- Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA
- Victor Horsley Department of Neurosurgery, National Hospital for Neurology and Neurosurgery, London, UK
- Division of Neurosurgery, University of California, San Diego, California, USA
- Department of Surgery, Division of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA
Correspondence Address:
Ekkehard M. Kasper
Department of Surgery, Division of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA
DOI:10.4103/2152-7806.194816
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shahzad Shaefi, Aaron M. Mittel, Jonathan A. Hyam, M. Dustin Boone, Clark C. Chen, Ekkehard M. Kasper. Hypothermia for severe traumatic brain injury in adults: Recent lessons from randomized controlled trials. 28-Nov-2016;7:103
How to cite this URL: Shahzad Shaefi, Aaron M. Mittel, Jonathan A. Hyam, M. Dustin Boone, Clark C. Chen, Ekkehard M. Kasper. Hypothermia for severe traumatic brain injury in adults: Recent lessons from randomized controlled trials. 28-Nov-2016;7:103. Available from: http://surgicalneurologyint.com/surgicalint_articles/hypothermia-for-severe-traumatic-brain-injury-in-adults-recent-lessons-from-randomized-controlled-trials/
Abstract
Background:Traumatic brain injury (TBI) is a worldwide health concern associated with significant morbidity and mortality. In the United States, severe TBI is managed according to recommendations set forth in 2007 by the Brain Trauma Foundation (BTF), which were based on relatively low quality clinical trials. These guidelines prescribed the use of hypothermia for the management of TBI. Several randomized controlled trials (RCTs) of hypothermia for TBI have since been conducted. Despite this new literature, there is ongoing controversy surrounding the use of hypothermia for the management of severe TBI.
Methods:We searched the PubMed database for all RCTs of hypothermia for TBI since 2007 with the intent to review the methodology outcomes of these trials. Furthermore, we aimed to develop evidence-based, expert opinions based on these recent studies.
Results:We identified 8 RCTs of therapeutic hypothermia published since 2007 that focused on changes in neurologic outcomes or mortality in patients with severe TBI. The majority of these trials did not identify improvement with the use of hypothermia, though there were subgroups of patients that may have benefited from hypothermia. Differences in methodology prevented direct comparison between studies.
Conclusions:A growing body of literature disfavors the use of hypothermia for the management of severe TBI. In general, empiric hypothermia for severe TBI should be avoided. However, based on the results of recent trials, there may be some patients, such as those in Asian centers or with focal neurologic injury, who may benefit from hypothermia.
Keywords: Critical care, expert opinion, hypothermia, traumatic brain injury, TBI
INTRODUCTION
Traumatic brain injury (TBI), broadly defined as “an alteration in brain function, or other evidence of brain pathology, caused by an external force,”[
Severe TBI is most frequently defined by a score of less than or equal to 8 on the Glasgow Coma Scale,[
The BTF guidelines do not provide definitive instructions for the use or avoidance of induced hypothermia for patients with severe TBI. Rather, similar to the remainder of the BTF stipulations, the recommendations pertaining to hypothermia are based on relatively low quality data and conflicting results of prior clinical trials. However, hypothermia has remained appealing because it confers several theoretical benefits in severe TBI, including the ability to reduce ICP, increase cerebral perfusion pressure, reduce cerebral oxygen consumption, reduce concentrations of excitatory neurotransmitters and inflammatory mediators in cerebrospinal fluid, and possibly maintain the integrity of the blood–brain barrier. Early trials in the 1990s showed promising results with employing hypothermia, though these were not always sustained in later studies.[
Given the discordant findings of available evidence at the time of the creation of the 2007 guidelines, the BTF authors performed a meta-analysis of randomized, controlled trials of moderate quality (there were no good quality trials), in an effort to create meaningful recommendations. Ultimately, their meta-analysis determined that the use of prophylactic hypothermia was not associated with a significant improvement in mortality, and thus cannot be recommended as a routine component of care for severe TBI. However, on secondary analysis, hypothermia maintained for more than 48 hours was associated with lower risk of death, an observation that was independent of the target temperature or the rate of re-warming. Furthermore, prophylactic hypothermia was associated with improved neurologic outcome, particularly when the target temperature was 32–33°C or 33–35°C. For both mortality and neurologic outcome scores, hypothermia was associated with better results when examining studies performed in single institutions rather than multicenter studies. The presence of hypothermia at the time of admission may have confounded the interpretation in analyzed trials, in part due to rather poorly understood physiologic effects of rewarming or continued hypothermia, and the possibility that hypothermia on admission may lead to sedation and therefore a relatively low Glasgow Coma Scale score despite the presence of less serious injuries compared to normothermic patients with the same score.[
The paucity of high quality studies and the observation that hypothermia may be associated with improved outcomes depending on duration, target temperature, rewarming rate, and time to initiation as well as center of the respective study, led to uncertainty regarding the significance of the 2007 guidelines. Subsequently, several RCTs of hypothermia for TBI have since been conducted, but these have also not been able to provide a basis for consensus.
