Research Article Open Access
Passive Response to Stress in Adolescent Female and Adult Male Mice after Intermittent Nicotine Exposure in Adolescence
Panayotis Thanos1,2*, Foteini Delis2, Lauren Rosko2 and Nora D Volkow11Laboratory of Neuroimaging, NIAAA, NIH, Department of Health and Human Services, Bethesda, MD, USA
2Behavioral Neuropharmacology & Neuroimaging Lab, Department of Medicine, Brookhaven National Laboratory, Upton, NY, USA
- *Corresponding Author:
- Dr. Panayotis Thanos
Behavioral Neuropharmacology & Neuroimaging Lab
Department of Medicine
Brookhaven National Laboratory
Upton, NY, USA
Tel: 631 344-7364
Fax: 631 344-5311
E-mail: thanos@bnl.gov
Received March 07, 2013; Accepted April 08, 2013; Published April 23, 2013
Citation: Thanos P, Delis F, Rosko L, Volkow ND (2013) Passive Response to Stress in Adolescent Female and Adult Male Mice after Intermittent Nicotine Exposure in Adolescence. J Addict Res Ther S6:007. doi:10.4172/2155-6105.S6-007
Copyright: © 2013 Thanos P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Smoking is frequently co-morbid with depression. Although it is recognized that depression increases the risk for smoking, it is unclear if early smoking exposure may increase the risk for depression. To test this possibility we assessed the effects of adolescent nicotine exposure on the Forced Swim Test (FST), which is used as a measure of passive coping, and depressive-like behavior in rodents, and on the open field test (OFT), which is used as a measure of locomotion and exploratory behavior. Male and female mice received daily saline or nicotine (0.3 or 0.6 mg/kg) injections from postnatal day (PD) 30 to PD 44. FST and OFT were performed either 1 or 30 days after the last injection (PD 45 and PD 74, respectively). In females, treatment with 0.3 mg/kg nicotine lead to increased FST immobility (64%) and decreased OFT locomotor activity (12%) one day following the last nicotine injection (PD 45); while no effects were observed in adulthood (PD 74). In contrast, on PD45, nicotine treatment did not change the male FST immobility but lead to lower OFT locomotor activity (0.6 mg/kg, 10%). In adulthood (PD 74), both nicotine doses lead to higher FST immobility (87%) in males while 0.6 mg/kg nicotine to lower OFT locomotor activity (13%). The results (i) identify females as more vulnerable to the immediate withdrawal that follows nicotine discontinuation in adolescence and (ii) suggest that adolescent nicotine exposure may enhance the risk for passive response towards unavoidable stress in adult males.
Keywords
Nicotine; Passive reaction; Depression; Adult; Male; Female
Abbreviations
FST: Forced Swim Test; OFT: Open Field Test; PD: Postnatal Day; NIC: Nicotine; F: Female; M: Male
Introduction
Tobacco smoking frequently starts in adolescence and is predominantly driven by nicotine’s rewarding effects [1,2]. Preclinical studies in rats show that nicotine exposure during early adolescence increases their intravenous nicotine self-administration as adults and renders them more sensitive to nicotine conditioned place preference [3-5], increases depression- and anxiety-like behaviors [6], and has long-term effects on cognitive performance [7,8]. Acutely, nicotine has anxiolytic and anti-depressant effects both in laboratory animals [9,10] and in humans [11], which could explain the high prevalence of smoking in depressed individuals who may use it to self-medicate [12,13]. However, studies also suggest that the high prevalence of smoking in depressed individuals, more frequent in women than men [14,15], could reflect a contributing role from early nicotine exposure [12,16,17]. Here we assessed if adolescent nicotine exposure has sex-specific effects on the forced swim test (FST), which is used as a measure of passive coping/depression-like behavior, and the open field test (OFT), which is used as a measure of locomotor activity and anxiety-like behavior, in adolescence and early adulthood. Adolescent male and female mice were exposed to daily nicotine injections during adolescence and OFT and FST measures were obtained after early (1 day post treatment) and protracted (30 days post treatment) withdrawal. We hypothesized that nicotine would affect both the FST and the OFT during early and protracted withdrawal and that females would be more sensitive than males.
Materials and Methods
Animals
Experiments were performed on 156 male and female C57 BL/6 mice that were 4 week old at the beginning of the experiment. Mice arrived at Brookhaven National Laboratory (BNL) on postnatal day (PD) 23 from Taconic (US, NY). They were individually housed under a 12 h-dark/12 h-light reverse cycle, lights on at 7:00 pm, with Purina rodent chow (food and water ad libitum). Body weight was monitored daily and food intake every three days. Experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and BNL Institutional Animal Care and Use Committee protocols.
Treatment
The mice were divided in three groups (with 12-14 mice per group) and received daily intraperitoneal (i.p.) injections of either vehicle (0.9% saline) or 0.3 mg/kg or 0.6 mg/kg nicotine solutions (calculated as free base, concentrations based on [18,19]. For the solutions, nicotine tartate (Sigma) was dissolved into sterile saline and the pH was adjusted to 7.4. On PD 29, mice were placed in the open-field arena and their baseline OFT behavior was recorded. Saline or nicotine treatment (1 i.p. injection per day, 9-11 am) started on PD30 and lasted until PD44. Twenty-four hours after the last nicotine injection (PD 45), half of the mice were tested in the OFT and 4 hours later in the FST and the other half were left undisturbed in their cages for one month, at which point (PD 74) they were tested in the OFT followed, 4 hours later, by the FST. Body weight was recorded daily during injections and twice a week during abstinence. Food intake was recorded twice a week throughout the experiment.
Forced swim test
For FST, each mouse was placed in a clear, Nalgene 20 cm diameter beaker (4 L, 20 cm diameter) containing 3 L water (15 cm height) at 25oC, for 6 minutes, after which it was removed from the water and placed under a heat lamp for 15 minutes. Tests were videotaped from the side of the cylinder and rated by two individuals blind to the experimental conditions according to previously published work [20,21]. The numbers express the total number of seconds the mice remained immobile. Immobility was defined as no movement or minimal movement of one limb in order to remain afloat.
Open field test
The OFT was performed on PD 30 (1 day prior to nicotine treatment initiation) and on PD 45 or PD 74. Mice were placed in a 16 inch3 arena with plexiglass walls (Tru Scan System, Coulbourn Instruments, PA, USA) that recorded horizontal and vertical motion through a series of 16 cross beams positioned 1 inch apart, allowing for 0.5 inch resolution. The animal was placed in the center of the open field arena for 30 minutes. The following movement parameters were recorded: movement time, rest time, time spent in the margin, time spent in center, total number of moves, average velocity and ambulatory velocity, number of center entries and rearing responses, total distance and ambulatory distance, distance and ambulatory distance traveled in the margins and the center of the field. Time measures are expressed in sec, and distance measures in cm.
Statistics
Three-way repeated-measures analysis of variance (ANOVA) was used to analyze body weight and food intake, with sex and treatment as between-subject and time as within-subject factor. Three-way ANOVA was used to analyze the FST scores with sex, treatment (saline - 0.3 mg/kg - 0.6 mg/kg nicotine), and withdrawal (1 day -30 days) as between-subjects factors. OFT measures were obtained twice for each animal, before the beginning and after the end of the treatment (1 or 30 days). Data were analyzed with four-way repeated measures ANOVA, with sex, treatment, and withdrawal (pertaining to 1 and 30 days withdrawal from the last injection) as between-subjects factors and time (pre-treatment - post-treatment) as within-subject factor. ANOVA was followed by Fisher’s post hoc test. Correlations between FST and OFT scores were assessed with the Pearson test. Overall level of significance was set at 0.05.
Results
Immobility scores (FST)
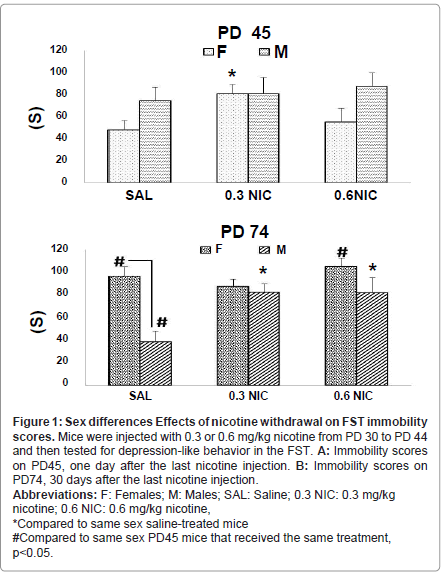
Withdrawal from nicotine had significant effects on FST immobility scores that differed between the sexes (Figure 1). Three-way ANOVA showed a significant main effect of treatment [F(2,144) = 3.70, p=0.02] and a sex by treatment interaction: [F(1,144) = 8.58, p= 0.004].
PD 45 (1 day post nicotine withdrawal): Female mice treated with 0.3 mg/kg nicotine had 64% higher FST immobility score than saline treated females (p=0.01; Figure 1). No differences were observed in females treated with 0.6 mg/kg nicotine, nor in male mice whether treated with 0.3 or 0.6 mg/kg nicotine. Comparisons between saline treated mice showed no differences between males and females.
PD 74 (30 days post nicotine withdrawal): Female mice treated with 0.3 or 0.6 mg/kg nicotine did not differ from saline treated mice on FST immobility scores. In contrast, male mice treated with 0.3 or 0.6 mg/kg nicotine had 87% higher FST immobility score than saline treated males (p=0.01, Figure 1). Comparisons between saline treated mice showed that females had 117% higher FST immobility score than age-matched males (p=0.003).
PD 74 vs. PD 45: Female mice treated with saline or 0.6 mg/kg nicotine had higher (92% and 74% respectively) immobility scores on PD 74, compared to PD 45 (p=0.02, p=0.007; Figure 1). No differences were observed for females injected with 0.3 mg /kg nicotine. In males, FST immobility scores were 40% lower in saline treated PD 74 males compared to PD 45 males (p=0.05) and no differences were observed for the nicotine treated male groups.
Locomotor activity (OFT)
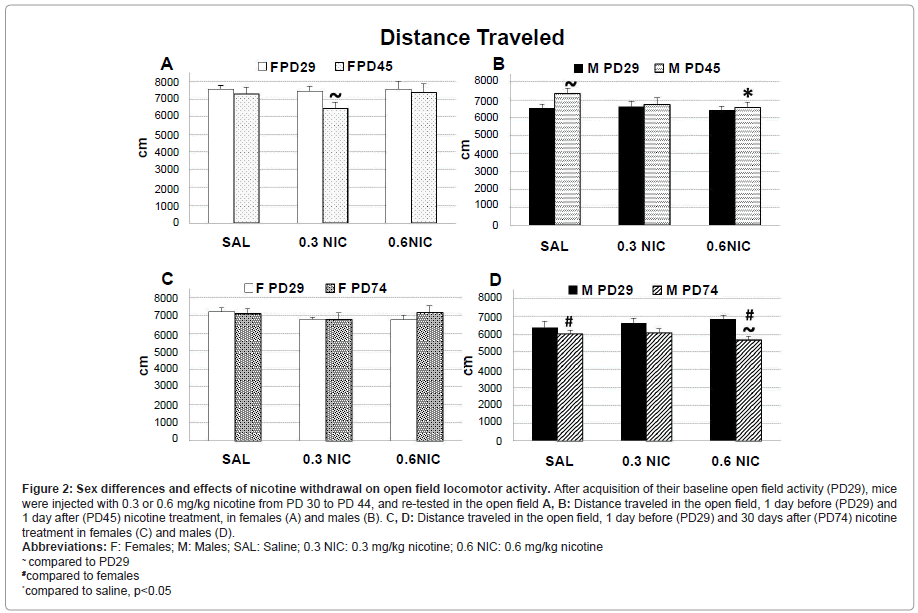
Locomotor activity was measured one day prior to treatment initiation (PD 29, baseline measure) and 1 (PD45) or 30 (PD74) days after the last nicotine injection (4 hrs prior to FST). Total horizontal, center, and margin distance, movement time and time spent in center and margin, total movements, center, and vertical plane entries, and velocity were recorded. Of all the OFT parameters examined, only horizontal distance and velocity showed significant differences and, thus, only these are described. The ANOVA on the OFT locomotor scores showed significant sex, treatment, and time effects.
Figure 1:Sex differences Effects of nicotine withdrawal on FST immobility
scores. Mice were injected with 0.3 or 0.6 mg/kg nicotine from PD 30 to PD 44
and then tested for depression-like behavior in the FST. A: Immobility scores
on PD45, one day after the last nicotine injection. B: Immobility scores on
PD74, 30 days after the last nicotine injection.
Abbreviations: F: Females; M: Males; SAL: Saline; 0.3 NIC: 0.3 mg/kg
nicotine; 0.6 NIC: 0.6 mg/kg nicotine,
*Compared to same sex saline-treated mice
#Compared to same sex PD45 mice that received the same treatment,
p<0.05.
Horizontal distance: Sex [F(1,144) = 17.6, p<0.001]; time x sex x treatment [F(2,144) = 3.1, p=0.04]; time x sex x withdrawal [F(1,144) = 16.4, p<0.001].
Velocity: Sex [F(1,144) = 55.5, p<0.001]; time x sex x withdrawal [F(1,144) = 13.0, p<0.001].
On PD 45, saline treated female and male mice had similar horizontal distance and velocity (Figures 2A and 2B, the same pattern was observed for velocity – data not shown). Female mice treated with 0.3 mg/kg nicotine (but not 0.6 mg/kg) showed 12% decreased horizontal distance and velocity when compared to baseline (PD 29) (p=0.02; Figure 2A) but not compared to saline. Nicotine treated male mice showed no changes in OFT when compared to baseline, but saline treated males showed higher horizontal distance and velocity (Figure 2B), compared to baseline (11%, p<0.001) and to nicotine treated mice (10%, 0.6 mg/kg; p=0.01)
On PD 74, saline treated male mice had 12% lower horizontal distance in OFT compared to saline treated females (p=0.03; Figure 2C). Male mice treated with 0.6 mg/kg nicotine (but not 0.3 mg/kg) decreased their horizontal distance by 13% (and velocity, data not shown) compared to their baseline measures (p<0.001; Figures 2C and 2D) and by 17% compared to similarly treated females (p<0.001; Figures 2C and 2D).
Other analyses
No significant differences between saline and nicotine treated mice were observed on body weight and food intake either on PD45 or PD74 (data not shown). Correlation analyses between the FST and OFT measures were not significant (data not shown).
Discussion
Following intermittent nicotine exposure in adolescence, female mice, during acute withdrawal, show increased immobility in FST and decreased activity in OFT but no long lasting effects when tested 30 days later, whereas males showed no changes during acute withdrawal but had increased immobility in FST and decreased activity in OFT after 30 days of nicotine discontinuation.
In animals, passive coping strategies such as immobility in the FST are phenotypes of depressive-like behavior; they are associated with and potentiated by high stress, by elevated corticosterone and low testosterone levels [22-27] and they are more pronounced in females than males [23]. FST immobility scores decrease with antidepressant treatment [28] whereas they increase after acute [29,30] and chronic stress [31,32] as well as in mice with that are genetically prone to depression-like behaviors [33].
Short term nicotine withdrawal affects female FST
An enhanced sensitivity of female mice to short-term nicotine withdrawal is consistent with clinical findings. Overnight abstinence produces greater mood changes in women than in men [34]. Women experience a greater difficulty to abstain from smoking [35], they have higher negative affective response at 24 hrs of withdrawal [36], and have more severe nicotine craving [37] than men. Our findings are also consistent with preclinical studies showing that upon nicotine discontinuation, adult female rats have more withdrawal symptoms than males [38], that 2 days after nicotine discontinuation, adult female rats increased their FST immobility scores [38,39], and that 5 days after nicotine withdrawal adolescent female mice had higher FST immobility than controls [40]. Thus, our findings together with previous studies, suggest that early nicotine withdrawal induces distress in female mice, which may occur both in adolescence (current findings and [40]) adulthood [38,39] and may appear as early as 24 hrs after nicotine discontinuation (current finding) and extend to 2 or 5 days post discontinuation [38,39].
Figure 2:Sex differences and effects of nicotine withdrawal on open field locomotor activity. After acquisition of their baseline open field activity (PD29), mice
were injected with 0.3 or 0.6 mg/kg nicotine from PD 30 to PD 44, and re-tested in the open field A, B: Distance traveled in the open field, 1 day before (PD29) and
1 day after (PD45) nicotine treatment, in females (A) and males (B). C, D: Distance traveled in the open field, 1 day before (PD29) and 30 days after (PD74) nicotine
treatment in females (C) and males (D).
Abbreviations: F: Females; M: Males; SAL: Saline; 0.3 NIC: 0.3 mg/kg nicotine; 0.6 NIC: 0.6 mg/kg nicotine
~ compared to PD29
#compared to females
*compared to saline, p<0.05.
The higher vulnerability of females to early nicotine withdrawal along with womens’ greater conditioning to sensory aspects of smoking [41] could explain the greater difficulty they experience when abstaining and their higher relapse rates than males [35]. The higher sensitivity to acute withdrawal is clinically relevant since among short-term abstainers, dysphoria and negative emotions during the first week, but not the fourth, consistently predict the urge to smoke and the rate of relapse [42]. Our findings suggest that interventions to prevent acute withdrawal upon nicotine discontinuation may be particularly important for females.
The extent to which early withdrawal from nicotine exposure has more detrimental effects on females that were exposed in adolescence than on females exposed as adults remains to be established in the mouse. We observed changes in FST (and OFT) during 1-day withdrawal in adolescent females treated with 0.3 mg/kg but not 0.6 mg/kg nicotine, which we interpret to reflect the longer duration of action of the higher nicotine dose that delayed the emergence of withdrawal symptoms. However future studies are needed to assess if after >24 hrs females treated with 0.6 mg/kg nicotine also show increased immobility in FST.
Long-term nicotine withdrawal affects male FST
Nicotine treated males (0.3 and 0.6 mg/kg) tested at 30 days of abstinence showed increased immobility in FST when compared with non-treated males. These results are in agreement with previously published findings in the rat [6], in contrast to findings in the mouse [40], and analogous to lower sucrose preference measures in the mouse [40]. The difference between our findings and previously reported negative findings in the male FST could be due to the different routes of nicotine administration (i.p. vs. oral), resulting in different pharmacokinetics in males, and thereby affecting brain neuroplasticity differently. Our findings, showing long term effects from adolescent nicotine exposure in males, are analogous to the increased FST immobility scores after 15-60 days of withdrawal in adult Swiss male mice treated with nicotine as adults [43], which suggests that the enhanced vulnerability for FST immobility triggered by long-term nicotine withdrawal may occur not only after adolescent exposure but also after exposure in adulthood.
Sex differences in FST
A comparison between saline treated male and female mice revealed that on PD 74 female mice showed higher FST immobility scores than males. This is in agreement with prior findings in rats [44] and is consistent with the higher vulnerability to depression in women than in men [45,46] and with the higher prevalence of passive coping strategy adoption by women, compared to men [47-51].
In humans, girls’ depression scores are slightly lower than boys’ during childhood; the phenotype reverses during adolescence while in adulthood substantially more women are depressed than men [45,52,53]. In the current study, while adult mice (PD 74) exhibited similar behavior as adult humans, with females showing higher depression-like score than males, late adolescent mice (PD 45) were more similar to human children than human adolescents, with males exhibiting slightly higher (although not statistically significant) immobility scores than females. In addition, in humans the adult gender difference is primarily due to changes in female depression scores while in mice to changes in both female and male immobility scores. These discrepancies suggest that biological factors or the FST alone cannot explain the transitions of human depressive behavior in its entirety, although the gross picture is similar between the 2 species.
Preclinical and clinical studies point to the suggestion that males are more vulnerable to the long term effects of nicotine exposure than females and that this difference could be due to sex-specific adaptations of the β2-expressing nicotinic acetylcholine receptor. Studies in rodents have shown that repeated nicotine exposure increases nicotinic receptor levels in males more than in females [54,55]. This sex difference persists for up to 1 month when nicotine exposure occurs in adolescence [56], possibly because adolescent rodents express significantly higher levels of the a4 β2 high affinity receptor subtype [57,58] which renders them particularly sensitive to nicotine treatment. Periadolescent nicotine exposure also induces a short- and long-term cross-sensitization to amphetamine in males, but not in females [59] while sex differences in nicotine receptor sensitivity have also been reported for younger animals (PD 5 –PD30), with males still being more sensitive to nicotine treatment than females [60-62]. Studies in human smokers showed that long-term (up to 1 month) rather than short-term (1 day) abstinence was associated with increased and persistent brain β2 nicotinic receptor availability in men, but not in women [63,64]. Based on the above, we may suppose that in the current study, the greater sensitivity of nicotine-treated male mice after 30 days of nicotine abstinence may be due to sex-specific modifications in β2-expressing nicotinic receptors in limbic brain regions.
The long term effects of adolescent nicotine exposure in males, but not in females, could also result from sex differences in nicotine metabolism, in hormone levels, in genetic factors or in neurotransmission adaptations. Female mice eliminate nicotine faster than males and have lower brain nicotine levels [65], which could result in a lower sensitivity of the female brain for the development of persisting neuroplastic changes. In males, castration increases immobility scores in FST and prevents the antidepressant effects of acute nicotine, effects that are reversed by testosterone replacement treatment [22,66]. The risk for nicotine dependence is affected by gene x environment interactions in a sexually dimorphic way [67,68], while early (in utero) nicotine exposure impairs neurotransmission in adult males [69].
Considerations and Limitations
It is possible that higher FST immobility scores do not reflect a passive response to an unavoidable stressor, but simply reflect locomotor impairments. However the lack of a correlation between the FST and OFT scores in the current as well as in previous studies [70] does not support this suggestion. In addition, whereas on PD 45 females treated with 0.3 mg/kg show higher FST immobility scores and lower OFT mobility, the males had no changes in FST but had lower OFT mobility. After protracted withdrawal (PD74), males treated with nicotine (0.3 and 0.6 mg/kg) showed higher immobility FST scores but only the 0.6 mg/kg group showed lower OFT mobility, compared to the baseline measures. And on PD74, saline treated females -when compared to saline treated males- had higher FST immobility scores but they also had higher OFT mobility.
Since nicotine intake is associated with a higher risk for anxiety disorders [71] we were surprised by not finding any significant differences between saline and nicotine treated mice on center entries, center time, and thigmotaxis measures of the OFT test. This indicates that the effects of nicotine withdrawal on the FST are specific to this test and do not generalize to a test of anxiety-like behavior. Further studies using other paradigms to assess anxiety behaviors (elevated plus maze) are needed to rule out the contribution of nicotine withdrawal in sex-specific changes of anxiety-related behaviors.
The estrous cycle of the female mice was not controlled in this study. Experiments in animals have shown that the estrous cycle does not affect the outcome of nicotine self-administration [72], conditioned place preference [73] or withdrawal [38] behaviors while human studies are still inconclusive [74,75]. Conflicting data are reported on the effects of the estrous cycle on FST scores [70,76,77], while no effects on the distance traveled in the OFT have been reported [78,79].
Conclusion
The current study identifies adolescent females as being more vulnerable to the immediate withdrawal that follows nicotine discontinuation and suggests that early exposure to nicotine promotes an increased risk for passive coping strategies in adult male, but not in female mice.
Acknowledgements
This work was supported by the NIAAA (AA 11034 & AA07574, AA07611).
References
- Benowitz NL (2010) Nicotine addiction. N Engl J Med 362: 2295-2303.
- Wittchen HU, Behrendt S, Höfler M, Perkonigg A, Lieb R, et al. (2008) What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. Int J Methods Psychiatr Res 17 Suppl 1: S16-29.
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, et al. (2003) Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 23: 4712-4716.
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM (2004) Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 174: 389-395.
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS (2003) Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 169: 141-149.
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, et al. (2009) Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology 34: 1609-1624.
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, et al. (2009) Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology 34: 299-306.
- Trauth JA, Seidler FJ, Slotkin TA (2000) Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res 880: 167-172.
- Semba J, Mataki C, Yamada S, Nankai M, Toru M (1998) Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry 43: 389-391.
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E Jr, et al. (1999) Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 142: 193-199.
- Mineur YS, Picciotto MR (2010) Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31: 580-586.
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P (1998) Major depression and stages of smoking. A longitudinal investigation. Arch Gen Psychiatry 55: 161-166.
- Needham BL (2007) Gender differences in trajectories of depressive symptomatology and substance use during the transition from adolescence to young adulthood. Soc Sci Med 65: 1166-1179.
- Husky MM, Mazure CM, Paliwal P, McKee SA (2008) Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend 93: 176-179.
- Poulin C, Hand D, Boudreau B, Santor D (2005) Gender differences in the association between substance use and elevated depressive symptoms in a general adolescent population. Addiction 100: 525-535.
- Boden JM, Fergusson DM, Horwood LJ (2010) Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br J Psychiatry 196: 440-446.
- Wu LT, Anthony JC (1999) Tobacco smoking and depressed mood in late childhood and early adolescence. Am J Public Health 89: 1837-1840.
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV (2006) Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 184: 382-390.
- Kota D, Robinson SE, Imad Damaj M (2009) Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol 78: 873-879.
- Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23: 238-245.
- Sunal R, Gümüşel B, Kayaalp SO (1994) Effect of changes in swimming area on results of "behavioral despair test". Pharmacol Biochem Behav 49: 891-896.
- Bernardi M, Genedani S, Tagliavini S, Bertolini A (1989) Effect of castration and testosterone in experimental models of depression in mice. Behav Neurosci 103: 1148-1150.
- Goel N, Bale TL (2008) Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology 149: 6399-6405.
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE (2005) Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 156: 105-114.
- Hill MN, Brotto LA, Lee TT, Gorzalka BB (2003) Corticosterone attenuates the antidepressant-like effects elicited by melatonin in the forced swim test in both male and female rats. Prog Neuropsychopharmacol Biol Psychiatry 27: 905-911.
- Johnson SA, Fournier NM, Kalynchuk LE (2006) Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res 168: 280-288.
- Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, et al. (2009) Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav Genet 39: 192-201.
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730-732.
- Bernal-Morales B, Contreras CM, Cueto-Escobedo J (2009) Acute restraint stress produces behavioral despair in weanling rats in the forced swim test. Behav Processes 82: 219-222.
- Suvrathan A, Tomar A, Chattarji S (2010) Effects of chronic and acute stress on rat behaviour in the forced-swim test. Stress 13: 533-540.
- Swiergiel AH, Leskov IL, Dunn AJ (2008) Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav Brain Res 186: 32-40.
- Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52: 90-110.
- Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, et al. (2009) Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci 29: 1937-1946.
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, et al. (2008) Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res 10: 1653-1661.
- Perkins KA (2001) Smoking cessation in women. Special considerations. CNS Drugs 15: 391-411.
- Hogle JM, Curtin JJ (2006) Sex differences in negative affective response during nicotine withdrawal. Psychophysiology 43: 344-356.
- Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK (2009) Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addict Behav 34: 620-623.
- Hamilton KR, Berger SS, Perry ME, Grunberg NE (2009) Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacol Biochem Behav 92: 51-59.
- Djurić VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M (1999) Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav 67: 533-537.
- Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhães AC, et al. (2011) Exposure to nicotine and ethanol in adolescent mice: effects on depressive-like behavior during exposure and withdrawal. Behav Brain Res 221: 282-289.
- Perkins KA, Donny E, Caggiula AR (1999) Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1: 301-315.
- Doherty K, Kinnunen T, Militello FS, Garvey AJ (1995) Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 119: 171-178.
- Mannucci C, Tedesco M, Bellomo M, Caputi AP, Calapai G (2006) Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochem Int 49: 481-486.
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, et al. (2004) Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience 126: 849-857.
- Hyde JS, Mezulis AH, Abramson LY (2008) The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev 115: 291-313.
- Kessler RC (2003) Epidemiology of women and depression. J Affect Disord 74: 5-13.
- Calvete E, Camara M, Estevez A, Villardón L (2011) The role of coping with social stressors in the development of depressive symptoms: gender differences. Anxiety Stress Coping 24: 387-406.
- Flynn SM, Schipper LJ, Roach AR, Segerstrom SC (2009) Gender differences in delayed-type hypersensitivity response: effects of stress and coping in first-year law students. Brain Behav Immun 23: 672-676.
- Gerdes EP, Ping G (1994) Coping differences between college women and men in China and the United States. Genet Soc Gen Psychol Monogr 120: 169-198.
- Nolen-Hoeksema S (2000) The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol 109: 504-511.
- Nolen-Hoeksema S, Larson J, Grayson C (1999) Explaining the gender difference in depressive symptoms. J Pers Soc Psychol 77: 1061-1072.
- Cyranowski JM, Frank E, Young E, Shear MK (2000) Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry 57: 21-27.
- Twenge JM, Nolen-Hoeksema S (2002) Age, gender, race, socioeconomic status, and birth cohort differences on the children's depression inventory: a meta-analysis. J Abnorm Psychol 111: 578-588.
- Koylu E, Demirgoren S, London ED, Poqun S (1997) Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci 61: PL185-190.
- Mochizuki T, Villemagne VL, Scheffel U, Dannals RF, Finley P, et al. (1998) Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse 30: 116-118.
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA (1999) Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res 851: 9-19.
- Doura MB, Gold AB, Keller AB, Perry DC (2008) Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res 1215: 40-52.
- Zoli M, Léna C, Picciotto MR, Changeux JP (1998) Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci 18: 4461-4472.
- Collins SL, Montano R, Izenwasser S (2004) Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res 153: 175-187.
- Alves NC, Bailey CD, Nashmi R, Lambe EK (2010) Developmental sex differences in nicotinic currents of prefrontal layer VI neurons in mice and rats. PLoS One 5: e9261.
- Damborsky JC, Winzer-Serhan UH (2012) Effects of sex and chronic neonatal nicotine treatment on Na²â�?º/Kâ�?º/Clâ�?» co-transporter 1, Kâ�?º/Clâ�?» co-transporter 2, brain-derived neurotrophic factor, NMDA receptor subunit 2A and NMDA receptor subunit 2B mRNA expression in the postnatal rat hippocampus. Neuroscience 225: 105-117.
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, et al. (2009) Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res 196: 207-213.
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, et al. (2009) beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 66: 666-676.
- Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, et al. (2012) Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry 69: 418-427.
- Hatchell PC, Collins AC (1980) The influence of genotype and sex on behavioral sensitivity to nicotine in mice. Psychopharmacology (Berl) 71: 45-49.
- Bonilla-Jaime H, Limón-Morales O, Arteaga-Silva M, Hernández-González M, Guadarrama-Cruz G, et al. (2010) Orchiectomy modifies the antidepressant-like response of nicotine in the forced swimming test. Physiol Behav 101: 456-461.
- Xie P, Kranzler HR, Krauthammer M, Cosgrove KP, Oslin D, et al. (2011) Rare nonsynonymous variants in alpha-4 nicotinic acetylcholine receptor gene protect against nicotine dependence. Biol Psychiatry 70: 528-536.
- Xie P, Kranzler HR, Zhang H, Oslin D, Anton RF, et al. (2012) Childhood adversity increases risk for nicotine dependence and interacts with α5 nicotinic acetylcholine receptor genotype specifically in males. Neuropsychopharmacology 37: 669-676.
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, et al. (2007) Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology 32: 1082-1097.
- Alonso SJ, Castellano MA, Afonso D, Rodriguez M (1991) Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav 49: 69-72.
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT (2007) Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychol Bull 133: 245-272.
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, et al. (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 151: 392-405.
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE (2009) Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 206: 303-312.
- Pauly JR (2008) Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci 13: 505-516.
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, et al. (2005) Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res 7: 91-102.
- Consoli D, Fedotova J, Micale V, Sapronov NS, Drago F (2005) Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test). Eur J Pharmacol 520: 100-107.
- Frye CA, Walf AA (2002) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41: 306-315.
- Gray P, Cooney J (1982) Stress-induced responses and open-field behavior in estrous and nonestrous mice. Physiol Behav 29: 287-292.
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W (2007) Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6: 192-200.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 13954
- [From(publication date):
specialissue-2013 - Jun 03, 2024] - Breakdown by view type
- HTML page views : 9585
- PDF downloads : 4369