Published online Oct 15, 2014. doi: 10.4239/wjd.v5.i5.724
Revised: June 26, 2014
Accepted: July 15, 2014
Published online: October 15, 2014
AIM: To describe the en bloc perfluorodissection (EBPD) technique and to demonstrate the applicability of using preoperative intravitreal bevacizumab during small-gauge vitreoretinal surgery (23-gauge transconjunctival sutureless vitrectomy) in eyes with advanced proliferative diabetic retinopathy (PDR) with tractional retinal detachment (TRD).
METHODS: This is a prospective, interventional case series. Participants included 114 (eyes) with advanced proliferative diabetic retinopathy and TRD. EBPD was performed in 114 eyes (consecutive patients) during 23-gauge vitrectomy with the utilization of preoperative bevacizumab (1.25 mg/0.05 mL). Patients mean age was 45 years (range, 21-85 years). Surgical time had a mean of 55 min (Range, 25-85 min). Mean follow up of this group of patients was 24 mo (range, 12-32 mo). Main outcome measures included best-corrected visual acuity (BCVA), retinal reattachment, and complications.
RESULTS: Anatomic success occurred in 100% (114/114) of eyes. Significant visual improvement [≥ 2 Early Treatment Diabetic Retinopathy Study (ETDRS) lines] was obtained in 69.2% (79/114), in 26 eyes (22.8%) BCVA remained stable, and in 8 eyes (7%) BCVA decreased (≥ 2 ETDRS lines). Final BCVA was 20/50 or better in 24% of eyes, between 20/60 and 20/400 in 46% of eyes, and worse than 20/400 in 30% of eyes. Complications included cataract in 32 (28%) eyes, iatrogenic retinal breaks in 9 (7.8%) eyes, vitreous hemorrhage requiring another procedure in 7 (6.1%) eyes, and phthisis bulbi in 1 (0.9%) eye.
CONCLUSION: This study demonstrates the usefulness of using preoperative intravitreal bevacizumab and EBPD during small-gauge vitreoretinal surgery in eyes with TRD in PDR.
Core tip: En bloc perfluorodissection and preoperative intravitreal bevacizumab use for small-gauge vitrectomy in patients with proliferative diabetic retinopathy and tractional retinal detachment are very useful, the combination reduces complications and operative time. En bloc perfluorodissection and preoperative intravitreal bevacizumab use seems to have many advantages including that the retina remains stable during vitrectomy, better visibility of the ocular structures in the vitreous cavity, immediate reattachment of the retina, bleeding control, subretinal fluid reabsorbsion and drainage, bleeding sites’ tamponade, and easier dissection of epiretinal tissues.
- Citation: Arevalo JF, Serrano MA, Arias JD. Perfluorocarbon in vitreoretinal surgery and preoperative bevacizumab in diabetic tractional retinal detachment. World J Diabetes 2014; 5(5): 724-729
- URL: https://www.wjgnet.com/1948-9358/full/v5/i5/724.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i5.724
Pars plana vitrectomy is a successful surgical technique for the complications of proliferative diabetic retinopathy (PDR)[1,2]. It is usually necessary within one year in up to 10% of patients presenting with PDR[3]. The commonest indication for surgery is non-clearing vitreous hemorrhage. Unfortunately[1,2], postoperative vitreous hemorrhage is a significant complication occurring in about 20% to 30% of cases[4-10].
Some advances in surgical techniques and instrumentation, such as; en bloc dissection, delamination, segmentation, and bimanual surgical techniques, have allowed better results in the treatment of severe PDR[11-13]. Viscodissection, described by Stenkula and Tornquist[12], and the use of perfluorocarbon liquids (PFCL), introduced as a surgical adjuvant in vitrectomy in 1987 by Chang et al[14], facilitate removal of epiretinal membranes, the management of proliferative vitreoretinopathy (PVR) with retinal detachment, tractional retinal detachments in diabetics, and control of intraoperative hemorrhage.
Quiroz-Mercado et al[15,16] published a technique called perfluorocarbon-perfused vitrectomy (PCPV). In their technique, PFCL is used in the infusion in a continuous way during vitrectomy. In selected cases PFCL may offer several advantages over saline solution, because of their properties including gravitational forces, immiscibility with fluids, and ability to transport oxygen[15,16]. Regardless of PFCL’s advantages, the use of PCPV has not extended worldwide. In addition, PCPV utilizes a considerable amount of PFCL, and membranes may be pushed against the retina during PCPV.
We have previously described “En bloc perfluorodissection” (EBPD), which combines the advantages of viscodissection and PCPV. EBPD helps the surgeon during removal of membranes over the retina and to create a posterior vitreous detachment by injecting PFCL between the retina and the posterior hyaloid separating tissues over the retina[17,18]. In addition, identification and removal of all posterior vitreoretinal traction is very important. Furthermore, vitreoschisis can also occur in patients with PDR, it is important to identify this feature and to perform dissection in the true vitreoretinal plane, to avoid recurrent traction and postoperative bleeding from retinal neovascularization[19].
Postoperative vitreous cavity hemorrhage is a significant complication following vitrectomy for the treatment of PDR. It has two main forms, “early” when hemorrhage (bleeding) is present in the first few postoperative days and “late”, when hemorrhage occurs a number of months after surgery. The presence of postoperative vitreous hemorrhage delays visual recovery can lead to elevated pressure within the eye and can make further treatment for diabetic retinopathy difficult. Revision surgery is required in 10% of patients, which has significant implications for resources, time and cost. The use of anti-vascular endothelial growth factor (anti-VEGF) before surgery (preoperatively) has been proposed as an intervention to reduce the incidence of postoperative vitreous hemorrhage[20].
Recently, it has been reported that intravitreal bevacizumab in patients with vitreous hemorrhage and PDR resulted in regression of retinal neovascularization and resolution of vitreous hemorrhage[21]. Chen et al[22] and Avery et al[23], have reported that preoperative intravitreal bevacizumab (Avastin®, Genentech Inc., San Francisco, CA) reduce the risk of bleeding during vitrectomy facilitating the removal of fibrovascular tissues.
The aim of this article is to describe the surgical technique and demonstrate the usefulness of combining en bloc perfluorodissection and preoperative intravitreal bevacizumab use for membrane peeling in tractional retinal detachment in advanced diabetic retinopathy with small-gauge vitreoretinal surgery (23-gauge transconjunctival sutureless vitrectomy).
This is a prospective, interventional case series. One hundred fourteen (eyes) with tractional retinal detachment (TRD) in PDR participated. The authors performed EBPD in 114 eyes (consecutive patients) during 23-gauge transconjunctival sutureless vitrectomy for tractional retinal detachment in severe PDR with the utilization of preoperative bevacizumab (1.25 mg/0.05 mL). Main outcome measures were best-corrected visual acuity (BCVA), retinal status, and complications. This study has been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and it was approved by the Institution’s Ethics Committee.
An aliquot of commercially available bevacizumab was prepared for each patient and placed in a tuberculin syringe using aseptic techniques. Four days before vitrectomy, after preparation of the eye using 5% povidone/iodine, an eyelid speculum was used to open the eyelids, and the injection of 1.25 mg (0.05 mL) of bevacizumab was performed 4 mm posterior to the limbus, through the superotemporal or inferotemporal pars plana with a 30-gauge needle under topical anesthesia. After the injection, retinal artery perfusion was checked with the indirect ophthalmoscope. In none of our cases an anterior chamber paracenthesis was necessary. No topical antibiotics were administered preoperatively.
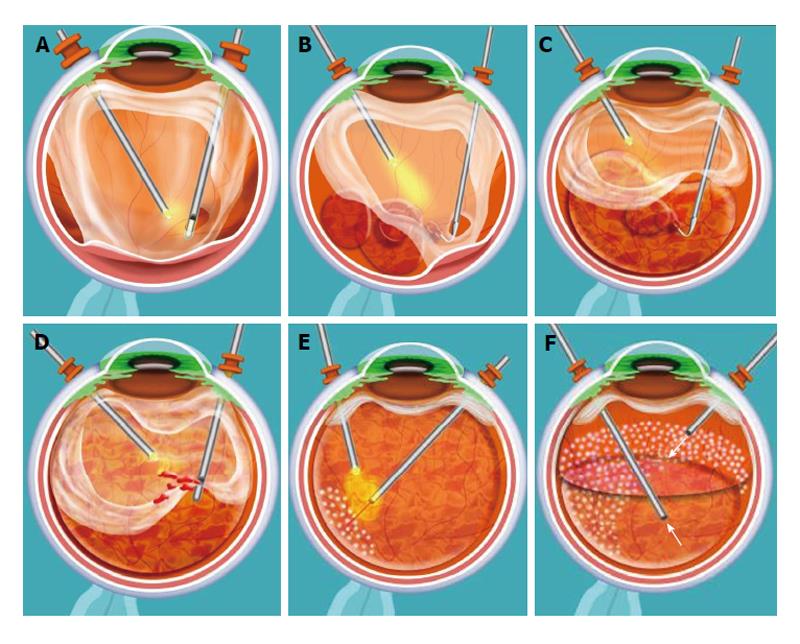
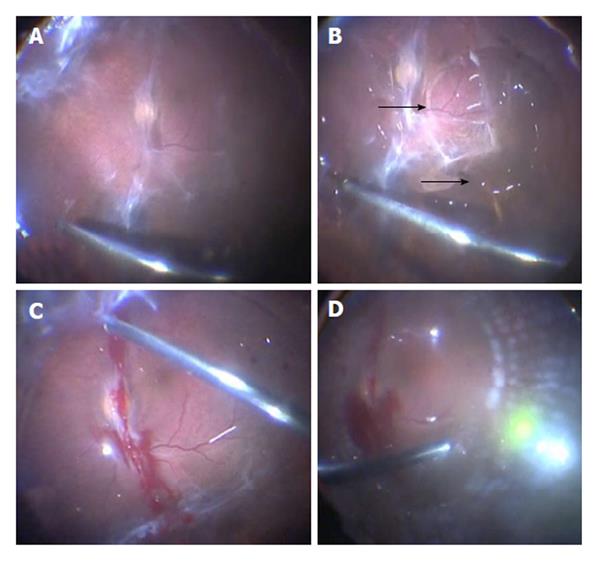
A 23-gauge transconjunctival sutureless vitrectomy was performed in all cases. A core vitrectomy is done first to clear any vitreous hemorrhage present. A hole is then made in the mid-peripheral posterior hyaloid (Figures 1A and 2A) to inject the perfluorocarbon liquid (PFCL) [Perfluorooctane (C8F18)] and mechanically detach the posterior hyaloid from the retina (Figures 1B, 1C and 2B). We use a 23-gauge Dual Bore cannula (Dual Bore cannula 0.6 mm, MedOne, Sarasota, FL) attached to a 5 cc syringe filled with PFCL to separate the posterior hyaloid and membranes from the retina. After all the membranes and posterior hyaloid have been separated from the retina, vitrectomy is completed up to the periphery (Figures 1D and 2C), endolaser is applied (Figures 1E and 2D), an air-fluid and air-gas [Perfluoropropane (C3F8), Escalon Medical Corporation, New Berlin, WI] exchange is performed to finish the case (Figure 1F).
Non-illuminated instrumentation was usually used in our cases[7] combined with a non-contact wide-angle viewing system (BIOM, Oculus, Wetzlar, Germany). An illuminated cannula was utilized (25ga, Awh chandelier, Synergetics Inc., O’Fallon, MO) in some cases for bimanual surgery.
Patients were prospectively enrolled from January 2006 to January 2010 at Clinica Oftalmologica Centro Caracas in Caracas, Venezuela. Inclusion criteria included patients with TRD in advanced PDR and macular involvement or impending macular involvement with or without vitreous hemorrhage. EBPD was performed in 114 consecutive eyes (patients) during small-gauge vitrectomy for severe PDR with TRD. The mean age of the patients was 45 years (range, 21-85 years). Surgical time had a mean of 55 min (Range, 25-85 min). Mean follow up of our patients was 24 mo (range: 12-32 mo).
Each patient underwent BCVA measurement with ETDRS. Patients were followed postoperatively on day 1, at one week, at three weeks, at 7 wk, and every 3 mo with complete eye examination at each visit, including BCVA, anterior segment examination, IOP determination, and fundus biomicroscopy. Patients were included only with a minimum 12 mo of follow-up. An increase or decrease in BCVA was considered to have occurred if there was a change of two or more Early Treatment Diabetic Retinopathy Study (ETDRS) lines. Main outcome measures were changes in BCVA, and retinal reattachment.
En bloc perfluorodissection was performed using a mean volume of PFCL of 4 mL (range: 3 to 8 mL). No patients in our series have shown ocular hypertension or inflammation. Anatomic success occurred in 100% (114/114) of eyes. Significant visual improvement (≥ 2 ETDRS lines) was seen in 69.2% (79/114), in 26 eyes (22.8%) BCVA remained stable, and in 8 eyes (7%) BCVA decreased (≥ 2 ETDRS lines). Final BCVA was 20/50 or better in 24%, between 20/60 and 20/400 in 46%, and worse than 20/400 in 30%. Complications included cataract in 32 (28%) eyes, iatrogenic retinal breaks in 9 (7.8%) eyes, vitreous hemorrhage requiring another procedure in 7 (6.1%) eyes, and phthisis bulbi in 1 (0.9%) eye.
