Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1190
Peer-review started: May 7, 2021
First decision: June 6, 2021
Revised: July 9, 2021
Accepted: September 7, 2021
Article in press: September 7, 2021
Published online: October 27, 2021
Along with the unceasing progress of medicine, Crohn's disease (CD), especially complex CD, is no longer a taboo for minimally invasive surgery. However, considering its special disease characteristics, more clinical trials are needed to confirm the safety and feasibility of laparoscopic surgery for CD.
To investigate the safety and feasibility of laparoscopic enterectomy for CD, assess the advantages of laparoscopy over laparotomy in patients with CD, and discuss comprehensive minimally invasive surgical techniques in complex CD.
This study prospectively collected clinical data from patients with CD who underwent enterectomy from January 2017 to January 2020. It was registered in the Chinese clinical trial database with the registration number ChiCTR-INR-16009321. Patients were divided into a laparoscopy group and a traditional laparotomy group according to the surgical method. The baseline characteristics, operation time, intraoperative blood loss, temporary stoma, levels of abdominal adhesion, pathological characteristics, days to flatus and soft diet, postoperative complications, hospitalization time, readmission rate within 30 d, and hospitalization cost were compared between the two groups.
A total of 120 eligible patients were enrolled into the pre-standardized groups, including 100 in the laparoscopy group and 20 in the laparotomy group. Compared with the laparotomy group, the postoperative hospitalization time in the laparoscopy group was shorter (9.1 ± 3.9 d vs 11.0 ± 1.6 d, P < 0.05), the days to flatus were fewer (2.8 ± 0.8 d vs 3.5 ± 0.7 d, P < 0.05), the days to soft diet were fewer (4.2 ± 2.4 d vs 6.2 ± 2.0 d, P < 0.05) and the intraoperative blood loss was less (103.3 ± 80.42 mL vs 169.5 ± 100.42 mL, P < 0.05). There were no statistically significant differences between the two groups in preoperative clinical data, operation time (149.0 ± 43.8 min vs 159.2 ± 40.0 min), stoma rate, levels of abdominal adhesion, total cost of hospitalization, incidence of postoperative complications [8.0% (8/100) vs 15.0% (3/20)], or readmission rate within 30 days [1.0% (1/100) vs 0.00 (0/20)].
Compared with laparotomy, laparoscopic enterectomy promotes the recovery of gastrointestinal function, shortens the postoperative hospitalization time, and does not increase the incidence of postoperative complications. Laparoscopic enterectomy combined with varieties of minimally invasive surgical techniques is a safe and acceptable therapeutic method for CD patients with enteric fistulas.
Core Tip: The purpose of this research was to investigate the safety, feasibility, and short-term efficacy of laparoscopic enterectomy for Crohn's disease (CD). For this purpose, we analyzed the clinical data of CD patients treated at our center over the past 4 years. Compared with the laparotomy group, the postoperative hospitalization time in the laparoscopy group was shorter, the days to flatus and soft diet were fewer, and the intraoperative blood loss was less. Also, the application of pre-operative ultrasound and intraoperative balloon dilatation for CD was explored specifically in the research.
- Citation: Wan J, Liu C, Yuan XQ, Yang MQ, Wu XC, Gao RY, Yin L, Chen CQ. Laparoscopy for Crohn's disease: A comprehensive exploration of minimally invasive surgical techniques. World J Gastrointest Surg 2021; 13(10): 1190-1201
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1190.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1190
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) that affects the entire gastrointestinal tract, especially the terminal ileum and cecum[1]. Despite considerable progress in drug therapy, 70%-90% of patients need to undergo at least one surgical treatment in their lifetime due to the progression of CD[2]. Moreover, the postoperative recurrence rate and reoperation rate are increasing year by year, which seriously endangers the physical and mental health of patients and brings a heavy burden to society[3]. The 2016 edition of the European Guidelines for CD indicates that laparoscopic surgery should be given priority for patients with ileocecal disease in experienced surgical centers[4]. Compared with laparotomy, laparoscopic surgery for CD has the advantages of less injury, less pain, faster return of enteric function, and shorter postoperative hospitalization stay[5]. In addition, patients with CD are often at risk of recurrence and multiple operations. Laparoscopic surgery can reduce abdominal adhesions and improve conditions for reoperation[6]. However, complex CD was considered a contraindication to laparoscopic surgery due to the extensive inflammation, abdominal abscess, enteric fistulas, and even enteric fistulas between adjacent organs (such as the bladder and vagina) that made the operation difficult[7]. Over the years, with the rapid advances in medical technology and the improvement in surgical skills, there is no longer an untouchable taboo associated with complex CD[8,9].
In patients with CD disease who have undergone multiple operations, extensive intraperitoneal adhesions often occur and the "dark chamber" is formed simultaneously. In such cases, it is often difficult to successfully insert the first laparoscopic trocar without damaging the abdominal organs and blood vessels. Previous studies have shown that ultrasound (US) can be used to assess preoperatively the degree of intraperitoneal adhesion, which is beneficial to laparoscopic surgery[10]. The activity of the abdominal wall and internal organs can be identified by US to determine the degree of adhesion, so as to locate the puncture point, thus alleviating the difficulty of laparoscopic surgery for CD. At the same time, CD patients often have multiple intestinal strictures. In order to retain more of the intestine during the operation, an ileus tube can be inserted through the nasal or small intestinal stoma, and the strictured intestine can be expanded using a balloon, thus promoting the remission of CD.
The purpose of this research was to investigate the safety, feasibility, and short-term efficacy of laparoscopic enterectomy for CD. For this purpose, we analyzed the clinical data of CD patients treated at our center over the past 4 years. By comparing the short-term efficacy of laparoscopic enterectomy with traditional laparotomy, the clinical advantages of laparoscopic enterectomy for CD were evaluated. Finally, we summarize the experience with laparoscopic enterectomy for CD in our center.
