Published online Apr 15, 2021. doi: 10.4239/wjd.v12.i4.331
Peer-review started: January 9, 2021
First decision: January 25, 2021
Revised: January 25, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: April 15, 2021
This review aims to summarize the health benefits of exposure to hypoxic conditions during exercise in patients with type 2 diabetes mellitus (T2DM). Exposure to hypoxic conditions during exercise training positively changes the physiological response in healthy subjects. Exposure to hypoxic conditions during exercise could markedly increase skeletal muscle glucose uptake compared to that in normoxic conditions. Furthermore, post-exercise insulin sensitivity of T2DM patients increases more when exercising under hypoxic than under normoxic conditions. Regular exercise under short-term hypoxic conditions can improve blood glucose control at lower workloads than in normoxic conditions. Addi
Core Tip: Current research shows that exercise interventions performed under hypoxic conditions have positive effects on healthy subjects and athletes. Exercise intervention under hypoxic conditions can be beneficial as a new treatment for patients, including those with diabetes. This review summarizes recent studies on the potential cause‒effect relationship for exercise interventions under hypoxic conditions in type 2 diabetes mellitus patients and discusses health benefits and risk factors.
- Citation: Kim SW, Jung WS, Chung S, Park HY. Exercise intervention under hypoxic condition as a new therapeutic paradigm for type 2 diabetes mellitus: A narrative review. World J Diabetes 2021; 12(4): 331-343
- URL: https://www.wjgnet.com/1948-9358/full/v12/i4/331.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i4.331
Type 2 diabetes mellitus (T2DM) is a severe global public health problem. The worldwide diabetes prevalence in 2019 was estimated to be 463 million people, with a projected increase to 578 million by 2030 and 700 million by 2045[1]. Diabetes is a primary cause of preventable lower limb amputations, blindness, and end-stage renal failure[2]. It is also associated with increased cardiovascular complications and premature death[3].
Insulin is the main hormone produced by pancreatic β-cells in the islets of Langerhans. It reduces blood glucose by stimulating glucose absorption into tissues, including fat and muscles. Insulin action refers to insulin signal cascade activation that is stimulated by insulin binding to its receptor, causing glucose and lipid absorption and metabolism, gene expression and protein synthesis, cell growth, and survival[4]. Skeletal muscle is the leading glucose-uptake site[5], one of the principal organs of insulin action, and is involved in glucose homeostasis regulation in healthy and diabetic conditions[6]. In hyperinsulinemia, uptake of insulin-mediated blood glucose in skeletal muscle accounts for about 95% of whole-body-based glucose disposal during hyperglycemia[5]. Glucose requires specific transfer proteins to be transported across the cell membrane[7].
Glucose transporter 4 (GLUT4) is primarily responsible for this action[8-10]. GLUT4 is the primary transporter of glucose in skeletal muscle[9]. Increased GLUT4 expression in the skeletal muscle membrane is an essential indicator of exercise-related improvement[11]. The carrying rate of glucose in skeletal muscle is the limiting stage for glucose uptake at rest, and the translation and expression of GLUT4 in response to exercise determine the acute regulation of glucose uptake[11]. Previous studies have reported impaired insulin-stimulated glucose uptake and a decreased rate of glycogen synthesis in insulin-resistant muscles[12,13]. The reduction of glycogen synthesis due to insulin-stimulated glucose transport disorder plays an important role in muscle insulin-resistance development[14]. Therefore, skeletal muscle insulin-resistance has been regarded as a significant defect in T2DM[12]. The precise mechanism underlying insulin-resistance in skeletal muscle has not yet been fully elucidated[15]. According to previous reports, the decrease in insulin-stimulated glucose uptake in skeletal muscle is caused by the degradation of insulin signals and a defect in multicellular cascades, such as glucose transport inhibition, glucose phosphorylation, and the reduction of glucose-oxidizing glycogen synthesis, which plays a decisive role in the development of insulin-resistance in skeletal muscle[16]. Thus, it has been reported that the decrease in insulin action in insulin-resistant skeletal muscle is related to the decrease in glycogen synthesis, caused by insulin-stimulated glucose transport disorder and decreased mitochondrial oxidation related to physical inactivity[12,17,18].
It has been established that exercise intervention can enhance insulin action and glycemic regulation ability in individuals with T2DM, which may be due to the increased oxidation capacity of skeletal muscle, resulting in improved β-cell function[19-22]. Patients with T2DM do not release enough insulin to control glucose due to loss of cells, deterioration of their function, or both[23]. Obesity increases the risk of T2DM[24,25] partly by decreasing insulin sensitivity, i.e., insulin does not cause a normal reduction in blood glucose.
Given the relationship between diabetes, increased cardiovascular disease, and decreased life expectancy[26], the concept of effective treatment for diabetes is important. Exercise interventions promise to enhance glycemic control and cardiovascular condition[19]. It is important to find a more effective strategy for treating this metabolic disease[27]. Recently, many studies have investigated treatment of diabetes by widely applying hypoxic conditions, based on studies that showed that exercising at high altitudes reduced the risk of diabetes, cardiovascular disease, and obesity-related diseases, as compared to exercising at sea level[27-31]. Hypoxic therapy is a novel treatment used as a common practice in many developed countries[28,32-35]. Hypoxic therapies such as hypoxic exposure or hypoxic exercise intervention have been recommended to treat and prevent diabetes by affecting glucose uptake, insulin sensitivity, and vascular health[27].
This narrative review aims to summarize any possible benefits of exposure and exercise under artificially generated hypoxic conditions in T2DM patients.
Short- or/and long-term exposure to hypoxic conditions causes extensive physiological changes[36]. Normobaric (i.e., simulated altitude) and hypobaric hypoxia (i.e., real and simulated altitude) can reduce oxygen partial pressure in tissues and in blood. The acute compensatory response activates the sympathetic nerves and increases ventilation, and causes an altitude-dependent increased cardiac output upon exposure of inhabitants of low-lying areas to high altitudes[37]. Hyperventilation is one of the essential processes involved in supplying sufficient oxygen to tissues[38]. Moreover, peripheral chemical receptors in the carotid body react to reduced arterial partial pressure of oxygen[39]. When a decrease in arterial oxygen saturation is detected by these receptors, the signal leads to sympathetic nerve activation and stimulation of ventilation, increasing the metabolic demand[40]. Exposure to dry and cold environments may increase water loss as ventilation increases[41]. As such, ventilation and cardiovascular reactions ensure that tissues’ metabolic demands are met at rest and during exercise at high altitudes. Sustained exposure to hypoxia results in a reduced cardiac output to a similar level as under normoxia. These adaptive responses are facilitated by an increased stimulated red cell mass and further increases in ventilator responses to hypoxia[36].
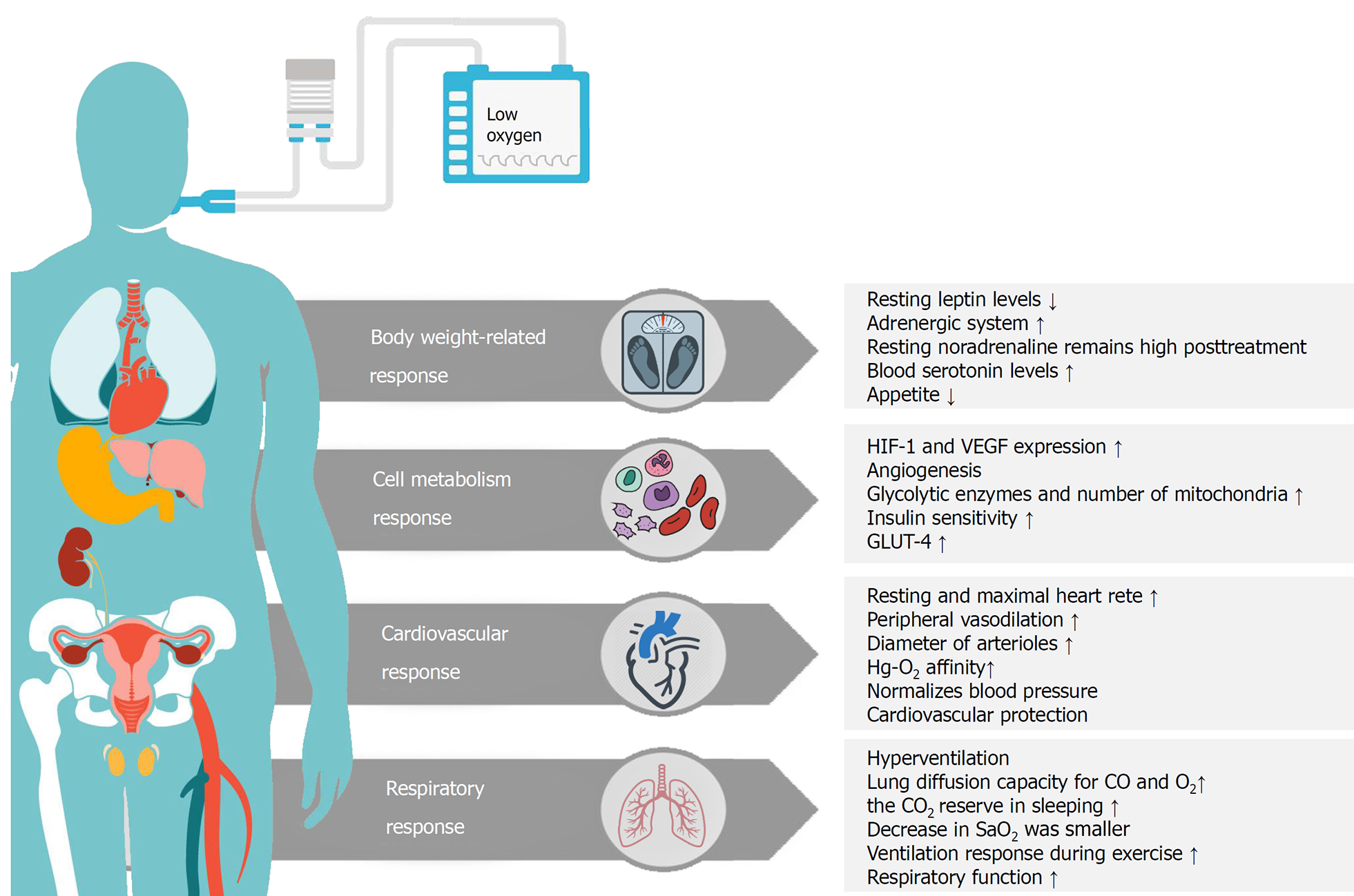
Intermittent hypoxia intervention has been studied over the past decades as a means of treatment for various health conditions[42-44]. It has been suggested that exposure to mild hypoxia for 1 h, with or without simultaneous exercise, had an acute effect on insulin-resistance and blood glucose level in T2DM patients[45,46]. In addition to these adaptations, exposure to the hypoxic environment positively affected body composition (e.g., fat mass, percent body fat, and fat-free mass)[28,30]. It has also been proven that several weeks of exercise under moderate hypoxia resulted in more weight loss in obese individuals than did exercising at the same or higher intensity under normoxia[29,30,47,48]. Previous studies have also reported the beneficial effects of repeated exposure to intermittent hypoxic interventions for a few weeks, in the absence of other types of intervention, on fasting blood glucose and insulin levels[49,50]. However, the fundamental mechanism underlying changes in glycemic control and insulin sensitivity due to hypoxia have been unclear[51]. In addition to the insulin-dependent regulatory mechanism, it has been speculated that hypoxia may affect glucose uptake in a way similar to exercise[51]. Therefore, exposure to hypoxic conditions is a new method of intervention for health-promotion and prevention or treatment of chronic diseases by improving body weight, cell metabolism, cardiovascular, and respiratory function (Figure 1)[32,52].
Review and meta-analysis suggest that exposure to hypoxic conditions during exercise can improve insulin sensitivity and enhance cardiovascular health more than exposure to normoxic conditions[28,53-55]. Furthermore, exposure to hypoxic conditions has been shown to increase endurance performance in athletes[56-58]. Exercising under hypoxic conditions can enhance the exercise adaptations and exercise tolerance of T2DM patients[27]. Previous studies of exposure to hypoxia during acute and chronic exercise in T2DM and insulin-resistant patients are summarized in detail in Tables 1 and 2.
| Ref. | Participants | Design and protocol | Exercise intensity | Main results |
| Mackenzie et al[63] (2011)1 | n = 8; sex: Male; age: 58 ± 4.0 yr; BMI: 29.2 ± 6.7 kg/m2 | (1) 60 min rest in normoxia; (2) 60 min rest in hypoxia normobaric hypoxia (FiO2: 14.6%, simulated altitude: ca.3100 m); (3) 60 min cycling in normoxia; and (4) 60 min cycling in hypoxia (normobaric hypoxia: FiO2: 14.6%) | (3) and (4): 90% lactate threshold | Blood lactate: ↔ (1), (2); ↑ (3), (4). Blood glucose: ↔ (1); ↓ (2), (3), (4). Insulin sensitivity (during glucose tolerance test): (4) > (3) > (2) > (1) |
| Mackenzie et al[46] (2012)1 | n = 8; sex: Male; age: 58.7 ± 2.2 yr; BMI: 28.3 ± 2.1 kg/m2 | (1) 60 min continuous cycling in hypoxia (normobaric hypoxia: FiO2: 14.7%, simulated altitude: ca.3100 m); (2) 60 min interval training with passive recovery (5:5 min) in hypoxia (normobaric hypoxia: FiO2: 14.7%); and (3) 60 min interval training with passive recovery (5:5 min) in normoxia | (1): 90% lactate threshold; (2): 120% lactate threshold; (3): 120% lactate threshold | HR and blood lactate: ↑ (1), (2), (3). Blood glucose decrease (pre- to post-exercise): (1) > (2). Glucose disappearance: ↑ (1); ↔ (2), (3). HOMA-IR index improved after 24 h: ↑ (1), (2); ↔ (3); after 48 h: ↑ (1); ↔ (3) |
| Brinkmann et al[76] (2017)2 | n = 8; sex: Male; age: 58.0 ± 15.0 yr; BMI: 33.0 ± 6.0 kg/m2 | 40 min cycling: (1) Normoxia; (2) Hypoxia (normobaric hypoxia: FiO2: 14%, simulated altitude: ca. 3400 m); and (3) Hypoxia (normobaric hypoxia: FiO2: 14%) + hyperoxia (normobaric hyperoxia: FiO2: 30%) intervals (5:5 min) | Blood lactate: 2.5 mmol/L | Blood lactate (post-exercise lower): (3) > (2). BORG RPE: ↔ (1), (2), (3). Pro-angiogenic factors: VEGF: ↑ (2), (3). Anti-angiogenic factor: endostatin: ↑ (2), (3) |
| Ref. | Participants | Intervention | Intensity | Frequency and duration | Main results |
| Wiesner et al[69] (2010)1 | n = 45. NTG: sex: 8 male, 13 females; age: 42.1 ± 1.7yr; BMI: 32.5 ± 0.8. HTG: sex: 10 male, 14 females; age: 42.2 ± 1.2 yr; BMI: 33.1 ± 0.3 | 60 min running on a treadmill; normobaric hypoxia: simulated altitude: ca. 2740 m | VO2peak: 65% | 3 d/wk, 4 wk | Lactate levels at the anaerobic threshold: ↓ HTG; fasting insulin, HOMA-IR: ↓ NTG, HTG; body fat decreased: HTG > NTG; BP, LDL-c: ↔ NTG, HTG |
| Schreuder et al[66] (2014)2 | n = 19. NTG: sex: 5 male, 4 females; age: 52.0 ± 8.0 yr; BMI: 36.0 ± 6.5 kg/m2. HTG: sex: 9 male, 1 female; age: 57.0 ± 6.0 yr; BMI: 30.9 ± 4.1 kg/m2 | 45 min endurance training (cycling) + series of strength training exercises; normobaric hypoxia: FiO2: 16.5%: simulated altitude: ca. 2100 m | HRR: 70%-75% | 3 d/wk, 8 wk | VO2max: ↑ NTG, HTG; BMI, BP, HOMA-IR, HDL-c, LDL-c, TC, TG, fasting glucose, HbA1c: ↔ NTG, HTG; Vasodilatory function: ↔ NTG, HTG |
To date, various technical equipment has been developed to create hypoxic conditions artificially. Artificially produced hypoxia can be obtained by changing barometric pressure (hypobaric hypoxia) or by changing the fraction of oxygen (FiO2) (normobaric hypoxia). FiO2 is always constant at sea level (ca. 21%), and barometric pressure decreases with higher altitude. Hypoxic conditions can be created by using a special chamber at rest or during exercise. Such an environment control chamber is shown in Figure 2.
Effects of exercise intervention under hypoxic conditions on glucose uptake and insulin sensitivity: Exposure to hypoxia increases glucose uptake in the skeletal muscles of healthy and obese adults[59]. Brooks et al[60] demonstrated that 3 wk of hypoxic exposure at an altitude of ca. 4300 m improves glucose turnover and decreases blood glucose in healthy males. Lippl et al[61] showed that short-term hypoxic exposure (1 wk at an altitude of ca. 2650 m) decreased glycated hemoglobin (HbA1c) levels in obese males. Exposure to hypoxia can markedly improve glucose uptake, and exposure continuously improves blood glucose regulation over the long term.
A previous review article reported that regular physical activity and exercise could markedly increase peripheral glucose uptake and improve blood glucose regulation[62]. It is necessary to clarify whether exercise and hypoxia can have positive combined effects in T2DM and whether patients with this condition can benefit from short- or long-term exposure to hypoxia during exercise. Mackenzie et al[63] examined short-term hypoxic exposure during acute exercise on the glucose homeostasis in T2DM patients. They proved that glucose loss and sustained glucose infusion during cycling under hypoxic conditions were more significantly increased than under normoxic conditions[46,63]. Glucose tolerance tests performed immediately after cycling showed that blood glucose regulation improved more under hypoxic conditions. Insulin sensitivity increased only after 24 h and 48 h after exercise under hypoxic conditions. It also reported that exercising at a continuous submaximal intensity under hypoxic conditions effectively increased glucose uptake and insulin sensitivity than interval training[46]. Thus, previous research suggests that acute exercise under hypoxic conditions positively affects glucose uptake and insulin sensitivity in T2DM patients. The increase in glucose uptake during hypoxia has been thought to be due to the upregulation in the glycolytic energy pathway, which compensates for decreased energy production by the aerobic system[64]. Katayama et al[65] reported that the respiratory exchange ratio was lower during submaximal cycling exercise for 30 min at sea levels in healthy males than similar cycling under hypoxic conditions (at an altitude of ca. 2000 m). However, Schreuder et al[66] found no change in insulin sensitivity and blood glucose regulation in T2DM patients after exercise training under normoxia or short-term hypoxia.
Previous studies were also conducted on insulin-resistance in healthy adults and T2DM patients. Haufe et al[67] reported significant improvements in the values for the homeostatic model assessment of insulin-resistance (HOMA-IR) index in healthy males, only during exercise under hypoxic conditions. However, Lecoultre et al[68] showed increased glucose and insulin concentrations and higher insulin-to-glucagon rates after exercise training under hypoxic conditions, as compared to under normoxic conditions. Wiesner et al[69] showed that exercise training performed under short-term hypoxia did not change the HOMA-IR index in overweight and obese subjects. However, the training workload was significantly lower in the hypoxic group than in the general group, and exercise under hypoxic conditions was a more efficient method. These previous studies also demonstrated improvements in the HOMA-IR index with reduced body fat in the hypoxia groups[67,69]. Decreasing body fat helps to increase insulin sensitivity, particularly in T2DM patients, because there is a positive association between body fat, peripheral insulin-resistance, and pro-inflammatory conditions[70].
Thus, the combination of short-term hypoxia exposure and exercise has a beneficial effect. Nevertheless, there is a lack of biochemical evidence in human research. Therefore, further studies are needed to clarify whether exercise under short-term hypoxia can effectively increase blood glucose uptake and insulin sensitivity, as compared to exercise under normoxia[27].
Effects of exercise intervention under hypoxic conditions on skeletal muscle: The decrease in skeletal muscle capillarization can have a negative effect on blood glucose regulation, and a negative relationship between skeletal muscle capillary density and insulin concentration has been shown previously[71]. Regular physical activity has been shown to have a positive effect skeletal muscle capillaries[53]. Lundby et al[72] concluded in a previous review that combining exercise and hypoxia may accelerate structural and functional adaptation. In contrast, prolonged exposure to hypoxia does not result in significant changes in human capillarization during rest[72]. Mizuno et al[73] reported that exposure to ca. 5300 m for 75 d did not change the ratio between capillaries and muscle fibers. However, because the reduction in fiber size can be adapted to hypoxic exposure, capillaries per region were increased at a similar altitude[74]. Recent meta-analysis studies have shown that exercise under hypoxic conditions positively affects the skeletal muscle capillaries and function of the vascular dilator[53].
A temporary increase in pro-angiogenic factors due to acute exercise may be related to the initiation and control of angiogenesis[75]. Brinkmann et al[76] found that acute exercise under hypoxia could lead to upregulation of serum pro-angiogenic factors, as compared to exercise under normoxia, in T2DM patients. Additionally, there are other mechanisms that contribute to increased pro-angiogenic regulator release after exercise under hypoxic conditions. First, tissue hypoxia during exercise can be enhanced by environmental hypoxic conditions and is increased by activation of hypoxia-induced factor-1α (HIF-1α), which initiates expression of proteins related to angiogenesis regulation[77]. However, responses to hypoxia in diabetes have been impaired, and hyperglycemia is a very important result in the HIF-1α regulation[78]. The second mechanism is increased sympathetic nerve activity and increased skeletal muscle blood flow and shear stress at the vessel walls, which induce intracellular signal transduction through mechanical stimulation of capillaries, thereby increasing angiogenesis via vascular formation[79].
Effects of exercise intervention under hypoxic conditions on vascular health: Exercise can help prevent disease progression and protect T2DM patients from secondary complications by improving long-term increases in skeletal muscle capillarization and vascular function. Patients with diabetes develop not only abnormal angiogenesis but also macroscopic and microscopic angiogenesis with endothelial dysfunction[80]. The current meta-analysis suggested that exercise improves vascular dilation when performed under hypoxia than under normoxia[53]. Exercise under hypoxic conditions is associated with a compensatory increase in blood flow to active muscles to meet the oxygen demand[81,82]. Exercise-induced blood flow is important in inducing vascular adaptation. The combination of exercise and hypoxia can positively affect vascular adaptation in normoxic exercise training, particularly in T2DM patients who typically exhibit attenuated exercise-induced blood flow[83]. However, Schreuder et al[66] showed no effect of training on the vascular dilation in T2DM patients, both when exercise was performed in normoxia and hypoxia. These differences may be due to the different training protocols or oxygen concentrations used, and other possible adaptation mechanisms in T2DM patients[84].
The effects of hypoxia and exercise may be related to subsequent increased blood flow to muscles, and high shear stress, nitric oxide (NO), and oxygen tension reduction[81,83,85,86]. While the specific mechanisms that underlie the effects of exercising under hypoxia remain unclear, it has been demonstrated that exposure of endothelial cells to hypoxia increases[87]. In this regard, the expression and activation of endothelial NO synthase (eNOS) as a potent vascular dilator can be increased and produce eNOS and NO levels. Therefore, exercise under hypoxic conditions is thought to have high potential to improve vascular health. However, further studies are needed to confirm that long-term exercise under hypoxia can improve skeletal muscle capillarization more significantly than exercise under normoxia in T2DM patients. Previous studies can also demonstrate how training protocols should be modified to induce effective adaptation of physiological variables. Schreuder et al[66] have reported that the vascular dilation in T2DM patients could be positively affected by exercise training under short-term hypoxic conditions. However, additional studies should be conducted to apply various training protocols and oxygen concentrations.
Effects of exercise intervention under hypoxic conditions on body composition: There are positive correlations between increased fat mass, insulin-resistance, chronic inflammation, and cardiovascular disease[70]. Decreasing fat mass is one of the important goals in the treatment of overweight and obese patients with T2DM. Previous studies have reported that exercise under hypoxia can help reduce body weight and body fat mass in overweight and obese patients with T2DM[27,88,89]. Kong et al[47] showed that combined aerobics and strength training under hypoxic conditions (simulated altitude: ca. 2100-3200 m, FiO2: 14.5%-16.5%) for 4 wk (11 sessions/wk) decreased weight more than under normoxic conditions in obese young adults. Wiesner et al[69] reported that hypoxia exercise training for 4 wk improved body composition in obese men and women without diabetes and with insulin-resistance. Acute exposure to hypoxia (2 h) at a simulated altitude of 4300 m has been reported to decrease the leptin reaction to glucose uptake in healthy humans[90]. The results of previous studies may be related to changes in hormones that control appetite. However, the effects of acute exposure to hypoxia on leptin levels and appetite in T2DM patients have not been demonstrated. Therefore, a future study on appetite regulation mechanisms is needed, considering that T2DM patients have leptin resistance[91].
Effects of exercise intervention under hypoxic conditions on blood lipids and oxidative stress: Another perspective of the effect of exercise under hypoxic conditions is its effects on blood lipid variables in patients with diabetes. Simpson et al[92] examined how exposure to hypoxic conditions during moderate exercise, as well as 16 d of rest in normoxia and continuous normobaric hypoxia (simulated altitude: ca. 3400 m, FiO2: 14%), changes total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels in healthy men. Exposure to hypoxic conditions during moderate exercise significantly reduced total cholesterol, HDL, and LDL levels. Schreuder et al[66] and Wiesner et al[69] reported no positive effect of exposure to hypoxic conditions during exercise on blood lipids variables in overweight and obese patients with T2DM and insulin-resistance. To date, there have been no studies providing evidence of an effect of exposure to hypoxic conditions during exercise on blood lipids.
T2DM patients may experience increased oxidative stress as free radicals increase, further exacerbating insulin-resistance or causing cardiovascular disease[93]. However, more research is needed on whether exercise under short-term hypoxic conditions can reduce oxidative stress in T2DM patients and protect against secondary complications caused by free radicals than exercise performed under normoxic conditions. A single bout of interval training under hypoxic conditions (simulated altitude: ca. 4000 m, FiO2: 13%) has been reported to increase ventilatory responses in T2DM patients[45]. These results demonstrate the potential of such training to benefit individuals with diabetes with autonomic regulation imbalances. Future research requires verification of the effectiveness of exercise under short-term hypoxic conditions in improving blood lipid levels and oxidative stress.
This narrative review describes some health risks that may arise when exercising under hypoxic conditions. The definition of the range of oxygen-availability under which exercise can be performed under hypoxic conditions without negatively affecting health needs to be defined. Previous studies have set short-term hypoxic conditions similar to simulated altitudes of up to ca. 4000 m for healthy participants and ca. 3400 m for T2DM patients. These hypoxic conditions did not result in health problems in previous studies. However, breathing air with rapidly reduced oxygen levels or prolonged exposure to very high altitude conditions increases the risk of neurocognitive impairment, myocardial infarction, and stroke[94]. Clinical effects on the human body have not been apparent, but T2DM patients have been shown to respond to hypoxic conditions with low ventilation reactions[95]. A previous study has suggested that cardiac output and heart rate is changed in T2DM patients upon exposure to hypoxic conditions[84]. In particular, T2DM patients with neurological disorders could be negatively affected during exercise involving exposure to hypoxic conditions. In terms of body composition, exposure to long-term hypoxic conditions may facilitate reduction in body weight and fat mass, but exposure to extreme altitude (> 5000 m) has been shown to affect fat-free mass negatively[96]. The effect of increased capillarization in skeletal muscle after exercise training under hypoxic conditions requires further study, and the clinical relevance of excessive abnormal angiogenesis in diabetes needs to be shown[80].
Oxidative stress is exacerbated under hypoxic conditions by both intense and long-term exercise[97]. The actual protocol for exposure to hypoxia has varied significantly across studies in terms of cycle length (e.g., weeks), the duration of exposure (e.g., minutes and hours), the number of exposures per day (e.g., session), and the number of days. Exposure to extreme acute hypoxic conditions may be similar to the findings obtained with animal models of ischemia or reperfusion, with acute release of excessive free radicals and decreased antioxidant capacity[98]. Oxidative stress can cause cell and tissue damage and be harmful to the human body. However, redox balance changes can play a positive role as a potential stimulus for adaptation to prolonged exercise[99]. Previous studies have shown that regular moderate exercise can weaken oxidative stress associated with hypoxia[100-103]. Therefore, there is potential health risks when T2DM patients exercise under hypoxic conditions. However, its value as an effective treatment method would be marked, if appropriate safety precautions are implemented.
Short- and long-term exposure to hypoxic conditions during exercise may improve glucose uptake and insulin sensitivity in T2DM patients more than when exercising under normoxic conditions. Additionally, exercising under hypoxic conditions could help reduce body weight and fat mass in overweight and obese patients with T2DM. Several previous studies have reported positive effects of exercise training under hypoxic conditions on the bodies of T2DM patients. However, there is currently a lack of research on the long-term adverse effects of exposure to hypoxic conditions during exercise training in T2DM patients. Future studies should evaluate the potential benefits of exposure to hypoxic conditions during exercise, to design new intervention methods (normobaric hypoxia vs hypobaric hypoxia) for treating T2DM patients. Overall, exposure to hypoxic conditions during exercise in T2DM patients have the potential value of adaptation to stress stimulation in terms of clinical treatment, which can protect against pathological biology and other stresses in diabetes. Overall, the literature suggests that exposure to hypoxic conditions during exercise (simulated altitude of ca. 3000 m) is highly likely to improve the health condition of patients with diabetes. However, there is insufficient evidence for the safety of exposure to short-term hypoxic conditions during exercise in T2DM patients, and further research is needed to develop suitable interventions. Thus, exposure to hypoxic conditions during exercise should be performed with consideration of safety precautions, and patients should be advised by a medical doctor before undertaking exposure to hypoxic conditions.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang LL S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5345] [Cited by in F6Publishing: 4598] [Article Influence: 919.6] [Reference Citation Analysis (8)] |
| 2. | Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res Clin Pract. 2010;87:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Guilherme A, Henriques F, Bedard AH, Czech MP. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol. 2019;15:207-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769-E774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 277] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 7. | Bouché C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004;25:807-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda). 2005;20:271-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 917] [Cited by in F6Publishing: 920] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 10. | Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem. 2019;294:11369-11381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 11. | Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 758] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 12. | DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157-S163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1183] [Cited by in F6Publishing: 1250] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 13. | da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8:e14607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Wen L, Zhou S, Zhang Y, Wang XH, He YY, Davie A, Broadbent S. Effects of four weeks intermittent hypoxia intervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and Akt activity in skeletal muscle of obese mice with type 2 diabetes. PLoS One. 2018;13:e0203551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 17. | Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 Suppl 3:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 860] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 18. | Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 325] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ; American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42:2282-2303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 20. | Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142-2147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985). 2011;111:1554-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 22. | Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1024-E1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Gunton JE. Hypoxia-inducible factors and diabetes. J Clin Invest. 2020;130:5063-5073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 24. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3146] [Cited by in F6Publishing: 3350] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 25. | Kubota T, Kubota N, Kadowaki T. Imbalanced Insulin Actions in Obesity and Type 2 Diabetes: Key Mouse Models of Insulin Signaling Pathway. Cell Metab. 2017;25:797-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Wareham NJ, Pfister R. Diabetes: glycated hemoglobin is a marker of diabetes and CVD risk. Nat Rev Cardiol. 2010;7:367-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Brinkmann C, Bloch W, Brixius K. Exercise during short-term exposure to hypoxia or hyperoxia - novel treatment strategies for type 2 diabetic patients? Scand J Med Sci Sports. 2018;28:549-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Park HY, Kim J, Park MY, Chung N, Hwang H, Nam SS, Lim K. Exposure and Exercise Training in Hypoxic Conditions as a New Obesity Therapeutic Modality: A Mini Review. J Obes Metab Syndr. 2018;27:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 29. | Park HY, Jung WS, Kim J, Lim K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: A randomized controlled trial. Geriatr Gerontol Int. 2019;19:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Jung K, Kim J, Park HY, Jung WS, Lim K. Hypoxic Pilates Intervention for Obesity: A Randomized Controlled Trial. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Park Y, Jang I, Park HY, Kim J, Lim K. Hypoxic exposure can improve blood glycemic control in high-fat diet-induced obese mice. Phys Act Nutr. 2020;24:19-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Urdampilleta A, González-Muniesa P, Portillo MP, Martínez JA. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J Physiol Biochem. 2012;68:289-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Gerber PA, Rutter GA. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid Redox Signal. 2017;26:501-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 34. | Karwasik-Kajszczarek K, Chmiel-Perzyńska I, Robak JM, Billewicz-Kraczkowska A, Pedrycz A, Smoleń A, Kraczkowski JJ. Impact of experimental diabetes and chronic hypoxia on rat fetal body weight. Ginekol Pol. 2018;89:20-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Xi L, Chow CM, Kong X. Role of Tissue and Systemic Hypoxia in Obesity and Type 2 Diabetes. J Diabetes Res. 2016;2016:1527852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Swenson ER, Bärtsch P. High altitude. Switzerland: Springer; 2016. [Cited in This Article: ] |
| 37. | Riley CJ, Gavin M. Physiological Changes to the Cardiovascular System at High Altitude and Its Effects on Cardiovascular Disease. High Alt Med Biol. 2017;18:102-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Gradwell DP. Hypoxia and hyperventilation. 4th ed. London: Hodder Arnold; 2006: 41-56. [Cited in This Article: ] |
| 39. | Prabhakar NR, Peng YJ, Kumar GK, Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol. 2015;5:561-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Amann M, Kayser B. Nervous system function during exercise in hypoxia. High Alt Med Biol. 2009;10:149-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Kayser B. Nutrition and energetics of exercise at altitude. Theory and possible practical implications. Sports Med. 1994;17:309-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Meeuwsen T, Hendriksen IJ, Holewijn M. Training-induced increases in sea-level performance are enhanced by acute intermittent hypobaric hypoxia. Eur J Appl Physiol. 2001;84:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Viscor G, Torrella JR, Corral L, Ricart A, Javierre C, Pages T, Ventura JL. Physiological and Biological Responses to Short-Term Intermittent Hypobaric Hypoxia Exposure: From Sports and Mountain Medicine to New Biomedical Applications. Front Physiol. 2018;9:814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Lüneburg N, Siques P, Brito J, Arriaza K, Pena E, Klose H, Leon-Velarde F, Böger RH. Long-Term Chronic Intermittent Hypobaric Hypoxia in Rats Causes an Imbalance in the Asymmetric Dimethylarginine/Nitric Oxide Pathway and ROS Activity: A Possible Synergistic Mechanism for Altitude Pulmonary Hypertension? Pulm Med. 2016;2016:6578578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Duennwald T, Gatterer H, Groop PH, Burtscher M, Bernardi L. Effects of a single bout of interval hypoxia on cardiorespiratory control and blood glucose in patients with type 2 diabetes. Diabetes Care. 2013;36:2183-2189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E546-E555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Kong Z, Zang Y, Hu Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 2014;18:591-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | González-Muniesa P, Quintero P, De Andrés J, Martínez JA. Hypoxia: a consequence of obesity and also a tool to treat excessive weight loss. Sleep Breath. 2015;19:7-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Serebrovska TV, Portnychenko AG, Drevytska TI, Portnichenko VI, Xi L, Egorov E, Gavalko AV, Naskalova S, Chizhova V, Shatylo VB. Intermittent hypoxia training in prediabetes patients: Beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp Biol Med (Maywood). 2017;242:1542-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Hobbins L, Hunter S, Gaoua N, Girard O. Normobaric hypoxic conditioning to maximize weight loss and ameliorate cardio-metabolic health in obese populations: a systematic review. Am J Physiol Regul Integr Comp Physiol. 2017;313:R251-R264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Mackenzie RW, Watt P. A Molecular and Whole Body Insight of the Mechanisms Surrounding Glucose Disposal and Insulin Resistance with Hypoxic Treatment in Skeletal Muscle. J Diabetes Res. 2016;2016:6934937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Verges S, Chacaroun S, Godin-Ribuot D, Baillieul S. Hypoxic Conditioning as a New Therapeutic Modality. Front Pediatr. 2015;3:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Montero D, Lundby C. Effects of Exercise Training in Hypoxia Versus Normoxia on Vascular Health. Sports Med. 2016;46:1725-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Mallette MM, Stewart DG, Cheung SS. The Effects of Hyperoxia on Sea-Level Exercise Performance, Training, and Recovery: A Meta-Analysis. Sports Med. 2018;48:153-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Storz JF, Scott GR. Life Ascending: Mechanism and Process in Physiological Adaptation to High-Altitude Hypoxia. Annu Rev Ecol Evol Syst. 2019;50:503-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 56. | Park HY, Shin C, Lim K. Intermittent hypoxic training for 6 wk in 3000 m hypobaric hypoxia conditions enhances exercise economy and aerobic exercise performance in moderately trained swimmers. Biol Sport. 2018;35:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Jung WS, Kim SW, Park HY. Interval Hypoxic Training Enhances Athletic Performance and Does Not Adversely Affect Immune Function in Middle- and Long-Distance Runners. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Park HY, Jung WS, Kim J, Hwang H, Kim SW, An Y, Lee H, Jeon S, Lim K. Effects of 2-Week Exercise Training in Hypobaric Hypoxic Conditions on Exercise Performance and Immune Function in Korean National Cycling Athletes with Disabilities: A Case Report. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Azevedo JL Jr, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44:695-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT. Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol (1985). 1991;70:919-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 176] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Lippl FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring). 2010;18:675-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1262] [Cited by in F6Publishing: 1305] [Article Influence: 163.1] [Reference Citation Analysis (1)] |
| 63. | Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27:94-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Goda N, Kanai M. Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol. 2012;95:457-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 65. | Katayama K, Goto K, Ishida K, Ogita F. Substrate utilization during exercise and recovery at moderate altitude. Metabolism. 2010;59:959-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Schreuder TH, Nyakayiru J, Houben J, Thijssen DH, Hopman MT. Impact of hypoxic vs normoxic training on physical fitness and vasculature in diabetes. High Alt Med Biol. 2014;15:349-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Haufe S, Wiesner S, Engeli S, Luft FC, Jordan J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med Sci Sports Exerc. 2008;40:1939-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Lecoultre V, Boss A, Tappy L, Borrani F, Tran C, Schneiter P, Schutz Y. Training in hypoxia fails to further enhance endurance performance and lactate clearance in well-trained men and impairs glucose metabolism during prolonged exercise. Exp Physiol. 2010;95:315-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Wiesner S, Haufe S, Engeli S, Mutschler H, Haas U, Luft FC, Jordan J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity (Silver Spring). 2010;18:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 71. | Eriksson KF, Saltin B, Lindgärde F. Increased skeletal muscle capillary density precedes diabetes development in men with impaired glucose tolerance. A 15-year follow-up. Diabetes. 1994;43:805-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Lundby C, Calbet JA, Robach P. The response of human skeletal muscle tissue to hypoxia. Cell Mol Life Sci. 2009;66:3615-3623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Mizuno M, Savard GK, Areskog NH, Lundby C, Saltin B. Skeletal muscle adaptations to prolonged exposure to extreme altitude: a role of physical activity? High Alt Med Biol. 2008;9:311-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001;204:3133-3139. [PubMed] [Cited in This Article: ] |
| 75. | Colville-Nash PR, Willoughby DA. Growth factors in angiogenesis: current interest and therapeutic potential. Mol Med Today. 1997;3:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Brinkmann C, Metten A, Scriba P, Tagarakis CV, Wahl P, Latsch J, Brixius K, Bloch W. Hypoxia and Hyperoxia Affect Serum Angiogenic Regulators in T2DM Men during Cycling. Int J Sports Med. 2017;38:92-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-4613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2798] [Cited by in F6Publishing: 2834] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 78. | Bento CF, Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54:1946-1956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 331] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 81. | Casey DP, Joyner MJ. Compensatory vasodilatation during hypoxic exercise: mechanisms responsible for matching oxygen supply to demand. J Physiol. 2012;590:6321-6326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 82. | González-Alonso J. ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J Physiol. 2012;590:5001-5013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 84. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1571] [Cited by in F6Publishing: 1566] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 85. | Hellsten Y, Hoier B. Capillary growth in human skeletal muscle: physiological factors and the balance between pro-angiogenic and angiostatic factors. Biochem Soc Trans. 2014;42:1616-1622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA. 2005;102:13147-13152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 87. | Presley T, Vedam K, Velayutham M, Zweier JL, Ilangovan G. Activation of Hsp90-eNOS and increased NO generation attenuate respiration of hypoxia-treated endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1281-C1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Westerterp KR, Kayser B, Wouters L, Le Trong JL, Richalet JP. Energy balance at high altitude of 6,542 m. J Appl Physiol (1985). 1994;77:862-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Millet GP, Debevec T, Brocherie F, Malatesta D, Girard O. Therapeutic Use of Exercising in Hypoxia: Promises and Limitations. Front Physiol. 2016;7:224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 90. | Kelly KR, Williamson DL, Fealy CE, Kriz DA, Krishnan RK, Huang H, Ahn J, Loomis JL, Kirwan JP. Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism. 2010;59:200-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 92. | Simpson EJ, Debevec T, Eiken O, Mekjavic I, Macdonald IA. PlanHab: the combined and separate effects of 16 days of bed rest and normobaric hypoxic confinement on circulating lipids and indices of insulin sensitivity in healthy men. J Appl Physiol (1985). 2016;120:947-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Watson JD. Type 2 diabetes as a redox disease. Lancet. 2014;383:841-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 94. | de Mol P, de Vries ST, de Koning EJ, Gans RO, Bilo HJ, Tack CJ. Physical activity at altitude: challenges for people with diabetes: a review. Diabetes Care. 2014;37:2404-2413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Weisbrod CJ, Eastwood PR, O'Driscoll G, Green DJ. Abnormal ventilatory responses to hypoxia in Type 2 diabetes. Diabet Med. 2005;22:563-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Wagner PD. Operation Everest II. High Alt Med Biol. 2010;11:111-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Quindry J, Dumke C, Slivka D, Ruby B. Impact of extreme exercise at high altitude on oxidative stress in humans. J Physiol. 2016;594:5093-5104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 98. | Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Sachdev S, Davies KJ. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 100. | Debevec T, Pialoux V, Mekjavic IB, Eiken O, Mury P, Millet GP. Moderate exercise blunts oxidative stress induced by normobaric hypoxic confinement. Med Sci Sports Exerc. 2014;46:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Debevec T, Pialoux V, Ehrström S, Ribon A, Eiken O, Mekjavic IB, Millet GP. FemHab: The effects of bed rest and hypoxia on oxidative stress in healthy women. J Appl Physiol (1985). 2016;120:930-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | Debevec T, Millet GP, Pialoux V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front Physiol. 2017;8:84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 103. | Boccatonda A, Tripaldi R, Davì G, Santilli F. Oxidative Stress Modulation Through Habitual Physical Activity. Curr Pharm Des. 2016;22:3648-3680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |