1. Introduction
The taxonomic identity of the small African Barbus (Presently known as Enteromius) [2] species remains unresolved [3]. However, the uncertainty around the taxonomy of the genus used to be deeper, as the genus formerly consisted of more unrelated members from Europe, Asia, and the Mediterranean compared to the present composition. The earlier composition was due to the morphological criteria used which turned out to be of less systematic value. The criterion used to group the species were possession of two pairs of barbels and the presence/absence of ossified and serrated rays in the dorsal fin [4].
On the basis of body size, African Barbus are generally recognized as either large or small. Large Barbus is characterized by an adult body size greater than 20 cm standard length (SL) and the presence of parallel or converging striae on their scales. In contrast, small African Barbus usually reach an adult size of less than 20 cm SL and have divergent scale striae [5]. Studies by [5] [6] and [7] showed that the small and large African Barbus are distantly related to each other; and that the large African Barbus is closely related to the European Barbus while the small African Barbus is related to Asian Barbus genus Puntius and allied genera.
Karyological data became a valuable tool in understanding the internal relationships within the small African Barbus when [4] successfully divided the members into either diploid or tetraploid lineages, although, the third lineage of African hexaploid Barbus was later reported by [7]. The small African Barbus was found to be diploid while the large African Barbus were either tetraploid or hexaploid. [8] confirmed the groupings made along ploidy lines, thereby further demonstrating the importance of karyological data in Barbus taxonomy [9]. [8] proposed the revalidation of the genusEnteromius to accommodate all African diploid “Barbus” species.
Despite the importance of karyological data in the taxonomy of the genus, such data are scarce, as it is only available for very few of the 300 African Barbus species recognized [10]. There are about 24 small African Enteromius species in Nigeria [11] but only the karyotypes of 3 have been assessed [12]. This study assessed the karyotype of E. parablabes [1].
2. Materials and Methods
Samples of Enteromius parablabes were collected from Aho stream, (7˚31'23.7"N and 4˚31'44.5"E) using frame nets and fish traps and kept alive in sets of aquaria at the Department of Zoology, O.A.U., Ile-Ife, Osun State. The identity of E. parablabes was determined based on diagnostic characters provided by [11]. The diagnostic characters are: two pairs of barbels; the anterior pair may extend beyond the anterior margin of the eye, and the posterior may reach to, or extend beyond, the posterior eye margin. The standard scale count of E. parablabes is 3.5 scales between lateral line and origin of the first dorsal fin; 24 to 26 scales on the lateral line; 3.5 to 4.5 scales between lateral line and ventral body profile; 2.5 scales between lateral line and pelvic-fin base and 12 scales around caudal peduncle. It also has a dorsal spine count of three and a dorsal fin ray of eight. The anal fin spine is equally three and the anal fin ray is five. The pectoral fins are 15 while the pelvic fins spine and ray are one and between 7 and 8 respectively. In terms of general description, its dorsal profile is convex; the head slightly is rounded while the mouth is sub-terminal. The lateral line is complete and covered by the black longitudinal band that runs from the gill cover to the end of the caudal peduncle.
The sexes of the samples were determined majorly by cutting them opened and examining the gonads. Cell division was arrested by injecting the fishes with 0.02 ml of colchicine per gram wet weight. The specimens were sacrificed three hours after the colchicine treatment and the gills removed. The tissues from the specimen of each sex were treated separately. The tissues excised were placed in a hypotonic solution of 0.56% KCl for 30 minutes. The pellets were suspended in freshly prepared Carnoy’s fixative. Cell suspension was dropped on a clean, cold and wet glass microscope slide and dried on Photax Dish warmer 2a Model slide warmer set at a temperature of 60˚C for about 24 hours. The cells were stained with 6% stock Giemsa stain. The slides were viewed under the Omax G013055005 Model trinocular light microscope while photomicrography of the spreads were done using Omax A3514OU Model camera attached to the microscope. The morphology, length of each chromosome and the idiogram were determined using Karyotype software (Version 2.0). Chromosomes were classified according to centromere position [13] as metacentric (m), submetacentric (sm) and telocentric (t) and subtelocentric (st). Metacentric and submetacentric chromosomes are grouped together as metacentric while telocentric and subtelocentric are grouped together as telocentric.
3. Results
The chromosome spread obtained for the male and female E. parablabes is shown in Plate 1 and Plate 3 while Plate 2 and Plate 4 shows their karyotype respectively. The diploid chromosome number of both is 50 while the autosomal fundamental number (NFa) for the male and female is 81 and 98 respectively. The chromosome nomenclature shows that the male’s chromosomes 1 - 10 are metacentric; 11, 12, 21, 22, 25 - 28, 31 - 34, 39, and 43 - 50 are sub-telocentric while 13 - 20, 23, 24, 29, 30, 35 - 38, and 40 - 42 are telocentric. On the other hand, chromosomes 1, 3 - 6, 9 - 17, 19 - 24, 27 - 35, and 37 - 46 of the female are metacentric; 2, 7, 8, 18, 25, 26, 36 are sub-metacentric; 49 and 50 are sub-telocentric while chromosomes 47 and 48 are telocentric. The morphology of the chromosomes of the male and female sexes in form of an ideogram is presented in Figure 1 and Figure 2 respectively.
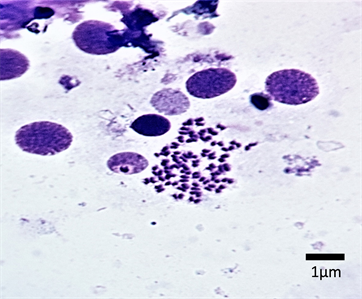
Plate 1. Mitotic chromosomes spread of male E. parablabes (2n = 50).
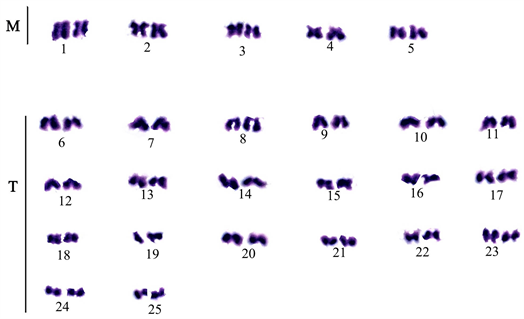
Plate 2. Karyotype of E. parablabes male.
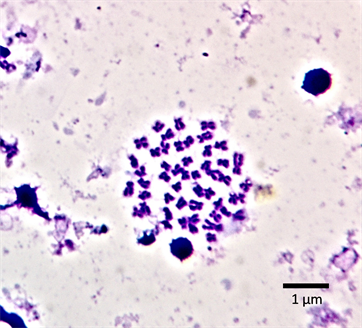
Plate 3. Mitotic chromosomes spread of female Enteromius parablabes (2n = 50).
![]()
Plate 4. Karyotype of Enteromius parablabes female.
![]()
Figure 1. An ideogram of the karyotype of male Enteromius parablabes.
![]()
Figure 2. An ideogram of the karyotype of female Enteromius parablabes.
4. Discussion
The diploid chromosome number of 50 exhibited by both sexes of the E. parablabes is consistent with the reported chromosome number of the species in the genus Enteromius. Studies undertaken so far regarding the karyotype of cyprinids including the genus Enteromius, have shown a very low variability of their chromosome number (48 and 50) [14] as the majority of the species examined presented a diploid number of 50. The high level of conservation of the karyological pattern of the majority of the cytogenetically analyzed fishes is, however, a great departure from their speciation and high morphological diversity [15].
In addition, karyotyped diploid cyprinids have been found to be mostly made up of small-sized chromosomes and this makes identifying their morphological orientations difficult [16]. Another challenge in karyotyping cyprinid species is chromosome arm contraction due to temporal and dose colchicine treatment (eg variations from 0.02 ml of colchicine per gram wet weight) [17]. Like other cyprinid species, the chromosomes of the E. parablabes, both male and female were very small, and karyotyping was difficult. Our final karyotype for the male E. parablabes was arrived at with great difficulty as some of the chromosomes were too small for us to assign them to a precise category. However, we suspect that more of the chromosome of the male E. parablabes will ultimately have their centromere at the terminal region. On the other hand, the chromosomes of the female E. parablabes are clearer and legible. The female chromosomes are mostly (92%) with their centromere at the median region, while only a few (8%) have their centromere at the terminal region. This karyotypic composition of both male and female E. parablabes falls within the range described for cyprinid fishes. The composition of the karyotype of cyprinids consists of the centromere positions being placed gradually from a median point to a terminal point [14] [17] [18]. A typical karyotype for the cyprinids consists of 6 - 8 pairs of metacentric chromosomes (m), 12 - 17 pairs of submeta- and subtelocentric chromosomes (sm, st), and 3 - 4 pairs of acrocentric chromosomes
Majority of cyprinids, including small African barbs present karyotypes rich in metacentric and sub-metacentric chromosomes [14] [19]. However, a telocentric rich karyotype was reported for B. callipterus by [20]. The differences in karyotype composition of the male and female E. parablabes could have been precipitated by chromosomal rearrangements, such as centric fusions and pericentric inversions, which have played an important role in karyotype evolution. Such chromosomal evolution has been shown to lead to numerous chromosomal rearrangements in the position of the centromere on the chromosome and in chromosome numbers. The incongruence between the chromosome morphology of the male with previous reports might also be attributed to population differences. Similar differences in the karyotype of male and female fish species have been reported by [21]. However, a distinctly large metacentric chromosome suspected to be a marker element for the small African Barbus [17], was found in both the male and female chromosome spread.
Sexual dimorphism at the chromosome level has been characterized among organisms [22]. There are the XX and XO, XY and XX and ZZ, and ZW types. In the XY and XO types, XX is female while XY is male. In this present study, both sexes have the same diploid chromosome number of 2n = 50 but although the earlier report did not find distinguishable sexual dimorphic chromosomes in Enteromius species, the result of this study suggests otherwise. Two chromosomes, which are telocentric and acrocentric in the female, are thought to be sex chromosomes. However, caution is generally suggested in the determination of sexual systems in cyprinids due to their characteristic small-sized chromosomes [17] (Rab et al., 1995). A more advanced cytogenetic approach like chromosome painting and banding techniques are therefore suggested to confirm the sexual dimorphism of E. parablabes at the chromosome level.