This is a prospective cohort study, with continuous enrollment of CD patients who underwent surgery at our center from January 2017 to January 2020. The inclusion criteria for patients were: (1) CD combined with ileus, stenosis, fistula, abscess, and ineffective conservative treatment causing hemorrhage of the digestive tract requiring excision of the ileum and colon anastomosis; (2) Age 18-75 years; (3) Females without pregnancy plans and strict birth control; and (4) Agreement to participate in the study and signing the consent form. The exclusion criteria were: (1) Rapidly deteriorating or end-stage disease, which may increase the risk of death during the study procedure; (2) Enrollment in another clinical trial; or (3) Infliximab use before surgery. All operations were performed by two experienced laparoscopic colorectal surgeons, and standardized treatment regimens were used during the perioperative period. The study was registered in the Chinese clinical trial database with the registration number ChicTR-InR-16009321 and approved by the Ethics Committee of the Shanghai Tenth People’s Hospital affiliated to Tongji University School of Medicine. Data collection included general information [gender, age, body mass index (BMI), smoking history, American Society of Anesthesiologists (ASA) class, CD duration, and family history], clinical characteristics, laboratory indexes (WBC, CRP, ESR, ALB, HB, PLT, PT, and APTT), imaging evaluation, operation and pathologic data, and postoperative treatment.
Preoperative preparation included physical examination, computed tomography, magnetic resonance imaging, ultrasonography, and colonoscopy. Indications for surgery included drug treatment failure, enterostenosis, intestinal obstruction, intraperitoneal abscess, internal and external fistula, perforation, bleeding, and cancerization. For patients with preoperative malnutrition or severe intestinal inflammation, more than 2 wk of enteral or parenteral nutritional support was administered. In patients with long-term hormone use, the dosage was gradually reduced until the hormone was discontinued. For intraperitoneal abscess, percutaneous drainage or double cannula flushing was performed to relieve local infection and inflammatory edema before surgery. All patients received 200 mL of 10% glucose orally at 10 h and 2 h before surgery, unless contraindicated.
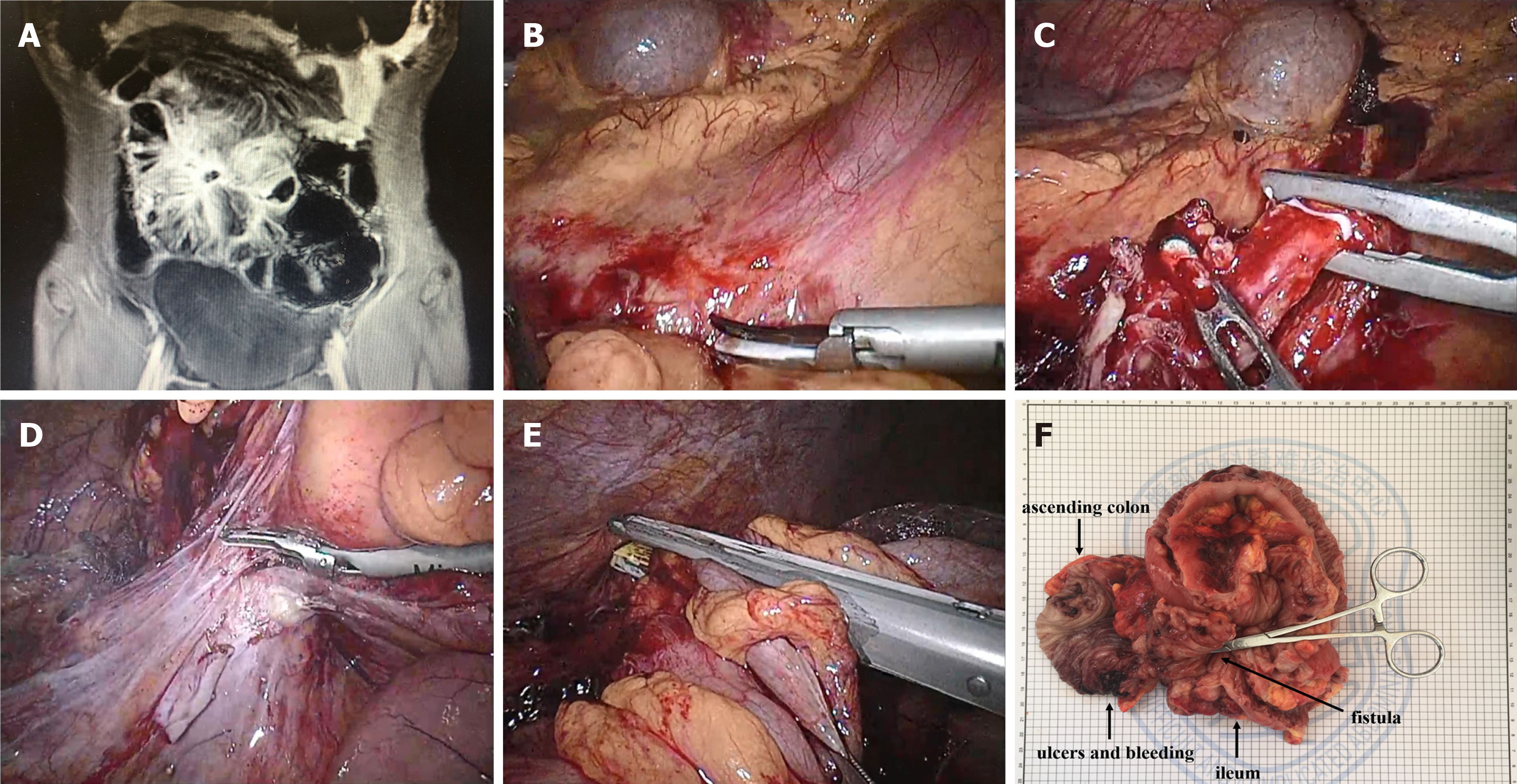
The laparotomy was performed routinely. For laparoscopic surgery, preoperative abdominal US was performed to evaluate the degree of abdominal adhesion, as well as the range of diseased bowel to determine the position of the trocar. The diseased bowel and its mesenteric vasculature were isolated in the abdominal cavity, and then the bowel was exteriorized through the auxiliary incision for resection and anastomosis (Figure 1). Conversion to laparotomy was defined when the length of the incision was greater than 7 cm or larger than the size required for the resection of the intestines outside the abdominal cavity.