MATERIALS AND METHODS
This review of recent trials and updated meta-analyses provides insight into the ongoing uncertainty surrounding the use of hypothermia for severe TBI. Expert opinions at the end of this review inform and provide recommendations for clinicians. Publications were selected for inclusion by searching the PubMed database in April 2016 using the following search terms: “(hypothermia) AND (traumatic brain injury),” limited to RCTs, meta-analyses, or systematic reviews conducted on adult (age more than 19 years) patients which were available in English. Studies published prior to 2007 were omitted from further review, as they had been analyzed within the aforementioned BTF guidelines and subsequent systematic reviews. This search strategy yielded 27 publications of interest. After reviewing the abstracts, we excluded feasibility studies, protocol descriptions of ongoing trials, or those performing post-hoc analysis of previously published trials of hypothermia. We also excluded those that involved pediatric populations, did not focus on TBI outcomes, or did not publish mortality or neurologic function outcomes. This approach excluded 20 of the original 27 publications; the remaining 7 manuscripts were included in the study. Review of the respective references of these studies identified one additional trial which was also appropriate for inclusion.[
RESULTS AND DISCUSSION
Review of randomized controlled trials of hypothermia for traumatic brain injury
Following the publication of the aforementioned meta-analyses, two trials in 2009 were performed at single center institutions that had previous experience with hypothermia for TBI. Both trials were conducted in an effort to precisely determine the influence of hypothermia within specific contexts. The first of these trials, conducted at a center in China which had routinely used 48–72 hours of induced hypothermia to 33°C from 1994 to 1999, investigated the impact of more moderate cooling in similar patients enrolled after the year 2000. Hypothermia in the more recent study population was induced for 48–72 hours, though only to 35°C. The authors found that mean ICP did not significantly differ between the groups, though CRP levels were lower in the 35°C group. Complication rates were comparable in both the groups, leading the authors to conclude that more moderate hypothermia was equally effective for ICP control and may be associated with less inflammatory response. Nevertheless, it is hard to interpret outcome data in these patients given the potential for changes in other aspects of critical care treatment (e.g. early goal-directed sepsis treatment) during the time between each group's enrolment.[
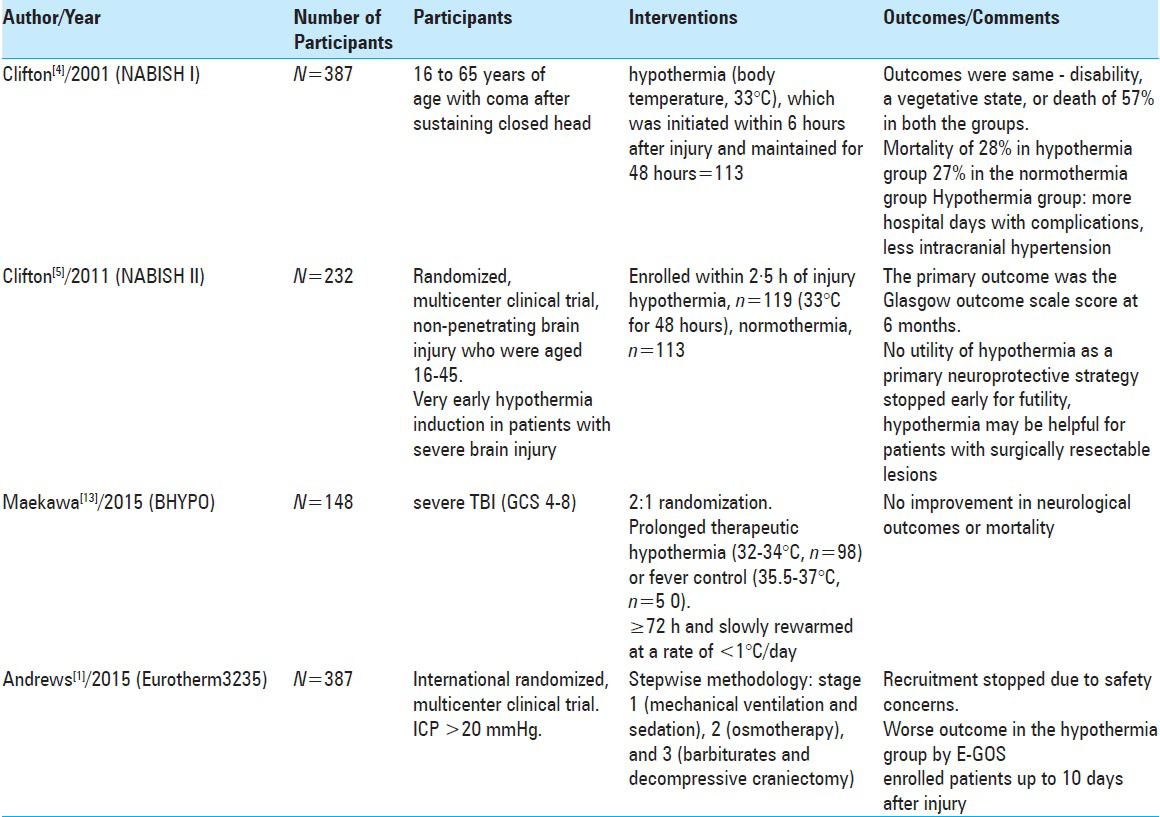
In 2001, the NABISH I trial identified a possible trend toward improved outcome in patients who were hypothermic at the time of admission when treated with ongoing hypothermia. The fact that a higher rate of hypotension was observed in the hypothermic group in NABISH I was thought to possibly confound the findings, preventing the identification of a durable improvement in outcome.[
These findings are in contrast to another Chinese RCT also published in 2011 that determined that hypothermia led to favorable neurologic outcome. In this study, hypothermia was induced within hours of enrolment (though not necessarily within hours of injury) to a goal of 32–33°C for at least 72 hours. However, this was a relatively small cohort study (81 patients) with the primary objective to examine mean glucose and lactate levels between hypothermic and euthermic groups rather than to identify neurologic or mortality benefit from hypothermia. Both laboratory parameters were found to be lower in the hypothermic group – a finding that is more likely to be spurious.[
More recently, two relevant trials (BHYPO and EuroTherm3235) were published in 2015 and have added to the emerging concerns surrounding hypothermia for TBI. BHYPO was a multicenter Japanese trial in which patients were rapidly cooled (32–34°C) or kept euthermic (35.5–37°C) within 6 hours of injury. This trial was notable for a relatively long duration of cooling of at least 72 hours and a slow rewarming speed (<1°C/day). Only 150 of a planned 300 patients were enrolled from 2002 to 2008; the trial was stopped early due to a combination of low enrolment and likely futility. There were no significant differences in neurologic outcome (the primary outcome of interest) or mortality (secondary outcome) between groups, even though the trial was relatively underpowered.[
Meta-analyses of these results in addition to those of prior well-conducted trials have yet to be performed. As of now, a 2014 systematic review by Crossley found that treatment with hypothermia in TBI patients is associated with a reduced risk of mortality or poor outcome. This finding is in direct contradiction to the results of most of the prior meta-analyses, and thus may be perceived with some skepticism. It must be noted that Crossley's review contained a relatively low number of well-conducted trials, and hence could not exclude the possibility of bias affecting the results.[
Ultimately, well-designed RCTs with therapeutic hypothermia below 35°C for severe TBI have mostly failed to show a significant improvement in mortality rates. It is important to note that hyperthermia is significantly associated with poor outcomes, and thus temperature control remains a critical component of neurointensive care. Theoretically, modest cooling (i.e., 35–37°C) may provide some of the putative neuroprotective effects of hypothermia while avoiding sequelae with a negative impact on outcome. Unfortunately, as of now there are no RCTs evaluating the effect of modest cooling compared to normothermia.[
Expert opinions
As there is a lack of consensus among experts regarding the use of hypothermia for the management of severe TBI, the authors advocate that before considering induction of hypothermia for severe TBI, the following viewpoints should be taken into account:
Dr. Clark Chen, Department of Neurosurgery; University of California San Diego (USA): Hypothermia should be avoided when treating severe TBI.
Hypothermia for severe TBI is an attractive therapy, but has repeatedly failed to meet the expectations of clinicians hoping to improve a patient's chance of death or neurologic outcome. The controversy surrounding the use of hypothermia is driven by the occasional report of clinical benefit of cooling in one trial versus harm in another. However, it is of utmost importance to recognize that there has yet to be a well-designed clinical trial that definitively favors the use of hypothermia. The large, well-conducted trials published within the last 5 years, specifically NABISH II, BHYPO, and EuroTherm3235, have all convincingly proven that moderate hypothermia is associated with deterioration in neurologic outcomes and an increase in mortality. Operating on the basis of these results, therapeutic hypothermia for the empiric treatment of severe TBI should be reserved for experimental use only, pending results from forthcoming studies.
Hypothermia on admission after TBI has been shown to be a predictor of poor outcome. In a retrospective cohort study of 110,000 admissions to 384 ICUs across UK and Australasia with stroke, TBI, or intracranial infection, peak temperature below 37°C within the first 24 hours of admission after TBI was associated with an increased risk of death compared to normothermia.[
Sometimes we do things in medicine not because it works well but because there are frankly no better options. Hypothermia as treatment for severe traumatic brain injury (TBI) patients is a case in point. It is a fact that the four well-designed, albeit admittedly imperfect, RCTs (NABISH I, NABISH II, B-HYPO, Eurotherm3235) summing to >1100 enrolled patients showed that hypothermia is not effective in improving the clinical outcome of severe TBI patients when the trial results were assessed based on the predetermined statistical measures and primary end-points. No amount of post-hoc analysis, meta-analysis, statistical manipulation, or intellectual rationalization changes this fact. It is also a fact that RCTs are designed to yield evidence when the outcomes are strictly interpreted based on the primary end-points. While it is acceptable to use post-hoc analysis as means of hypothesis generation, it is not acceptable to suggest that these exploratory observations are conclusive. When evaluated in this context, the conclusion from the available RCTs is necessarily that treatment with hypothermia do not significantly alter the clinical outcome of severe TBI patients. That said, nothing modern medicine offers alter the clinical course of severe TBI patients. In this context, hypothermia remains a treatment option. There will always be patients who show remarkable recovery from a severe TBI, hypothermia, or not. The fundamental question in the modern era of health care cost-containment is how many well-designed (and necessarily imperfect) RCTs will it take to divert financial resources from an ineffectual treatment into another societal need? How many prayers must go unanswered before a faith is abandoned?
Dr. Jonathan Hyam, National Hospital for Neurology and Neurosurgery, Queen Square; London (UK): Hypothermia is an acceptable component of care for the patients with severe TBI.
Despite the high morbidity and mortality associated with TBI, the number of therapies available to the neurotraumatologist is limited and their indications for optimal use unclear. As such, therapeutic hypothermia should not be dismissed without strong evidence against its place within the medical armamentarium.
The results of NABISH II, BHYPO, and EuroTherm3235 have all seemingly added to the growing consensus that use of hypothermia for the patient who has suffered severe TBI is harmful. However, each of these trials (and their predecessors) have sought to assess changes in mortality or neurologic outcome using disparate approaches to the management of severe TBI, including the implementation of hypothermia. Specifically, the questions of when to induce hypothermia, how cold to target, and long to cool the patient have never been precisely answered [
Furthermore, there are specific clinical scenarios in which therapeutic hypothermia can tip the balance toward the patient and clinician. An example is in intractably raised ICP. In a patient who has raised ICP despite basic therapy (including removal of any evacuatable mass lesions, sedation with targeted pCO2 control, etc.) the advanced therapeutic options become much narrower.
EuroTherm 3235 concluded that the hypothermia group's neurological outcome was poorer than the control group when patients with an ICP >20 mmHg for more than 5 minutes were entered into the trial and indeed the study was stopped prematurely. Although an excellently executed trial, there are several limitations, many of which the authors acknowledge. First, therapeutic hypothermia was used alone in the intervention group as a second-line of therapy, without “Stage 2” therapy, i.e., hyperosmolar agents/inotropes, whereas the control group received these as a second-line of therapy. Second, in the hypothermia group there were far fewer first occurrences of raised ICP, suggesting a beneficial effect at least on ICP. As a result of this, fewer hypothermia patients received the benefits of hyperosmolar therapy.
The authors acknowledge that the study did not address patients with intractable raised ICP resistant to stage 2 therapy. Significantly fewer patients in the hypothermia group received barbiturates. The neuroprotective properties of barbiturate therapy may, therefore, have skewed the neurological outcome results away from the hypothermia group.
Therapeutic hypothermia should therefore be considered as part of a multimodal treatment in addition to hyperosmolar agents and inotropes, not as a substitute for them. If control over ICP is not obtained after basic interventions, there is a danger of progression in a vicious cycle of high pressure, vascular and parenchymal compression, ischemia and further increases in ICP/swelling. If this cycle can be interrupted in a timely fashion with a combination of available second-line therapies, this could avoid progression toward the most aggressive therapies such as decompressive craniectomy and the risk of the significant adverse effects associated with this intervention.
Recommendations
The lack of available high quality evidence is a reflection of the challenges associated with treating severe TBI. Pragmatic study designs have led to discrepancies in approaches to hypothermia implementation and toward goals to be achieved. This applies to the question as to when (e.g., within hours of injury or within 10 days of injury), over what period (e.g., 24, 48, 72, or more hours), or to what target value (ICP) or outcome measure (e.g., improvement in neurologic outcome, or reduction in mortality) hypothermia should be induced.
EXECUTIVE SUMMARY
Well-designed studies which have been rigorously executed are only few in number. However, those that were incorporated in the 2007 BTF guidelines or have been published since generally do not favor use of hypothermia for severe TBI. Specific subgroups of patients, such as those with focal hematomas that can be surgically removed or perhaps patients treated in Asian centers, may benefit from moderate (32–35°C) hypothermia, if such treatment is implemented early and maintained for at least 48 hours. However, this remains speculative at this point. At present, neurointensivists should keep temperature control at their focus with the intent to avoid hyperthermia while limiting hypothermia unless ICP is exceedingly difficult to control.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015. 373: 2403-12
2. Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012. 9: CD004128-
3. Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007. 24: S1-106
4. Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001. 344: 556-63
5. Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): A randomised trial. Lancet Neurol. 2011. 10: 131-9
6. Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care. 2014. 18: R75-
7. El-Fiki M. The need for WFNS standard simplified guidelines for the management of severe traumatic brain injuries. World Neurosurg. 2011. 75: 458-61
8. Ghajar J. Traumatic brain injury. Lancet. 2000. 356: 932-9
9. Harris OA, Muh CR, Surles MC, Pan Y, Rozycki G, Macleod J. Discrete cerebral hypothermia in the management of traumatic brain injury: A randomized controlled trial. J Neurosurg. 2009. 110: 1256-64
10. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2008. 22: 341-53
11. Lei J, Gao G, Mao Q, Feng J, Wang L, You W. Rationale, methodology, and implementation of a nationwide multicenter randomized controlled trial of long-term mild hypothermia for severe traumatic brain injury (the LTH-1 trial). Contemp Clin Trials. 2015. 40: 9-14
12. Li P, Yang C. Moderate hypothermia treatment in adult patients with severe traumatic brain injury: A meta-analysis. Brain Inj. 2014. 28: 1036-41
13. Maekawa T, Yamashita S, Nagao S, Hayashi N, Ohashi Y. Brain-Hypothermia Study Group. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: A randomized controlled trial. J Neurotrauma. 2015. 32: 422-9
14. Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997. 336: 540-6
15. Menon DK, Schwab K, Wright DW, Maas AI. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: Definition of traumatic brain injury. Arch Phys Med Rehabil. 2010. 91: 1637-40
16. Nichol A, Gantner D, Presneill J, Murray L, Trapani T, Bernard S. Protocol for a multicentre randomised controlled trial of early and sustained prophylactic hypothermia in the management of traumatic brain injury. Crit Care Resusc. 2015. 17: 92-100
17. Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: A systematic review and meta-analysis. J Neurotrauma. 2008. 25: 62-71
18. Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database Sys Rev. 2012. 12: CD000033-
19. Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013. 9: 231-6
20. Saxena M, Andrews PJ, Cheng A, Deol K, Hammond N. Modest cooling therapies (35-37.5) for traumatic brain injury. Cochrane Database Sys Rev. 2014. 8: CD006811-
21. Saxena MKLast accessed on 2014 Oct 26. Details available at https://clinicaltrials.gov/ct2/show/NCT01231139 .
22. Saxena M, Young P, Pilcher D, Bailey M, Harrison D, Bellomo R. Early temperature and mortality in critically ill patients with acute neurological diseases: Trauma and stroke differ from infection. Intensive Care Med. 2015. 41: 823-32
23. Schierhout G, Roberts I. Hyperventilation therapy for acute traumatic brain injury. Cochrane Database Sys Rev. 2000. 2: CD000566-
24. Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Sys Rev. 2009. 2: CD001048-
25. Taylor CA, Greenspan AI, Xu L, Kresnow M. Comparability of national estimates for traumatic brain injury-related medical encounters. J Head Trauma Rehabil. 2015. 30: 150-9
26. Tokutomi T, Miyagi T, Takeuchi Y, Karukaya T, Katsuki H, Shigemori M. Effect of 35 degrees C hypothermia on intracranial pressure and clinical outcome in patients with severe traumatic brain injury. J Trauma. 2009. 66: 166-73
27. Wagner AK, Fabio A, Puccio AM, Hirschberg R, Li W, Zafonte RD. Gender associations with cerebrospinal fluid glutamate and lactate/pyruvate levels after severe traumatic brain injury. Crit Care Med. 2005. 33: 407-13
28. Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Sys Rev. 2013. 8: CD001049-
29. Zhao QJ, Zhang XG, Wang LX. Mild hypothermia therapy reduces blood glucose and lactate and improves neurologic outcomes in patients with severe traumatic brain injury. J Crit Care. 2011. 26: 311-5