Abstract
Coronary artery disease (CAD) and aortic valve stenosis (AS) are frequently coexisting. It has been reported that CAD is present in 40% of patients with AS undergoing surgical aortic valve replacement, and in up to 60% of patients with AS undergoing transcatheter aortic valve implantation (TAVI). Elderly patients with CAD and AS are characterised by higher baseline risk profiles as compared to patients with isolated AS, increasing the complexity of their therapeutic management. In patients with CAD and AS the combination of coronary artery bypass grafting (CABG) and surgical aortic valve replacement has been shown to improve survival. Therefore, CABG is recommended in patients with CAD and AS undergoing surgical aortic valve replacement according to current guidelines of the European Society of Cardiology (ESC) and of the American College of Cardiology Foundation/American Heart Association (ACCF/AHA). Conversely, whether the presence of CAD has any prognostic implications in elderly patients with severe AS undergoing TAVI is still a matter of debate. Of note, according to the most recent ESC guidelines on myocardial revascularisation, percutaneous revascularisation should be considered in patients undergoing TAVI with a stenosis >70% in proximal coronary segments (class IIa, level of evidence C). The aim of this article is to provide an overview of evidence supporting the need for coronary revascularisation in patients with severe AS and CAD undergoing TAVI, and to summarise optimal timing and treatment modalities for percutaneous coronary interventions in these patients.
Introduction
Coronary artery disease (CAD) and aortic valve stenosis (AS) frequently coexist1,2. This association is related, at least in part, to a similar pathogenesis as well as to risk factors shared by these two disease entities1,3. Inflammatory reactions due to subendothelial infiltration of oxidised low-density lipoproteins represent the common initiating pathophysiologic factor, with subsequent endothelial dysfunction, fibrosis and calcification, followed by disease progression that is accelerated by mechanical stress in both CAD and AS4-6. In addition, CAD and AS have a number of risk factors in common, including male gender, diabetes mellitus, arterial hypertension, chronic kidney disease, and age3,7,8. It has been reported that 40% of patients with AS undergoing surgical aortic valve replacement have concomitant CAD9-11. However, the prevalence of CAD rises to 60% in elderly patients with severe AS undergoing transcatheter aortic valve implantation (TAVI) (Figure 1)12-16. It is noteworthy that elderly patients with concomitant CAD and AS are characterised by higher baseline risk profiles as compared to patients with isolated AS9, increasing the complexity of their therapeutic management.
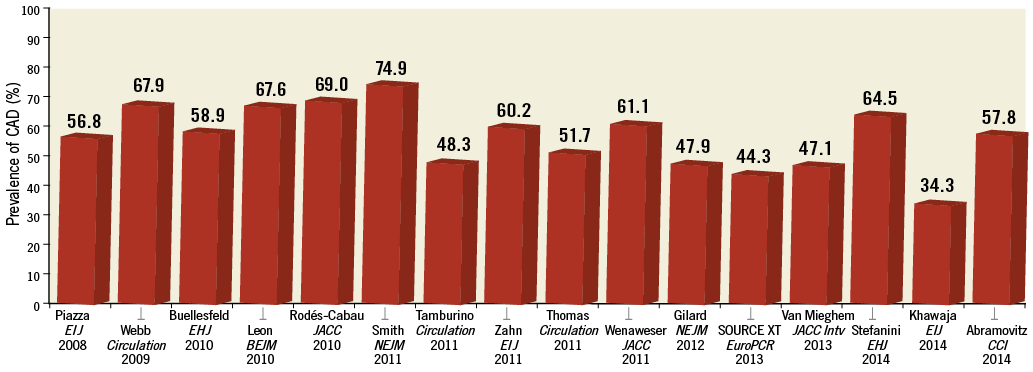
Figure 1. Prevalence of coronary artery disease in patients with aortic stenosis undergoing TAVI.
