Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Case Report
Novel Mutation inPRKAR1A in Carney Complex - Ko Un Park, Hyun-Sook Kim1, Seung Kwan Lee1, Woon-Won Jung2, Yong-Koo Park3
-
Korean Journal of Pathology 2012;46(6):595-600.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.595
Published online: December 26, 2012
Indiana University School of Medicine, Indianapolis, IN, USA.
1Department of Biomedical Science, College of Health Science, Korea University, Seoul, Korea.
2Research Institute of Health Science, College of Health Science, Korea University, Seoul, Korea.
3Department of Pathology, Kyung Hee University School of Medicine, Seoul, Korea.
- Corresponding Author: Yong-Koo Park, M.D. Department of Pathology, Kyung Hee University Hospital, Kyung Hee University School of Medicine, 23 Kyunghee-daero, Dongdaemun-gu, Seoul 130-872, Korea. Tel: +82-2-958-8742, Fax: +82-2-957-0489, 'ykpark@khmc.or.kr'
• Received: February 3, 2012 • Revised: April 17, 2012 • Accepted: May 7, 2012
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- A case of Carney complex in a Korean patient is presented. The patient had the characteristics of Carney complex including skin lesions, positive family history, and multiple myxomas including a superficial angiomyxoma in the perianal area. An extensive genetic analysis revealed a novel mutation in the protein kinase A type I-a regulatory subunit (PRKAR1A) gene, but not in the phosphodiesterase type 11A (PDE11A) gene. This is the first case wherein extensive genetic studies were performed in a patient with Carney complex in Korea.
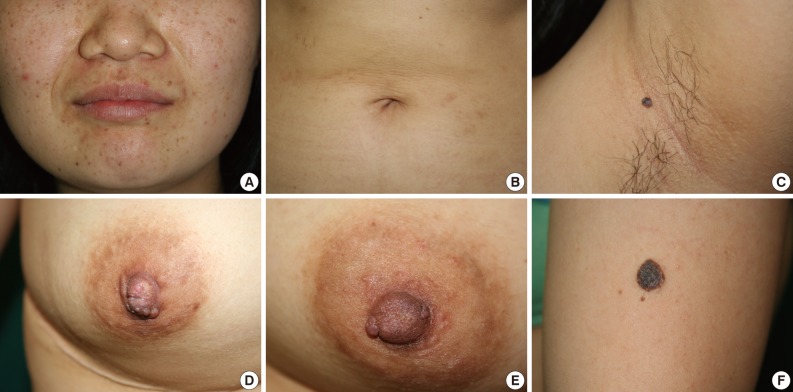
- A 19-year-old woman was admitted due to slowly progressing right hemiparesis. At the time of admission, apart from a slightly drowsy mental status, her presentation was unremarkable with stable vital signs and no past medical history. On physical examination, she had multiple tiny brown pigmented macules on her face and abdomen, and a bean-shaped brownish papule on the axilla and left upper arm. She also had groups of brownish papules on both nipples (Fig. 1). Her mother and elder sister had a history of cardiac myxoma.
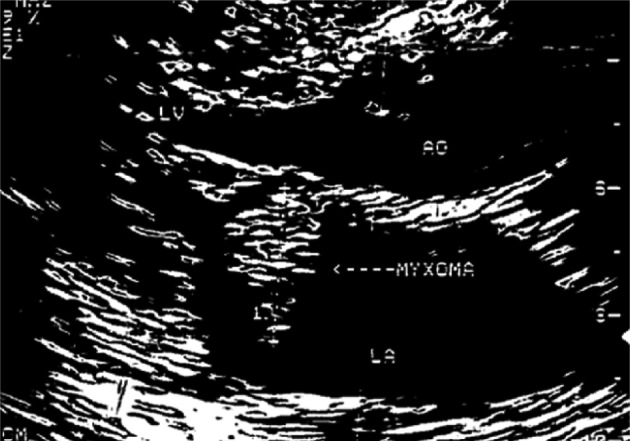
- Magnetic resonance imaging (MRI) of the brain demonstrated diffuse hemorrhagic infarction in the middle cerebral artery territory. Echocardiography showed a 3×2.5×2 cm echogenic mass in her left atrium (Fig. 2). The cardiac mass was resected through an open thoracotomy, and the masses on both nipples were also excised during the operation. The histologic diagnosis of all three masses was myxoma. Her multiple myxomas, skin pigmentation, and familial history of cardiac myxoma were compatible with CNC. Upon evaluation for endocrinologic derangement, the laboratory data including adrenocorticotropic hormone, cortisol, estradiol, and testosterone were within the normal range. Follow-up was planned for the patient with regular echocardiography and ultrasonography (US) on her breasts and thyroid glands.
- During 5 years of follow-up, US demonstrated several isoechoic or hypoechoic nodules in both of her breasts. US-guided core needle biopsy of these lesions revealed fibroadenomas. The number of nodules on her breasts gradually increased, and numerous nodules of various sizes were found on her breasts and axilla. Her thyroid glands had several tiny cystic nodules on both lobes, but the overall number or size of the nodules remained unchanged.
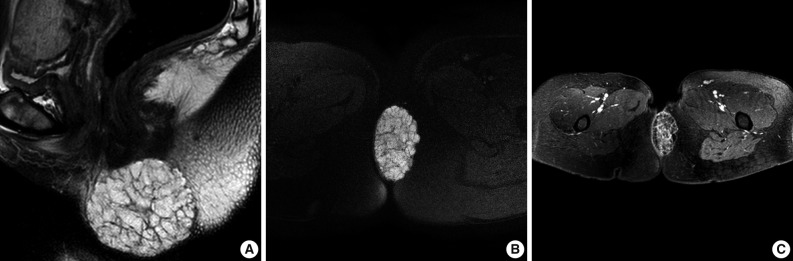
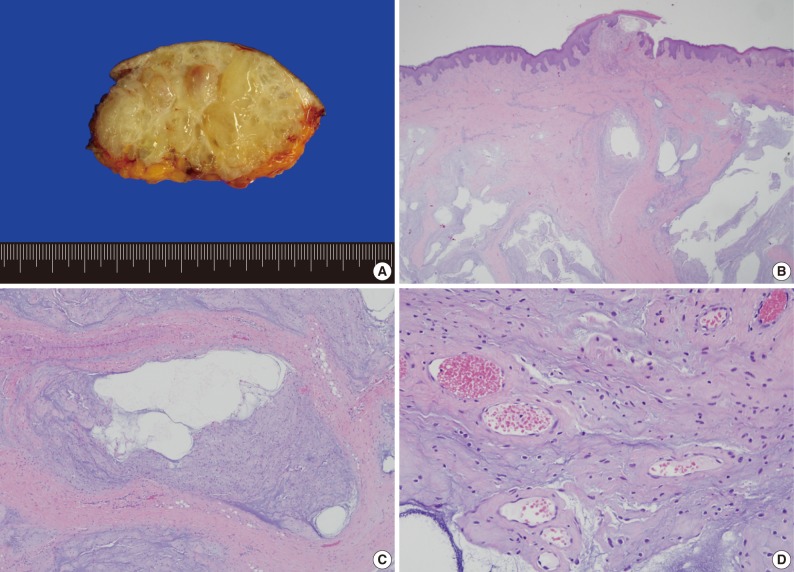
- Most recently, the patient was admitted due to a perianal mass that she had for 1 year. It did not cause any pain, tenderness, or dyschezia. MRI demonstrated a 6.7×5.8×4.0 cm round mass occupying the subcutaneous soft tissue, abutting the inferior border of the internal sphincter. The mass showed a low signal intensity on T1-weighted imaging (WI) and a bright high signal intensity on T2WI, and on fat suppression T2WI. With gadolinium enhancement, the mass revealed multiple enhancing internal septa (Fig. 3). With the presumptive diagnosis of a malignant mucinous neoplasm, surgical resection was performed. Grossly, multiple thin fibrous septa were identified within the gray to white gelatinous surface. Histologic examination of the tumor showed a multilobular growth of myxoid stroma and loosely deposited spindle cells. The thin fibrous septa traversed the tumor parenchyma. Occasional prominent arborizing blood vessels were also identified (Fig. 4). These findings were consistent with superficial angiomyxoma.
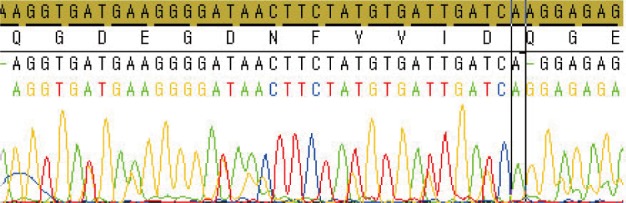
- DNA was extracted using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) from the perianal specimen. Polymerase chain reaction was performed using a thermal cycler (GeneAmp PCR System 9600, Perkin-Elmer, Wellesley, MA, USA) for which the primers of 10 exons of PRKAR1A,2 exon 16 of myosin, heavy chain 8, skeletal muscle, perinatal (MYH8),3 and 7 exons of PDE11A4 were prepared (Table 1). The cycle sequencing procedure was followed by the use of the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's recommendations, and the reaction mixture was analyzed on an ABI Prism 3100 DNA sequencer (Applied Biosystems). The genetic analysis found a novel deletion mutation (c.537delA) in the PRKAR1A gene in exon 6. This resulted in a frameshift mutation and introduced a premature stop codon downstream in exon 7 (Fig. 5). Genetic studies of the PDE11A gene showed mutations in exons 1 and 20, but these mutations were also found in the normal control DNA; we used stored normal DNA that was extracted from a normal volunteer. No mutations were found in the MYH8 gene.
CASE REPORT
- Over 500 patients of diverse ethnicity with CNC from all continents have been registered by the National Institutes of Health (NIH)-Mayo Clinic (USA) and the Cochin Hospital (France), and though this is the largest registry for CNC to date, it does not include patients from a Korean heritage.5 One case of a sporadic form of CNC in a Korean patient that manifested as a rare psammomatous melanotic schwannoma has been reported, in which molecular studies demonstrated a loss of heterozygosity at the 17q22-24 locus.6 However, to our knowledge, our case is the first case of CNC in a Korean patient where extensive genetic studies have been performed.
- CNC is diagnosed in patients with at least two of the main criteria.7,8 In the present case the patient had the characteristics of CNC including skin pigmentation, cardiac myxoma, cutaneous myxoma, breast fibroadenoma, and blue nevus. Cardiac myxomas are the most common noncutaneous lesions occuring in the context of CNC.7 The mass can occur in multiples with recurrences, and it does not have a predilection for the left atrium though it did occur in the left atrium of the present case.9
- A study of 353 patients in the CNC consortium found that CNC can be further classified into 3 subgroups based on the PKA R-Ia (PRKAR1A) gene mutation status and the presence of primary pigmented nodular adrenocortical disease (PPNAD).10 Although CNC shows vast genetic heterogeneity, the disease can be grouped based on phenotype to genotype correlation. According to this subclassification, the carriers of the PRKAR1A mutation were grouped as CNC1, and this phenotype correlated with disease occurrence at a younger age with a higher frequency of myxomas, schwannomas, and thyroid and gonadal tumors than those without the mutation. Based on these criteria, the present case would be classified as CNC type 1 given the PRKAR1A mutation. CNC type 2 shows a strong linkage to the short arm of chromosome 2 (2p16), but is beyond the context of this paper and was not further studied.
- The PRKAR1A gene encodes the type 1A regulatory subunit of the cAMP-dependent protein kinase, or PKA.11 To date, a total of 117 different PRKAR1A mutations have been identified (online database: http://prkar1a.nichd.nih.gov) in 387 unrelated families of various ethnic origins.12 PRKAR1A mutations are spread along the entire coding sequence without predilection for a particular exon, although several 'hot spots' for sequence changes have been identified. Not surprisingly, the novel mutation found in our patient occurred in exon 6, which encompasses part of the cAMP functional domain. The previously mentioned CNC consortium also found that although numerous pathogenic variants have been identified, the majority of PRKAR1A defects were premature stop codons generated by nonsense or frameshift mutations.10 The instability introduced from the premature stop codon leads to nonsense mRNA mediated decay and results in either an absence or reduction in the mutant protein level.10,13 Much like the majority of the PRKAR1A mutations that have been identified, the mutation type of the novel mutation found in our case is a premature stop codon. Interestingly, the premature stop codon did not result directly from the amino acid change in exon 6, but rather downstream in exon 7 due to the frameshift change.
- However, despite these extensive genetic studies on PRKAR1A, its role in this complex is largely unknown. Given the multiple interactions with major signaling pathways and sometimes opposing effects on cellular functions, PRKAR1A is not a tumor suppressor gene in its classic sense.14 Thus, the exact role of PRKAR1A in tumorigenesis remains an area for further investigation.
- Among the endocrinologic manifestations of CNC, the most common involves the adrenal gland, more specifically in the form of PPNAD.1,15 This is a rare cause of Cushing's syndrome that is pituitary-independent but adrenal-dependent.1,15,16 The genetic mutation culprit linked to PPNAD has been identified as protein-truncating mutations of PDE11A.17 PDE11A is expressed by zona fasciculata cells in the adrenal cortex that mainly produce cortisol. CNC patients without PPNAD had PDE11A sequence variants not significantly different in frequency from healthy controls.4 Not surprisingly, our patient did not have PPNAD, but had PDE11A sequence variations that are commonly found in the control group of healthy adults.
- Another unique aspect of this case was the indolent perianal mass. Previous case series have failed to establish CNC in patients with superficial angiomyxoma, thus concluding that CNC is only weakly linked to these types of myxomas.18 Superficial angiomyxomas, also called cutaneous myxomas, that have been previously described in association with CNC were often small (less than 1 cm in diameter) and occurred most commonly in the eyelids, ears, and nipples.19 Superficial angiomyxomas could range from very small sessile papules to large pedunculated polypoid masses. The present case is noteworthy because the superficial angiomyxoma was large and occurred in the deep internal anal sphincter area, which is an unusual location.
- In conclusion, the presence of multiple myxomas and lentiginosis should be an indication to clinicians to further investigate for the presence of CNC. Numerous advancements in genetic studies have helped identify the PRKAR1A mutation as the culprit. As of now, several hundreds of various genetic mutations in PRKAR1A have been identified including our novel deletion mutation (c.537delA) in exon 6. Despite the numerous genetic mutations identified in recent years, new treatment for the disease remains to be elucidated. Understanding how the diverse mutations converge into a common syndrome of phenotypes may be critical in elucidating a cure.
DISCUSSION
- 1. Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985; 64: 270–283. PMID: 4010501. ArticlePubMed
- 2. Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet 2000; 9: 3037–3046. PMID: 11115848. ArticlePubMed
- 3. Veugelers M, Bressan M, McDermott DA, et al. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. N Engl J Med 2004; 351: 460–469. PMID: 15282353. ArticlePubMed
- 4. Libe R, Horvath A, Vezzosi D, et al. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab 2011; 96: E208–E214. PMID: 21047926. ArticlePubMedPMC
- 5. Rothenbuhler A, Stratakis CA. Clinical and molecular genetics of Carney complex. Best Pract Res Clin Endocrinol Metab 2010; 24: 389–399. PMID: 20833331. ArticlePubMed
- 6. Kim MJ, Choi J, Khang SK, Kim JS, Lee JS, Cho KJ. Primary intraosseous melanotic schwannoma of the fibula associated with the Carney complex. Pathol Int 2006; 56: 538–542. PMID: 16930334. ArticlePubMed
- 7. Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 2001; 86: 4041–4046. PMID: 11549623. ArticlePubMed
- 8. Stratakis CA, Kirschner LS, Carney JA. Carney complex: diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas. Am J Med Genet 1998; 80: 183–185. PMID: 9805140. ArticlePubMed
- 9. Boikos SA, Stratakis CA. Carney complex: the first 20 years. Curr Opin Oncol 2007; 19: 24–29. PMID: 17133108. ArticlePubMed
- 10. Almeida MQ, Stratakis CA. Carney complex and other conditions associated with micronodular adrenal hyperplasias. Best Pract Res Clin Endocrinol Metab 2010; 24: 907–914. PMID: 21115159. ArticlePubMedPMC
- 11. Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 2000; 26: 89–92. PMID: 10973256. ArticlePubMed
- 12. Horvath A, Bertherat J, Groussin L, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat 2010; 31: 369–379. PMID: 20358582. ArticlePubMedPMC
- 13. Kirschner LS. PRKAR1A and the evolution of pituitary tumors. Mol Cell Endocrinol 2010; 326: 3–7. PMID: 20451576. ArticlePubMedPMC
- 14. Bossis I, Voutetakis A, Bei T, Sandrini F, Griffin KJ, Stratakis CA. Protein kinase A and its role in human neoplasia: the Carney complex paradigm. Endocr Relat Cancer 2004; 11: 265–280. PMID: 15163302. ArticlePubMed
- 15. Carney JA, Young WF Jr. Primary pigmented nodular adrenocortical disease and its associated conditions. Endocrinologist 1992; 2: 6–21. Article
- 16. Park YK, Kim YW, Kim JW, et al. Bilateral primary pigmented nodular adrenocortical disease: a case of report describing a rare cause of Cushing's syndrome. J Korean Med Sci 1994; 9: 450–457. PMID: 7786440. ArticlePubMedPMC
- 17. Horvath A, Boikos S, Giatzakis C, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 2006; 38: 794–800. PMID: 16767104. ArticlePubMed
- 18. Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components: report of 30 tumors in 28 patients. Am J Surg Pathol 1988; 12: 519–530. PMID: 3389450. ArticlePubMed
- 19. Carney JA, Headington JT, Su WP. Cutaneous myxomas: a major component of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Arch Dermatol 1986; 122: 790–798. PMID: 3729510. ArticlePubMed
References
Fig. 1Pigmented skin manifestations. Generalized scattered brownish macules on the face and abdomen (A, B). A solitary bean-sized pedunculated papule with a blue-grayish hue in the left axilla (C). Grouped brownish papules with a lobulated contour on both nipples (D, E). A solitary coin-sized, dark-brownish patch on the left upper arm (F).


Fig. 3(A-C) Magnetic resonance imaging demonstrated a 6.7×5.8×4.0 cm multilocular cystic mass with multiple septa in the perianal area. The mass shows a bright high signal intensity on both T2-weighted imaging (T2WI) and fat suppression T2WI.


Fig. 4Grossly, the mass is well circumscribed and has a gray to white, gelatinous cut surface with multiple thin fibrous septa (A). Histologically, the tumor shows a multilobular appearance with an infiltrative border (B). The fibrous septa traverse the tumor into multiple lobules (C). Spindled to stellate-shaped tumor cells are loosely deposited in a myxoid stroma. Prominent vasculature is identified in this area (D).


Figure & Data
References
Citations
Citations to this article as recorded by 

- Structures of the PKA RIα Holoenzyme with the FLHCC Driver J-PKAcα or Wild-Type PKAcα
Baohua Cao, Tsan-Wen Lu, Juliana A. Martinez Fiesco, Michael Tomasini, Lixin Fan, Sanford M. Simon, Susan S. Taylor, Ping Zhang
Structure.2019; 27(5): 816. CrossRef - Carney Complex with Multiple Cardiac Myxomas, Pigmented Nodular Adrenocortical Hyperplasia, Epithelioid Blue Nevus, and Multiple Calcified Lesions of the Testis: A Case Report
Hyunchul Kim, Hyun-Yee Cho, Jeong Nam Lee, Kook-Yang Park
Journal of Pathology and Translational Medicine.2016; 50(4): 312. CrossRef

 E-submission
E-submission



 PubReader
PubReader Cite this Article
Cite this Article