1. Introduction
The synthesis of ferrate (VI) appears to be very delicate due to the instability conferred by their high oxidizing power. Although the existence of alkali ferrate is cited for a century [1] [2] , they have not been the subject of a considerable number of studies, because of the instability and difficulties encountered during their preparation.
The synthesis of iron (VI) was studied by several authors [3] -[12] . But the importance of the reactions for the preparation of dry ferrates lies mainly in their ability to produce alkaline ferrate (IV) or (VI) directly from cheap and easily available products (such as iron oxide) without complicated electrochemical processing or preliminary preparation of reagents [13] . Martinez-Tamayo et al. [14] have studied the performances and conduct of the Na2O2-FeSO4 system; their study includes the results obtained from the use of infrared spectrometry, X-ray diffraction and differential thermal analysis. They obtained ferrate (V) and (VI), the nature of which depends on the molar ratio of the initial reactants. Kisselev et al. [15] have studied extensively the Na-Fe-O system and have shown that we could prepare the ferrate (IV) of pure sodium Na2FeO3 formula and ferrate (VI) of formula Na4FeO5. In their study, they obtained sodium ferrate Na2FeO3 heating the mixture of Na2O2-Fe2O3 in oxygen at a temperature of 400˚C, the molar ratio Na/Fe leading to better results is 2.
Kopelev et al. [16] prepared the sodium ferrate Na4FeO5 and Na2FeO3 according to the procedure of Kiselev et al. [15] . The ferrates (VI) were also prepared from galvanized waste [17] : the waste was mixed with ferric oxide in an oven at 800˚C. The sample was cooled and mixed with sodium peroxide solid, and then gradually heated for a few minutes. Among the methods of synthesis by electrochemical and wet means, the dry method avoids the ferrate reaction with water. This preparation process of ferrate is considered a green technology recycling of various iron waste compounds [18] .
This work aims at preparing ferrate (VI) (Na2FeO4) stable at dry ambient optimizing the parameters influencing the phase synthesis and stability performance at room temperature to reduce costs, and facilitate storage and transportation for a long period.
2. Materials and Methods
Pure Fe2O3 and Na2O2 were mixed in a platinum crucible to avoid side reactions. The resulting mixture was placed in an oven at a temperature of 700˚C during 13 hours. The molten mixture was cooled in a ball desiccator to avoid moisture absorption and above we worked at a temperature of 700˚C. According to Jiang and Lloyd [19] , the dry synthesis at a temperature above or equal to 500˚C seems unconvincing because of the explosion of the reaction medium and makes the synthetic route very dangerous at high temperature. The found phase was analyzed and monitored over time with UV spectrophotometry by measuring the optical density at 507 nm.
According to Sapin et al. [20] , measuring the optical density of the solution of ferrate (VI) is performed at the wavelength 507 nm with a pH greater than 10.
The characteristic peak of iron (VI) comes out at this wavelength.
The synthesis reaction is the following:

3. Results
The measurement of the optical density of the solution of ferrate (VI) synthesized in stage Na2FeO4 has a wavelength of 507 nm depending on the ratio of Na/Fe and it gives an idea about the assessment of the synthesis reaction (Figure 1).
According to the curve (Figure 1), we see that the optical density of the resulting ferrate (VI) increases with the Na/Fe ratio till the peak of value of 4 with OD = 2.65, which implies a change in the performance of ferrate (VI) that depends on the Na/Fe ratio.
From this curve (Figure 2), we see that the optical density of ferrate (VI) increases with temperature up to 700˚C which is an optimum temperature for the production of stable at ambient ferrate (VI) with a substantial output of a synthesis reaction whose optical density is of the order of 2.60.
According to these results (Figure 3), the optical density peaks at 2.59 for a period of 13 h. This explains the importance of duration of the synthesis reaction in the production of stable at ambient iron (VI) as well as in its performance.
4. Monitoring Ferrate Degradation throughout Time
The results of ferrate degradation monitoring over time is shown in the following Figure 4.
According to these results (Figure 4), it is found that after optimization of parameters essential for the production of ferrate (VI) is stable in ambient dry, the period of storage up to 13 months.
The degradation rate of iron (VI) in the first six months does not exceed 20.53%.
The relation used to calculate the percentage of degradation of iron (VI) is given by the following formula:
![]()
Figure 1. Optical density of the solution of ferrate (VI) (Na2FeO4) at 507 nm versus Na/Fe ratio (t = 13 h, T = 700˚C).
![]()
Figure 2. Optical density of the solution of ferrate (VI) (Na2FeO4) at 507 nm versus temperature (Na/Fe = 4, t = 13 h).
![]()
Figure 3. Optical density of the solution of ferrate (VI) (Na2FeO4) at 507 nm versus time (T = 700˚C and Na/Fe = 4).
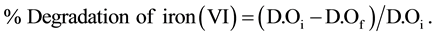
D.Oi: Optical density of iron (VI) in the initial state.
D.Of: Optical density of iron (VI) in the final state.
The calculation of the rate of degradation between the months and the status of the production of ferrate (VI) and different months of storage is given by the following Table 1.
From these results (Table 1), we can deduce that the rate of degradation of iron (VI) remains variable over time and varies differently from one month to the other during storage, which means that climate change impacts the degradation rate of ferrate (VI) due to variations in humidity.
![]()
Figure 4. Optical density of the solution of ferrate (VI) (Na2FeO4) to 507 nm depending on the month of storage at room temperature.
![]()
Table 1. Optical density of the synthesized phase Na2FeO4 ferrate (VI) solution based on the rate of degradation between the initial state of the production and the different months of storage of ferrate (VI) (%) and on the rate of degradation between month storage ferrate (VI) (%).
5. Discussion
According to our results, the Na/Fe ratio required for the synthesis of stable at room iron (VI) for significant performance is of the order of 4 (Figure 1), as is consistent with the results of different prior studies [11] [12] , which shows that the Na/Fe ratio is ideal for the synthesis of iron (VI) by a dry process is greater than 2.
The optimum temperature (T = 700˚C) for the synthesis of ferrate (VI) (Figure 2) represent a positive step forward towards better development of industrial processes for the production of ferrate (VI). This result confirms studies done by Martinez-Tamayo et al. [11] .
The reaction time of the synthesis (t = 13 h) is an important parameter for the production of dry stable at ambient ferrate (VI) [12] . And also climate change impacts the degradation rate and the duration of at room temperature storage of ferrate (VI).
6. Conclusions
This manuscript reviews the most appropriate steady method for the synthesis of (Na2FeO4) alkaline ambient ferrate (VI) from the reaction of Fe2O3 with Na2O2 with Na/Fe ratio = 4, a temperature of 700˚C and a reaction duration of about 13 hours.
The method of synthesis of dry ferrate (VI) is a very easy and very promising method, although there is still a need for more technical and economic improvements on the implementation of industrial policy.
NOTES
*Corresponding author.