In selected cases en bloc perfluorodissection during vitrectomy in eyes with TRD in PDR and preoperative use of intravitreal bevacizumab, we can obtained an anatomic (100%) and functional success (69.2%). Other benefits of this technique include that the retina remains stable during vitrectomy, less blood in the vitreous cavity, rapid retinal reattachment, better visualization of vitreous and intraocular structures, blood confinement, and easier dissection of epiretinal membranes.
In our study, the authors have not seen any difficulties with the technique. However, in one case PFCL was injected within a vitreous schisis. After a short amount of instillation (1 mL) that situation was apparent, and PFCL was aspirated and a new hole in the posterior hyaloid was made at another location making sure that the proper plane was found between the posterior hyaloid and the retina this time. No complications rose from this event. In addition, there were 2 eyes (1.7%) with subretinal PCL that were solved with a peripheral retinotomy, aspiration with an extrusion cannulae, and the injection of additional PCL in the posterior pole. In our study the prevalence of postoperative vitreous hemorrhage was lower (6.1%) than that reported in other studies (20% to 30%)[4-10] which can be explained by the use of intravitreal bevacizumab 4 d preoperatively.
Surgeons with extensive experience can manage complex retinal detachments in patients with TRD using either viscodissection or conventional techniques with pick and scissors. Thus, surgeons should deal with these cases selectively according to their level of experience. An ideal case for EBPD might be one in which there is a TRD with no tears, with limited posterior vitreous detachment, and relatively loose attachment of the posterior hyaloid to the retina. We use a combination of several techniques in our cases including EBPFD, and the use of picks and forceps with bimanual surgery. Currently, the use of small-gauge vitreoretinal surgery (23-gauge transconjunctival sutureless vitrectomy) and preoperative intravitreal bevacizumab for TRD in diabetics have improved our surgical time and results.
In the future, MIVS with 23-gauge transconjunctival sutureless vitrectomy techniques will be increasingly performed in diabetic patients due to the increased incidence of diabetes and its complications. In the coming years we will use techniques that are less invasive in vitreoretinal surgery such as 25+, and 27-gauge. We will have available other anti-VEGF antibodies capable of blocking all types of VEGF isoforms before and after surgery, reducing intraoperative bleeding, and postoperative inflammation. It is likely that the use of preoperative agents that promote the detachment of the posterior hyaloid and facilitate the removal of membranes will become routine. They will facilitate surgery of complex cases such as PDR cases. Optical coherence tomography equipment will be available in the operating room and that will facilitate intraoperative tissue differentiation, and help us get better functional results. The advent of new lasers will permit us faster retinal photocoagulation, and will minimize collateral damage of the retina.
In summary, EBPD and preoperative intravitreal bevacizumab use for vitrectomy in eyes with TRD in PDR it is very useful. En bloc perfluorodissection and preoperative intravitreal bevacizumab use seems to have many advantages including that the retina remains stable during vitrectomy, better visibility of intraocular structures, immediate reattachment of the retina, bleeding control, reabsorbsion and drainage of subretinal fluid, bleeding sites’ tamponade, and easier dissection of epiretinal tissues.
Dr. Arevalo is a PhD student at Faculty of Health Sciences, Stellenbosch University, Stellenbosch, South Africa. This article is part of his PhD thesis on “Intravitreal Bevacizumab as Anti-Vascular Endothelial Growth Factor in the Management of Complications of Diabetic Retinopathy”.
Authors have previously described a new surgical dissection technique, namely “En bloc perfluorodissection” (EBPD), which combines the advantages of viscodissection and perfluorocarbon-perfused vitrectomy. EBPD helps the surgeon during removal of epiretinal membranes and to detach the posterior hyaloid by injecting perfluorocarbon liquid between the retina and the posterior hyaloid to separate the epiretinal tissues from the retina.
The objective of this article is to describe the surgical technique and demonstrate the usefulness of combining en bloc perfluorodissection and preoperative intravitreal bevacizumab use for membrane peeling in tractional retinal detachment in advanced diabetic retinopathy with small-gauge vitreoretinal surgery (23-gauge transconjunctival sutureless vitrectomy).
En bloc perfluorodissection and preoperative intravitreal bevacizumab use seems to have many advantages including that the retina remains stable during vitrectomy, better visibility of vitreous and intraocular structures, immediate retinal reattachment, bleeding control in the vitreous cavity, subretinal fluid reabsorbsion and drainage, bleeding sites’ tamponade, and easier dissection of epiretinal tissues.
En bloc perfluorodissection and preoperative intravitreal bevacizumab use for vitrectomy in eyes with tractional retinal detachment in advanced proliferative diabetic retinopathy it is very useful technique, reduces complication and operative time.
The report is interesting, well documented, and the paper should be published.
P- Reviewer: Koleva-Georgieva DN, Velez-Montoya R S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Ho T, Smiddy WE, Flynn HW. Vitrectomy in the management of diabetic eye disease. Surv Ophthalmol. 1992;190-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | McLeod D. Microsurgical management of neovascularisation secondary to posterior segment ischaemia. Eye (Lond). 1991;5:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Kaiser RS, Maguire MG, Grunwald JE, Lieb D, Jani B, Brucker AJ, Maguire AM, Ho AC, Fine SL. One-year outcomes of panretinal photocoagulation in proliferative diabetic retinopathy. Am J Ophthalmol. 2000;129:178-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Benson WE, Brown GC, Tasman W, McNamara JA. Complications of vitrectomy for non-clearing vitreous hemorrhage in diabetic patients. Ophthalmic Surg. 1988;19:862-864. [PubMed] [Cited in This Article: ] |
| 5. | Blankenship GW. Management of vitreous cavity hemorrhage following pars plana vitrectomy for diabetic retinopathy. Ophthalmology. 1986;93:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Liggett PE, Lean JS, Barlow WE, Ryan SJ. Intraoperative argon endophotocoagulation for recurrent vitreous hemorrhage after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1987;103:146-149. [PubMed] [Cited in This Article: ] |
| 7. | Novak MA, Rice TA, Michels RG, Auer C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91:1485-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Tolentino FI, Cajita VN, Gancayco T, Skates S. Vitreous hemorrhage after closed vitrectomy for proliferative diabetic retinopathy. Ophthalmology. 1989;96:1495-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Virata SR, Kylstra JA. Postoperative complications following vitrectomy for proliferative diabetic retinopathy with sew-on and noncontact wide-angle viewing lenses. Ophthalmic Surg Lasers. 2001;193-197. [PubMed] [Cited in This Article: ] |
| 10. | Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:365-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Abrams GW, Williams GA. “En bloc” excision of diabetic membranes. Am J Ophthalmol. 1987;103:302-308. [PubMed] [Cited in This Article: ] |
| 12. | Stenkula S, Tornquist R. Healon: a guide to its use in ophthalmic surgery. Use of healon in vitrectomy and difficult retinal detachments. New York: Wiley 1983; 207-221. [Cited in This Article: ] |
| 13. | Grigorian RA, Castellarin A, Bhagat N, Del Priore L, Von Hagen S, Zarbin MA. Use of viscodissection and silicone oil in vitrectomy for severe diabetic retinopathy. Semin Ophthalmol. 2003;18:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Chang S, Zimmerman NJ, Iwamoto T, Ortiz R, Faris D. Experimental vitreous replacement with perfluorotributylamine. Am J Ophthalmol. 1987;103:29-37. [PubMed] [Cited in This Article: ] |
| 15. | Quiroz-Mercado H, Guerrero-Naranjo J, Agurto-Rivera R, Leizaola-Fernández C, Suárez-Tatá L, Murillo-López S, Reategui-Escalante G, García-Aguirre G, Fromow-Guerra J. Perfluorocarbon-perfused vitrectomy: a new method for vitrectomy--a safety and feasibility study. Graefes Arch Clin Exp Ophthalmol. 2005;243:551-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Quiroz-Mercado H, Garcia-Aguirre G, Ustáriz-González O, Martín-Avià J, Martinez-Jardon S. Perfluorocarbon-perfused vitrectomy using a transconjunctival 25-gauge system. Retina. 2007;27:926-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Arevalo JF. En bloc perfluorodissection for tractional retinal detachment in proliferative diabetic retinopathy. Ophthalmology. 2008;115:e21-e25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Arevalo JF. En bloc perfluorodissection in vitreoretinal surgery: a new surgical technique. Retina. 2008;28:653-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Schwatz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology. 1996;103:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B, Essex R, McCombe M, Qureshi S, Campbell WG. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin Experiment Ophthalmol. 2008;36:449-454. [PubMed] [Cited in This Article: ] |
| 21. | Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 345] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 22. | Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006;26:699-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1-1695.15. [PubMed] [Cited in This Article: ] |