Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1824
Peer-review started: August 22, 2023
First decision: September 18, 2023
Revised: September 28, 2023
Accepted: November 25, 2023
Article in press: November 25, 2023
Published online: December 15, 2023
Fibroblast growth factor 21 (FGF21), primarily secreted by the pancreas, liver, and adipose tissues, plays a pivotal role in regulating glucose and lipid metabolism. Acute pancreatitis (AP) is a common inflammatory disease with specific clinical manifestations. Many patients with diabetes present with concurrent inflammatory symptoms. Diabetes exacerbates intestinal permeability and intestinal inflammation, thus leading to the progression to AP. Our previous study indicated that FGF21 significantly attenuated susceptibility to AP in mice.
To investigate the potential protective role of FGF21 against AP in diabetic mice.
In the present study, a mouse model of AP was established in diabetic (db)/db diabetic mice through ceruletide injections. Thereafter, the protective effects of recombinant FGF21 protein against AP were evaluated, with an emphasis on examining serum amylase (AMS) levels and pancreatic and intestinal inflammatory cytokines [interleukin (IL)-6, tumor necrosis factor-alpha (TNF-), and intestinal IL-1β]. Additionally, the impact of this treatment on the histopathologic changes of the pancreas and small intestinal was examined to elucidate the role of FGF21 in diabetic mice with AP. An antibiotic (Abx) cocktail was administered in combination with FGF21 therapy to investigate whether the effect of FGF21 on AP in diabetic mice with AP was mediated through the modulation of the gut microbiota. Subsequently, the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt), a bioinformatics software package, was used to predict different pathways between the groups and to explore the potential mechanisms by which the gut microbiota influenced the protective effect of FGF21.
The results indicated that FGF21 notably diminished the levels of serum AMS (944.5 ± 15.9 vs 1732 ± 83.9, P < 0.01) and inflammatory factors including IL-6 (0.2400 ± 0.55 vs 1.233 ± 0.053, P < 0.01), TNF- (0.7067 ± 0.22 vs 1.433 ± 0.051, P < 0.01), and IL-1β (1.377 ± 0.069 vs 0.3328 ± 0.02542, P < 0.01) in diabetic mice with AP. Moreover, notable signs of recovery were observed in the pancreatic structure of the mice. The histologic evidence of inflammation in the small intestine, including edema and villous damage, was significantly alleviated. FGF21 also significantly altered the composition of the gut microbiota, reestablishing the Bacteroidetes/Firmicutes ratio. Upon treatment with an Abx cocktail to deplete the gut microbiota, the FGF21 + Abx group showed lower levels of serum AMS (0.9328 ± 0.075 vs 0.2249 ± 0.023, P < 0.01) and inflammatory factors (1.083 ± 0.12 vs 0.2799 ± 0.032, p < 0.01) than the FGF21 group. Furthermore, the FGF21 + Abx group exhibited diminished injury to the pancreatic and small intestinal tissues, accompanied by a significant decrease in blood glucose levels (17.50 ± 1.1 vs 9.817 ± 0.69 mmol/L, P < 0.001). These findings underscored the superior protective effects of the combination therapy involving an Abx cocktail with FGF21 over the FGF21 treatment alone in diabetic mice with AP. The gut microbiota composition across different groups was further characterized, and a differential expression analysis of gene functions was undertaken using the PICRUSt2 prediction method. These findings suggested that FGF21 could potentially confer therapeutic effects on diabetic mice with AP by modulating the sulfate reduction I pathway and the superpathway of n-acetylceramide degradation in the gut microbiota.
This study reveals the potential of FGF21 in improving pancreatic and intestinal damage recovery, reducing blood glucose levels, and reshaping gut microbiota composition in diabetic mice with AP. Notably, the protective effects of FGF21 are augmented when combined with the Abx cocktail.
Core Tip: This study reveals the potential of facilitates fibroblast growth factor 21 (FGF21) in improving pancreatic and intestinal damage recovery, reducing blood glucose levels, and reshaping gut microbiota composition in diabetic mice with acute pancreatitis (AP). Notably, the protective effects of FGF21 are augmented when combined with the Abx cocktail. These findings provide new insights into the prevention and treatment of diabetes complicated by AP.
- Citation: Sun QY, Wang XY, Huang ZP, Song J, Zheng ED, Gong FH, Huang XW. Depletion of gut microbiota facilitates fibroblast growth factor 21-mediated protection against acute pancreatitis in diabetic mice. World J Diabetes 2023; 14(12): 1824-1838
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1824.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1824
Acute pancreatitis (AP) is a local inflammatory disorder of the pancreas caused by aberrant activation of pancreatic proteases due to various contributing factors. The global annual incidence of AP is estimated to be approximately 34 cases per 100000 individuals, leading to many hospitalizations, high medical costs, and long-term sequelae for patients worldwide[1,2]. Diabetes is a chronic metabolic disorder caused by insufficient secretion or impaired action of insulin, leading to elevated blood glucose levels. Inflammation-related symptoms are commonly observed in many diabetic patients. Chronic inflammation is a complication of diabetes and other diseases, contributes to the occurrence and progression of diabetes and the associated conditions. The occurrence of diabetes has also been indicated to exacerbate the development of AP. Recent evidence further suggests that obesity aggravates the severity of AP, increases intestinal permeability, and facilitates intestinal inflammation[3]. Additionally, the analysis of the fecal microbiota composition revealed a reduction in the abundance of bacteria in obese rats with AP compared with rats with a normal body weight[4,5].
The imbalance in the gut microbiota composition and the reduction in microbial diversity in the intestine may lead to an increase in the pathogenic bacterial count and the disruption of cellular integrity. These alterations can contribute to an increase in intestinal leakage and permeability, leading to the subsequent development of intestinal inflammation and a reduction or disturbance in the immune response of the intestinal mucosa[6]. Prior research has highlighted that rats with type 2 diabetes and AP undergo changes in the structure of their gut microbiota, which increases the susceptibility to complex AP injury. It is interesting to note that fecal microbiota transplantation effectively mitigates intestinal mucosal injury and reduces inflammatory cell infiltration in mice[7]. Another study has proposed the prognosis of AP could be moderately facilitated through probiotic therapy[8]. Probiotic strains can enhance the production of interleukin (IL)-10, a pivotal regulatory and anti-inflammatory cytokine in diabetic mice. IL-10 suppresses pro-inflammatory cytokines, such as interferon-gamma and IL-2/IL-1β, thereby impeding the development of low-grade inflammation and diabetes[9,10].
Fibroblast growth factor 21 (FGF21), a recently identified metabolic regulator secreted by the liver, adipose tissue, and pancreas, has shown potent anti-inflammatory effects in animal experiments. It can downregulate the expression of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-) and IL-6[11]. Our preliminary research underscored a significant upregulation of FGF21 expression in the context of AP. Exogenous administration of FGF21 has been indicated to curtails pancreatic injury, aberrant expression of digestive enzymes, and inflammatory response, thus impeding the occurrence of AP[12]. However, it is important to further explore the potential of FGF21 to ameliorate local or systemic inflammation and diminish blood glucose levels in mice with diabetes complicated by AP. Additionally, the involvement of the gut microbiota in the protective effects of FGF21 in diabetic mice with AP warrants further investigation.
