The Composition and Structure of Reef Community at Tho Chu Island (South China Sea) after Ketsana Typhoon ()
1. Introduction
Coral reefs are under threat worldwide. An estimated 58% of reefs are classified as threatened [1], and 11% of the original extent of coral reefs have already been lost [2]. The composition of remaining coral reefs is also changing rapidly. For example, coral cover on reefs across the Caribbean has decreased by 80% in the past three decades [3], and some formerly abundant coral species have almost disappeared from the region [4]. The causes of coral decline are thought to include a combination of direct anthropogenic factors, such as overfishing, pollution, and sedimentation [5]. Hurricanes and tropical storms are perhaps the most obvious and frequent natural disturbances affecting reef communities. They have long been recognized as being important determinants of both the structure and function [6-8] of reef ecosystems. A number of studies have documented the severe immediate consequences of hurricane impacts at single sites in terms of reduced coral cover [9,10], highlighting the effects as being impressive in magnitude, speed, and patchiness.
Vietnamese island of the Gulf of Thailand for the most part is open all year co wind waves in all directions. Their slopes are formed by underwater boulders and boulder heaps of moving from deep stony deposits, and on to the platform of sand and silt. Openness and vulnerability of islands, shallow water of the coastal zone make them prone to intense excitement even during strong winds. In times of typhoons, such excitement leads to severe physical damage and destroying fragile coral colonies falling asleep and their bottom sediments. This peculiarity of the coastal geomorphology and hydrology of the region affects the formation of his few reefs, which are also often subject to strong typhoons. So in October 2009, the Vietnam landfall of typhoon “Ketsana”, which reached gusts of wind up to 165 km/h corresponded to category 2 on a scale Saffir-Simpson Hurricane. Typhoon was accompanied by heavy rainfall (about 200 mm of rain), and the excitement of the sea more than two meters tall. After comparable in strength typhoon corals are destroyed at depths greater than 12 m—60% to 80%—between 12 m and 30 m and 100%—beyond 35 m, where as earlier living coral cover age ranged from 60 to 75% in these zones [11]. As is well known, flows of storm (flash flood) of wastewater and desalination are characterized by strong turbidity due to the large number of different particulate matter, which have a detrimental effect on the existence of coral communities, leading to their partial and sometimes total loss [12,13]. Most of the reefs of the island Tho Chu were virtually destroyed. When the coastal zone at depth of 5.3 m, 40% of its corals were destroyed; at depth of 5 - 8 m in the settlement of Acropora—100%; at depth of 8 - 12 m—60%; 5.3 m, 40% of its corals were destroyed; at depth of 5 - 8 m in the settlement of Acropora—100%; at depth of 8 - 12 m—60%; at depth of 12 m—10%. In this regard, it is appropriate to go back briefly to the information about the state of these reefs of quarter century ago.
First reefs in this region have been studied in detail in the joint Soviet-Vietnamese expeditions in 1986 and 2007, and then World Wildlife Fund (WWF) in 1992- 1993 [14-17]. There are clarified species composition, population density of dominant species of macrobenthos and the degree of coverage of the substrate corals and macrophytes. Through identifying these reefs based on the data points of the coral community, it shows that the reefs are quite satisfactory and similar with those of North and South Vietnam and the Indo-Pacific [18-21].
Tho Chu Island (9˚18'N, 103˚28'E), together with surrounding rocks and small islets is about 11 km in diameter (Figure 1). The relief of the island represents a high plateau bordered by steep abrasion denudation slopes. It is a remnant of a large sub platform structure destroyed in the Pleistocene because of a tectonic immersion of the bottom in the Gulf of Siam. The island consists of coarse layered sub horizontal sandstones and conglomerates of coastal-marine genesis, which is evidenced by well-rolled gravels, shoestring distribution of pebbles, and the pattern of rhythmic and textured sediments that is characteristic of coastal shallows. The development of the ruinedrocky submarine relief is, to a significant degree, due to these geomorphologic peculiarities. The submarine slopes represent boulder-block bottoms transforming in deeper depths into stony and gravel deposits, which, in deeper depths, replaced by sandy-corallogenic deposits with great amounts of organic detritus. The northern and northeastern slopes have ratios of 0.05 - 0.08 going down to a platform at a depth of 35 - 40 m. In the south and southeast, the slope ratio is 0.032 - 0.044 and the slope transforms into the platform at a depth of 23 - 25 m. An ingressive sandy bay with a slope ratio of 0.026 and a wide zone of wave accumulation in the innermost part is set into the western coast.

Figure 1. Schematized map showing the location of Tho Chu Island (a) and the position of transects 1-4 (b).
The re-examination reefs in 2010 and 2013 on the same cuts as in the 80 s of the last century have been found significant changes in the composition and structure of reef communities. Therefore, these results of the studied reefs in the region, in our view, are interesting, and as an independent study, they can also be used as material for comparison with the changes taking place on the reefs not only in the central and southern Vietnam, but also throughout the entire Pacific.
2. Materials and Methods
Using SCUBA equipment, we investigated the composition, distribution of scleractinian corals and mass species of macrobenthos, structure of communities in reef zones at four sections in sandy and stony inlets and near rocky coasts (Figure 1). Investigations were carried out in accordance with the standard hydrobiological technique using quadrats and transects [22,23]. Abundance of mass species of mollusks and echinoderms, branched, massive, encrusted and funnel-shaped scleractinian colonies, as well as the degree of substrate cover by corals were estimated along a 150 m transect frame divided into 100 squares with the area of 10 cm2 each. Photographing of reef landscapes, and their flora and fauna was conducted. More than 750 photos by Olympus and Lumix digital cameras were made for later analysis of species composition [24, 25] and structure of community of coral reef survey methods [26-28]. Coefficients of species diversity corals were calculated by the formula: 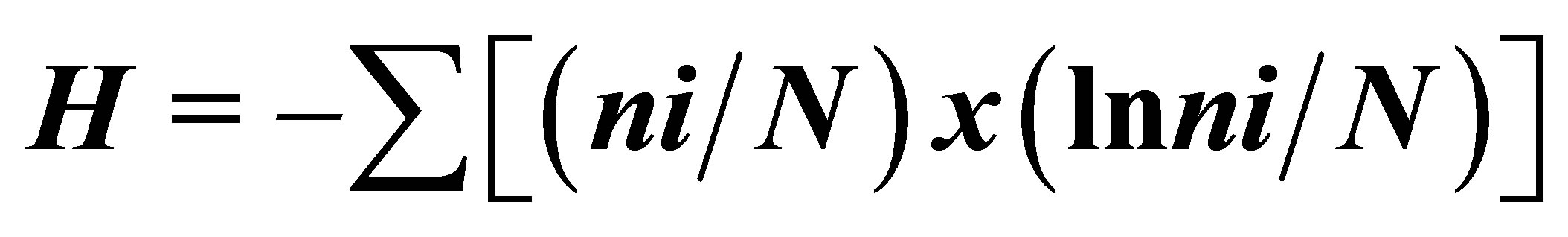 , where H—Shannon Diversity Index, ni—number of individuals belonging to i species, N—total number of individuals [29].
, where H—Shannon Diversity Index, ni—number of individuals belonging to i species, N—total number of individuals [29].
3. Results and Discussion
In 1986, the diversity of species on different reefs varied from 335 to 387 species, of which 275 species in total were scleractinian. Ubiquitous and often dominated by 37 species: sponge Petrozia testudinaria; corals Sarcophyton trocheliophorum, Sinularia dura, Lobophytum pauciflorum, Junceella fragilis, Seriatopora hystrix, Pocillopora damicornis, Acropora nobilis, A. cytherea, Pavona decussata, Pachyseris rugosa, Diaseris distorta, Polyphyllia talpina, Galaxea fascicularis, Lobophyllia hemprichii, L. hattai, Goniopora stokesi, Platygyr daedalea, Diploastrea helioporaи Millepora platyphylla; mollusks Cyprea tigris, Beguina semiorbiculata и Malleus malleus; echinoderms Holothuria atra, H. edulis, Stichopus variegatus, Bohadshia graeffei, Diadema setosum, Echinotrix diadema, Culcita novaeguineae и Linckia laevigata; polychaetes Spirobranchus giganteus; and algae Turbinaria ornata, Padina australis, Laurencia papilosa, Caulerpa racemosaи Sargassum duplicatum.
Distribution of certain dominant species or groups of the same species in different zone of the same reefs revealed several macrobenthos of communities. The composition and characteristics of formation of which are discussed below.
In the coastal zone of the studied island, except the southern sandy bay, a coral formed polyspecific community arises extending through 15 - 35 m from the shoreline to a depth of 2 m. Usually, in this area, there are separate spots of settlements with an area of up to a few tens of square meters. In terms of frequency of occurrence (75% - 100%) predominated scleractinian P. daedalea, P. verrucosa, Acropora millepora, A. robusta and hydroid M. platyphylla, were also distributed to individual colonies and settlements of soft corals S. trocheliophorum (biomass of 3050 g/m2) and L. pauciflorum (2680 g/m2). The largest coral cover substrate does not exceed 40%. Constant components of the coral community were algae L. papilosa and T. ornata, with a predominance of the first of them (up to 376 spec./m2 at biomass 3516 g/m2), clam C. arabica, echinoderms D. setosum and N. atra. A polychetes S. giganteus (up to 179 spec./m2) is always present in the branches of the colonies hydroid M. platyphylla.
The coastal polyspecific coral-algal community is replaced by a community of Acropora + Diploastrea, which develops in the area from the reef front zone down to the lower part of reef slope, extending for 50 - 100 m along the slope, in depths of 2 to 15 m deep. No absolute domination of any Acropora species is observed there. This community represents extended spots of solid populations, with 100% substrate coverage by one of the following species: A. nobilis, A. cytherea, A. rnicrophthalma, A. divaricata, or A. florida. The subdominants are D. heliopora (large massive colonies up to 2 - 3 m in diameter) and encrusting-lamellate colonies of Euphyllia orphensis (up to 2 m in diameter); they encountered in 70% - 100% of cases. This community is characterized by rich taxonomic diversity of scleractinian (more than 60% of the total species diversity). Each of the genera Montipora, Pontes, Fungia, Symphyllia, Favia, Favites, Pavona, Echinophyllia, Montastrea, and Lobophyllia is often represented there by several species. The degree of substrate coverage by corals totals, as a rule, more than 60% and reaches 100% in the spots of monosettlements. In the windward reefs of the northern side of the island, in the community of Acropora + Diploastrea, on a submerged terrace, a facies of A. nobilis + A. microphthalma has developed with almost solid coverage by substrate by the corals of these two species.
A community of Junceella fragilis + Diaseris distorta develops in gravel-sandy bottoms with numerous small coral fragments, at a depth of 15 - 18 m. The basis of this community is constituted by gorgonian J. fragilis and single mushroom-shaped corals D. distorta that dominate all other macrobenthos species by population density (18 and 20 spec./m2 respectively). Besides the dominating species, constant components of this community are other species:corals Verrucella umbraculum, S. dura, Cycloseris costulata, Fungia fungites, and Polyphyllia talpina, sponge P. testudinaria, echinoderms B. graeffei, H. edulis and Toxopneustes pileolus. Among the associated macrobenthos, the corals Turbinaria peltata, S. hystrix, Goniopora stokesi, Leptastrea pruinosa, and Favia speciosa; holothurians S. variegates and Halodeima edulis, seastar Culcita novaeguineae and gastropods Cyprea tigris and Cassis cornuta are most often encountered.
A community of the reef slope is characterized by a great degree of substrate coverage (75% - 100%) and the greatest species diversity of macrobenthos (about 70% of the registered species diversity), which is characteristic of the reef zone in the Indo-Pacific [14,20,30-35]. It is formed in the depth of 3 - 12 m and extends for 120 - 150 m along the reef slope. The upper part of the slope, as in the reef flat, is dominated by A. cytherea, A. hyacinthus, and A. nasuta. Large lamellate colonies (1.2 - 2 m in diameter) of M. aequituberculata and M. hispida distributed amongst the colonies of these three species. The area of coverage by monospecific settlements of P. rus and large colonies of D. heliopora increases up to 30% - 40% in the middle part of the reef slope. The diversity of other scleractinian species (faviids, fungiids, and encrusting-lamellate colonies of the genera Euphyllia, Echinipora, Pachyseris, Micedium, and Merulina) increases and reaches 147 species. The dominance of any coral species is not observed in the lower part of the slope. A significant role-played in this part of the reef by funnel form and encrusting species and colonies with rather large corallites is represented by different species of the genera Turbinaria, Pavona, Euphyllia, Physogyra, Galaxea, Lobophyllia, and Symphyllia. The degree of substrate coverage by corals falls down to 20% - 30%. In the massive colonies of the corals Porites, Platygyra, and Astreopora, there are settlements of mollusks B. semiorbiculata, A. ventricosa, and P. pinguin with the mean density of 2.8 - 4.2 spec./m2 and the domination of A. ventricosa (up to 10.9 spec./m2, with the biomass of 455.6 g/m2). The oyster L cristagalli (0.5 spec./m2) and the mollusk T. squamosa (1.5 - 2.0 spec./m2) are continuously encountered. The sea urchins D. setosum and E. diadema and holothurians S. variegatus and B. graejfei are common in the community of the reef slope in this part of Tho Chu Island, as well as in the communities described above.
Typhoon caused significant damage of richness scleractinian species declined by almost a third (from 275 to 95 species). There are decreased by 2 - 3 times of substrate coral cover as well as an index of species diversity (Figures 2 and 3). The number of related corallobionts: clams, gastropods, sea cucumbers and sea stars, sea urchins, with the exception of D. setosum, E.


Figure 2. Coverage of the substrate corals: A. Common, B. Branched (solid line) and massive (dashed line) colonies.
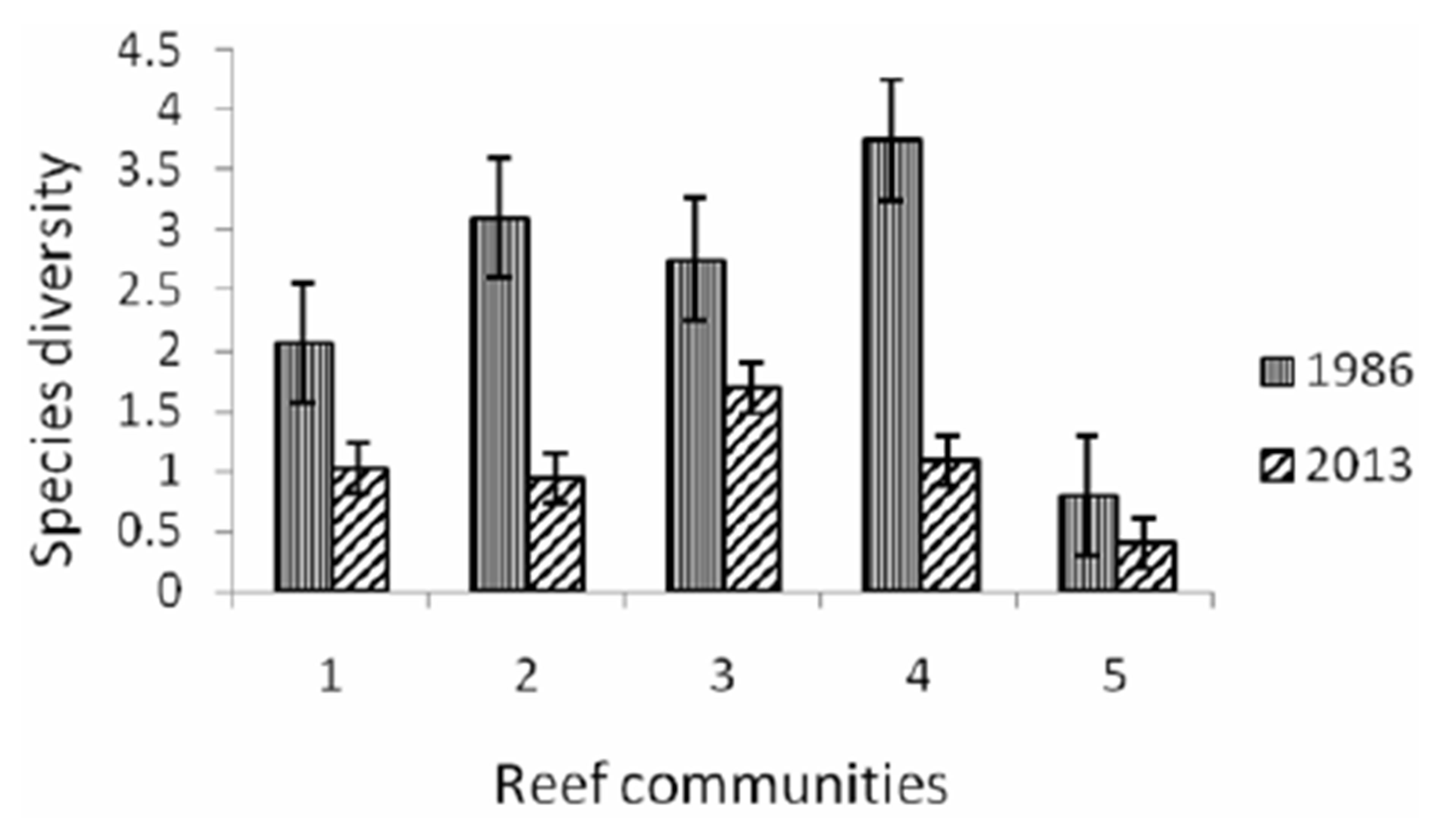
Figure 3. Variations of a specific diversity on the investigated reefs. 1. coastal polyspecific community; 2. fascia A. nobilis + A. microphthalma; 3. community Acropora + Diploastrea; 4. community of reef slope; 5. community Junceella fragilis + Diaseris distorta.
diadema and T. pileolus is also reduced. Although this reduction is probably not due to the influence of the typhoon, and the omnipresence of the hose diving equipment, with which listed aquatic actively fished by all Vietnamese reefs. Recovery of structure reefs zone, composition and structure of coral communities occurred mainly in the south-eastern side of the island (transect 3, 9˚18'N., 108˚29'E). This typhoon outlives specimen of corals with large corallites: Diploastrea heliopora, Turbinaria peltata, Astreopora microphthalma, up to 2 - 3 species from Euphyllidae (Euphyllia, Plerogyra) and Mussidae (Lobophyllia, Symphyllia), large specimen of Fungia, and large massive colonies of Porites. The abundance of poritids accounted for by their ability to secrete a firm mucous covering and starter production 1 - 2 months earlier than other coral species. These peculiarities favor their better adaptation to water overheating, and desalination under other stressful conditions of silted shallow water [36-39]. Scleractinian and several species of Pocillopora come off outlive remnant animated due to its farness from shorefront to depth 12 - 15 meters. This is known that Pocillopora even damaged 25% preserve to two thirds of productive capacity and settling [40].
The community Junceella fragilis + Diaseris distorta of for reef platform (depth of 16 m, 125 m from the shoreline) was transformed into settlements that are only one of the gorgonian with a density of 4.5 to 12.0 spec./m2 and individual colonies Sinularia, Astreopora, Pocillopora, Porites, Favia, Favites and single young Fungia, still attached to the substrate. Here there are uncommon sponge P. testudinaria and sea urchins E. diadema and T. pileolus, single starfish C. novaeguineae. Dominated in this community fungiids D. distorta, as well as the formerly common species of 4 - 5 other single mushroom scleractinian corals were not met.
At a depth of 6 - 8 meters and a distance of 50 - 40 meters from the shoreline spreads the zone of dead Acropora (Figure 4) spreads on the site of pre-existing fasces A. nobilis + A. microphthalma. Currently, there is formed polyspecific settlement of corals survived the typhoon and re-inhabiting species of scleractinian and alcyonarian. The most common here are Faviidae (D. heliopora, Favia—5 species, Favites—3 species), Poritidae—7 species, Fungiidae—6 species for 3 species of genus Lobophyllia, Symphyllia and Pavona. The most diverse are coral genus Acropora—12 species, but they met only unitary colonies, with the exception of A. cytherea and A. gemmifera, 4 and 3 colonies are observed in the meter area transect. Here, there are settlements of soft corals S. trocheliophorum, covering the surface of the substrate to a rock 40% - 70%. On clearing free of dead Acropora formed settlements of various Fungia (14 - 16 specimen/m2 and 4 - 6 species) of different age and size characteristics.

Figure 4. Completely destroyed by the typhoon Acropora from the former fasces A. nobilis + A. microphthalma, depth 4 m.
There are also visible remains of the dead fungiids (Figure 5). In general, there is another stage of succession reef community—its rebirth. Ubiquitous young colonies (size 3 - 5 cm) Acropora, Favia, Favites, Goniastrea, and Fungia settle on a free dead Acropora substrate. So, newly settled Acropora successfully grow (Figure 6).
With 100 meters of the coast at a depth of 12 meters is now formed community Pocillopora + Junceella, which is based on the survival of the typhoon and re-settling colonies of these cnidarians (Figure 7). This community extends over a distance of 40 - 60 meters to a depth of 8 meters with a population density of gorgonians to 25 spec./m2 and coating the surface of the substrate of Pocillopora to 70%. Besides the three species of Pocillopora (damicornis, meandrina and verrucosa) and large colonies Diploasrea heliopora in this community are common, individual colonies include Porites australiensis, Lobophyllia robusta, L. hemprichii, L. flabelliformis, Galaxea fascicularis, Euphyllia divisa, Fungia fungites, Pavona explanulata and

Figure 5. Settlement of Fungia, seen dead corals, depth 5 m.

Figure 6. Scleractinian settlement on the free hard substrate in the former zone of Acropora + Diploastrea, depth 3 m.

Figure 7. Recovery settlements of Pocillopora (existed in 1986), coated surface of the substrate of Pocillopora to 70%, depth 11.5 m.
single Acropora abrolhosensis, A. gemmifera, and Montipora grisea. Concomitant spread of macrobenthos sponge P. testudinaria is up to 1.2 meters high and 0.6 meters in diameter, sea urchins D. setosum to 14 spc./m2, E. diadema to 2 spec./m2, T. pileolus—0.2 spec./m2, as well as individual Tridacna crocea, Trochus niloticus, Pteria penguin, Mauritia arabica and Holothuria atra.
Rocky coastal area was the most taxonomically rich. It was most likely to be preserved corals with massive and encrusting forms colonies between large rocky boulders several feet across. This area is still formed polyspecific community scleractinian and alcyonarian consisting of 40 - 50 species, of which the most common are Acropora millepora, Diploastrea heliopora, Goniastrea aspera, Echinopora lammelosa, Euphyllia divisa, Lobophyllia robusta, Montipora grisea, Pavona decussata, Plerogysa sinuosa, Symphyllia recta, and by 2 - 3 species of Favia and Porites. There are also common colony S. troheliophorum, Lobophytum pauciflorum and Zoanthus ssp. near the water’s edge marked by some bushes macrophytes T. ornatum and Laurencia papilosa. In the colonies of Porites constantly meet bivalve’s B. semiorbiculata (from two to seven individuals per colony) and the polychetes S. giganteus and 12 individuals per colony. Distributed earlier hydroid M. platyphylla was not met in any area of the transect, or in the immediate vicinity.
The reefs on the Tho Chu now meet only a few members of the family Acroporidae, which are the basic element of all living reefs and usually form the basis of their (reef) species diversity and a high degree of coverage of live coral substrate [35,39-43]. Species diversity of corals, their coverage of the substrate and slow of their growth with increasing amounts of sediment have been noted in many publications [16,44-48]. Further, the strong deposit may hold coral larvae from settling and cause high mortality after attachment to the substrate by mechanical abrasion. All this leads to a fundamental change in the community scleractinian and possible power macrophytes and other competitors of corals [49].
In general, clearly visible irregularities and changes are observed in the zonal structure of the reef, so the composition and structure of reef communities of the island Tho Chu are changed. Nevertheless, it is equally obvious that reef communities are gradually recovering. In addition, it is hoped that after some time it will return to its previous state and species diversity, which consisted of more than five hundred species of coral and abundant species associated macrobenthos.
Especially because there are, opportunities to restore reefs through the replenishment of coral, larvae and aquatic organisms associated with other reef community. To the south of the island (in the distance of one mile 9˚16'N, 103˚21'E) there is a bank with depths ranging from 4 to 13 meters from the optimally developed noticeably damaged by typhoon coral community. There’s the usual form for the settlement of various coral reefs more than 100 species, many common, at least 20 species Acropora, (Figure 8) and related corallobionts typical Vietnamese optimal reef community: T. crocea, T. squamosa, L. cristagalli, A. ventricosa Lambis lambis, L. hiragra, C. tigris, M. arabica, Acantaster planci, C. novaeguineae, L. laevigata, H. atra, S. variegatus. D. setosum, E. diadema and so on. In 2 - 3 years after calcareous substrate formation the dominance of reef building coral colonies becomes appreciable. The climax of the secondary succession, where Acropora community is formed, seems to be reach 4 - 6 years after the start of the succession, as long as the coral reefs remain in good health and are not subjected to large disturbance such as coral bleaching, sedimentation, typhoons or other bad factors [50,51].
4. Acknowledgements
The author is grateful to I. Budin, A. G. Goloseev, A. A. Gutnick, N. I. Selin, Tran Dinh Nam, Dao Tan Ho for his

Figure 8. Reef communities in optimal conditions at the bank, depth 7 m.
help in the study communities, the definition of flora and fauna.