SPSS 24 statistical software was used to analyze the data. Quantitative data are expressed as the mean ± SD (range). Student’s t, Kruskal-Wallis, and χ2 tests were used to analyze the data. P < 0.05 was considered statistically significant.
A total of 120 patients diagnosed with CD were included in the study: 100 who underwent laparoscopic surgery and 20 who underwent open surgery. There were no statistically significant differences between the two groups in gender, age, BMI, smoking history, ASA class, CD duration, past medical history, or hematologic examination (Table 1). However, for indications of surgery, we tended to choose open surgery for patients with intestinal perforation because such patients tend to have unstable vital signs.
| Variable | Laparoscopy (n = 100) | Laparotomy (n = 20) | P value |
| Age, yr, mean (range) | 40.0 (19-71) | 41.0 (19-72) | NS |
| Gender, male | 71 | 14 | NS |
| BMI, kg/m2, mean ± SD | 19.08 ± 2.60 | 20.88 ± 3.70 | NS |
| Smoking history, n (%) | 25 (25.0) | 6 (30.0) | NS |
| ASA class, n (%) | NS | ||
| I | 18 (18.0) | 3 (15.0) | |
| II | 70 (70.0) | 12 (60.0) | |
| III | 12 (12.0) | 5 (25.0) | |
| Crohn’s duration, mo, mean ± SD (range) | 76.2 ± 56.0 (1-240) | 59.1 ± 52.8 (1-144) | NS |
| Past medical history, n (%) | |||
| Enterectomy | 32 (32.0) | 3 (15.0) | NS |
| Anal fistula | 19 (19.0) | 3 (15.0) | NS |
| Appendicectomy | 13 (13.0) | 1 (5.0) | NS |
| Others | 18 (18.0) | 2 (10.0) | NS |
| Indications for resection, n (%) | |||
| Enterostenosis | 50 (50.0) | 5 (25.0) | NS |
| Intestinal obstruction | 42 (42.0) | 4 (20.0) | NS |
| Intraperitoneal abscess | 13 (13.0) | 2 (10.0) | NS |
| Fistula | 36 (36.0) | 7 (35.0) | NS |
| Intestinal perforation | 4 (4.0) | 5 (25.0) | P < 0.05 |
| Hematologic examination | |||
| WBC (/L) | 6.4 ± 2.69 | 9.59 ± 6.50 | NS |
| CRP (mg/L) | 36.3 ± 44.23 | 56.49 ± 72.47 | NS |
| ESR (mm) | 33.0 ± 20.22 | 29.0 ± 20.43 | NS |
| ALB (g/L) | 38.1 ± 6.56 | 38.99 ± 6.82 | NS |
| Hb (g/L) | 116.0 ± 21.32 | 125.6 ± 26.89 | NS |
| PLT (/L) | 298.0 ± 115.10 | 272.1 ± 226.50 | NS |
| PT (s) | 12.5 ± 1.28 | 13.90 ± 6.37 | NS |
| APTT (s) | 31.4 ± 5.73 | 35.1 ± 20.03 | NS |
The laparoscopy group was superior to the laparotomy group in terms of intraoperative blood loss (103.3 ± 80.42 mL in the laparoscopy group vs 169.5 ± 100.42 mL in the laparotomy group), days to flatus (2.8 ± 0.8 d vs 3.5 ± 0.7 d), days to soft diet (4.2 ± 2.4 d vs 6.2 ± 2.0 d), and length of postoperative hospitalization stay (9.1 ± 3.9 d vs 11.0 ± 1.6 d) (P < 0.05) (Tables 2 and 3). There were no statistically significant differences in the operation time, stoma rate, levels of abdominal adhesion, hospital cost, or total postoperative complications between the two groups. Except for one patient in the laparoscopy group who received surgical treatment again due to anastomotic fistula, all of the other complications were cured by conservative treatment. Only one patient in the laparoscopy group was readmitted 30 d after discharge, and this was because of non-specific abdominal pain.
| Variable | Laparoscopy (n = 100) | Laparotomy (n = 20) | P value |
| Operative time, min | 149.0 ± 43.8 (70-300) | 159.2 ± 40.0 (90-260) | NS |
| Estimated blood loss, mL | 103.3 ± 80.42 (20-400) | 169.5 ± 100.42 (50-400) | P < 0.05 |
| Extent of surgery, n (%) | |||
| Ileal resection | 13 (13.0) | 5 (25.0) | NS |
| Ileo-colic resection | 70 (70.0) | 11 (55.0) | NS |
| Colonic resection | 15 (15.0) | 3 (15.0) | NS |
| En bloc resection with pelvic organ | 2 (2.0) | 1 (5.0) | NS |
| Intestinal resection with temporary stoma | 49 | 5 | NS |
| Levels of abdominal adhesion | NS | ||
| Level 0 | 19 | 2 | |
| Level 1 | 17 | 3 | |
| Level 2 | 43 | 8 | |
| Level 3 | 15 | 5 | |
| Level 4 | 6 | 2 | |
| Clinicopathologic features | |||
| Stricture | 50 | 5 | NS |
| Proximal dilatation | 43 | 4 | NS |
| Fistula | 36 | 7 | NS |
| Intestinal perforation | 4 | 5 | P < 0.05 |
| Variable | Laparoscopy (n = 100) | Laparotomy (n = 20) | P value |
| Days to | |||
| Flatus, d, mean ± SD (range) | 2.8 ± 0.8 (1-4) | 3.5 ± 0.7 (2-5) | P < 0.05 |
| Soft diet, d, mean ± SD (range) | 4.2 ± 2.4 (2-8) | 6.2 ± 2.0 (3-12) | P < 0.05 |
| Total postoperative complication, n (%) | 8 (8.0) | 3 (15.0) | NS |
| Anastomotic hemorrhage | 1 | 0 | |
| Anastomotic leakage | 1 | 0 | |
| Ileus | 0 | 0 | |
| Intraabdominal abscess | 0 | 0 | |
| Pelvic effusion | 2 | 0 | |
| Wound infection | 2 | 2 | |
| Urinary tract infection | 0 | 1 | |
| Reoperation | 1 | 0 | |
| Readmission after discharge | 1 | 0 | NS |
| Length of stay, d, mean ± SD (range) | 9.1 ± 3.9 (4-36) | 11.0 ± 1.6 (9-15) | P < 0.05 |
| Cost (RMB) | 72534.6 | 75032.6 | NS |
At present, most researchers believe that laparoscopic surgery affords a rapid recovery and diminishes immune and inflammatory responses, thus reducing the risk of postoperative recurrence, and possibly reducing postoperative abdominal adhesion[11,12]. The prospective cohort study in our center showed that laparoscopic surgery is safe and feasible for both simple and complex CD. Compared with open surgery, laparoscopic enterectomy can promote the recovery of gastrointestinal function, shorten the postoperative hospitalization stay, and does not increase the incidence of postoperative complications. In conclusion, laparoscopy is a safe and effective method for the treatment of CD complicated with enteric fistulas.
Due to the special disease characteristics of CD, some patients had to undergo surgical treatment to alleviate their condition due to drug treatment failure, intestinal stricture, intestinal obstruction, internal and external fistula, perforation, bleeding and/or cancerization[13]. For CD patients with multiple surgical histories, the abdominal cavity has lost its normal anatomical structure, so that the usual surgical techniques of interstitial separation are often difficult to follow. In addition, the associated complications such as abdominal abscess, internal and external intestinal fistula, and inflammatory mass may increase the difficulty of surgery. The intestinal mesentery of CD patients is thickened with contracture and prone to bleeding, which aggravates the difficulty of separating the blood vessels. A limited visual field and poor exposure are often caused by thickened inflammatory mesenteric blood vessels and a dilated bowel. These difficulties are not only a great challenge for open surgery, but also for laparoscopic surgery[14,15].