In the present study, a mouse model of AP was induced in diabetic (db)/db diabetic mice using ceruletide injections. The subsequent investigation focused on evaluating the protective effects of recombinant FGF21 protein on serum amylase (AMS) and pancreatic and intestinal inflammatory cytokines (IL-6, TNF-, and intestinal IL-1β). Additionally, we assessed the impact of this treatment on histopathologic changes in the pancreas and small intestine, aiming to enhance understanding of the role of FGF21 in diabetic mice with AP. The study proceeded by administering a combination of FGF21 therapy and an antibiotic (Abx) cocktail to assess the involvement of gut microbiota in the potential impact of FGF21 on AP in diabetic mice. Subsequently, the application of Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt), a bioinformatics software package, enabled us to predict different pathways between the groups. The objective was to explore the potential mechanisms by which the gut microbiota influenced the protective effect of FGF21.
Male diabetic mice (db/db), aged 10 wk and weighing 40-55 g, were purchased from GemPharmatech Co., Ltd. (Nanjing, Jiangsu, China) and housed at the Experimental Animal Center of Wenzhou Medical University (Zhejiang, China). All experimental protocols involving animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Committee on Animal Health and Care of Wenzhou Medical University. Before the experiment, all animals were provided with a normal diet and allowed to acclimatize for 1 wk under a 12:12 Light-dark cycle at room temperature (23 ± 1°C) and approximately 60% humidity. Before AP modeling, the animals were subjected to a 12-h fasting period and had ad libitum access to drinking water.
Mice with fasting blood glucose levels > 16.7 mmol/L were regarded as diabetic mice and were randomly divided into the following groups (n = 5 per group): Diabetic mouse group (db), ceruletide-induced AP model group (AP), FGF21 treatment group (FGF21), and FGF21 combined with Abx cocktail treatment group (FGF21 + Abx). AP model was established in mice of the AP, FGF21, and FGF21 + Abx groups, wherein each mouse received seven intraperitoneal injections of ceruletide (50 μg/kg), at hourly intervals[13,14]. The mice in the db group received intraperitoneal injections of the same volume of normal saline as a control. The successful establishment of the AP mouse model was confirmed based on the following criteria: (1) Increased activity of serum AMS released from the pancreas, wherein the enzyme was detected using an enzyme-linked immunosorbent assay kit after AP induction; (2) no increase in pancreatic AMS levels; and (3) pancreatic tissues not meeting the diagnostic criteria for pancreatitis according to the modified Schmidt scoring system.
The animals were euthanized 6 h after the final injection of ceruletide. Serum and pancreatic and intestinal tissues were collected from mice of each group to determine the serum AMS levels and the ratio of pancreas weight to body weight. The collected tissues were embedded in paraffin, sliced, and stained with hematoxylin and eosin (HE), followed by a microscopic examination to observe the morphological changes in pancreatic tissues. 16S rRNA sequencing was performed to observe changes in the gut microbiota.
Experimental mice in the FGF21 and FGF21 + Abx groups received an intraperitoneal injection of FGF21 (1 mg/kg) 1 h before the ceruletide injection. In the same manner, mice from the db and AP groups received intraperitoneal injections of normal saline as a control.
Mice in the FGF21 + Abx group were orally administered with an Abx cocktail of non-absorbable Abx (ampicillin, neomycin, metronidazole, and vancomycin). The Abx cocktail was prepared at concentrations of 1 g/L, 1 g/L, 1 g/L, and 0.5 mg/L for each of these Abx, respectively. The Abx cocktail solution was freshly prepared every 2 d and administered continuously for 3 wk.
After 3 wk of Abx cocktail treatment, fecal samples were collected from mice in the FGF21 + Abx group for fecal DNA extraction[13], followed by the detection of bacteria in the intestines using universal primers[14]. After depletion of the majority of bacteria in the mouse intestine, the mice in the FGF21 + Abx group received an intraperitoneal injection of FGF21, followed by an intraperitoneal injection of ceruletide 1 h later to establish the AP model in diabetic mice.
Total protein was extracted from the pancreatic and intestinal tissues of mice, and the protein concentration was determined using a bicinchoninic acid assay. The protein samples were separated through sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). The membranes were then blocked with 10% skim milk–Tris-buffered saline with Tween 20 (TBST) solution at room temperature for 1.5 h, followed by an overnight incubation at 4 °C with the corresponding primary antibodies (IL-6 antibody diluted at 1:1000, IL-1β antibody diluted at 1:1000, TNF- antibody diluted at 1:2000, β-actin antibody diluted at 1:5000, and GAPDH antibody diluted at 1:5000, all purchased from Proteintech). The membranes were rinsed with TBST solution in triplicate and then incubated with the corresponding secondary antibodies conjugated with horseradish peroxidase at room temperature for 1 h. Chemiluminescent signals were detected using the Tanon-5200 chemiluminescence imaging system. The signal from each protein band was quantified using the ImageJ software.
Before histological analysis, mouse pancreatic and small intestinal tissues were fixed with 4% paraformaldehyde for more than 24 h. The fixed tissues were then embedded in paraffin, sliced at a thickness of 5 µm, and subjected to HE staining. The stained tissue sections were mounted with neutral resin and observed under a light microscope. The modified Schmidt scoring system was applied for the quantitative evaluation of pancreatic tissue damage.
The diversity of the gut microbiota in clinical or laboratory animal samples was analyzed using 16S rRNA sequencing and the next-generation microbiome bioinformatics platform QIIME 2[15]. The latest version of the QIIME 2 platform, together with the DADA2 software package, was used to denoise the sequence data using approximately 100% similarity, with an operational taxonomic unit (OTU) clustering at 97% similarity[16]. Redundancy was then removed to obtain feature data (representative sequences) for comparison with the 16S database (132 version) and NT-16. This comparison aimed to identify and annotate all 16S rRNA sequences detected in the samples, including taxonomic categories of kingdom, phylum, class, order, family, genus, and species.
Statistical analysis of data was performed using GraphPad Prism 6.0 software (GraphPad Software, San Diego, CA). Data were presented as mean ± SEM. Statistical significance was determined using Student's t-test (for comparisons between two experimental conditions) or analysis of variance (ANOVA) (for comparisons among three or more experimental conditions). Pearson analysis was used to determine linear correlations between variables. A P-value of less than 0.05 was considered to indicate statistical significance.
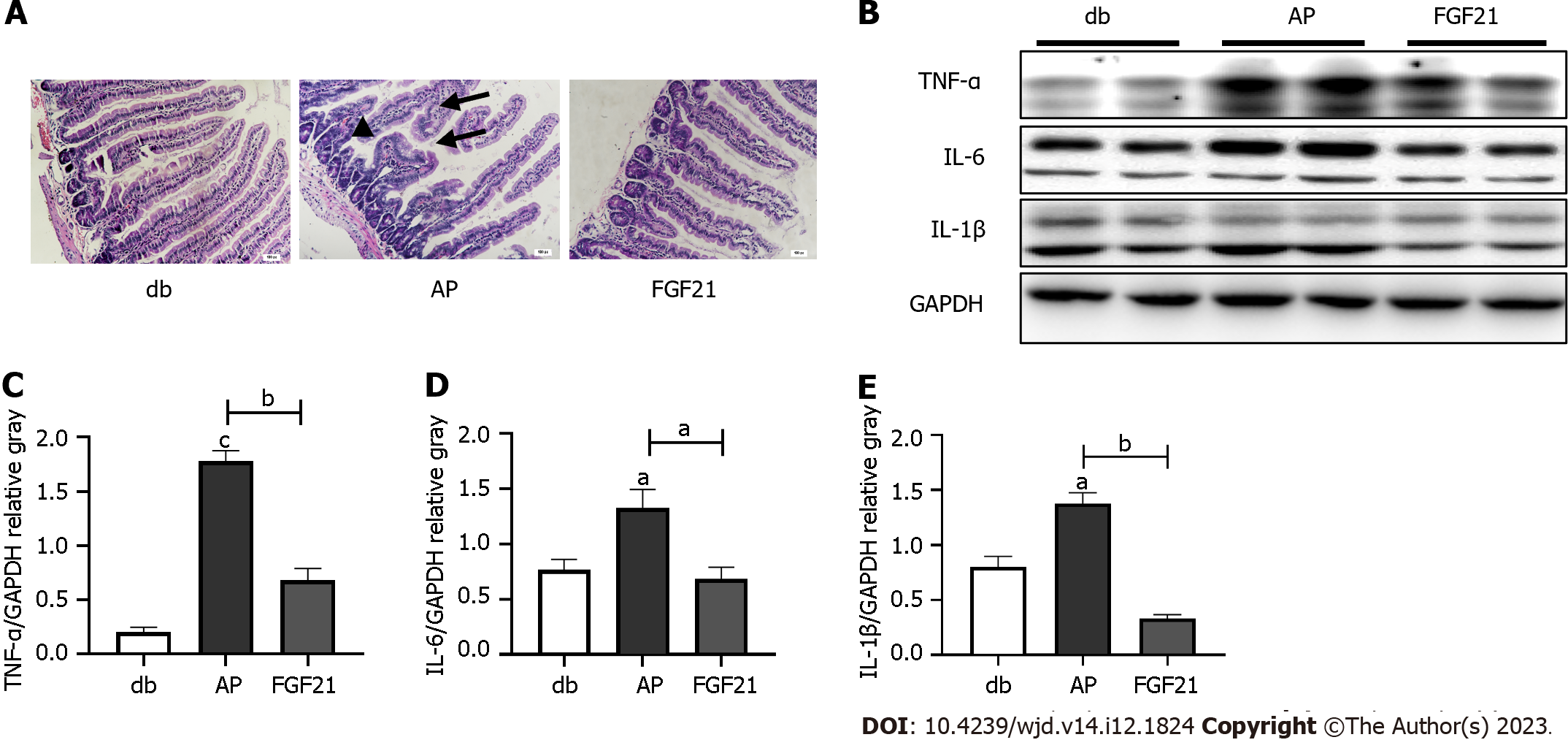
To investigate the impact of FGF21 on AP in diabetic mice, we established an AP model in diabetic mice by administering ceruletide injections. Following the ceruletide injection, the ratio of pancreas weight to body weight of diabetic mice was notably reduced by approximately 22.1% (Figure 1A, P < 0.01). Serum levels of AMS in diabetic mice were found to be twice as high as those in the db group (Figure 1B, P < 0.01). Concurrently, the concentration of the inflammatory cytokine IL-6 in diabetic mice increased to five times of that in the db group (Figure 1C and D, P < 0.01), while the TNF- level showed a significantly elevated to twice that of the db group (Figure 1C and E, P < 0.01). Following intraperitoneal injection of recombinant human FGF21 protein, the FGF21 group demonstrated an elevated ratio of pancreas weight to body weight, measuring at 19.3% (Figure 1A, P < 0.05). This result was accompanied by a reduction in the levels of serum AMS and inflammatory cytokines IL-6 and TNF- (Figure 1C). Specifically, the serum levels of AMS decreased by 40.1% (Figure 1B, P < 0.001), IL-6 levels decreased by 24.4% (Figure 1D, P < 0.01), and TNF- levels decreased to 65.1% of those in the AP group (Figure 1E, P < 0.05). Furthermore, diabetic mice with AP displayed pathological changes in pancreatic tissues, such as pancreatic edema, extensive intracellular vacuolation, and cellular necrosis (Figure 1F). Conversely, histological tissue sections of mice in the FGF21 group exhibited a significant reduction in tissue damage (Figure 1F).
These findings highlight that ceruletide injections induce pancreatic injury and inflammation in diabetic mice, while FGF21 treatment mitigates these symptoms in diabetic mice with AP.
To explore gut microbiota alterations in the context of AP, we assessed intestinal tissue damage in mice. The mice in the AP group showed exacerbated histologic evidence of inflammation in the small intestine, characterized by tissue edema, increased villus width, and villus damage (Figure 2A). After FGF21 treatment, noticeable reductions in the levels of inflammatory factors were observed in the small intestinal tissue (Figure 2B), with TNF- levels decreasing to 38.4% of that in the AP group (Figure 2C, P < 0.01), IL-6 levels decreasing by half (Figure 2D, P < 0.05), and IL-1β levels decreasing to 24.2% of that in the AP group (Figure 2E, P < 0.01), indicating a significant alleviation of intestinal tissue damage.
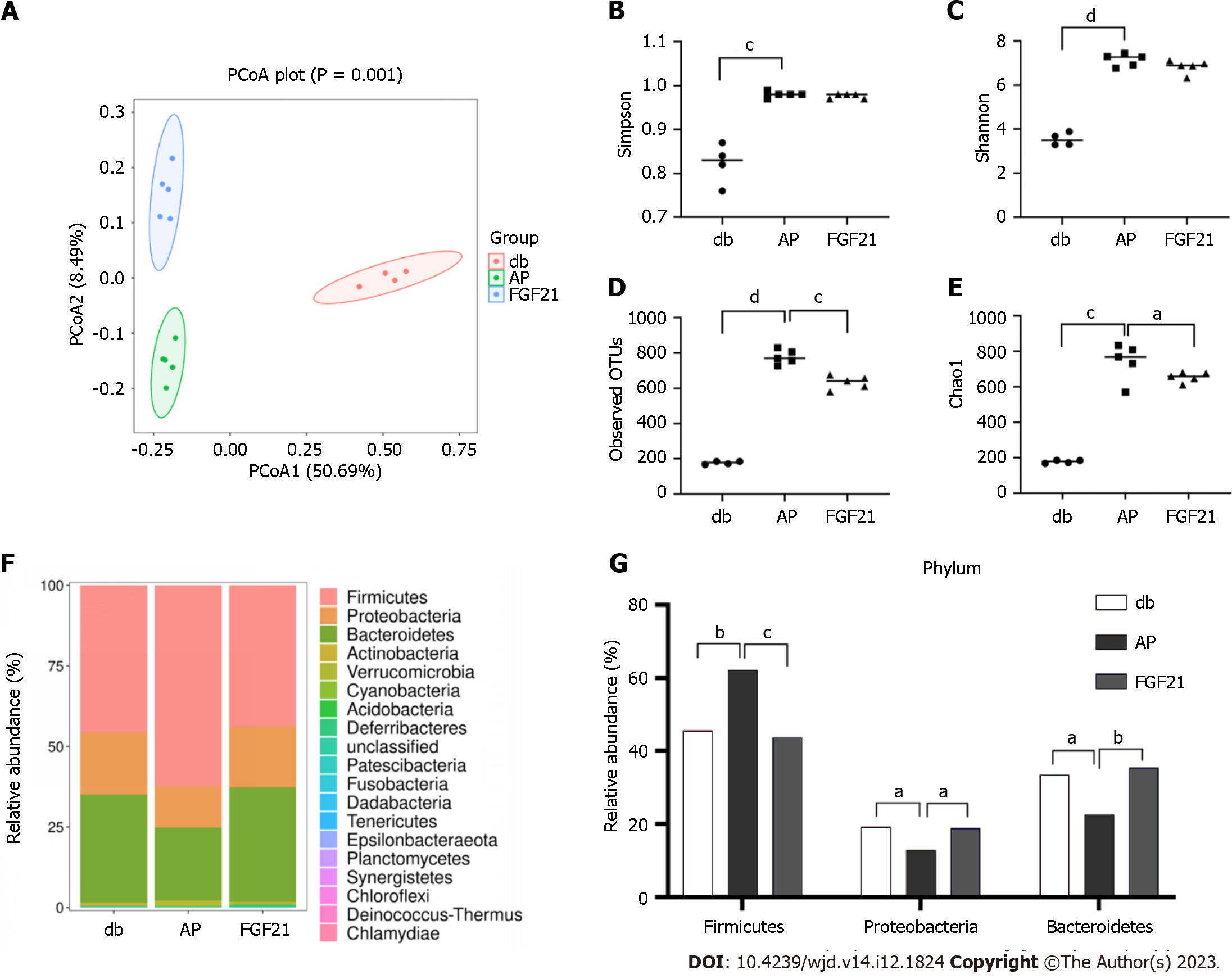
Next, 16S rRNA sequencing was performed to examine whether FGF21 altered the composition of the gut microbiota in diabetic mice while alleviating intestinal damage. Principal coordinate analysis (PCoA) results demonstrated distinct segregation of the microbial communities among the db, AP, and FGF21 groups, underscoring differences in the gut microbiota composition among the three groups (Figure 3A, P < 0.01). Alpha diversity of the gut microbiota, which serves as a comprehensive index of species abundance and evenness in community ecology, was assessed using four commonly used indices: observed OTUs, Chao1, Shannon, and Simpson indices. The observed OTUs and Chao1 indices reflect the species abundance in a sample, while the Shannon and Simpson indices reflect both the species abundance and evenness. When compared with the db group, all four indices showed significant increases in the AP group (P < 0.001). The FGF21 group exhibited notable decreases in OTUs and Chao1 indices when compared with the AP group (P < 0.05), with Shannon and Simpson indices showing a nonsignificant decrease (Figure 3B-E). These results indicate an increase in gut microbiota abundance in diabetic mice with AP, and FGF21 treatment could reverse this change. The gut microbiota plays a pivotal role in the occurrence of AP in diabetic mice.
We further compared the alterations in bacterial communities among the three groups, with the stacked bar chart illustrating bacterial species distribution and changes in species composition and distribution within each group. The experimental results highlighted distinct variations in the highest relative abundance of the top 30 species at different taxonomic levels of the samples. At the phylum level, Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria emerged as major taxa in abundance across all samples (Figure 3F). Notably, phylum Firmicutes exhibited a marked elevation in the AP group (Figure 3G, P < 0.01), with significant decreases in the FGF21 group (Figure 3G, P < 0.001). Meanwhile, phyla Proteobacteria and Bacteroidetes showed significant decreases in the AP group (Figure 3G, P < 0.05) and significant increases in the FGF21 group (Figure 3G, P < 0.05). Moreover, Firmicutes was found to be the most abundant phylum across all samples. This study found that the Bacteroides/Firmicutes ratio decreased in the AP group, with a rebound in the FGF21 group.
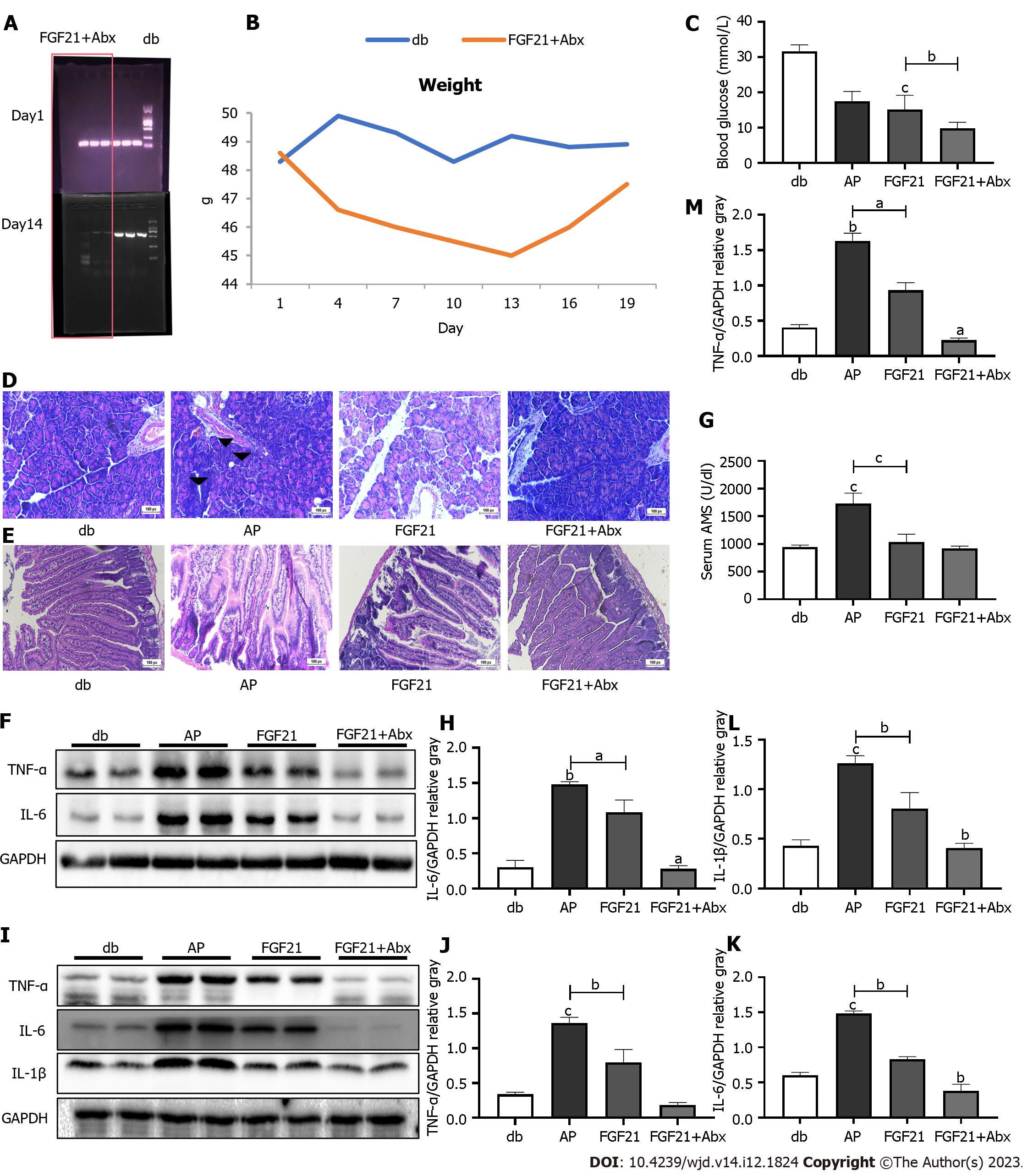
Upon disrupting the gut microbiota through an Abx cocktail, a notable decrease in bacterial abundance was observed in the mouse feces (Figure 4A). Following the commencement of Abx cocktail treatment, the body weight of mice decreased, reaching the lowest point on day 13, with an average body weight of 45 g. However, following adaptation to the Abx cocktail feeding, the body weight of mice gradually increased and reached 48 g (Figure 4B). In subsequent experiments, when compared with the AP group, the FGF21 + Abx group demonstrated a significant decrease in serum AMS levels (Figure 4C, P < 0.0001), with minimal damage observed in pancreatic and intestinal tissue sections (Figure 4D and E). Immunoblotting analysis revealed further reductions in the levels of the pro-inflammatory cytokines TNF- and IL-6 in the FGF21 + Abx group in the pancreatic tissue