs. 120 μm) and the dosage of sirolimus of the BuMA stent is much lower than that in the EXCEL stent (6-8 μg/mm on both sides of the struts vs. abluminal 13-14 μg/mm, respectively). Therefore, beneficial vascular responses following BuMA stent implantation seen at early follow-up in our study might be attributed to the novel PLGA polymer with a shorter biodegradation duration and to the eG coating layer easing re-endothelialisation.
Although limited by the short-term (≤1 year) span for OCT evaluation of biodegradable polymer DES, a recent randomised study comparing BioMatrix biodegradable polymer BES versus durable polymer SES showed no significant difference in the percentage of uncovered struts (14.7% vs. 8.6%, p=0.98); however, there was a significantly lower percentage of uncovered struts at 12 months in the BES relative to the SES group (2.6% vs. 6.2%, p=0.028)7. In our study, the rate of uncovered struts in the EXCEL stent at three months was 10.0%, which is consistent with the data for BES, although the eluted drug was different. In the LEADERS OCT substudy (26 BES and 30 SES), biodegradable polymer BES was associated with overall lesser proportions of uncovered (0.6% vs. 2.1%, p=0.04) and malapposed struts (0.2% vs. 0.4%, p=0.08) than durable polymer SES at nine-month follow-up22. The discrepancy between the LEADERS substudy and the present study might be attributed to the higher risk profile of enrolled patients and longer follow-up in the LEADERS all-comers trial. Early OCT evaluation for strut coverage might play a crucial role in predicting late clinical events (i.e., late ST), and further studies with longer follow-up are warranted.
The premise that early vascular healing might shorten DAPT treatment has driven the design of several stent platforms. In the Cre8 study, Prati et al reported that the new Cre8™ thin-strut cobalt-chromium polymer-free DES (CID, Saluggia, Italy) is non-inferior to BMS in terms of strut coverage with superiority of Cre8 in reducing neointimal thickness (0.08±0.03 mm at three months vs. BMS: 0.18±0.10 mm at one month, p<0.001)23. The authors proposed that it might be safe to discontinue DAPT at three months after Cre8 implantation. Recently, the novel COMBO dual therapy stent (OrbusNeich, Wanchai, Hong Kong, China) using “pro-healing technology” has also shown an overall low rate of clinical events, without any ST up to 12 months24. The ongoing Nano plus OCT trial in Europe is aimed at assessing if Nano™ polymer-free SES (Lepu Medical, Beijing, China) has an improved early arterial healing and consequently if DAPT treatment can be discontinued at three months after PCI (ClinicalTrials.gov Identifier: NCT01925027). Although the latter study is expected to provide insights into the relationship between early OCT strut coverage and the safety of stopping DAPT, studies aimed at identifying potential risk factors for ST are warranted. To this end, the recently published MOST study showed that subacute ST was associated with significant stent underexpansion while late/very late ST was associated with greater stent strut malapposition distance25. In the present study, OCT results following BuMA stent implantation appear favourably optimal; however, several considerations might affect the validity and clinical usefulness of this finding: 1) despite the study’s randomised design, the lack of baseline OCT examination precludes ruling out baseline differences in strut apposition and plaque type; 2) the percentage of cross-sections with RUTTS score >30% and that of uncovered struts >10% per lesion were relatively high (5.03% and 8.11%, respectively); and 3) although a registry study documented that use of OCT guidance with its much higher resolution significantly reduces the rate of cardiac death or MI25, improvement in patient outcomes needs to be confirmed in randomised studies. Nonetheless, the results of the present study might allow us to determine better and to customise the duration and composition of dual antiplatelet therapy after implantation of the novel BuMA DES.
Limitations
Our study has several limitations, notably its small sample size. Despite its randomised design with sample size calculation, differing baseline confounders between groups, such as the higher rate of post-dilation with larger balloons in the BuMA group and the greater stent length in the EXCEL group, may have influenced the OCT-evaluated results. Second, differences in OCT results between the two stents at three-month follow-up can partially be driven by differences in baseline strut apposition and plaque type, which could not be ruled out because of the lack of baseline OCT examination. Third, the possible underlying mechanisms discussed for favourable vascular responses are speculative. However, experimental studies have shown that the BuMA stent with its unique base layer design is associated with excellent re-endothelialisation without necrosis, vascular tumours or in-stent thrombosis. Finally, the clinical relevance of OCT-evaluated early vascular responses remains to be proven.
Conclusions
BuMA-OCT is the first randomised trial to compare early neointimal coverage between two differently designed biodegradable polymer DES. The proportion of covered struts, as primary endpoint, was higher following BuMA than EXCEL stent implantation. A large-scale trial is warranted to assess the prognostic value and impact on adjunctive pharmacotherapy use of the difference observed between these two biodegradable polymer DES.
| Impact on daily practice The combination of metallic backbone with a biodegradable polymer coating that is fully absorbed after elution of the active drug is an intuitively attractive approach avoiding the sequela of chronic exposure to drug and polymer residue in the coronary artery. The BuMA stent, compared to the EXCEL stent, has a similar 316L stainless steel stent platform and sirolimus drug but has a different polymer, which changes the drug elution kinetics and led to faster re-endothelialisation in this study, verified by three-month optical coherence tomography examination. A large-scale trial is therefore warranted to assess the prognostic value, and impact on adjunctive pharmacotherapy use, of the difference observed between these two biodegradable polymer drug-eluting stents. |
Funding
This study was sponsored by SINOMED, Beijing, China.
Conflict of interest statement
The authors have no conflicts of interest to declare.