Currently, many clinical studies have shown that laparoscopic ileocecal resection can be used for treating CD[16,17], and it has several advantages. First, patients with CD frequently are in poor general health, and often receive hormone and immunosuppressive therapy, resulting in low immune function and poor ability to fight infection. The minimally invasive method of laparoscopy can reduce the stress response brought on by the surgery as much as possible, which is conducive to a rapid recovery[18]. Second, less invasive laparoscopic surgery can reduce the incidence of abdominal adhesions, which is more conducive to secondary abdominal surgery should it be required by CD patients. Third, laparoscopic surgery is associated with less postoperative pain, faster recovery of intestinal peristalsis, earlier oral intake, and shorter postoperative hospitalization stay. Fourth, most CD patients are young and laparoscopic surgery meets the aesthetic requirements of the incision[19].
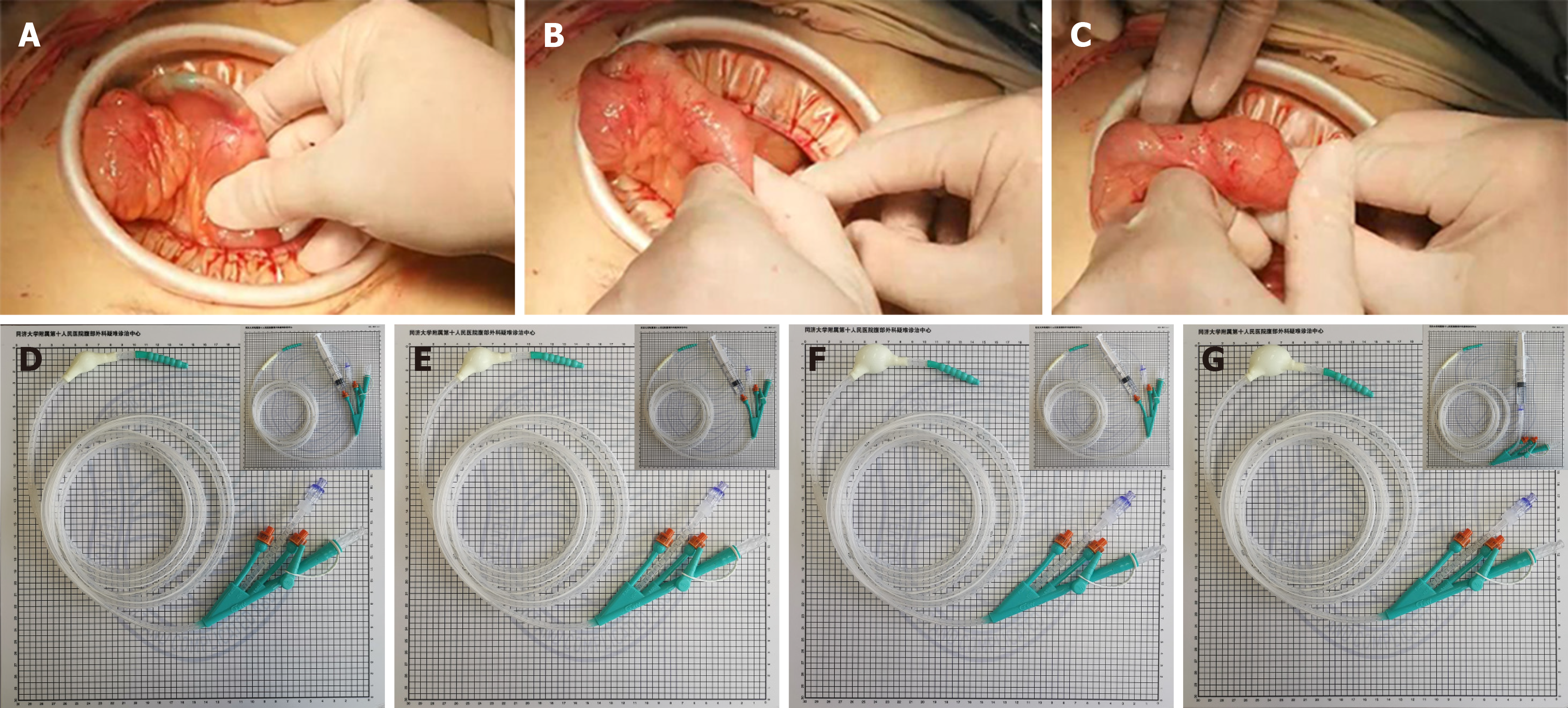
At present, in our center, the use of laparoscopy can greatly accelerate the recovery of patients after surgery, and achieve comprehensive and systematic treatment of the complications of CD in the gastrointestinal tract. In addition, the minimally invasive incision minimizes the rates of infection and pain at the surgical incision, so as to speed up rehabilitation training. Since CD affects the whole digestive system, strictures in the intestine can be treated minimally through stricturoplasty and intraoperative balloon dilatation. Although enterotomy can completely remove the strictured intestine, for patients with CD who have had multiple operations, there is a risk of developing short bowel syndrome with repeated resections. For stricturoplasty, conventional techniques such as HM strictureplasty and Finney strictureplasty are the commonly used methods. Although complications such as anastomotic leakage, fistula, and abscess formation may occur, some reports have shown no statistical difference in CD recurrence rates between strictureplasty and enterostomy[20]. For intraoperative balloon dilatation, an obstruction catheter is inserted through the intestinal stoma or the nose, and the size of the balloon is determined according to the degree of intestinal stenosis (Figure 2). Importantly, compared with traditional endoscopic balloon dilatation[21], this method is safe for continuous dilatation of the bowel with multiple narrow segments under direct vision. At the same time, an ileus tube has a good protective effect in patients with extensive abdominal adhesions[22]. If an ileus tube is required, we will intubate through the enterostomy to the flexor ligament, which not only provides conditions for enteral nutrition, but also avoids pneumonia caused by nasal insertion. According to our statistics, about 70% (37/50) of people with CD have pulmonary ventilation dysfunction.
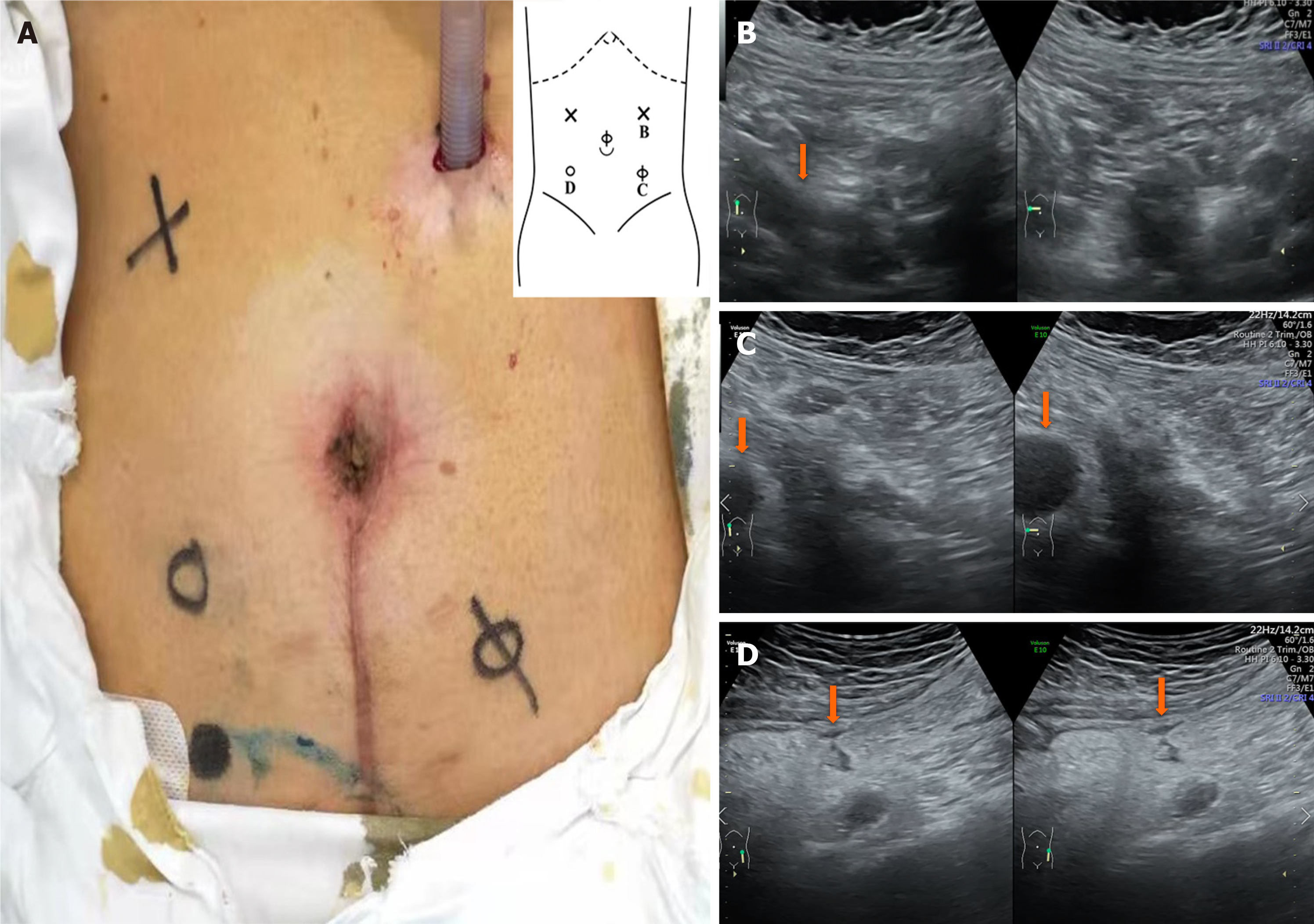
According to our experience, US is widely employed as an objective, accurate, non-invasive, and convenient examination method for evaluating CD[23]. To determine the first trocar position in patients with complex CD (Figure 3), preoperative abdominal US is useful for evaluating the degree and location of abdominal adhesions, as well as the range of diseased bowel[24]. In general, we will choose the area where the activity between the abdominal wall and viscera is greater than 30 mm to insert the first trocar.
