Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.1011
Peer-review started: February 11, 2024
First decision: February 19, 2024
Revised: March 6, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: May 15, 2024
Since adverse events during treatment affect adherence and subsequent glycemic control, understanding the safety profile of oral anti-diabetic drugs is imperative for type 2 diabetes mellitus (T2DM) therapy.
To evaluate the risk of infection in patients with T2DM treated with dipeptidyl-peptidase 4 (DPP-4) inhibitors.
Electronic databases were searched. The selection criteria included randomized controlled trials focused on cardiovascular outcomes. In these studies, the effects of DPP-4 inhibitors were directly compared to those of either other active anti-diabetic treatments or placebo. Six trials involving 53616 patients were deemed eligible. We calculated aggregate relative risks employing both random-effects and fixed-effects approaches, contingent upon the context.
The application of DPP-4 inhibitors showed no significant link to the overall infection risk [0.98 (0.95, 1.02)] or the risk of serious infections [0.96 (0.85, 1.08)], additionally, no significant associations were found with opportunistic infections [0.69 (0.46, 1.04)], site-specific infections [respiratory infection 0.99 (0.96, 1.03), urinary tract infections 1.02 (0.95, 1.10), abdominal and gastrointestinal infections 1.02 (0.83, 1.25), skin structure and soft tissue infections 0.81 (0.60, 1.09), bone infections 0.96 (0.68, 1.36), and bloodstream infections 0.97 (0.80, 1.18)].
This meta-analysis of data from cardiovascular outcome trials revealed no heightened infection risk in patients undergoing DPP-4 inhibitor therapy compared to control cohorts.
Core Tip: This meta-analysis revealed no significant correlation between dipeptidyl peptidase-4 (DPP-4) inhibitor usage and the risk of overall, serious, opportunistic, or site-specific infections in patients with type 2 diabetes participating in cardiovascular outcome trials. These findings suggest that DPP-4 inhibitors do not pose a heightened risk of infections compared to other treatments used for these patients. However, further long-term studies and real-world surveillance are warranted to examine the broader implications of DPP-4 inhibitors therapy on infection susceptibility in clinical settings.
- Citation: Yang N, He LY, Liu P, Li ZY, Yang YC, Ping F, Xu LL, Li W, Zhang HB, Li YX. Dipeptidyl peptidase-4 inhibitors and the risk of infection: A systematic review and meta-analysis of cardiovascular outcome trials. World J Diabetes 2024; 15(5): 1011-1020
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/1011.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.1011
Type 2 diabetes mellitus (T2DM) is an epidemic and a progressive ailment marked by deteriorating glycemic control. Approximately half of T2DM patients fail to achieve adequate glycemic management, and adverse events, are significant factors contributing to treatment discontinuation[1]. Consequently, comprehending the safety profile of oral anti-diabetic drugs remains pivotal in T2DM therapy, as adverse effects encountered during treatment can detrimentally affect adherence and subsequent glycemic control.
Dipeptidyl-peptidase 4 (DPP-4) inhibitors enhance blood glucose regulation by elevating circulating glucagon-like peptide-1 (GLP-1) levels[2]. GLP-1 prompts insulin secretion, thus curbing glucagon release, appeasing appetite, and retarding gastric emptying. Given their safety benefits and favorable reception among patients, DPP-4 inhibitors are widely used in clinical practice[3]. Beyond its role in inactivating incretin hormones, DPP-4 also participates in various biological processes due to its presence on diverse cell surfaces, where it potentially impacts immune function[4]. DPP4 inhibitors have been observed to dampen T cell proliferation and proinflammatory cytokine production, with lower levels of these factors correlating with increased infection severity[5]. As a result, attention has been paid to the relationship between DPP4 inhibitors and infection[1]. Additionally, the risk and prevalence of various infections are increased in diabetic patients because of their compromised immune responses[6]. Both innate and adaptive immune systems are affected in patients with uncontrolled diabetes[7], potentially increasing their vulnerability to various infections, including those of the urinary tract; dermal layers; and respiratory systems, such as pneumonia[8-10]. It is unclear whether the use of DPP4 inhibitors increases this risk.
Previous investigations have suggested that the use of DPP4 inhibitors use might increase the risk of nasopharyngeal inflammation and urinary tract infections[11-13], although contradictory findings have been reported[14,15]. Notably, a 2022 cross-sectional analysis of patients with T2DM with advanced chronic kidney disease (CKD) or end-stage kidney disease (ESKD) indicated a higher incidence of sepsis and infection-linked mortality in those treated with DPP-4 inhibitors than those treated with GLP-1 receptor agonists[13]. Nevertheless, the relationship between DPP4 inhibitors and infection risk remains controversial. To date, only one meta-analysis has presented overall infection risk estimates in patients undergoing DPP-4 inhibitors therapy, which indicated the overall risk of infections of DPP-4 inhibitors was not increased compared with control groups[16]. However, this meta-analysis included only approximately 30000 individuals and did not incorporate cardiovascular outcome trials (CVOTs). Combining data from CVOTs involving same class molecules in a meta-analysis can bolster statistical power, enabling the detection of subtle inter-group disparities. Importantly, most CVOTs have large sample sizes[17]. Moreover, previous studies of site-specific infections remain limited, as most studies have focused primarily on respiratory and urinary tract infections.
Consequently, the goal of this meta-analysis was to evaluate the potential infection risks, including overall infection risk and the risk of serious, opportunistic, and site-specific infections, associated with DPP-4 inhibitors treatment in patients with T2DM participating in CVOTs.
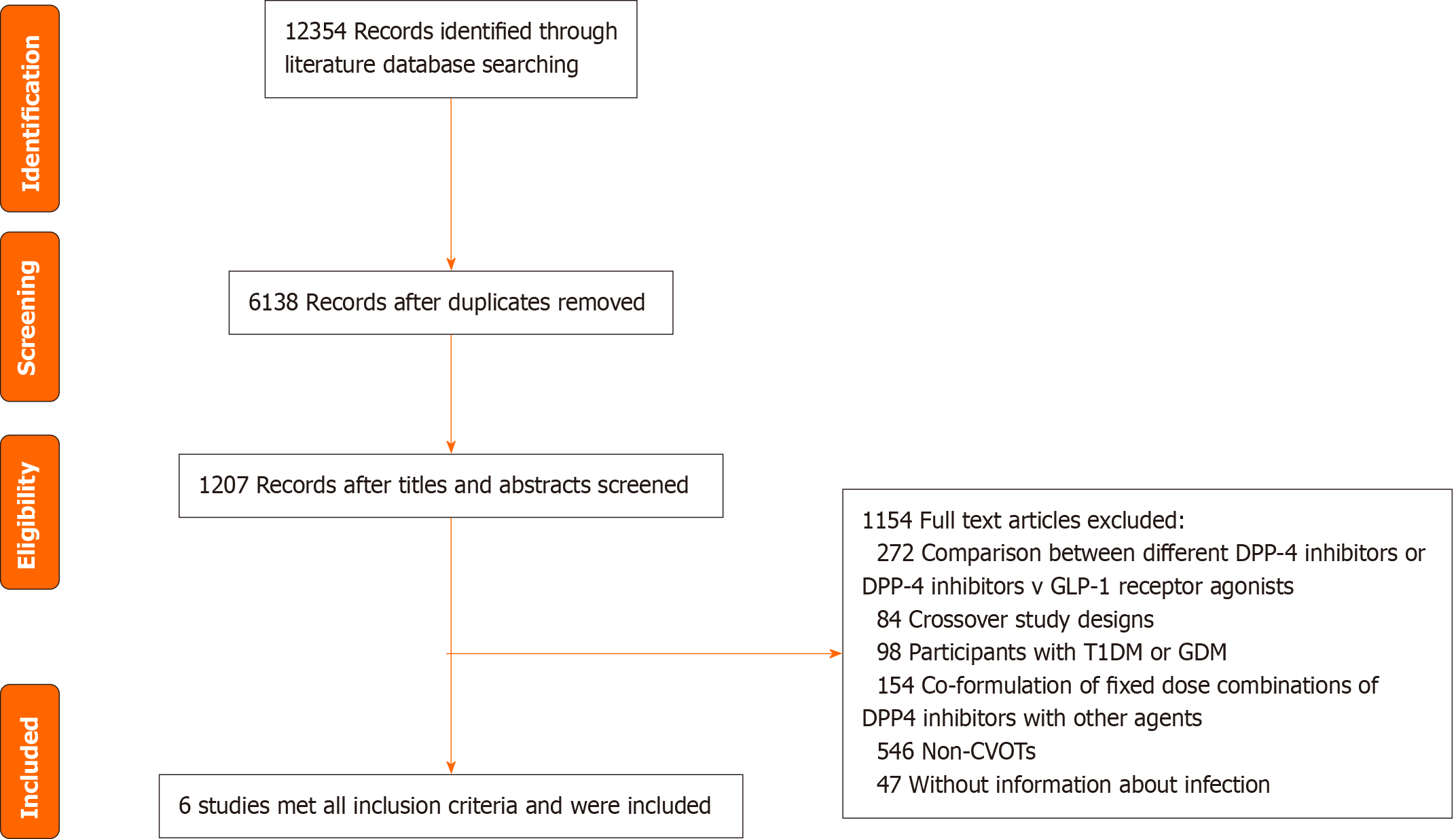
This systematic examination and meta-analysis followed stringent protocols, was registered under the International Prospective Register of Systematic Reviews (CRD42023411108). Additionally, it adhered to the PRISMA directives[18]. The need for an ethical review and informed consent was waived given the study's exclusive reliance on secondary data from previous research.