when compared with those in the FGF21 group (Figure 4F). Specifically, TNF- exhibited a significant decrease of 75.9% (Figure 4G, P < 0.01), and IL-6 showed a reduction to 25.8% of the FGF21 + Abx group (Figure 4H, P < 0.01). Similarly, noticeable reductions in the levels of inflammatory factors were observed in the small intestinal tissue (Figure 4I), with TNF- levels decreasing to 23.4% of that in the FGF21 group (Figure 4J, P < 0.001), IL-1β levels decreasing by half (Figure 4K, P < 0.05), and IL-6 levels decreasing to 45.6% (Figure 4L, P < 0.01). Notably, blood glucose levels significantly decreased from 17.50 ± 1.1 to 9.817 ± 0.69 mmol/L (Figure 4F, P < 0.001) in the FGF21 + Abx group, further decreasing from 15.14 ± 1.8 mmol/L in the FGF21 group (Figure 4M, P < 0.05). These experimental results demonstrate that the combination therapy of Abx cocktail with FGF21 exerts a more potent protective effect on AP in diabetic mice compared to FGF21 treatment alone. The Abx cocktail enhances the protective efficacy of FGF21 in diabetic mice with AP.
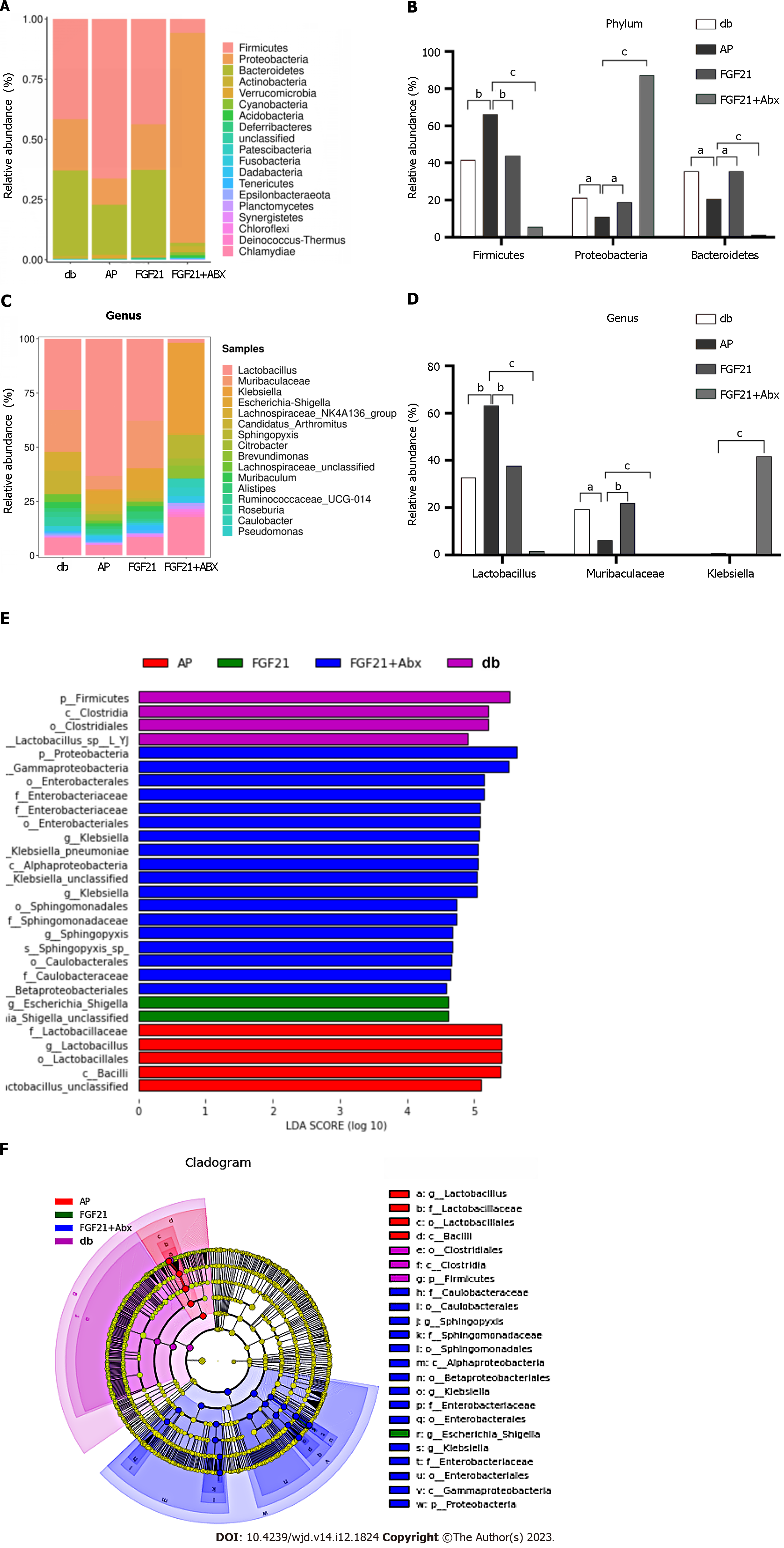
To further investigate the contribution of the microbiota to the protective effects of FGF21 in diabetic mice with AP, we compared the changes in bacterial communities among four groups of mice (Figure 5A and B). At the phylum level, Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria were the major taxa of the bacterial communities across all four groups, accounting for 96.6% of the total abundance. In comparison with the db group, phylum Firmicutes showed a substantial increase to 66.2% in the AP group (Figure 5B, P < 0.01), while it decreased to 43.7% in the FGF21 group, reaching normal levels, and further decreased to 5.7% in the FGF21 + Abx group. Relative to the db group, phyla Proteobacteria and Bacteroidetes significantly decreased to 10.9% and 20.7%, respectively, in the AP group (Figure 5B, P < 0.05). After FGF21 treatment, the abundance of Proteobacteria and Bacteroidetes significantly increased to 18.9% and 35.5%, respectively, in the FGF21 group (Figure 5B, P < 0.05), reaching normal levels. In contrast, in the FGF21 + Abx group, the phylum Proteobacteria significantly increased to 87.3% (Figure 5B, P < 0.001), while the phylum Bacteroidetes significantly decreased to 1.3% (Figure 5B, P < 0.001). Additionally, significant differences in gut microbiota composition were observed at the genus level among different groups (Figure 5C and D). The predominant taxa in the gut microbiota of the four groups of mice were Lactobacillus, Mucispirillum-Klebsiella, and Escherichia coli-Shigella, accounting for 44.3% in the FGF21 + Abx group. Moreover, the AP group exhibited the highest abundance of Lactobacillus, accounting for 53%. In the FGF21 + Abx group, Escherichia coli-Shigella accounted for 42%, while its abundance remained below 1% in the other three groups. Notably, prior research has highlighted an increase in the abundance of the phylum Firmicutes (gram-positive bacteria) and a decrease in the abundance of the phylum Bacteroidetes in obese mice[13]. After FGF21 treatment, the proportion of Firmicutes was significantly reduced, while that of Bacteroidetes was significantly increased in diabetic mice with AP, underscoring the effectiveness of FGF21 in alleviating diabetes conditions in diabetic mice with AP.
Through linear discriminant analysis (LDA) effect size (LEfSe), the biomarkers were compared among the four groups of samples. In this study, a threshold of > 4.5 LDA score and P < 0.05 were set for the LEfSe analysis. The variance in LDA scores across the four groups of microbiota samples revealed that the phylum Proteobacteria, order Enterobacteriales, and family Enterobacteriaceae were the most abundant species in the FGF21 + Abx group. Escherichia coli-Shigella was found to be the most abundant species in the FGF21 group. The phylum Firmicutes, class Clostridia, and order Clostridiales were most abundant in the db group. These bacteria may serve as potential targets for the treatment of diabetes complicated with AP. The order Lactobacillales, family Lactobacillaceae, and genus Lactobacillus were the most abundant in the AP group (Figure 5E). These bacteria belong to the class Bacilli. The phylogenetic tree in Figure 5F shows the origins of the microbiota at different taxonomic levels.
PICRUSt was used to predict the potential functions of microbial genes[17]. In this study, we adopted the PICRUSt2 prediction method to obtain gene function annotations from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Subsequently, statistical analysis of the metagenomic profiles (STAMP) was utilized for differential expression analysis[18] to identify significantly different gene functions among groups. Of note, the KEGG pathway database integrates current knowledge of molecular interaction networks, including biochemical processes such as metabolism, membrane translocation, signal transduction, cell cycle, and conserved subpathways in the same cell lineage.
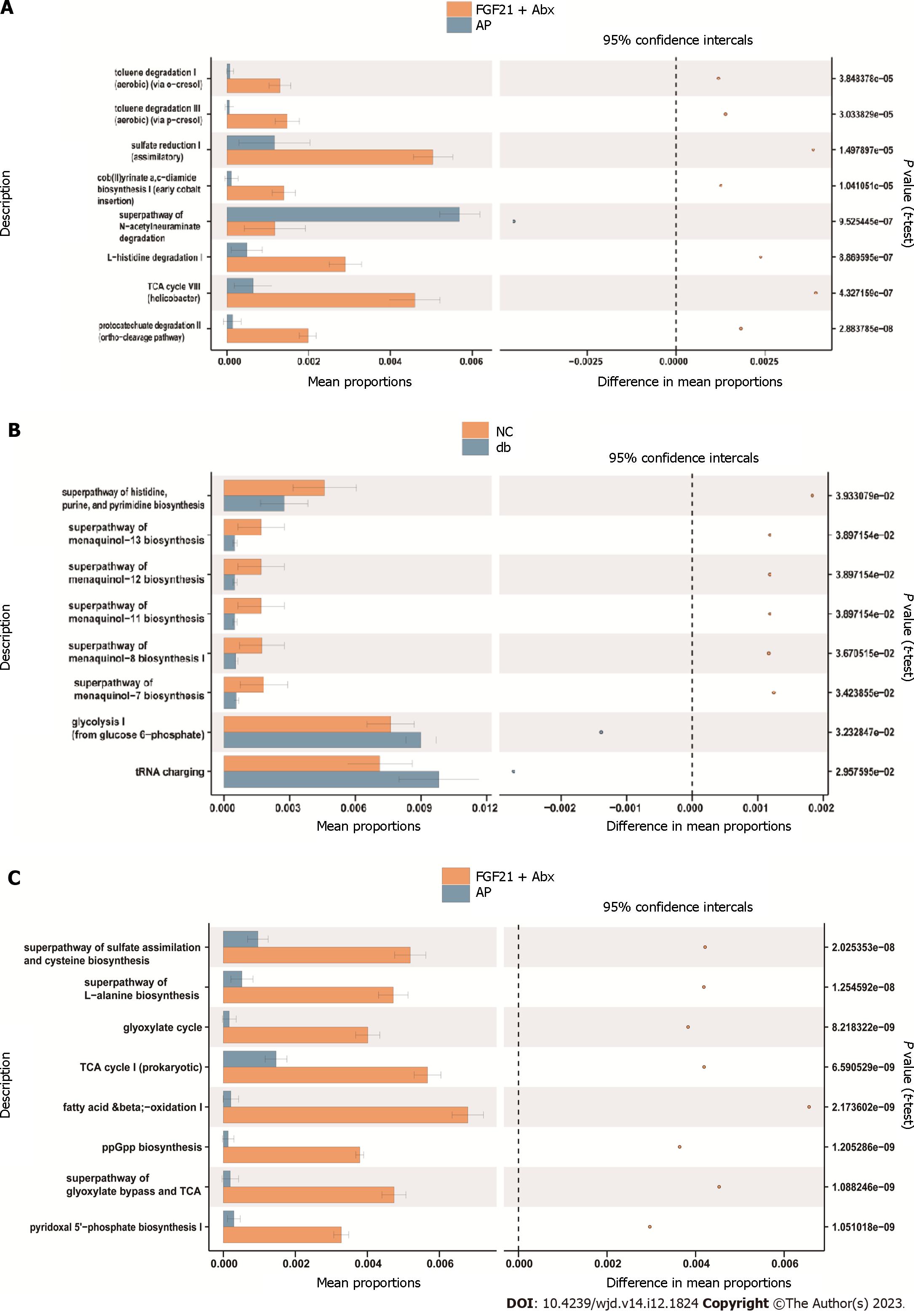
The obtained findings suggested that the differential pathways (P < 0.05) between the FGF21 + Abx group and the AP group included toluene degradation I (aerobic), toluene degradation III (aerobic), sulfate reduction I, cob(II) acetate a, c-diamine biosynthesis I, and the superpathway of n-acetylceramide degradation (Figure 6A). In addition, the differential pathways (P < 0.05) between the db and AP groups included histidine, purine, and pyrimidine biosynthesis, methoxy-13 biosynthesis, methoxy-12 biosynthesis, methoxy-11 biosynthesis, and methoxy-8 biosynthesis (Figure 6B). Furthermore, differential pathways (P < 0.05) between the db and FGF21 + Abx groups included the superpathway of sulfate assimilation and cysteine biosynthesis, superpathway of L-alanine biosynthesis, glyoxylate cycle, tricarboxylic acid cycle I prokaryote, and fatty acid β-oxidation I (Figure 6C). These distinct differentially expressed pathways may provide critical insights into the effects of AP treatment.
AP is a clinically prevalent inflammatory disorder. Previous animal experiments have revealed the potential of FGF21 to reduce the levels of digestive enzymes (AMS and lipase) in AP mice without affecting protein synthesis[19]. Additionally, FGF21 has been demonstrated to diminish the release of inflammatory cytokines, such as TNF- and IL-6, indicating a potent anti-inflammatory effect[11]. A previously reported study found that FGF21 transgenic mice showed significant improvements in pancreatic inflammation and fibrosis in a model of AP induced by ceruletide[20]. In our previous study, it was revealed that FGF21 exerted protective effects against AP through various mechanisms. These mechanisms included the stimulation of Sirt1 expression, the restoration of impaired mitochondria and lysosomes, the promotion of normal autophagic flux, and the suppression of aberrant expression of digestive enzymes in AP[12]. Furthermore, FGF21 was found to reduce inflammatory responses, thereby contributing to the amelioration of AP. In the current study, FGF21 treatment was found to ameliorate histopathological damage in the pancreatic tissues, reduce serum levels of AMS, and diminish levels of pro-inflammatory cytokines (IL-6 and TNF-) in diabetic mice with AP. These results confirmed the potential of FGF21 in decreasing the susceptibility to AP in diabetic mice. Moreover, both the FGF21 group and the FGF21+Abx group showed a decline in blood glucose levels, indicating that FGF21 and Abx cocktail therapy effectively alleviated both diabetes and AP in diabetic mice. These findings present a novel and enhanced pharmacological option for diabetic patients complicated by AP.
Notably, a previous comprehensive study was conducted using 16S rRNA sequencing technology to examine the bacteria associated with pancreatitis. It was found that 70% of patients with pancreatitis had various microbial DNA in their bloodstream[21]. The majority of these microbes exhibited similarity to those found in the gastrointestinal tract, suggesting a possible origin from the gut. Hence, we intended to explore whether the intestinal damage caused by diabetes and AP altered the composition of gut microbiota. To address this issue, the present study employed 16S rRNA sequencing technology to analyze the diversity of intestinal microbiota across three groups of mice. The analyses of beta and alpha diversity analyses revealed the differences in gut microbiota composition across the three groups. Notably, the AP group exhibited a significant increase in the abundance of gut microbiota of the AP group, which returned to normal levels after FGF21 treatment. Prior research has established significant differences in the composition of gut microbiota between obese mice and wild-type mice. Specifically, obese mice showed a notable abundance of bacteria from the phylum Firmicutes, while wild-type mice displayed a predominant abundance of bacteria belonging to the phylum Bacteroidetes[22]. Furthermore, it has been identified that a decline in the Bacteroides/Firmicutes ratio is associated with obesity[23]. Thus, the current study conducted a comparative analysis of bacterial community changes in the mice of three groups, revealing a diminished Bacteroides/Firmicutes ratio in the AP group, which was increased after FGF21 treatment. This finding suggests that AP exacerbates the changes in the gut microbiota of diabetic mice, and both AP and diabetes contribute to the increase in gut microbiota diversity and the decline in the Bacteroides/Firmicutes ratio. FGF21 treatment effectively ameliorates the alterations in gut microbiota, thereby facilitating the alleviation of diabetes and AP conditions.
Subsequently, we sought to elaborate on whether the dysbiosis of gut microbiota in diabetic mice with AP was a concomitant phenomenon or an influencing factor in the occurrence and development of AP in diabetic mice. Our investigation also aimed to illuminate whether FGF21 could ameliorate diabetes and AP condition through the modulation of the gut microbiota. The observations revealed a substantial protective effect of FGF21 against pancreatitis and intestinal inflammation symptoms. Nonetheless, the effects of Abx on diabetes and systemic inflammation have been a subject of contentious debate in many studies. Some studies have reported the protective effect of Abx against diabetes and systemic inflammation, while in other studies, Abx has been shown to exacerbate the disease[24]. Previous studies have provided evidence demonstrating a reduction in the occurrence of diabetes in mice receiving vancomycin treatment from birth to weaning[25]. Moreover, prior research has also suggested that an Abx cocktail (sulfamethoxazole, trimethoprim, and streptomycin sulfate) lowered the occurrence of diabetes and delayed its onset[26]. The timing of Abx administration, particularly before the onset of diabetes onset in mice, can disrupt the balance of healthy gut microbiota, which in turn could provide an explanation for the increased occurrence of diabetes observed in most studies[27]. Notably, the responses of female and male mice to the same Abx treatments might be significantly different. Therefore, multiple variables, such as Abx type, dosage, administration timing, and the specific animal model, may significantly influence the efficacy of Abx treatment. In this study, the use of an Abx cocktail after the onset of diabetes reduced inflammation in the pancreas and small intestines. The administration of Abx to the mice was beneficial as it facilitated the elimination of harmful bacteria, thereby supporting the protective effect of FGF21. Although Abx administration may disrupt the gut microbiota and cause damage to the intestine, FGF21 was reported to repair intestinal damage and effectively mitigate the adverse effects of Abx treatment on the intestine.
We also observed different microbial compositions in the db, AP, FGF21, and FGF21 + Abx groups. Importantly, the AP group showed a notable increase in the abundance of Lactobacillus. Lactobacillus, as a probiotic, has been extensively studied. For instance, a recent study has highlighted the role of Lactobacillus reuteri in establishing a balanced gut microbiota, thereby mitigating the intestinal permeability damage caused by bacterial translocation. This probiotic also enhances the secretion of IgA in the ileum and colon and increases the populations of CD4+ and CD8+ cells. Thus, Lactobacillus reuteri shows promise in ameliorating methotrexate-induced enterocolitis[28]. In this study, the LEfSe analysis revealed that the most abundant taxa in the FGF21 + Abx group were the phylum Proteobacteria, order Enterobacteriales, and family Enterobacteriaceae. The FGF21 group was predominantly enriched with Escherichia coli-Shigella. In contrast, the phylum Firmicutes, class Clostridia, and order Clostridiales were most abundant in the db group. These bacteria may serve as targets for the treatment of AP under diabetic conditions. For instance, the deficiency of antimicrobial peptides, which exhibits a negative correlation with the abundance of Escherichia coli and Shigella, has been linked to intestinal barrier dysfunction and bacterial translocation[29]. Enterobacter cloacae, a common type of Bacteroides, can trigger inflammation and promote lipid accumulation, thus the development of metabolic diseases and atherosclerosis[30]. Dysbiosis of various gut probiotics is tightly associated with the progression of diabetes and AP. For instance, the reduction of Faecalibacterium prausnitzii abundance has been observed in the gut microbiota of individuals with intestinal diseases and type 2 diabetes has been observed[31,32].
The present study adopted the PICRUSt2 prediction method to obtain gene functional annotations from the KEGG database. In addition, STAMP was employed to perform differential expression analysis and identify gene functions that exhibit significant differences between groups. The identification of differential pathways may provide pivotal insights for AP treatment and unravel the mechanisms whereby gut microbiota modulates the therapeutic effects of FGF21 on AP under diabetic conditions. Prior research has underscored a decrease in the levels of butyryl-CoA dehydrogenase in patients with diabetes relative to the control group, accompanied by a decrease in butyrate production in the gut microbiota[33]. In our study, FGF21 + Abx treatment significantly facilitated the sulfate reduction pathway and inhibited the superpathway of n-acetylceramide degradation. These findings suggest significant differences when compared with the AP group and call for further investigation in subsequent studies.
This study revealed the potential ability of FGF21 to enhance the recovery of pancreatic and intestinal damage recovery, reduce blood glucose levels, and modulate the composition of gut microbiota in diabetic mice with AP. Notably, the Abx cocktail therapy further influences the composition of the gut microbiota and enhances the protective effects of FGF21. These findings provide new insights into the prevention and treatment of diabetes complicated by AP. However, further investigation is required to elucidate the specific mechanisms by which the gut microbiota affects the protective effects of FGF21 against AP in diabetic mice.
Fibroblast growth factor 21 (FGF21) plays a pivotal role in regulating glucose and lipid metabolism. Acute pancreatitis (AP) is a common inflammatory disease with clinical manifestations. Diabetes exacerbates intestinal permeability and intestinal inflammation, thus leading to the progression to AP. Our previous study indicated that FGF21 significantly attenuated susceptibility to AP in mice.
Yet, whether FGF21 similarly protects AP in diabetic mice remains unexplored.
Herein, we were intrigued to investigate the potential protective role of FGF21 against AP in diabetic mice.
In the present study, a mouse model of AP was established in db/db diabetic mice through ceruletide injections. By comparing the differences in AP indicators between diabetic mouse group (db), ceruletide-induced AP model group (AP), FGF21 treatment group (FGF21), and FGF21 combined with an antibiotic (Abx) cocktail treatment group (FGF21 + Abx), we investigated the protective effect of recombinant FGF21 protein and investigated whether FGF21 plays its role in the treatment of diabetic mice with AP by modulating the gut microbiota.
FGF21 notably diminished the levels of serum amylase, inflammatory factors and the histological evidence of inflammation in the pancreas and the small intestine in diabetic mice with AP. FGF21 also significantly altered the composition of the gut microbiota, reestablishing the Bacteroidetes/Firmicutes ratio. Upon treatment with an Abx cocktail to deplete the gut microbiota, the FGF21 + Abx group showed superior protective effect. The gut microbiota composition across different groups was further characterized, and a differential expression analysis of gene functions was undertaken using the PICRUSt2 prediction method. These findings suggested that FGF21 could potentially confer therapeutic effects on diabetic mice with AP by modulating the sulfate reduction I pathway and the superpathway of n-acetylceramide degradation in the gut microbiota.
This study reveals the potential of FGF21 in improving pancreatic and intestinal damage recovery, reducing blood glucose levels, and reshaping gut microbiota composition in diabetic mice with AP. Notably, the protective effects of FGF21 are augmented when combined with the Abx cocktail. These findings provide new insights into the prevention and treatment of diabetes complicated by AP.
Further investigation is required to elucidate the specific mechanisms by which the gut microbiota affects the protective effects of FGF21 against AP in diabetic mice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Susak YM, Ukraine; Lu Cai, United States S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Ye C, Liu L, Ma X, Tong H, Gao J, Tai Y, Huang L, Tang C, Wang R. Obesity Aggravates Acute Pancreatitis via Damaging Intestinal Mucosal Barrier and Changing Microbiota Composition in Rats. Sci Rep. 2019;9:69. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260-270. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Paun A, Yau C, Danska JS. The Influence of the Microbiome on Type 1 Diabetes. J Immunol. 2017;198:590-595. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Li M, Liang P, Li Z, Wang Y, Zhang G, Gao H, Wen S, Tang L. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol. 2015;6:692. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103-1107. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Sanz Y, Rastmanesh R, Agostoni C. Understanding the role of gut microbes and probiotics in obesity: how far are we? Pharmacol Res. 2013;69:144-155. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring). 2013;21:2310-2321. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27-42. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Chen Q, Li J, Ma J, Yang X, Ni M, Zhang Y, Li X, Lin Z, Gong F. Fibroblast growth factor 21 alleviates acute pancreatitis via activation of the Sirt1-autophagy signalling pathway. J Cell Mol Med. 2020;24:5341-5351. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127-1151. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134-1144. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852-857. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581-583. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Douglas GM, Maffei VJ, Zaneveld J, Yurgel SN, Langille MGI. PICRUSt2: An improved and extensible approach for metagenome inference. 2020 Preprint. Available from: bioRxiv:672295. [DOI] [Cited in This Article: ] |
| 18. | Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123-3124. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Wang S, Wang Y, Zhang Z, Liu Q, Gu J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017;8:e3018. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Fortunato F, Bürgers H, Bergmann F, Rieger P, Büchler MW, Kroemer G, Werner J. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137:350-360, 360.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Li Q, Wang C, Tang C, He Q, Li N, Li J. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques*. Crit Care Med. 2013;41:1938-1950. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, Gorzelak MA, Chan Y, Chan JM, Lochner A, Dutz JP, Vallance BA, Gibson DL. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10:321-332. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, Buschard K, Hansen AK. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285-2294. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, Bos NA. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105-2108. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Lee JG, Eun CS, Jo SV, Lee AR, Park CH, Han DS. The impact of gut microbiota manipulation with antibiotics on colon tumorigenesis in a murine model. PLoS One. 2019;14:e0226907. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Zhang P, Meng X, Li D, Calderone R, Mao D, Sui B. Commensal Homeostasis of Gut Microbiota-Host for the Impact of Obesity. Front Physiol. 2017;8:1122. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154-1163. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Jin M, Zheng L, Wei Y, Cheng J, Zhang D, Yan S, Qin H, Wang Q, Ci X, Feng H. Enterobacter cloacae aggravates metabolic disease by inducing inflammation and lipid accumulation. Environ Toxicol Pharmacol. 2022;90:103819. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255-261. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. [PubMed] [DOI] [Cited in This Article: ] |