When establishing pneumoperitoneum, it is necessary to observe the pressure value carefully and determine whether the abdominal bulge is symmetrical. If there are any abnormalities, the lens may not penetrate into the abdominal cavity, but accidentally enter the adhesion site. For patients who have had multiple surgeries, it is not recommended to perform the procedure on a routine basis, as it usually results in intraoperative collateral damage.
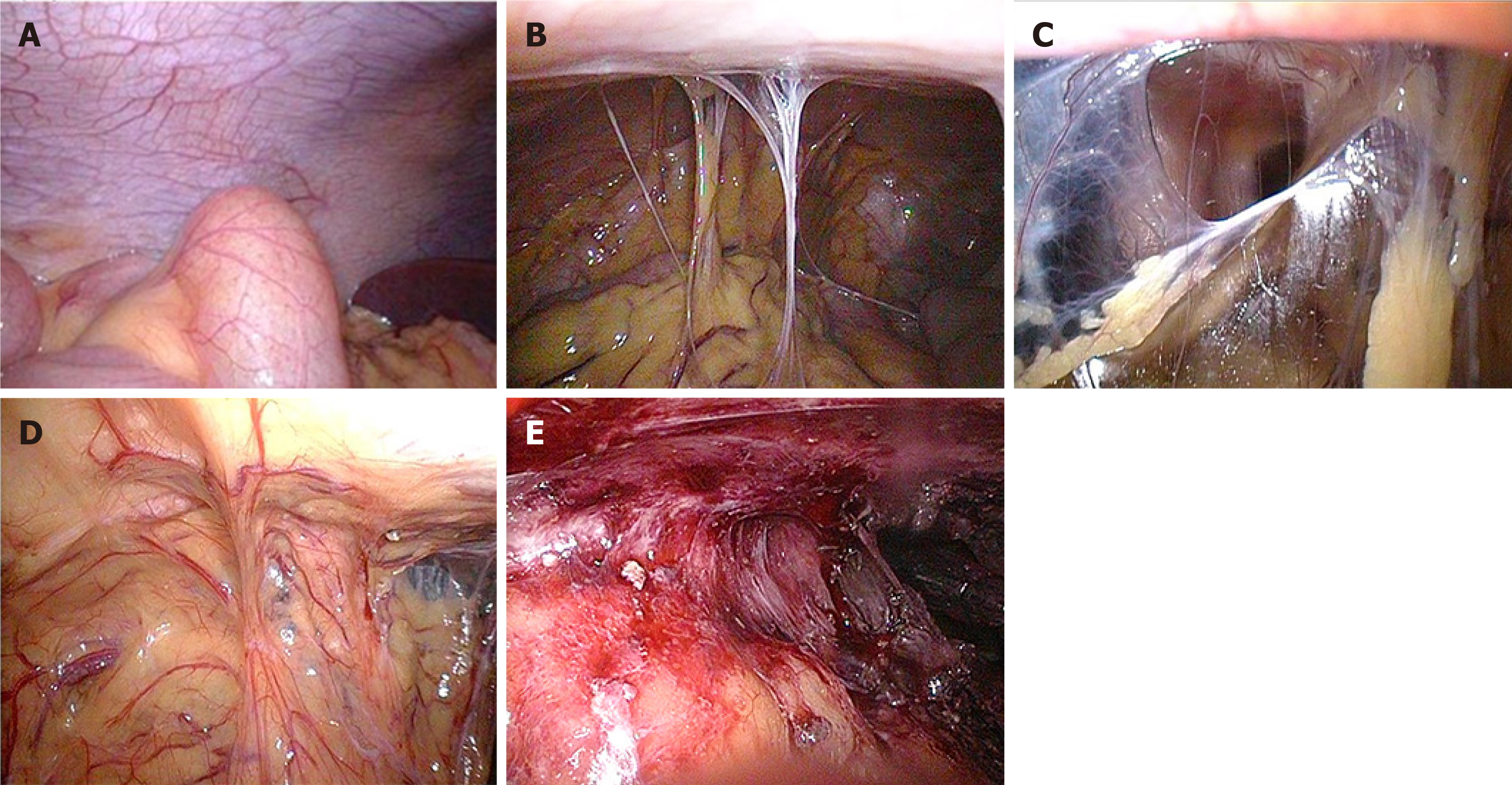
According to clinical practice, our center classifies abdominal adhesions into five levels (Figure 4): Level 0, no adhesion; level 1, slight adhesion (strip adhesion), which can be separated bluntly; level 2, moderate adhesion (membrane adhesion or tight adhesion), which can be directly sharply separated, without bleeding, or tight adhesion that can be seen between the bowel and abdomen, or between bowel and bowel, but there is a certain gap so that it can be cut with scissors but readily oozes blood; level 3, severe adhesion (fusion adhesion or complex adhesion), in which the naked eye is unable to distinguish the boundary between the intestines and the abdominal wall, and some intestines even fuse with the abdominal wall, so that adhesion separation can easily cause bleeding and intestinal damage; level 4, extremely heavy adhesion (wide compound adhesion), in which it is necessary to combine scissors and an ultrasonic knife to separate adhesions and intestinal damage is often inevitable. We believe that levels 1 and 2 adhesions are simple adhesions because the separation of adhesions via laparoscopy usually does not cause intestinal damage or rupture. However, levels 3 and 4 adhesions are complex adhesions. In those cases, a large amount of blood oozing and rupture of intestinal injury often occur, and the injured intestines often need to be repaired or excised.
Another important preoperative imaging concern is whether abdominal adhesion affects other organs. If necessary, a gastric tube, ileus tube, anal tube and/or ureteral catheter may be inserted as intraoperative guidelines for protection[25]. Regarding temporary stoma, we will refer to the patient’s condition and CDAI score, as well as a comprehensive assessment of the patient’s psychological status and acceptance level. In addition, after mesenteric vasculature and bowel dissociation under laparoscopy, the diseased intestinal segment is pulled out through the stoma or an incision of 3-5 cm close to the trocar for further careful examination, resection, and anastomosis. This not only simplifies the surgical procedure, but also shortens the operation time and reduces the cost of hospitalization.
Perioperative management is also important for CD patients. Sometimes, laparoscopic surgery takes a long time and requires a high level of physical condition. The situation regarding enteric fistulas should be fully evaluated before the operation. For patients with severe infection, percutaneous drainage or double cannula flushing should be considered first, and the surgery should be performed after the infection and inflammatory edema are alleviated. At the same time, antibiotic therapy should be used rationally and both abdominal and pulmonary infections should be considered, which provides a prerequisite for surgery. In brief, preoperative management of malnutrition and coexisting disease should be performed as much as possible before surgical intervention[26]. Surgery can only have the desired effect if the nutritional status is improved, the disease is in remission, and the coexisting diseases are controlled. The principle of damage control surgery should also be given full consideration for the surgery of enteric fistulas[27]. For seriously ill patients, one-stage operation is not considered and a temporary stoma should be performed first. Further treatment should be commenced after the condition of the body stabilizes.
The disadvantage of this research is that it is not a randomized controlled study. Currently, many surgeons prefer laparoscopic surgery, inevitably leading to selection bias. At the moment, most studies demonstrated that laparoscopic ileocolonic resection in CD is available. But for complex or recurrent CD, there is insufficient evidence to recommend laparoscopic surgery as the preferred technique. In the future, we will continue to explore the long-term follow-up of patients with complex or recurrent CD undergoing laparoscopic resection.
The inflammatory properties of CD lead to a certain particularity and complexity of the intraperitoneal anatomy, making it subject to numerous changes. According to our experience, laparoscopy for CD is safe and feasible, conducive to the postoperative rehabilitation of patients, and worthy of further promotion. Laparoscopic surgery for CD requires surgeons not only to have rich CD treatment experience in open surgery, but also advanced laparoscopic surgical skills[28]. Most importantly, if the abdominal cavity is found to contain freezing-like adhesions during the operation, resulting in anatomical difficulties, the procedure should be transferred to laparotomy in a timely manner to try to avoid collateral damage, bleeding, infections, and other complications.
Along with the unceasing progress of medicine, Crohn's disease (CD), especially complex CD, is no longer a taboo for minimally invasive surgery. However, considering its special disease characteristics, more clinical trials are needed to confirm the safety and feasibility of laparoscopic surgery for CD.
Although laparoscopic ileocolonic for CD is proved to be beneficial, for complex or recurrent CD, more minimally invasive surgical techniques need to be explored and applicated in laparoscopic surgery.
To investigate the safety and feasibility of laparoscopic enterectomy for CD, and to explore minimally invasive surgical techniques in complex CD.
This study prospectively collected clinical data from patients with CD who underwent enterectomy from January 2017 to January 2020. Patients were divided into a laparoscopy group and a traditional laparotomy group according to the surgical method. The baseline characteristics, operative and pathologic data, and short-term (30-d) outcomes were compared between the two groups.
A total of 120 eligible patients were enrolled into the pre-standardized groups, including 100 in the laparoscopy group and 20 in the laparotomy group. Compared with the laparotomy group, the patients in the laparoscopy group recovered more quickly, but had fewer postoperative complications.
Laparoscopic enterectomy combined with varieties of minimally invasive surgical techniques could promote the recovery of patients with CD.
The inflammatory properties of CD lead to a certain particularity and complexity of the intraperitoneal anatomy, making it subject to numerous changes. It requires surgeons not only to have rich CD treatment experience in open surgery, but also advanced laparoscopic surgical skills. Most importantly, if the abdominal cavity is found to contain severe adhesions, the procedure should be transferred to laparotomy in a timely manner to avoid collateral damage, bleeding, infections, and other complications.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim KT S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1121] [Cited by in F6Publishing: 1355] [Article Influence: 193.6] [Reference Citation Analysis (3)] |
| 2. | Toh JW, Stewart P, Rickard MJ, Leong R, Wang N, Young CJ. Indications and surgical options for small bowel, large bowel and perianal Crohn's disease. World J Gastroenterol. 2016;22:8892-8904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 3. | Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An update of the role of nutritional therapy in the management of Crohn's disease. J Gastroenterol. 2012;47:872-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S, Laureti S, Sampietro GM, Vucelic B, van der Woude CJ, Barreiro-de Acosta M, Maaser C, Portela F, Vavricka SR, Gomollón F; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 451] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 5. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic vs open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1030] [Cited by in F6Publishing: 883] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 6. | Sica GS, Biancone L. Surgery for inflammatory bowel disease in the era of laparoscopy. World J Gastroenterol. 2013;19:2445-2448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 7. | Nguyen SQ, Teitelbaum E, Sabnis AA, Bonaccorso A, Tabrizian P, Salky B. Laparoscopic resection for Crohn's disease: an experience with 335 cases. Surg Endosc. 2009;23:2380-2384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Panteleimonitis S, Ahmed J, Parker T, Qureshi T, Parvaiz A. Laparoscopic resection for primary and recurrent Crohn's disease: A case series of over 100 consecutive cases. Int J Surg. 2017;47:69-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Chaudhary B, Glancy D, Dixon AR. Laparoscopic surgery for recurrent ileocolic Crohn's disease is as safe and effective as primary resection. Colorectal Dis. 2011;13:1413-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Yasemin A, Mehmet B, Omer A. Assessment of the diagnostic efficacy of abdominal ultrasonography and cine magnetic resonance imaging in detecting abdominal adhesions: A double-blind research study. Eur J Radiol. 2020;126:108922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Marcello PW. Laparoscopy for inflammatory bowel disease: pushing the envelope. Clin Colon Rectal Surg. 2006;19:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Stevens TW, Haasnoot ML, D'Haens GR, Buskens CJ, de Groof EJ, Eshuis EJ, Gardenbroek TJ, Mol B, Stokkers PCF, Bemelman WA, Ponsioen CY; LIR!C study group. Laparoscopic ileocaecal resection vs infliximab for terminal ileitis in Crohn's disease: retrospective long-term follow-up of the LIR! Lancet Gastroenterol Hepatol. 2020;5:900-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Holder-Murray J, Marsicovetere P, Holubar SD. Minimally invasive surgery for inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1443-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Tavernier M, Lebreton G, Alves A. Laparoscopic surgery for complex Crohn's disease. J Visc Surg. 2013;150:389-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Sevim Y, Akyol C, Aytac E, Baca B, Bulut O, Remzi FH. Laparoscopic surgery for complex and recurrent Crohn's disease. World J Gastrointest Endosc. 2017;9:149-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Gardenbroek TJ, Verlaan T, Tanis PJ, Ponsioen CY, D'Haens GR, Buskens CJ, Bemelman WA. Single-port vs multiport laparoscopic ileocecal resection for Crohn's disease. J Crohns Colitis. 2013;7:e443-e448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Zhu Y, Xiang J, Liu W, Cao Q, Zhou W. Laparoscopy Combined with Enhanced Recovery Pathway in Ileocecal Resection for Crohn's Disease: A Randomized Study. Gastroenterol Res Pract. 2018;2018:9648674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Ren J, Liu S, Wang G, Gu G, Ren H, Hong Z, Li J. Laparoscopy improves clinical outcome of gastrointestinal fistula caused by Crohn's disease. J Surg Res. 2016;200:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Gardenbroek TJ, Tanis PJ, Buskens CJ, Bemelman WA. Surgery for Crohn's disease: new developments. Dig Surg. 2012;29:275-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ambe R, Campbell L, Cagir B. A comprehensive review of strictureplasty techniques in Crohn's disease: types, indications, comparisons, and safety. J Gastrointest Surg. 2012;16:209-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Bettenworth D, Gustavsson A, Atreja A, Lopez R, Tysk C, van Assche G, Rieder F. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn's Disease. Inflamm Bowel Dis. 2017;23:133-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Cui H, Jiang X, Li H. Adhesive small-bowel obstruction treatment using internal intestinal splinting with a nasointestinal ileus tube. Minerva Chir. 2015;70:327-330. [PubMed] [Cited in This Article: ] |
| 23. | Liu C, Ding SS, Zhang K, Liu LN, Guo LH, Sun LP, Zhang YF, Sun XM, Ren WW, Zhao CK, Li XL, Wang Q, Xu XR, Xu HX. Correlation between ultrasound consolidated score and simple endoscopic score for determining the activity of Crohn's disease. Br J Radiol. 2020;93:20190614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Bollegala N, Griller N, Bannerman H, Habal M, Nguyen GC. Ultrasound vs Endoscopy, Surgery, or Pathology for the Diagnosis of Small Bowel Crohn's Disease and its Complications. Inflamm Bowel Dis. 2019;25:1313-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Tsujinaka S, Wexner SD, DaSilva G, Sands DR, Weiss EG, Nogueras JJ, Efron J, Vernava AM 3rd. Prophylactic ureteric catheters in laparoscopic colorectal surgery. Tech Coloproctol. 2008;12:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 26. | Nickerson TP, Merchea A. Perioperative Considerations in Crohn Disease and Ulcerative Colitis. Clin Colon Rectal Surg. 2016;29:80-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Chamieh J, Prakash P, Symons WJ. Management of Destructive Colon Injuries after Damage Control Surgery. Clin Colon Rectal Surg. 2018;31:36-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Is laparoscopic ileocecal resection a safe option for Crohn's disease? Int J Surg. 2014;12:22-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |