An Insight into Podoplanin – Emphasizing its Role in Oral Diseases
Received: 24-Apr-2015 / Accepted Date: 25-May-2015 / Published Date: 27-May-2015 DOI: 10.4172/2161-0681.1000230
Abstract
Cancer is a heterogeneous and multistep disease process involving dysregulation of multiple pathways linked to cellular differentiation, cell cycle control, apoptosis, angiogenesis, and metastasis. Multiple genetic alterations causes’ unregulated proliferation of cells, influenced by genetic predisposition as well as by environmental influences, including tobacco, alcohol, chronic inflammation and viral infections. Most of the cancers arises de novo or is often preceded by potentially malignant disorders such as leukoplakia and OSMF. The potentially malignant disorders have shown to have a high risk of malignant transformation, on average about one percent of oral lesions transform into cancer annually [1].
Keywords: Marker; Oral disease; Podoplanin; Tumor
Introduction
Cancer is a heterogeneous and multistep disease process involving dysregulation of multiple pathways linked to cellular differentiation, cell cycle control, apoptosis, angiogenesis, and metastasis. Multiple genetic alterations causes’ unregulated proliferation of cells, influenced by genetic predisposition as well as by environmental influences, including tobacco, alcohol, chronic inflammation and viral infections. Most of the cancers arises de novo or is often preceded by potentially malignant disorders such as leukoplakia and OSMF. The potentially malignant disorders have shown to have a high risk of malignant transformation, on average about one percent of oral lesions transform into cancer annually [1].
Advancement in genomic and basic research has increased our understanding of the molecular processes governing tumor formation and progression. The most important factors which impacts the prognosis and therapeutic strategy in various types of cancer is the ‘lymph node status. It was thought that lymphatic borne metastases is a factor of relevance for the poor prognosis which occurred by a passive mechanism based on the apparently simpler structure of lymphatic capillaries as demonstrated by electron microscopy many years ago. The investigations of the lymphatic system at a molecular level stated almost 15 years ago and later with the introduction of first three markers LYVE-I, Prox1 and Podoplanin, it was established that lymphatic vessels are present in the tumor and peritumoral areas, and their number correlates with prognosis in some malignancies like malignant melanoma, squamous cell carcinoma of the head and neck, breast cancer and gastric adenocarcinoma [2,3].
Podoplanin has attracted interest as a marker for cancer diagnosis and prognosis as this field of study is not completely explored. Podoplanin is specifically expressed in lymphatic endothelial cells but not in blood endothelial cells and has been utilized as a specific marker for recognizing lymphatic vessels.
History of Podoplanin
Podoplanin is a 38-KDa mucin type I transmembrane glycoprotein consisting of 162 amino acids, nine of which form the intracellular domain. The extracellular domain is highly O-glycosylated with sialic acid, α-2, 3 linked to galactose, forming the main part of the protein carbohydrate moieties, specifically expressed by lymphatic but not blood vascular endothelial cells. Podoplanin was first identified in lymphatic endothelial cells and normal cells in vivo by Wetterwald and his colleagues, in the year 1996, and named it as “E11-antigen” since it is first expressed between E10.5-E11 (E-embryonic day) [3]. Later it was named as podoplanin because of its low level in kidney podocytes and thought to play a role in maintaining the shape of foot processes (flat-feet podoplanin) and glomerular permeability.
Genetic Control Of Podoplanin
The expression of podoplanin is regulated by homeobox gene Prox-1, a master gene that controls development of lymphatic progenitors from embryonic veins, and it is located on chromosome p36.21 and the name of the gene is “chymotrypsin C (caldecrin)”. Podoplanin contains a single transmembrane domain, a short, nine amino acid cytoplasmic tail, and a heavily glycosylated extracellular domain. Podoplanin possessed a disialyl-core structure in the PLAG (platelet aggregation) domain, which is necessary for the binding of podoplanin to its specific receptors CLEC-2 (a C type lectin like receptor-2) which are endogeneous receptor of podoplanin on platelets [3,4].
Cancer cells commonly exhibit changes in the expression of cell-surface mucins or their carbohydrate epitope exposure that are detected by specific monoclonal antibodies. These mucins act as anti-adhesive molecules due to their large rod-like extracellular domains and are thought to mediate invasion and metastasis when overexpressed in carcinoma cells.
Exact physiological function of podoplanin is not known but has been reported to be expressed in a variety of malignant tumors such as squamous cell carcinoma and mesothelioma. The lymphatic endothelial cell specific markers have multiple functions on physiological and pathological condition, and are helpful to identify changes related to lymphangiogenesis. By electron microscopy podoplanin is mainly expressed on the luminal surface of lymphatic endothelial cells and only rarely on the abluminal surface of the lateral domain in cytoplasmic organelles of endothelial cells.
Various Expression Pattern Of Podoplanin
Normal tissue
Podoplanin is remarkably an exclusive marker for lymphatic endothelium but also expressed in other normal human tissues like podocytes, osteoblastic cells, osteocytes, basal keratinocytes, choroid plexus epithelial cells, type I endothelial cells of the thymus, myoepithelial cells, myofibroblasts of the prostate, granulosa cells of ovary, follicular dendritic cells, and alveolar type I cells. Yuan et al. demonstrated that podoplanin is not detectable in normal squamous epithelium adjacent to the tumors, or its expression is low in basal cells, whereas a high expression was observed in some hyperplastic and dysplastic epithelial areas adjacent to the tumors [5]. Matsui et al. observed from his study that podoplanin plays a role in maintaining the unique shape of podocyte for processes and glomerular permeability and Schacht et al. proposed that podoplanin is required to control different aspects of normal lymphatic vasculature formation [6,7]. A strong ectoplasmic and membranous D2-40 staining of the basophilic germinative cells from the outer layer of normal sebaceous glands was detectable and intense immunoreactivity in the basal layer of the outer root sheets of human hair follicles, including the bulge area was observed by Margaritescu et al. [8].
Inflammation
The role of lymphangiogenesis in inflammatory diseases is not fully resolved. On one hand, lymphatic vessels reduce the edema and drain inflammatory cells and mediators associated with inflammation, thereby helping in the resolution of inflammation. On the other hand, they serve as a channel for carrying immune cells to the lymph nodes where adaptive immune responses take place, thereby facilitating inflammation. Miyazaki et al. observed the expression of podoplanin in inflamed gingivas which were evident in the cell membrane and cytoplasm of the basal cells of oral epithelium. In oral gingival epithelium, reactivity for podoplanin was in the basal cells when severe inflammatory changes are present in the connective tissue just beneath the epithelium. In addition, positivity was also detected in basal cell extensions. It has been reported that basal cell extensions are frequently observed in gingival epithelium, but not in other types of oral epithelium, which implies that the gingival epithelium may possess characteristics of odontogenic epithelium. Enhanced expression of podoplanin was especially evident in oral sulcular and junctional epithelia including the elongated rete pegs associated with severe inflammatory reaction in the connective tissue because the inflammatory stimuli from periodontopathic bacteria might induce podoplanin expression in the oral sulcular and junctional epithelia and increase the migratory capability essential for the progression of chronic periodontitis. These possibly conclude that the expression of podoplanin is directly related to the progression of inflammation [9].
Odontogenic cyst and tumors
Most of the uni or multicystic, intraosseous tumors of the odontogenic origin shows a potential aggressiveness and local invasion. Recent studies have correlated the podoplanin expression with the neoplastic character as a parameter for assessment of tumors of odontogenic origin. Okamoto et al. observed the podoplanin immunostaining in the cell membrane and the cytoplasm of the basal and suprabasal cells, peripheral cells of the satellite cyst and the proliferation epithelial buds of keratocystic odontogenic tumor whereas weak to moderate positivity of podoplanin was observed in the dentigerous cyst and other odontogenic cysts. He also observed strong podoplanin expression in the area of high inflammation however, the reason of why podoplanin expression is enhanced in the presence of inflammation remains unknown. Schacht et al. suggested that podoplanin might play an important role in mediating cellular contractile properties and cytoskeletal reorganization [9,10].
In odontogenic tumors, such as ameloblastomas, adenomatoid odontogenic tumors, odontomas and calcifying epithelial odontogenic tumors, the expression of podoplanin is predominantly observed in the epithelial odontogenic cells in the peripheral areas of the tumor. Experimental studies during the murine odontogenesis showed that the pre-ameloblasts exhibit a strong immunoreaction for podoplanin but as the pre-ameloblasts which dierentiate into secreting ameloblasts, showed drastically decreased podoplanin expression. Sawa et al. proposed that podoplanin, may be associated with ectodermal cell proliferation. Interestingly, both mitotic activity and podoplanin expression within the ameloblastoma were coincident in a study by Sandra et al. Sawa et al. and Imaizumi et al. witnessed that the protein is expressed during intense proliferative activity in odontogenic cells and when these cells reach maturity or a stable state, there is a reduction or lack of podoplanin immunoreactivity [11,12]. This was further supported by Gonza´ lez-Alva et al. who observed a strong podoplanin immunoreaction of the secreting ameloblast-like cells in odontomas whereas in non-secreting ameloblast-like cells, the expression was absent [13].
Damante and Fleury, observed podoplanin expression in the epithelial component of a dental follicle consisting of the epithelial rests of the dental lamina and the reduced enamel epithelium, both of which represent quiescent and mature odontogenic epithelium that has performed its function and remains dormant in the ectomesenchymal tissue. He observed weak/no expression in the areas of epithelial island thereby suggesting that podoplanin is necessary for the proliferative activity of odontogenic epithelium [14].
Premalignant lesions
Leukoplakia is potentially malignant lesion with a tendency of malignant transformation into squamous cell carcinoma. In several well conducted studies, the malignant transformation rate of oral leukoplakia were reported to range from 17% to 24% of the patients with median follow-up more than 7 years [15].
Tumourigenicity and capability of recapitulating human squamous cell carcinomas are by definition properties of tumor-initiating cells (TICs). Podoplanin has been identified as a marker of tumour-initiating cells (TICs) in squamous cell carcinomas. Premalignant lesions with podoplanin expression beyond the basal cell layer may represent truly early neoplastic lesions, enriched in TICs and with a higher risk of progression to invasive cancer. The expression in premalignant lesions such as oral leukoplakia determines its putative role as biomarker in predicting oral cancer development. Kawaguchi H et al. and Shi et al. observed negative expression of podoplanin in normal mucosa whereas basal and suprabasal layers of oral leukoplakia exhibited podoplanin expression. This led them to conclude that podoplanin is associated with the malignant transformation risk and is a potent biomarker for risk assessment in oral malignant transformation [15,16].
De Vincente JC et al. investigated the potential association between podoplanin and risk of malignant transformation of oral leukoplakia with epithelial dysplasia into squamous cell carcinoma. He observed the risk of malignant transformation increases with the increasing grades of epithelial dysplasia [17]. Severe dysplastic lesions presented strong podoplanin expression with increase intensity of cell proliferation and migration.
Oral malignancy
Lymphatic vessels and lymphangiogenesis are known for their role in cancer metastasis in many cancer types. Lymphangiogenic factors such as VEGF-C, VEGF-D, VEGF-A along with podoplanin are thought to be released by both tumor cells and stromal cells in the proximity of the tumor, which leads to lymphatic vessel enlargement and sprouting. Other growth factors that are involved in lymphatic vessel maturation are hepatocyte growth factor (HGF), fibroblast growth factor (FGF), platelet-derived growth factor-BB (PDGF BB) and Ang-1. Like inflammation, cancer can also induce lymphangiogenesis in the draining lymph nodes. Experimental study in mice by Hirakawa et al. showed overexpression of lymphatic markers in the skin and lymphangiogenesis in the sentinel lymph nodes indicated increase cancer metastasis to distant organs. Interestingly he suggested that, lymphangiogenesis in the sentinel lymph node starts prior to the arrival of cancer cells which indicated the release of factors by the cancer cells so as to prepare a pre-metastatic niche.
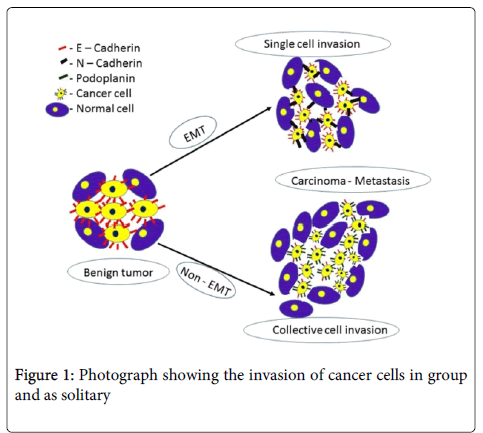
The correlation of tumor lymphangiogenesis with lymph node metastasis was assessed based on the lymph vessel density (LVD) in oral squamous cell carcinomas. The significantly high LVD were correlated with the lymph node status, tumor progression and poor prognosis. Also, LVD positivity was observed more in the tumor front of invasion. Lymphatic endothelial cells showed more tortuous and disorganized complex structure in intratumoral lymphatic vessels than in peritumoral ones whereas the lymphatic structure in the normal mucosa appeared less complex. Higher intratumoral lymphatic density was significantly associated with a higher incidence of intratumoral lymphatic invasion, peritumoral lymphatic invasion and recurrence of tumor. Thus, lymphangiogenesis in cancers might be used as an index to inflect the progression of the disease, to evaluate the status of lymphatic metastasis, to separate patients at higher risk of an adverse clinical outcome (Figure 1) [18-21].
The mechanism behind podoplanin-mediated tumor invasion and the molecular pathways leading to single and collective cell invasion has been revised in the literature [22]. Two mechanisms are suggested to be involved in the progression of a benign to malignant (a) either the tumors undergo EMT (Epithelial mesenchyme interaction), (b) or non-EMT. In EMT, the expression profile of adhesion molecules, components of the cytoskeleton and transcriptional regulators are changed. Although non-EMT pathways of tumor invasion and migration includes alterations of the cytoskeleton and the adhesive apparatus in addition with podoplanin and possibly other mucin-like transmembrane proteins. This allows the tumor cells to invade solitarily after loss of cell-cell adhesion, or as a group without losing cell-cell contacts.
Its involvement in tumour metastasis, however, has been demonstrated in an experimental model to be due to its platelet aggregation-inducing activity leading to pulmonary retention of carbohydrates in cells that overexpress podoplanin. It has also been demonstrated that podoplanin contributes to tumour invasion by binding ERM proteins to activate RhoA resulting in epithelial-mesenchymal transition Although podoplanin-positive TICs in squamous cell carcinomas may use these mechanisms to initiate and sustain tumour growth, and allow rapid proliferation through the activation of the SHH (sonic Hedgehog) signaling pathway. In addition to these intrinsic mechanisms, the microenvironment also influences the ability of TICs to generate tumours. These findings suggest a role of podoplanin in tissue development and repair as well as in carcinogenesis and malignant progression [23-26].
Salivary gland tumors
In normal salivary gland, only myoepithelial cells express podoplanin. These cells are contractile, showing frequent changes in shape mediated by myofilaments. Salivary gland myoepithelial cells have been reported to constantly produce podoplanin which are glycosylated with polysaccharides in the Golgi apparatus, and transported into the cell membrane. Podoplanin is involved in maintaining the homeostasis of myoepithelial cells through its characteristic mucin type transmembrane protein.
The tumor cells that differentiate into neoplastic myoepithelial cells, which is one of the unique pathological features of salivary gland tumors. Neoplastic myoepithelial cells by themselves do not demonstrate glandular formation, but are located around the ductal cells in the gland-forming tumors. The cells show various morphologies, such as epitheloid, spindle, plasmacytoid and clear cell features, and frequently produce a mucinous or basement membrane-like extracellular matrix. Various findings have suggested that podoplanin is an antigen for salivary gland myoepithelial cells and the immunostaining of podoplanin in salivary glands directly reflects myoepithelial cell shapes. Kanner et al. in their study showed that myopeithelial cells in breast and salivary gland and basal cells in prostrate consistently demonstrate podoplanin immune reactivity, but typically less intensely than lymphatic’s [27-29].
Conclusion
Emerging studies of podoplanin suggest that this molecule plays diverse role throughout the body. It is involved in the development, inflammatory diseases and metastases. Over expression of podoplanin in various cell lines results in increased motility and altered mesenchymal phenotype in vitro and increased metastases in vivo. Hence a better understanding of tumor markers growth factors and tumor stromal cells may lead to great insights in the diverse fields of development, cellular interactions in the immune system, and cancer progression and metastasis.
Acknowledgement
We would like to acknowledge the Management, Dean and Vice Dean of Buraydah Private Dental College for allowing us to carry out our work successfully.
References
- Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, et al. (2006) Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 107: 563-569.
- Rodrigo JP, García-Carracedo D, González MV, Mancebo G, Fresno MF, et al. (2010)Podoplanin expression in the development and progression of laryngeal squamous cell carcinomas. Mol Cancer 9: 48.
- Raica M, Cimpean AM, Ribatti D (2008) The role of podoplanin in tumor progression and metastasis. Anticancer Res 28: 2997-3006.
- Lingen MW, Pinto A, Mendes RA, Franchini R, Czerninski R, et al. (2011) Genetics/epigenetics of oral premalignancy: current status and future research. Oral Dis 17 Suppl 1: 7-22.
- Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, et al. (2006) Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 107: 563-569.
- Matsui K, Breiteneder-Geleff S, Kerjaschki D (1998) Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J Am SocNephrol 9: 2013-2026.
- Schacht V, Ramirez M I, YoungKwon H, Hirakawa S, Feng D, et al. (2003)T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J 22: 3546-3556.
- Margaritescu C, Raica M, Pirici D, Simionescu C, Mogoanta L, et al. (2010)Podoplanin expression in tumor-free resection margins of oral squamous cell carcinomas: an immunohistochemical and fractal analysis study. HistolHistopathol 25: 701-711.
- Miyazaki Y, Okamoto E, González-Alva P, Hayashi J, Ishige T, et al. (2009) The significance of podoplanin expression in human inflamed gingiva. J Oral Sci 51: 283-287.
- Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, et al. (2005) Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 166: 913-921.
- Sawa Y, Iwasawa K, Ishikawa H (2008) Expression of podoplanin in the mouse tooth germ and apical bud cells. ActaHistochemCytochem 41: 121-126.
- Imaizumi Y, Amano I, Tsuruga E, Kojima H, Sawa Y (2010)Immunohistochemical examination for the distribution of podoplanin-expressing cells in developing mouse molar tooth germs. Actahistochemicacytochem 43: 115-121.
- González-Alva P, Inoue H, Miyazaki Y, Tsuchiya H, Noguchi Y, et al. (2011)Podoplanin expression in odontomas: clinicopathological study and immunohistochemical analysis of 86 cases. J Oral Sci 53: 67-75.
- Damante JH, Fleury RN (2001) A contribution to the diagnosis of the small dentigerous cyst or the paradental cyst. PesquiOdontol Bras 15: 238-246.
- Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, et al. (2008)Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy. J ClinOncol 26: 354-360.
- Shi P, Liu W, Zhou ZT, He QB, Jiang WW (2010)Podoplanin and ABCG2: malignant transformation risk markers for oral lichen planus. Cancer Epidemiol Biomarkers Prev 19: 844-849.
- De Vicente JC, Rodrigo JP, Rodriguez-Santamarta T, Lequerica-Fernández P, Allonca E, et al. (2013)Podoplanin expression in oral leukoplakia: tumorigenic role. Oral Oncol 49: 598-603.
- Margaritescu C, Raica M, Pirici D, Simionescu C, Mogoanta L, et al. (2010)Podoplanin expression in tumor-free resection margins of oral squamous cell carcinomas: an immunohistochemical and fractal analysis study. HistolHistopathol 25: 701-711.
- Filho AL, Oliveira TG, Pinheiro C, De Carvalho MB, Curioni OA, et al.(2007)How useful is the assessment of lymphatic vascular density in oral carcinoma prognosis? World Journal of Surgical Oncology 5:140
- Miyahara M, Tanuma J, Sugihara K, Semba I (2007)Tumorlymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer 110: 1287-1294.
- Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, et al. (2006) Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 107: 563-569.
- Wicki A, Christofori G (2007) The potential role of podoplanin in tumour invasion. Br J Cancer 96: 1-5.
- Kunita A, Kashima TG, Morishita Y, Fukayama M, Kato Y, et al. (2007) The platelet aggregation-inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am J Pathol 170: 1337-1347.
- Martín-Villar E, Megías D, Castel S, Yurrita MM, Vilaró S, et al. (2006)Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci 119: 4541-4553.
- Atsumi N, Ishii G, Kojima M, Sanada M, Fujii S, et al. (2008)Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. BiochemBiophys Res Commun 373: 36-41.
- Wicki A, Christofori G (2007) The potential role of podoplanin in tumour invasion. Br J Cancer 96: 1-5.
- Hata M, Amano I, Tsuruga E, Kojima H, Sawa Y (2010)Immunoelectron microscopic study of podoplanin localization in mouse salivary gland myoepithelium. ActaHistochemCytochem 43: 77-82.
- Savera AT, Zarbo RJ (2004) Defining the role of myoepithelium in salivary gland neoplasia. AdvAnatPathol 11: 69-85.
- Kanner WA, Galgano MT, Atkins KA (2010)Podoplanin expression in basal and myoepithelial cells: utility and potential pitfalls. ApplImmunohistochemMolMorphol 18: 226-230.
Citation: Bina Kashyap, Sridhar Reddy (2015) An Insight into Podoplanin – Emphasizing its Role in Oral Diseases. J Clin Exp Pathol 5:230. Doi: 10.4172/2161-0681.1000230
Copyright: © 2015 Kashyap B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14795
- [From(publication date): 6-2015 - Apr 27, 2024]
- Breakdown by view type
- HTML page views: 10342
- PDF downloads: 4453