The presence of CAD has been consistently associated with impaired clinical outcomes in patients with AS undergoing surgical aortic valve replacement9-11. Among these patients, the combination of coronary artery bypass grafting (CABG) and surgical aortic valve replacement reduces the risks of perioperative myocardial infarction and mortality as well as late mortality and morbidity as compared with isolated CABG17-20. This combined operation, however, carries a twofold increased risk of mortality over isolated aortic valve replacement in patients without CAD9,21-23. Notably, the number of patients undergoing concomitant CABG and surgical aortic valve replacement has doubled during the last decade24. In line with this evidence, the guidelines of the European Society of Cardiology (ESC)25,26 and of the American College of Cardiology Foundation/American Heart Association (ACCF/AHA)27 recommend performing CABG in patients with a primary indication for aortic valve surgery with a coronary stenosis ≥70% (class I, level of evidence C), and considering CABG for coronary stenoses ≥50-70% (class IIa, level of evidence C).
Conversely, whether the presence of CAD has prognostic implications in elderly patients with severe AS undergoing TAVI is still a matter of debate. In view of the high baseline risk profile of patients undergoing TAVI, it is debated whether CAD has any impact on clinical outcomes or whether the valvular heart disease and the comorbid conditions overcome any negative prognostic impact of CAD.
The aim of this article is to provide an overview of evidence supporting the need for coronary revascularisation in patients with severe AS and CAD undergoing TAVI, and to summarise optimal timing and treatment modalities for percutaneous coronary interventions (PCI) in these patients.
Why to treat?
IMPACT OF CAD ON CLINICAL OUTCOMES
An increasing body of observational evidence indicates that the presence of CAD at baseline is associated with a higher risk of adverse events among patients with severe AS undergoing TAVI.
In a retrospective pooled analysis of two multicentre registries including 201 patients, Dewey and colleagues observed a significantly higher risk of all-cause mortality in patients with CAD as compared to patients without CAD undergoing TAVI at 30 days (13.1% vs. 1.2%, p=0.002) and at one-year follow-up (35.7% vs. 18.4%, p=0.01)16. Similarly, CAD at baseline has been identified as an independent predictor of all-cause mortality in the large-scale multicentre SOURCE XT TAVI registry (HR 1.38, 95% CI: 1.07-1.76)28. Conversely, Masson and colleagues observed no significant impact of CAD on mortality during one-year follow-up after TAVI in a single-centre study of 136 patients13. Nevertheless, rates of all-cause mortality were 1.5-fold higher in patients with CAD as compared to those without CAD (27.7% vs. 18.8%, p=0.63), a difference that may result in being significant in a larger patient population13.
However, in an analysis of 870 patients included in the United Kingdom TAVI Registry, the presence of CAD at baseline did not result in being an independent predictor of all-cause mortality at one year (HR 1.23, 95% CI: 0.88-1.73)29. Similar findings were described in a meta-analysis of seven observational studies including a total of 2,472 patients undergoing TAVI, which suggested no impact of CAD on all-cause mortality after TAVI (OR 1.0, 95% CI: 0.67-1.50)30.
The heterogeneity in terms of CAD extent and complexity in patients with severe AS undergoing TAVI may explain the inconsistency of these findings. Indeed, the severity of CAD among these patients may vary widely, ranging from one-vessel CAD with simple coronary anatomy to complex three-vessel CAD15. A recent analysis of 445 patients from the Bern TAVI registry showed that CAD extent and complexity –as quantified by the SYNTAX score– is associated with impaired clinical outcomes at one year after TAVI15. According to these findings, patients with SYNTAX score >22 at baseline have a higher risk of the composite of cardiovascular mortality, stroke or myocardial infarction compared with patients without CAD or with SYNTAX score ≤22 (no CAD: 12.5%, SYNTAX score ≤22: 16.1%, SYNTAX score >22: 29.6%; p=0.016). This difference was primarily driven by a higher risk of cardiovascular mortality in patients with a higher SYNTAX score (no CAD: 8.6%, SYNTAX score ≤22: 13.6%, SYNTAX score >22: 20.4%; p=0.029) (Figure 2). Comparable results were observed in a retrospective study of 288 patients undergoing TAVI at St. Thomas’ Hospital in London31. In this study, Khawaja and colleagues reported a higher risk of all-cause mortality in patients with SYNTAX scores in the higher tertile (>32) as compared to patients with intermediate (23-32) or low (≤22) SYNTAX scores (low SYNTAX score: 23.3%, intermediate SYNTAX score: 22.1%, high SYNTAX score: 57.1%; p=0.007)31.
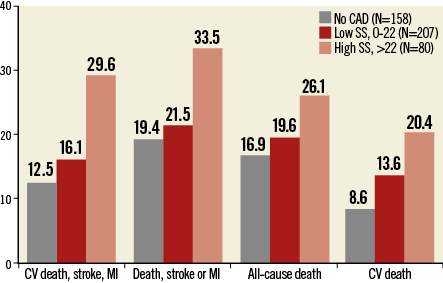
Figure 2. One-year outcomes in patients treated with TAVI according to baseline SYNTAX score. One-year outcomes in patients treated with TAVI according to coronary artery disease (CAD) severity quantified with the use of the SYNTAX score (SS) assessed at baseline. CV: cardiovascular; MI: myocardial infarction. Data from the Bern TAVI Registry15.
Overall, these findings suggest that the severity of CAD at baseline has prognostic implications among patients with severe AS undergoing TAVI, providing a strong rationale for performing coronary revascularisation in these patients in order to reduce the burden of myocardial ischaemia.
SAFETY AND EFFICACY OF PCI
A recent propensity score-matched analysis has shown that PCI can be safely and effectively performed in patients with severe AS and CAD32. In this analysis, Goel and colleagues compared the outcomes of 254 patients with AS and CAD treated with PCI with those of a matched group of patients without AS who underwent PCI during the same time period. All-cause mortality at 30 days was comparable between patients with AS and CAD treated with PCI and the control group (HR 0.93, 95% CI: 0.51-1.69; p=0.2)32.
A few observational studies have investigated the use of PCI in patients with severe AS undergoing TAVI. A study of 125 consecutive patients undergoing TAVI at a single centre applying a strategy of pre-procedural PCI of all coronary stenoses >50% was reported by Abdel-Wahab and colleagues33. Patients undergoing concomitant PCI and TAVI had a similar risk of all-cause mortality (2% vs. 6%, p=0.27), cardiovascular mortality (2% vs. 4%, p=0.44), myocardial infarction (0% vs. 0%), and stroke (4% vs. 5%, p=0.27) as compared to patients undergoing TAVI alone at 30-day follow-up33. In addition, the risk of all-cause mortality did not differ between patients undergoing PCI and TAVI as compared to patients undergoing TAVI alone at six-month follow-up (9% vs. 14%, p=0.42). Along this line, in an analysis of the Bern TAVI Registry, 167 out of 257 patients (64.9%) undergoing TAVI had CAD at baseline, and 59 of these (35.3%) underwent PCI and TAVI34. Patients undergoing PCI and TAVI had similar outcomes to patients undergoing TAVI alone in terms of all-cause mortality (10.2% vs. 5.6%, p=0.24) as well as myocardial infarction (0.5% vs. 0%, p=1.00) and major stroke (4.1% vs. 3.4%, p=1.00) at 30-day follow-up. Recently, Abramowitz and colleagues reported the findings of a cohort of 249 patients with severe AS undergoing TAVI at a single centre, stratified according to the presence of CAD and whether PCI was performed35. At 30 days, rates of all-cause mortality were numerically lower among patients with AS and CAD undergoing PCI and TAVI as compared to those undergoing TAVI alone (1.6% vs. 2.4%; p=1.00).
Taken together, this evidence supports the feasibility and safety of PCI in patients with severe AS and CAD undergoing TAVI.
What to treat?
The identification of coronary stenoses subtending myocardial ischaemia is not trivial in patients with severe AS. Non-invasive and invasive methods for functional evaluation of ischaemia have not been validated in these patients. Moreover, signs and symptoms related to AS are usually dominant in the clinical presentation, further increasing the challenge of evaluating myocardial ischaemia in this setting. Therefore, assessment of CAD in patients with severe AS is limited to an anatomical evaluation by invasive coronary angiography. At this point in time, it is unclear whether TAVI patients should undergo complete revascularisation of all anatomically significant coronary stenoses. In a propensity score-matched study, patients undergoing surgical aortic valve replacement and CABG had similar short- and long-term clinical outcomes compared with patients undergoing isolated surgical aortic valve replacement9. This suggests that CABG with complete revascularisation at the time of aortic valve replacement offsets the adverse effects related to CAD in patients with otherwise similar comorbidities9. However, a more selective strategy – with PCI of lesions located in proximal segments of major epicardial vessels only – has been proposed as a valid alternative in patients with CAD undergoing TAVI14. Of note, a selective revascularisation strategy allows the reduction of the contrast media load, the procedure time, and the risk of procedural complications. In a series of 263 consecutive patients with AS undergoing TAVI at the Erasmus Medical Center in Rotterdam, Van Mieghem and colleagues observed that a judicious revascularisation strategy based on Heart Team discussion can generate favourable midterm outcomes obviating the need for complete coronary revascularisation among appropriately selected TAVI patients14. Along this line, in the Bern TAVI Registry, patients with incomplete revascularisation with high residual SYNTAX score (>14) had impaired long-term clinical outcomes after TAVI, whereas patients with lower residual SYNTAX score (0-14) –indicating an acceptable extent of residual CAD after PCI– were associated with outcomes comparable to patients with complete revascularisation (Figure 3)15. Similarly, Khawaja and colleagues identified a residual SYNTAX score of 9 as the optimal threshold to predict 30-day and one-year mortality by receiver operating characteristic curves31, supporting the notion that complete revascularisation of all anatomically significant stenoses may not be necessary in TAVI patients.
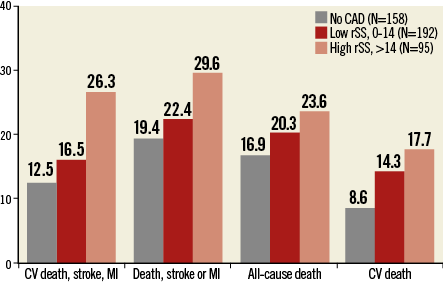
Figure 3. One-year outcomes in patients treated with TAVI according to residual SYNTAX score. One-year outcomes in patients treated with TAVI according to residual coronary artery disease (CAD) severity quantified with the use of the SYNTAX score (SS) assessed after coronary revascularisation. CV: cardiovascular; MI: myocardial infarction. Data from the Bern TAVI Registry15.
When to treat?
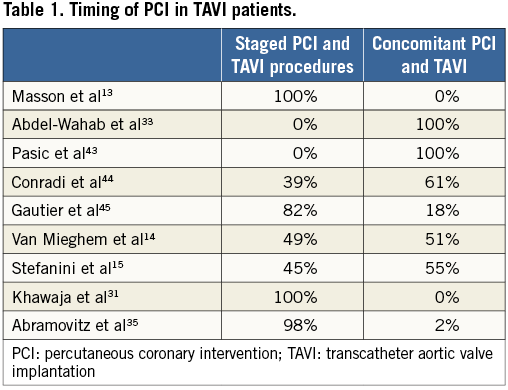
The timing of coronary revascularisation may be critical if the selected treatment strategy includes PCI and TAVI. Of note, patients evaluated for TAVI usually undergo invasive coronary angiography prior to the Heart Team discussion. Therefore, ad hoc PCI at the time of coronary angiography is an option only for patients known to be inoperable due to coexisting comorbid conditions. Little evidence is available on the optimal timing strategy for PCI in patients undergoing TAVI. As summarised in Table 1, the timing of PCI in TAVI patients varies between different institutions. A staged approach, with PCI and TAVI performed in two separate sessions, has some advantages, including a reduced duration of the TAVI procedure, an optimisation of the contrast media volume used, and a reduced risk of haemodynamic instability due to PCI-related complications during the TAVI session. In the above-mentioned study by Abdel-Wahab and colleagues33, PCI systematically performed prior to TAVI (i.e., 10 days median interval between PCI and TAVI) was described as a feasible strategy, resulting in similar risks of adverse events to TAVI alone. Nevertheless, performing PCI and TAVI during the same invasive session may be a more practical strategy and avoids the risks associated with an additional invasive procedure.
Since there are no conclusive data as to whether PCI before TAVI should be performed as a staged intervention or concomitantly during the same procedure, the timing of PCI should be individualised according to the leading clinical problem (aortic stenosis versus CAD), comorbidities, and complexity of the underlying CAD. Concomitant PCI and TAVI in patients with CAD requiring revascularisation may be considered if PCI complexity is expected to be low. Conversely, staged interventions should be considered among patients with more severe CAD for whom a higher PCI complexity is foreseen. Therapeutic strategies in this context should be based on the Heart Team discussion.
How to treat?
Coronary stent choice may also be critical for TAVI patients scheduled for PCI, due to the subsequent dual antiplatelet therapy. Drug-eluting stents have been shown to improve clinical outcomes compared with bare metal stents, primarily by markedly reducing the risk of restenosis36. In addition, drug-eluting stents have recently been associated with a reduced risk of stent thrombosis as compared to bare metal stents37,38. Undoubtedly, reducing the need for repeat interventions due to restenosis or stent thrombosis is important in TAVI patients. Nevertheless, up to 40% of TAVI patients have coexisting atrial fibrillation and may require long-term oral anticoagulation, incurring an increased bleeding risk in case of triple therapy with dual antiplatelet therapy in addition to oral anticoagulation39. The optimal duration of dual antiplatelet therapy after drug-eluting stent and bare metal stent implantation is still debated. Dual antiplatelet therapy should be continued for at least one month after bare metal stent implantation and for at least six months after drug-eluting stent implantation according to the most recent guidelines on myocardial revascularisation of the ESC25. However, shorter dual antiplatelet therapy regimens (<6 months) may be safe and effective after PCI with contemporary drug-eluting stents40,41. In addition, PCI with drug-eluting stents has been shown to be safe and effective in patients requiring long-term oral anticoagulation42. In view of these considerations, drug-eluting stents should be considered the standard of care in TAVI patients undergoing PCI in order to reduce the risk of repeat interventions.
Conclusions
In conclusion, in the light of the available evidence, coronary revascularisation should be attempted in patients with CAD and severe AS undergoing TAVI. The optimal strategy with respect to completeness of revascularisation in TAVI patients should be evaluated by the Heart Team on a case-by-case basis, taking into consideration the extent and complexity of CAD, the myocardium at risk and the anticipated complexity of PCI, as well as the comorbidities of each individual patient. Of note, the recently published ESC guidelines on myocardial revascularisation recommend PCI in CAD patients undergoing TAVI with a diameter stenosis >70% in proximal coronary segments (class IIa, level of evidence C)25. As it relates to the timing of revascularisation, both staged PCI followed by TAVI and concomitant PCI and TAVI represent valid strategies with advantages and disadvantages that need to be carefully weighed on an individual basis.
It must be underscored, however, that randomised trials with prospective planning of the revascularisation strategy are needed for definitive conclusions on optimal revascularisation strategies among patients with CAD and severe AS undergoing TAVI.
Conflict of interest statement
P. Wenaweser has received proctoring and lecture fees from Medtronic and Edwards Lifesciences, and research grants to the institution from Medtronic. S. Windecker has received research contracts to the institution from Biotronik and St Jude. The other authors have no conflicts of interest to declare.