We conducted extensive searches of several databases, including PubMed, Web of Science, Embase, Cochrane Library, and clinical trial registries (ClinicalTrials.gov), encompassing records from inception to April 1, 2023. References within relevant systematic reviews were examined, and manual scouting of gray literature was carried out in clinical trial databases. The literature review was independently executed by two reviewers (Yang N and Liu P) following specified search methodologies (Supplementary Table 1). Subsequently, two reviewers (Yang N and Liu P) independently id-entified eligible studies based on predefined inclusion and exclusion criteria (Supplementary Table 2). For inclusion, the studies had to be randomized controlled CVOTs that directly compared adults with T2DM who were administered DPP-4 inhibitors alongside those administered placebos or an active antidiabetic medication. Research involving pregnant females and subjects pre-treated with DPP-4 inhibitors or GLP-1 receptor agonists prior to the trial were excluded. Moreover, studies utilizing fixed-dose co-formulations of DPP4 inhibitors combined with other prevalent drugs were excluded to negate the influence of the additional medications. Any disparities were resolved through consensus by a third impartial investigator.
The primary outcome of this analysis was overall infections, indicating all types of infections. Secondary outcomes consisted of: (1) Severe infections, with varying definitions per study, mainly encompassing infections leading to hospitalization, intravenous antibiotic use, or mortality; (2) opportunistic infections, such as tuberculosis, JC virus, Nocardia, cytomegalovirus, Epstein-Barr virus, candidiasis of the mouth or esophagus, infections from varicella-zoster, herpes zoster, Pneumocystis jirovecii, Histoplasma capsulatum, Legionella pneumonia, herpes simplex, and other unspecified opportunistic infections; and (3) infections specific to sites such as the respiratory system, urinary tract, abdomen and gastrointestinal tract, skin structures and soft tissues, bones, and bloodstream (Supplementary Table 3 for infection definitions).
Information was collated from articles in the public domain and their accompanying supplementary materials. The extracted data included the trial name; year of publication; number of patients; duration of treatments; population characteristics; DPP4 inhibitors administered, comparator drugs administered; and the demographic details of the study subjects, including their age, sex, body mass index (BMI), and levels of glycated hemoglobin (HbA1c). In addition, public repositories (e.g., ClinicalTrials.gov), conference abstracts, study protocols, and clinical study reports were used as supplementary information sources to facilitate data extraction.
The likelihood of bias in the included studies was assessed using the updated Cochrane risk-of-bias instrument for randomized trials[19]. This task was independently carried out by two assessors (Yang N and He LY), with any disagreements settled by engaging a third evaluator. Evidence certainty for every reported outcome was gauged via the GRADE system[20], which accounts for factors such as bias risk, inaccuracy, variability, lack of directness, and publication bias within the studies considered. We evaluated publication bias visually by employing funnel plots and statistically through the application of Egger’s test.
We computed the combined relative risks and their corresponding 95% confidence interval (CI) utilizing either random-effects or fixed-effects models. We assessed statistical variation via Q-tests and the I2 index[21]. Mantel-Haenszel techniques and fixed-effects models were employed in instances of negligible heterogeneity instances (Q-tests, P > 0.05; I2 < 50%). Conversely, random-effects models were employed in cases of notable heterogeneity. To conduct sensitivity analyses, we systematically excluded trials individually, disregarded studies that used alternative anti-diabetic medi-cations as comparators, and eliminated various types of DPP4 inhibitors in isolation. All statistical analyses were executed utilizing Stata v.15.0, and R v.4.2.1 and statistical significance was determined as P < 0.05.
We included 6 CVOTs involving 53616 patients (Figure 1). The characteristics of the studies and their participants included in the meta-analysis are presented in Table 1[22-27]. The participants had an average age of 64.6 years, and 33.1% (n = 17738) were female. The mean BMI was 30.6 kg/m2, and the mean HbA1c level was 7.7%. Supplementary Table 4 illustrates the potential bias within the studies included in the analysis. The majority of the studies displayed minimal to moderate levels of bias within the five domains evaluated (Supplementary Table 4).
| Trials | No. of patients | Treatment duration | Population | Mean age (yr) | No. (%) of females | Mean BMI (kg/m2) | Mean HbA1c (%) | DPP-4 inhibitors treatment | Comparators |
| EXAMINE (2013) (NCT00968708)[22] | 5380 | 18 months | T2DM with OAD; with acute coronary syndrome | 61 | 1729 (32.1) | 28.7 | 8.0 | Alogliptin 25 mg | Placebo |
| SAVOR-TIMI 53 (2013) (NCT01107886)[23] | 16492 | 2.1 yr | T2DM with established CVD or multiple risk factors for vascular disease | 65.1 | 5455 (33.1) | 31.1 | 8.0 | Saxagliptin 5 mg | Placebo |
| TECOS (2015) (NCT00790205)[24] | 14540 | 3 yr | T2DM with established CVD; with one or two OAD; aged ≥ 50 yr | 65.4 | 4297 (29.3) | 30.2 | 7.2 | Sitagliptin 100 mg | Placebo |
| MK-3102-018 (2017) (NCT01703208)[25] | 4192 | 96 wk | T2DM with a history of established CVD; aged ≥ 40 yr | 63.7 | 1254 (29.8) | 31.3 | 8.0 | Omarigliptin 25 mg | Placebo |
| CARMELINA (2018) (NCT01897532)[27] | 6979 | 2.2 yr | T2DM with inadequate glycemic control; with high CV and renal risk | 65.8 | 2589 (37.1) | 31.4 | 7.9 | Linagliptin 5 mg | Placebo |
| CAROLINA (2019) (NCT01243424)[26] | 6033 | 6.3 yr | T2D with high cardiovascular risk | 64 | 2414 (40.0) | 30.1 | 7.2 | Linagliptin 5 mg | Glimepiride |
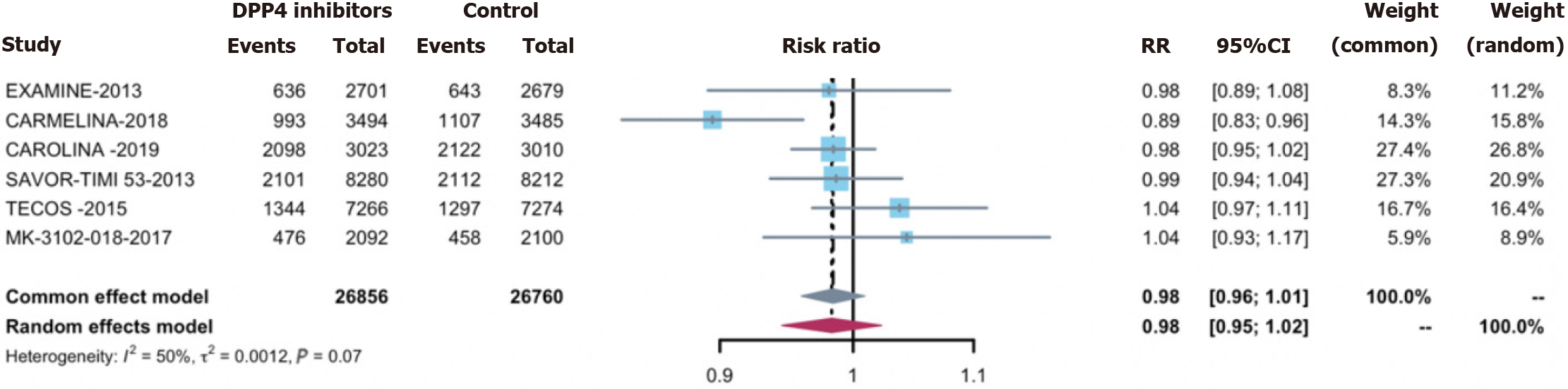
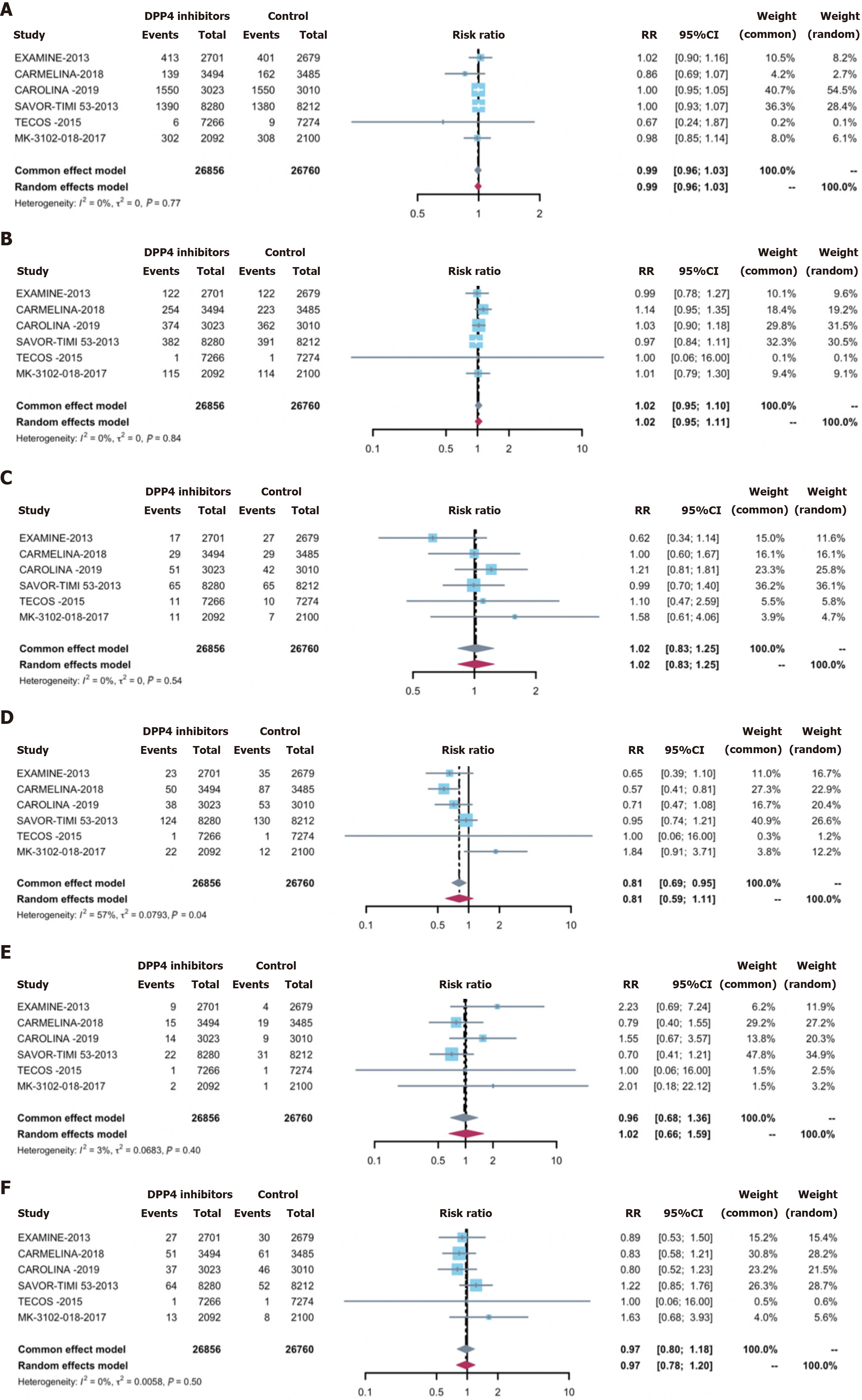
Overall infections were observed in 27.0% of the treatment group (7648 cases) and 27.3% of the control group (7739 cases). Employing DPP-4 inhibitors did not correlate with an elevated overall infection acquisition risk [0.98 (0.95, 1.02)] (Figure 2). Concerning secondary outcomes, DPP-4 inhibitors exhibited no significant associations with the risks of: (1) Serious infections [0.96 (0.85, 1.08)] (Supplementary Figure 1); (2) opportunistic infections [0.69 (0.46, 1.04)] (Sup
To execute sensitivity analyses, we excluded eligible trials individually while consistently obtaining estimates that aligned with the primary analyses of infection-related outcomes (Supplementary Figure 3). Furthermore, after excluding two studies of linagliptin, we observed no alterations in the outcomes (Supplementary Figure 3). Additionally, we excluded studies employing alternative anti-diabetic drugs as controls, demonstrating that DPP4i remained unassociated with infection (Supplementary Figure 3).
No indication of publication bias was found across studies assessing overall infection (P = 0.920), severe infection (P = 0.363), opportunistic infection (P = 0.286), or any site-specific infection (respiratory infection, P = 0.140; urinary tract infections, P = 0.932; abdominal and gastrointestinal infections, P = 0.952; skin structure and soft tissue infections, P = 0.845; bone infections, P = 0.212; bloodstream infection, P = 0.677), based on the Egger test. Moreover, funnel plots displayed no visual evidence of publication bias (Supplementary Figure 4).
Our meta-analysis entailed a thorough appraisal of the infection risks linked to the administration of DPP-4 inhibitors for the treatment of T2DM, involving a patient cohort numbering 53616. Our meta-analysis results decisively indicated that DPP-4 inhibitors treatment does not confer a significantly increased overall risk of infections, serious infections, opportunistic infections, or site-specific infections (including respiratory, urinary tract, abdominal, and gastrointestinal infections, skin structure and soft tissue infections, bone infections, and bloodstream infections). These conclusions hold some significance for the clinical application of DPP4 inhibitors, in that clinicians may be more confident in prescribing them to patients who are concerned about infection risks, especially those who have a high risk of cardiovascular events.
CD26/DPP-4, a membrane-bound glycoprotein prevalent across diverse immunocyte populations, including lymphocytes, plays a crucial role in regulating immune signaling processes, often linked to adenosine deaminase interactions[15]. CD26/DPP-4 exists as both membrane-bound and soluble forms, with the latter released into the circulation, potentially reaching diverse organs and tissues. In addition to influencing incretin hormones, DPP-4 interacts with various substrates, including cytokines and chemokines, thus impacting the body’s innate immune response and inflammatory processes. Pathophysiological investigations have suggested that CD26 inhibition may disrupt responses to severe infections. Consequently, concerns have arisen regarding the potential increase in infection occurrence owing to the use of DPP-4 inhibitors. However, only one meta-analysis has been directed at appraising the comprehensive infection risk associated with DPP-4 inhibitors treatment[16], with limited attention paid to site-specific infections beyond the respiratory and urinary contexts. Furthermore, prior meta-analyses were hampered by relatively small sample sizes and the lack of inclusion of CVOTs. To address these gaps, we conducted an extensive meta-analysis focusing on infection risk among individuals with T2DM undergoing DPP-4 inhibitors therapy within the CVOT context. Our comprehensive analysis demonstrated that treatment with DPP-4 inhibitors does not significantly increase the risk of overall, serious, opportunistic, or site-specific infections.
Prior investigations have examined the impact of DPP4 inhibitors on overall infection rates. Importantly, an embedded case-control investigation utilizing the WHO’s database Vigibase found an uptick in infection reports post-commercialization linked to the usage of DPP-4 inhibitors[28]. A previous meta-analysis from 2008 revealed a significant increase in the number of general infections following treatment with sitagliptin, in contrast to vildagliptin, which showed no marked increase[29]. A cross-sectional analysis of patients with T2DM with stage 5 CKD or ESKD, who were on DPP-4 inhibitors, noted an upsurge in mortality related to sepsis and infections when compared to those treated with GLP-1 receptor agonists[13]. Nevertheless, the relationship between DPP4 inhibitors and infection risk remains contentious, as certain meta-analyses align with our findings of no elevated risk of overall infections due to DPP4 inhibitors use. A 2012 meta-analysis reported an overall risk ratio of 0.98 (with 95%CI 0.93 to 1.15) for infections and infestations when comparing the totality of DPP-4 inhibitors against placebo[12]. Additionally, a meta-analysis evaluating alogliptin demonstrated no significant infection risk difference compared to controls[30]. Although our study suggested that DPP4 inhibitors usage did not increase the risk of infection, it remains plausible that distinct DPP4 inhibitors variations exert varying effects on infections, potentially impacting specific populations, particularly those with CKD. Further studies are important to confirm the risk mitigation aspects of DPP-4 inhibitors and to ascertain whether certain populations exhibit different risks of infection.
However, research regarding the relationship between DPP4 inhibitors administration and the risk of infections at specific sites is currently insufficient. Previous studies have predominantly concentrated on the respiratory and urinary systems, with a notable dearth of studies involving other specific sites and severe or opportunistic infections. Our study comprehensively addressed this research gap. Preliminary reports suggest a potential link between the relationship between DPP-4 inhibitors application and an increased incidence of upper respiratory tract infections (URIs)[31]. URI symptom prevalence, including pharyngitis, nasopharyngitis, cough, bronchitis, rhinitis, and sinusitis, is up to 11.8% in some cases[32], with vildagliptin- and saxagliptin-treated patients exhibiting elevated frequencies. Previous meta-analyses noted an increased risk of infections, including nasopharyngitis, compared to control groups[11,12,29]. Nonetheless, safety evaluations from clinical trials of sitagliptin and vildagliptin demonstrated no elevation in respiratory infection risk when measured against placebo or comparative control interventions[32-35]. The meta-analysis by Karagiannis likewise detected no link between the administration of DPP-4 inhibitors and the incidence of nasopharyngitis or infections of the upper respiratory tract relative to other antihyperglycemic agents in the comparator cohorts[36]. A recent meta-analysis based on CVOTs echoed our findings, showing no discernible impact of DPP-4 inhibitors on the likelihood of overall RI apart from coronavirus disease 2019[37]. Some studies have even suggested a potential reduction in pneumonia risk associated with DPP4 inhibitors use. A study from a United Kingdom primary care database suggested a reduction in pneumonia risk by 30% among patients using DPP-4 inhibitors[38]. Multiple studies have demonstrated that the utilization of DPP-4 inhibitors does not increase the risk of urinary tract infections compared to placebo or other control treatments[33,34,36]. In contrast, another meta-analytical examination focusing on sitagliptin and vildagliptin revealed a heightened risk for urinary tract infections[11]. Although it did not reach statistical significance, our meta-analysis indicated a slight increase in the risk of urinary tract infections compared to the control group. Based on the results of our study and previous studies, it is recommended that clinicians using DPP4 inhibitors in patients with T2DM pay attention to the risk of urinary tract infections. However, limited research has addressed other specific-site infections, leaving a significant research gap. Consequently, our study comprehensively explored the risks of serious, opportunistic, and specific-site infections (including abdominal, gastrointestinal, skin, soft tissue, bone, and bloodstream infections) in relation to DPP-4 inhibitors treatment, ultimately revealing no increased risk. Our research showed a slight increase in the risk of abdominal and gastrointestinal infections; however, this observation failed to achieve statistical significance. Since the studies included were not specifically designed to investigate abdominal and gastrointestinal infections, there may be bias in the event reports; therefore, relevant research is needed to investigate this.
Our meta-analysis has certain limitations. First, by aggregating all DPP-4 inhibitors and infection data, we acknowledge the possibility of differing effects among different DPP-4 inhibitors owing to their diverse mechanisms of action. This divergence arises from the use of different DPP-4 inhibitors in various CVOTs. Nevertheless, our sensitivity analysis, which excluded specific types of DPP-4 inhibitors, consistently revealed no increase in the risk of infection. Additionally, initial infection statuses were not consistently documented throughout the included studies, which may have influenced the interpretative validity of the results. Moreover, some studies omitted relevant factors influencing infection risk, such as the use of immunosuppressive agents. Finally, the included studies were not explicitly designed to assess infection risk associated with DPP-4 inhibitors use, and the inclusion and exclusion criteria for CVOTs were typically quite strict, which may limit the applicability of the findings to the general T2DM population.
Our comprehensive analysis suggested that DPP-4 inhibitors treatment does not increase the risk of infection when compared with placebo or active comparators. Nevertheless, evaluation of the long-term effects of DPP4 inhibition on infection necessitates studies with an extended duration, accompanied by post-approval surveillance in real-world clinical scenarios.
Inhibitors of dipeptidyl peptidase-4, also known as dipeptidyl-peptidase 4 (DPP-4) inhibitors, represent a widely adopted category of oral hypoglycemic medications favored for managing type 2 diabetes mellitus (T2DM), attributable to their efficacy in reducing blood sugar levels and favorable patient tolerability. However, given the role of DPP-4 in immune function, understanding the association between DPP-4 inhibitor use and infection risk is crucial for treatment adherence and long-term glycemic control.
Previous studies have reported conflicting results regarding the infection risk linked to the application of DPP-4 inhibitors in individuals with T2DM. With diabetes patients already at an increased risk for various infections, clarifying the safety profile of DPP-4 inhibitors in terms of infection risk is essential for informed clinical decision-making.
This meta-analysis aims to assess the risk of overall, serious, opportunistic, and site-specific infections in T2DM patients treated with DPP-4 inhibitors, using data extracted from cardiovascular outcome trials (CVOTs).
A literature search across multiple databases was conducted to identify randomized controlled CVOTs contrasting DPP-4 inhibitors against placebos or operative antidiabetic substances in adult T2DM patients. Cumulative relative risks were calculated employing both random-effects and fixed-effects frameworks, considering the diversity of the trials included.
Six trials involving 53616 patients were included. The assessment uncovered that DPP-4 inhibitors' usage did not markedly escalate the risk of overall, serious, opportunistic, or site-specific infections. This was consistent across various infection types, including respiratory, urinary tract, abdominal, gastrointestinal, skin, soft tissue, bone, and bloodstream infections.
The results suggest that DPP-4 inhibitors do not correlate with a heightened infection risk relative to control groups. This supports the continued use of DPP-4 inhibitors in T2DM therapy without added concerns for heightened infection risk.
While the current meta-analysis provides reassurance about the infection risk associated with DPP-4 inhibitors, further long-term studies and real-world data are essential to thoroughly grasp the consequences of DPP-4 inhibition concerning infection susceptibility. This will help refine treatment strategies for T2DM patients, particularly those at high risk for cardiovascular events.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lomeli SM, Mexico S-Editor: Qu XL L-Editor: A P-Editor: Guo X
| 1. | Kawalec P, Mikrut A, Łopuch S. The safety of dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter 2 (SGLT-2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30:269-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Yang Z, Lv Y, Yu M, Mei M, Xiang L, Zhao S, Li R. GLP-1 receptor agonist-associated tumor adverse events: A real-world study from 2004 to 2021 based on FAERS. Front Pharmacol. 2022;13:925377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | D'Andrea E, Wexler DJ, Kim SC, Paik JM, Alt E, Patorno E. Comparing Effectiveness and Safety of SGLT2 Inhibitors vs DPP-4 Inhibitors in Patients With Type 2 Diabetes and Varying Baseline HbA1c Levels. JAMA Intern Med. 2023;183:242-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 4. | Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, Wang M, Li S, Morita H, Altunbulakli C, Reiger M, Neumann AU, Lunjani N, Traidl-Hoffmann C, Nadeau KC, O'Mahony L, Akdis C, Sokolowska M. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75:2829-2845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 340] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 5. | Dalan R. Is DPP4 inhibition a comrade or adversary in COVID-19 infection. Diabetes Res Clin Pract. 2020;164:108216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18:525-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 180] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 7. | Jiang L, Cheng M. Impact of diabetes mellitus on outcomes of patients with sepsis: an updated systematic review and meta-analysis. Diabetol Metab Syndr. 2022;14:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Tegegne KD, Wagaw GB, Gebeyehu NA, Yirdaw LT, Shewangashaw NE, Kassaw MW. Prevalence of urinary tract infections and risk factors among diabetic patients in Ethiopia, a systematic review and meta-analysis. PLoS One. 2023;18:e0278028. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Rayate AS, Nagoba BS, Mumbre SS, Mavani HB, Gavkare AM, Deshpande AS. Current scenario of traditional medicines in management of diabetic foot ulcers: A review. World J Diabetes. 2023;14:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 10. | Ding HF, Li F, Xu YX, Wang F, Ding ZY. Changes in inflammatory markers and efficacy analysis of continuous subcutaneous insulin infusion in senior patients with type 2 diabetes mellitus hospitalized with community-acquired pneumonia: a randomized controlled trial. Eur Geriatr Med. 2024;15:519-525. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 858] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 12. | Gooßen K, Gräber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2012;14:1061-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Chen JJ, Wu CY, Jenq CC, Lee TH, Tsai CY, Tu HT, Huang YT, Yen CL, Yen TH, Chen YC, Tian YC, Yang CW, Yang HY. Association of Glucagon-Like Peptide-1 Receptor Agonist vs Dipeptidyl Peptidase-4 Inhibitor Use With Mortality Among Patients With Type 2 Diabetes and Advanced Chronic Kidney Disease. JAMA Netw Open. 2022;5:e221169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Wang M, Zhang X, Ni T, Wang Y, Wang X, Wu Y, Zhu Z, Li Q. Comparison of New Oral Hypoglycemic Agents on Risk of Urinary Tract and Genital Infections in Type 2 Diabetes: A Network Meta-analysis. Adv Ther. 2021;38:2840-2853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Morieri ML, Bonora BM, Longato E, Di Camilo B, Sparacino G, Tramontan L, Avogaro A, Fadini GP. Exposure to dipeptidyl-peptidase 4 inhibitors and the risk of pneumonia among people with type 2 diabetes: Retrospective cohort study and meta-analysis. Diabetes Obes Metab. 2020;22:1925-1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yang W, Cai X, Han X, Ji L. DPP-4 inhibitors and risk of infections: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2016;32:391-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Mannucci E, Mosenzon O, Avogaro A. Analyses of Results From Cardiovascular Safety Trials With DPP-4 Inhibitors: Cardiovascular Outcomes, Predefined Safety Outcomes, and Pooled Analysis and Meta-analysis. Diabetes Care. 2016;39 Suppl 2:S196-S204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17946] [Cited by in F6Publishing: 22751] [Article Influence: 7583.7] [Reference Citation Analysis (0)] |
| 19. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6581] [Cited by in F6Publishing: 9926] [Article Influence: 1985.2] [Reference Citation Analysis (0)] |
| 20. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11058] [Cited by in F6Publishing: 12662] [Article Influence: 791.4] [Reference Citation Analysis (0)] |
| 21. | Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane. 2022. Available from: https:// training.cochrane.org/handbook. [Cited in This Article: ] |
| 22. | Hwang YC, Morrow DA, Cannon CP, Liu Y, Bergenstal R, Heller S, Mehta C, Cushman W, Bakris GL, Zannad F, White WB. High-sensitivity C-reactive protein, low-density lipoprotein cholesterol and cardiovascular outcomes in patients with type 2 diabetes in the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial. Diabetes Obes Metab. 2018;20:654-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2548] [Cited by in F6Publishing: 2470] [Article Influence: 224.5] [Reference Citation Analysis (0)] |
| 24. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1831] [Cited by in F6Publishing: 1772] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 25. | Gantz I, Chen M, Suryawanshi S, Ntabadde C, Shah S, O'Neill EA, Engel SS, Kaufman KD, Lai E. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A, Mattheus M, Baanstra D, Meinicke T, George JT, von Eynatten M, McGuire DK, Marx N; CAROLINA Investigators. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA. 2019;322:1155-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 27. | Wanner C, Cooper ME, Johansen OE, Toto R, Rosenstock J, McGuire DK, Kahn SE, Pfarr E, Schnaidt S, von Eynatten M, George JT, Gollop ND, Marx N, Alexander JH, Zinman B, Perkovic V; CARMELINA investigators. Effect of linagliptin versus placebo on cardiovascular and kidney outcomes in nephrotic-range proteinuria and type 2 diabetes: the CARMELINA randomized controlled trial. Clin Kidney J. 2021;14:226-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Willemen MJ, Mantel-Teeuwisse AK, Straus SM, Meyboom RH, Egberts TC, Leufkens HG. Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care. 2011;34:369-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;2008:CD006739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Berhan A, Berhan Y. Efficacy of alogliptin in type 2 diabetes treatment: a meta-analysis of randomized double-blind controlled studies. BMC Endocr Disord. 2013;13:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Scheen AJ. Efficacy / safety balance of DPP-4 inhibitors versus SGLT2 inhibitors in elderly patients with type 2 diabetes. Diabetes Metab. 2021;47:101275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Richard KR, Shelburne JS, Kirk JK. Tolerability of dipeptidyl peptidase-4 inhibitors: a review. Clin Ther. 2011;33:1609-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Engel SS, Round E, Golm GT, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther. 2013;4:119-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Ligueros-Saylan M, Foley JE, Schweizer A, Couturier A, Kothny W. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes Metab. 2010;12:495-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Cai L, Cai Y, Lu ZJ, Zhang Y, Liu P. The efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2012;37:386-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 37. | Grenet G, Mekhaldi S, Mainbourg S, Auffret M, Cornu C, Cracowski JL, Gueyffier F, Lega JC, Cucherat M. DPP-4 Inhibitors and Respiratory Infection: A Systematic Review and Meta-analysis of the Cardiovascular Outcomes Trials. Diabetes Care. 2021;44:e36-e37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Wvan der Zanden R, de Vries F, Lalmohamed A, Driessen JH, de Boer A, Rohde G, Neef C, den Heijer C. Use of Dipeptidyl-Peptidase-4 Inhibitors and the Risk of Pneumonia: A Population-Based Cohort Study. PLoS One. 2015;10:e0139367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |