Integrative and complementary therapies for patients with advanced cancer
Introduction
One in four deaths is due to cancer, and the lifetime probability of developing cancer is 45% for men and 38% for women. Lung, prostate, breast and colorectal cancers account for 48% of cancers. Palliative care is especially needed in the care of these patients (1). Palliative care, combined with integrative holistic medicine and conventional cancer care, can create a very effective approach to treating the whole person with cancer. This integration of care modalities addresses all appropriate treatments of the cancer, alleviating the challenging symptoms of cancer and its treatment, as well as helping patients thrive rather than merely survive their cancer journey. In integrative medicine, well-being is emphasized, and in palliative care, quality of life (QOL) is a similar concept or goal. Both can occur despite advanced cancer. Integrative medicine serves to combine the best of alternative, complementary and conventional therapies to optimize well-being and QOL, whether or not a person is at the end of life. When integrative medicine is combined with palliative care modalities, the toolbox to provide symptom control and well-being or QOL is increased or broadened. Palliative care and integrative medicine are best provided early in the trajectory of illness such as cancer, and increase in amount as the illness progresses toward end of life. Goals of care change as the disease progresses, and a patient’s unique situation creates a different balance of integrative and conventional therapies. This review focuses on how integrative and complementary modalities can be included in comprehensive palliative care for patients with advanced malignancies.
Higher quality integrated cancer services can result in cost savings and can enhance cancer care. In palliative care research, it is recognized that higher costs and more conventional interventions do not necessarily increase the quality of care and often decrease it with unnecessary interventions with their own adverse effects (2). Inpatients of the oncology unit at Beth Israel Medical Center were part of an integrative medicine study called the Urban Zen Initiative. This included yoga therapy, holistic nursing techniques and attention to a healing environment. Cost data was analyzed via the electronic medical record and cost database. Eighty-five patients were in the non-intervention group and 72 in the intervention group. Researchers found that the integrative medicine group used fewer medications for symptoms, which resulted in substantial cost savings (3). In another retrospective cohort study of cancer survivors, those who used complementary and alternative medicine (CAM) therapies combined with conventional therapies were compared with those who did not use CAM. Baseline characteristics from a survey were compared with measurements from a chart review six months later. Results indicated that infections and hospitalizations were significantly reduced with CAM use (4). Many CAM therapies such as music, aromatherapy, exercise, relaxation, and mindfulness practices are inexpensive, accessible, effective, and easily incorporated into holistic and integrated treatment plans in conventional cancer centers and in palliative care services.
In a study of 17 patients who use CAM and conventional modalities and 20 alternative practitioners, themes of holism, empowerment, access and legitimization emerged from the analysis of semi-structured interviews. Conventional modalities were often more accessible to those who had insurance and were often more socially legitimate but often left patients disempowered with fragmented care. CAM modalities, because they were often paid out of pocket, felt less accessible, less legitimate by conventional medicine standards, but were more holistic and empowering. All who participated advocated for the integration of both conventional and CAM approaches (5).
Integrative and complementary modalities are commonly used by patients with advanced malignancies but often not reported to conventional medicine practitioners. Reasons for this vary considerably, and conventional practitioners may consider integrative modalities as interfering with conventional therapies, ineffective, a source of false hope, poorly researched, or harmful. Many practitioners also question the quality of research done on integrative modalities. Resources such as Inspire Health, an organization that provides quality reviews of the latest research in integrative cancer care, do provide patients and practitioners important evidence based information in addressing concerns of patients such as symptom management from advanced cancer and cancer treatments. Uninformed use of CAM therapies such as using an herb with a chemotherapy agent that interacts negatively with that herb can lead to toxicities of the conventional drug, or render it less effective. Who will inform the patient? Oncologists, palliative care practitioners, integrative health practitioners, and other health professionals can incorporate evidence based CAM modalities into their palliative and cancer care, and serve patients in a more open-minded and empowering way. If patients are doing their own research, clinicians can invite them to share their evidence and critically examine their data and come to a plan of care that is safe and effective. Left on their own, patients especially with cancer, are vulnerable to anecdotal, non-evidence based claims for CAM modalities that are potentially useless, expensive and harmful, and they may not be directed to sources of information on CAM modalities that are effective and more appropriate for a particular indication. The Society of Integrative Oncology has developed guidelines on evidence-based integrative oncology practice (6,7) (Table 1).
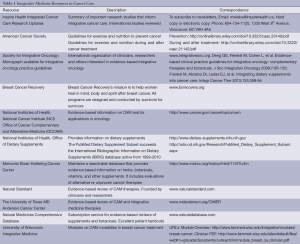
Full table
Integrative modalities increase the number of available options in the palliative care toolbox to treat patients with advanced cancer. The plan of care meetings common in palliative care can include queries on what patients feel are important in their cancer care, and this may include integrative modalities.
A comprehensive review of all effective integrative and complementary therapies is impossible given the enormous diversity in this area. This review will concentrate on modalities such as nutrition, movement, mind-body modalities, music, aromatherapy, massage, select supplements, and acupuncture that have been recently researched in cancer survivors to give readers the most up to date research findings. Many of these modalities are quite effective for a number of symptoms in palliative care and have been extensively studied in non-cancer populations. CAM modalities used in relieving common symptoms addressed in palliative care are numerous and include systematic reviews of useful research in the Cochrane database and in peer reviewed journals (Table 2).
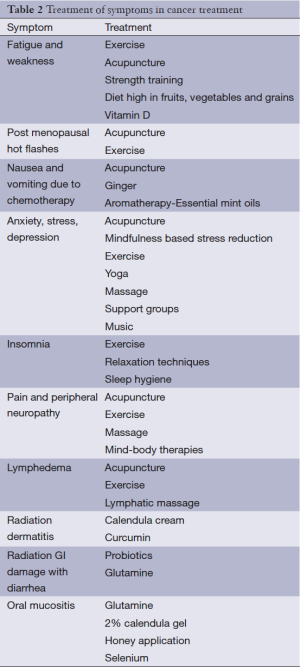
Full table
Nutrition
The American Cancer Society convened a panel of experts to review the research on nutrition and exercise for cancer survivors. Their first report and guidelines were published in 2006 and updated in 2012. Overall, the guidelines recommend maintaining a healthy weight, engaging in regular exercise including movement and strength training, and eating a diet high in fruits, vegetables and whole grains. Regular exercise may reduce cancer recurrence and cancer related mortality, increase QOL, decrease fatigue, anxiety, depression, and increase muscle strength and tone, and fitness. The guidelines also provide cautions about exercise during cancer treatment, such as avoiding pools and exercise in public places when immune deficient, avoiding chlorine exposure to irradiated skin, and paying special attention to balance if experiencing peripheral neuropathy. Food sources of nutrients are usually superior to supplement intake, and supplements not adequately found in foods such as vitamin D need to be carefully supplemented. The guidelines cover specific cancers and often-asked questions about controversial topics such as supplements. This is a well referenced evidence-based resource for patients and clinicians (8). Despite the existence of a large evidence-based literature on the benefits of healthy diet in cancer survivors, in one study, not one patient of 40 recruited received information from conventional clinicians on healthy eating (9).
A study by Zick et al. reported in 2013 examined associations of diet and fatigue in cancer survivors; those that had higher intake of whole grains, vegetables, particularly green leafy vegetables and tomatoes, reported less fatigue than those that had lower intake of these foods (10). Cruciferous vegetable intake in a 2012 study by Richman et al. decreased progression of prostate cancer (11).
Soy food intake in patients with breast cancer has in the past been controversial, but a number of studies more recently have shown a protective effect in breast cancer patients reducing both mortality and recurrence of cancer (12,13). In a study of soy food consumption of >10 mg isoflavones in breast cancer survivors from data of U.S. and Chinese women, there was a nonsignificant reduction in cancer related mortality but significant reduction in risk of recurrence (14).
A high fat dairy diet was associated in one study of breast cancer survivors with increased cancer and non-cancer mortality, but low fat dairy had no effect on mortality (15). In 4,577 patients with non-metastatic prostate cancer, intake of animal fat and trans fats increased all cause mortality. Richman et al. reported that replacing 10% of animal fat with vegetable fat reduced the risk of developing lethal prostate cancer (16). In another study of milk fat content, ingestion of whole milk was associated with a higher risk of prostate cancer mortality and a higher incidence of more aggressive and fatal prostate cancer, while skim or low fat milk was associated with greater risk of non-aggressive prostate cancer. Increased intake of dairy foods was associated with increased prostate cancer incidence (17). Intake of processed meats increases the recurrence, mortality and metastatic activity of colorectal cancer (18).
Exercise and movement
Patients with cancer are often not encouraged to engage in exercise during cancer treatment, but the positive effects on QOL, weakness, fatigue and mood are documented in a number of studies.
In a 10-year longitudinal study of post-treatment breast cancer patients, pain was significantly reduced in patients who maintained regular physical activity and an ideal body weight, as opposed to those women who had a weight gain of >5%, who were obese or overweight, and had a sedentary lifestyle (19). In one small study of patients with non-small cell lung cancer, eight weeks of aerobic exercise training decreased fatigue and dyspnea, and increased exercise capacity even in patients with higher QOL (20). In Taiwanese lung cancer patients, light to moderate physical activity improved QOL during active treatment compared with sedentary patients. There were no significant differences in QOL in patients not in active treatment (21). A home based 8-week exercise program for post-mastectomy breast cancer patients with lymphedema showed a significant reduction in lymphedema and increase in QOL (22). Breast cancer patients in one study who exercised more had fewer symptoms, and patients that were more sedentary had higher rates of shoulder limitations, chest wall pain, weight gain, lymphedema, and breathlessness (23). An exercise intervention in a randomized controlled trial (RCT) of patients with breast cancer showed an increase in physical activity and more positive mood. These positive results persisted 60 months after the intervention (24). In a systematic review of 40 exercise interventions, a small overall decrease in depressive symptoms was found. Among cancer survivors, this response was greater depending on the amount of exercise; more was better than less. Improvements were greater for breast cancer patients, cancer survivors 47-62 years, and when exercise sessions were supervised (25).
Decreased functional capacity, weakness and fatigue, which lead to poor QOL, are common issues in patients with cancer or in cancer treatment. The Danish Head and Neck Cancer Group found that 12 weeks of progressive resistance training helped to build lean body mass, increased functional capacity and improved QOL (26). Another study of a home based light exercise program for patients after thoracotomy for lung cancer showed improvements in cancer related fatigue (27). A recent Cochrane Review also supported the findings that exercise can relieve cancer related fatigue (28). In another systematic review, Grade A evidence was found for exercise improving endurance, QOL, and reducing fatigue in prostate cancer patients. Grade B evidence exists for improving muscle mass, strength, functional capacity, health, social and physical QOL (29). The UW WELL-FIT program of aerobic exercise, strength training, and stretching in a supervised setting, demonstrated improved physical function and QOL in patients undergoing active cancer treatment (30).
Dragon boat racing, a popular team activity among cancer patients, especially breast cancer patients, helps improve QOL, fatigue, functional capacity, emotional and spiritual well-being (31).
In one small study of tai chi/qigong training, cancer survivors had increased shoulder mobility, strength and functional well being than the control group without the training (32).
A meta-analysis of 13 RCTs of yoga in cancer survivors documented significant reductions in distress, anxiety, and depression; moderate reductions in fatigue; moderate increases in social and emotional function, and QOL; and a small increase in functional well-being (33). Bower et al. also showed a reduction in fatigue in breast cancer survivors in a yoga program (34). Yoga breathing or pranayama can assist cancer patients during chemotherapy with sleep, anxiety, and QOL (35).
One novel approach for increasing fruit and vegetable intake, increasing physical activity, agility, strength, and endurance is gardening for cancer survivors. In a study reported by Blair et al. in 2013, a master gardener mentor was paired with a cancer survivor for 1 year. The intervention included planting gardens, harvesting plants, and troubleshooting gardening problems (36).
Herbs and supplements
Herbs and supplements may be useful for a number of symptoms that occur with cancer treatment such as chemotherapy or radiation therapy. A key resource for assessing whether an herb or supplement may interfere with a particular chemotherapy agent or radiation therapy is the text “The definitive guide to cancer: an integrative approach to prevention, treatment, and healing” by Alschuler and Gazella. Some botanicals may interfere with the metabolism and action of chemotherapeutic agents such as taxanes, platinum-based drugs, cyclophosphamide, doxorubicin, etoposide, and irinotecan. Herbs and supplements might increase or decrease the action of a chemotherapeutic drug, by causing toxicity if too much drug becomes available, or becoming less effective if the drug’s metabolism is increased. Botanicals generally do not affect radiation therapy, but antioxidant supplements may or may not interfere with the action of radiation or chemotherapy. Antioxidants in food are not a concern (37). Frenkel et al. and the organization, The Society of Integrative Oncology, have created an evidence-based document on herbs and supplements such as curcumin, glutamine, vitamin D, Maitake mushrooms, fish oil, green tea, milk thistle, Astragalus, melatonin and probiotics, found to be most beneficial in cancer care. This document is recommended for a more complete review of these beneficial herbs and supplements (6). Many of our patients with advanced malignancies or who are elderly may be indoors due to illness, or cancer treatment effects such as weakness or fatigue. Vitamin D levels in this population may be low and put patients at risk for complications of vitamin D deficiency such as immune deficiency, fatigue, decreased bone mineralization, muscle pain, and weakness. One study found that 79.5% of patients with advanced breast cancer were vitamin D deficient (<30 ng/mL 25 hydroxy-vitamin D level) and at the end of neoadjuvant chemotherapy, 97.4% of patients were deficient (38). In a study of colorectal patients given 2,000 units of vitamin D3 daily, a rise of >10 nm/mL in 25-hydroxy vitamin D levels was found in 92% of chemo free patients versus 39% of patients undergoing chemotherapy. It was hypothesized that chemotherapy can attenuate the effects of vitamin D supplementation (39). Optimal levels for serum vitamin D levels are controversial. In a 9-year [1991-2000] follow-up analysis of the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994), a serum 25-hydroxy vitamin D level of 81 nmol/L was the nadir level of all-cause mortality with higher and lower levels of vitamin D showing a J shaped association of higher levels of all-cause mortality. This association held up for ages 20-64 years, and in non-Hispanic whites, but not in the elderly (40). Vitamin D levels in the highest normal quartile increased survival in patients with breast, colon, lung cancers and lymphoma (41). Suggested dosages are 800-2,000 units daily, and dosing can be checked with a 25-hydroxy vitamin D level to insure patients are not deficient or toxic according to lab reference values.
The use of antioxidant supplements during chemotherapy and radiation therapy in cancer patients remains controversial since theoretically antioxidants can decrease the effectiveness of these conventional therapies by neutralizing the cancer-damaging free radicals caused by these therapies. One study showed that breast cancer and chemotherapy decreased antioxidants enzymes and glutathione significantly. Women in the study who received 500 mg of vitamin C and 400 mg of vitamin E had more normal levels of antioxidant enzymes, less DNA damage, and more normal glutathione levels (42). The life after cancer epidemiology (LACE) study demonstrated that 81% of patients with breast cancer used antioxidant supplements after diagnosis. Among these women, those who used vitamin C or E had a lower rate of recurrence of cancer, and vitamin E users had lower all cause mortality. Those who used mixed carotenoids did not, and had an increase in recurrence (43).
Glutamine is protective for the upper and lower gastrointestinal (GI) tracts. Patients taking 15 grams of oral glutamine three times daily versus a placebo group had significantly less severe diarrhea caused by radiation therapy. No patients had severe diarrhea in the glutamine treated group, versus 69% of patients in the placebo group (44).
In another RTC, selenium twice daily was found to significantly decrease the severity and duration of mucositis in patients undergoing high dose chemotherapy and stem cell transplantation for leukemia (45). Two percent calendula gel can also decrease the intensity of oral mucositis in patients with head and neck cancers (46). The application of honey can also improve radiation-induced oral mucositis in head and neck cancer patients. This was shown in two studies (47,48).
Vitamin E 800 units per day can help prevent radiation induced salivary gland dysfunction in patients undergoing single dose radiation therapy for thyroid cancer (49). Probiotics, in a meta-analysis of six studies and systematic review of ten, significantly decreased radiation induced-diarrhea, and loperamide use decreased as well (50). Probiotics used peri-operatively during colectomy for colorectal cancer decreased post-operative infection (51).
Ginger is a botanical traditionally used for nausea and vomiting from a number of causes. It is efficacious for nausea associated with chemotherapy. Its mechanism of action is unknown (52). This herb is consumed as a tea (boiling ginger root in water), ginger root extract 500-1,000 mg every 4-6 hours as needed, or eating 1 teaspoon or 5 grams of crystallized ginger every 2-3 hours as needed. Too much can cause heartburn (53). In a large RTC of ginger at various doses versus placebo, ginger at the dose of 0.5-1 mg daily gave optimal effective control of chemotherapy-induced nausea (54,55).
Curcumin taken at dose of 6 grams a day significantly decreased radiation dermatitis in breast cancer patients (55). Calendula cream applied several times a day reduced radiation dermatitis in breast cancer patients (56).
Life review and cognitive/behavioral modalities and mindfulness-based-stress-reduction program (MBSR)
Psychological, social and spiritual health assists patients in thriving in their cancer journeys, as opposed to merely surviving. Psycho-oncology is a growing field with more cancer centers offering these services. This area of care includes cognitive behavioral interventions (either group or individual), relaxation techniques, breath work, mindfulness meditation, peer support groups, and prayer. Since so many cancer centers now offer these supportive services, the boundaries between integrative, complementary and conventional modalities are blurred. In a study of newly diagnosed multiple myeloma patients, those with depression and younger in age preferred psychosocial interventions such as peer support, relaxation techniques, and counseling (57). One study compared aromatherapy and massage with cognitive behavioral therapy (CBT) in a variety of cancer patients receiving conventional cancer care. Patients preferred the aromatherapy/massage intervention, and both interventions had similar improvements on depression, mood and anxiety scores (58). A brief mindfulness based CBT improved sexual functioning and sexual health in women with gynecological cancer (59).
One study using a mindfulness-based-stress-reduction program (MBSR) with breast cancer patients showed an increase in coping, healthy relationships, calmness, peacefulness, and acceptance (60). In another study of patients with breast cancer who are post surgery, chemo and radiation therapy, MBSR improved mood, QOL and well-being (61). MBSRs and mindfulness based cognitive therapy decrease anxiety and depression in breast cancer survivors (62). In one study of prostate cancer patients, an 8-week group cognitive behavioral intervention improved psycho-sexual well-being in patients following radical prostatectomy (63). In a pilot RCT of terminal cancer patients, brief CBT tailored to the issues most relevant to patients significantly reduced anxiety. This modality worked on relaxation skills, coping with concerns of cancer, and pacing activity (64). In another study using cognitive based stress management, prostate cancer survivors, in a 10-week group program, had increased levels of emotional well-being. This occurred even in the participants experiencing sexual and urinary dysfunction (65).
In rural and underserved populations without access to in-person support groups and emotional support services, online professionally lead support groups can also be beneficial (66). One study found that men prefer online communication in larger groups, and women prefer same gender, more intimate social support groups (67).
Dignity-enhancing therapy is a modality pioneered by Dr. Harvey Chochinov in Canada and used within palliative care. In his interviews of patients that had advanced cancer, he found that loss of dignity was common in these patients. A dignity model emerged from the research, and a dignity-enhancing intervention was created from the model which includes a brief life review interview guided by a trained interviewer. The interview is then transcribed and edited into a generativity document that can help not only the patient with emotional, psychological and spiritual issues, but also family and friends. In the beginning, this intervention was used with people in hospice nearing end of life. Although acceptability of the intervention and perceived benefit are measured in non RCTs, effect on outcome measures is less than robust in randomized control trials of the intervention (68-71).
Other studies have focused on advanced cancer patients. One RTC found that the dignity therapy increased hope in advanced cancer patients. Both the intervention and control group scored low on dignity related distress, and there was no significant change in both groups; however, the intervention group scored higher on hope at one and four week follow up, but the control group did not. The groups were not significantly different on measures of anxiety, depression, QOL or other palliative care measures (72). One pilot study of advanced cancer patients and life review combined with online educational resources found the interview and receipt of an edited life story was helpful and meaningful. Most participants that were older than 70 years did not use the online resources (73). In a RTC of a dignity-enhancing life review combined with online support, cancer patients in the intervention group had a greater sense of peace (74).
Aromatherapy and essential oils
In a RCT of spearmint and peppermint oils in the treatment of chemotherapy-induced nausea and vomiting, these essential oils were significantly effective in reducing emetic events without adverse effects. Cost of overall treatment was decreased with essential oil use over the placebo or control, conventional treatment groups (75).
Massage
Massage may help cancer patients with reductions in anxiety, pain, and nausea. No effect on depression was found in a Cochrane Review on the topic of massage in cancer patients (76). In a population of veterans with cancer, massage provided by personal caregivers (81% spouses, 78% female), decreased pain, stress, anxiety, and fatigue. A total of 27 dyads of patient/caregiver were recruited, and 11 dyads completed the study. Attrition reported was due to caregiver burden. Massage was taught with video and written instructional materials, and done weekly by caregivers. Patients reported high levels of satisfaction with this touch therapy (77).
Acupuncture
At the Sao Paulo Cancer Institute, 183 patients were enrolled in an acupuncture study with 30% receiving active treatment with chemotherapy or radiation, 16% receiving hormonal treatment, and 55% in remission. Patients’ main symptoms were cancer pain, chemo-toxicity, lumbar pain, chronic postoperative pain. Acupuncture treatment significantly reduced the mean symptom severity scores from 7.04 to 2.56 (P<0.001) (78).
In a study of 33 patients with breast cancer related lymphedema of the arm, acupuncture significantly reduced arm circumference and was safe (79). Acupuncture can also reduce hot flashes and sleep disturbance in breast cancer survivors (80).
In 36 patients with postsurgical gastroparesis in abdominal cancers, 27 were cured, 6 improved and 3 not improved, with a combination of acupuncture, moxibustion, and cupping (81).
In a study of patients undergoing radiation therapy for nasopharyngeal cancer, acupuncture during radiation therapy reduced xerostomia and improved QOL (82).
Music
A Cochrane systematic review of music therapy in cancer patients found that music has positive effects on anxiety, pain, mood and QOL measures. There was no evidence to support positive effect on relief of depression (83).
Summary
Patients with advanced malignancies are best served by using an integrated, holistic, person centered approach using conventional and CAM therapies. The boundaries between CAM therapies and conventional therapies are often blurred especially as CAM therapies, such as vitamin D, accumulate scientific, evidence based research to demonstrate their usefulness even to those conventional practitioners cautious about their use. Nutritional and movement interventions with a plethora of quality research demonstrating positive outcomes for patients, are still not readily incorporated into all conventional oncology care. Palliative medicine can provide the bridge to patients with advanced malignancies to receive integrative services that provide higher QOL, decreased symptom burden, emotional and spiritual well being, hope, empowerment, reduced recurrence and mortality in some cancers, lower overall costs of care, and increased completion of conventional treatment sometimes not possible due to symptom burden and adverse effects. It is also our responsibility to discuss possible adverse effects of some CAM therapies when combined with certain conventional therapies. The balance of CAM and conventional therapies will continue to depend on the receptivity of individual patients, families, practitioners and health system priorities, and ongoing research efforts. CAM and conventional modalities can be combined in potentially synergistic ways to decrease the amount of medication needed to treat a symptom such as anxiety when a modality such as music or meditation is also used. Palliative care practitioners, with open hearts and open minds, must be guided by evidence, creativity and person centered care.
Acknowledgements
Thank you to Char Luchterhand, MSSW, and Adrianne Gasper, APNP, who reviewed this manuscript.
Disclosure: The author declares no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009;169:480-8. [PubMed]
- Kligler B, Homel P, Harrison LB, et al. Cost savings in inpatient oncology through an integrative medicine approach. Am J Manag Care 2011;17:779-84. [PubMed]
- Chan A, Lin TH, Shih V, et al. Clinical outcomes for cancer patients using complementary and alternative medicine. Altern Ther Health Med 2012;18:12-7. [PubMed]
- Barrett B, Marchand L, Scheder J, et al. Bridging the gap between conventional and alternative medicine. J Fam Pract 2000;49:234-9. [PubMed]
- Frenkel M, Abrams DI, Ladas EJ, et al. Integrating dietary supplements into cancer care. Integr Cancer Ther 2013;12:369-84. [PubMed]
- Deng GE, Frenkel M, Cohen L, et al. Evidence based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J Soc Integr Oncol 2009;7:85-120. [PubMed]
- Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243-74. [PubMed]
- Pullar JM, Chisholm A, Jackson C. Dietary information for colorectal cancer survivors: an unmet need. N Z Med J 2012;125:27-37. [PubMed]
- Zick SM, Sen A, Han-Markey TL, et al. Examination of the association of diet and persistent cancer related fatigue: a pilot study. Oncol Nurs Forum 2013;40:E41-9. [PubMed]
- Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer 2012;131:201-10. [PubMed]
- Chi F, Wu R, Zeng YC, et al. Post-diagnosis soy food intake and breast cancer survival: a meta analysis of cohort studies. Asian Pac J Cancer Prev 2013;14:2407-12. [PubMed]
- Zhang YF, Kang HB, Li BL, et al. Positive effects of soy isoflavones food on survival of breast cancer patients in china. Asian Pac J Cancer Prev 2012;13:479-82. [PubMed]
- Nechuta SJ, Caan BJ, Chen WY, et al. Soy food intake after diagnosis of breast cancer and survival: an in depth analysis of combined evidence from cohort studies of UA and Chinese women. Am J Clin Nutr 2012;96:123-32. [PubMed]
- Kroenke CH, Kwan ML, Sweeney C, et al. High and low fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J Natl Cancer Inst 2013;105:616-23. [PubMed]
- Richman EL, Kenfield SA, Chavarro JE, et al. Fat Intake after diagnosis and risk of lethal prostate cancer and all cause mortality. JAMA Intern Med 2013;173:1318-26. [PubMed]
- Song Y, Chavarro JE, Cao Y, et al. Whole milk intake is associated with prostate cancer specific mortality among US male physicians. J Nutr 2013;143:189-96. [PubMed]
- Zhu Y, Wu H, Wang PP, et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open 2013;3:e002270. [PubMed]
- Forsythe LP, Alfano CM, George SM, et al. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behaviors. Breast Cancer Res Treat 2013;137:617-30. [PubMed]
- Hwang CL, Yu CJ, Shih JY, et al. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer 2012;20:3169-77. [PubMed]
- Lin YY, Wu YC, Rau KM, et al. Effects of physical activity on the quality of life in Taiwanese lung cancer patients receiving active treatment or off treatment. Cancer Nurs 2013;36:E35-41. [PubMed]
- Gautam AP, Maiya AG, Vidyasagar MS. Effect on home-based exercise program on lymphedema and quality of life in female post-mastectomy patients: pre-post intervention study. J Rehabil Res Dev 2011;48:1261-8. [PubMed]
- Gho SA, Steele JR, Jones SC, et al. Self reported side effects of breast cancer treatment: a cross sectional study of incidence, associations, and the influence of exercise. Cancer Causes Control 2013;24:517-28. [PubMed]
- Mutrie N, Campbell A, Barry S, et al. Five year follow up of participants in a randomized controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects? J Cancer Surviv 2012;6:420-30. [PubMed]
- Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta analysis. PloS ONE 2012;7:e30955. [PubMed]
- Lønbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy – results from the randomized DAHANCA 25B trial. Radiother Oncol 2013;108:314-9. [PubMed]
- Hoffman AJ, Brinthall RA, Brown JK, et al. Too sick not to exercise: using a 6 week home based exercise intervention for cancer related fatigue self management for postsurgical non-small cell lung cancer patients. Cancer Nurs 2013;36:175-88. [PubMed]
- Cramp F, Byron-Daniel J. Exercise for the management of cancer related fatigue in adults. Cochrane Database Syst Rev 2012;11:CD006145. [PubMed]
- Keogh JW, Macleod RD. Body composition, physical fitness, functional performance, quality of life and fatigue benefits of exercise in prostate cancer patients: a systematic review. J Pain Symptom Manage 2012;43:96-110. [PubMed]
- Noble M, Russell C, Kraemer L, et al. UW WELL-FIT: the impact of supervised exercise programs on physical capacity and quality of life in individuals receiving treatment for cancer. Support Care Cancer 2012;20:865-73. [PubMed]
- Ray HA, Verhoef MJ. Dragon boat racing and health-related quality of life of breast cancer survivors: a mixed methods evaluation. BMC Complement Altern Med 2013;13:205. [PubMed]
- Fong SS, Ng SS, Luk WS, et al. Shoulder mobility, muscular strength, and quality of life in breast cancer survivors with and without tai chi qigong training. Evid Based Complement Alternat Med 2013;2013:787169.
- Buffart LM, Van Uffelen JGZ, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2012;12:559. [PubMed]
- Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer 2012;118:3766-75. [PubMed]
- Dhruva A, Miaskowski C, Abrams D. Yoga breathing for cancer chemotherapy- associated symptoms and quality of life: results of a pilot randomized controlled trial. J Altern Complement Med 2012;18:473-9. [PubMed]
- Blair CK, Madan-Swain A, Locher JL, et al. Harvest for health gardening intervention feasibility study for cancer survivors. Acta Oncol 2013;52:1110-8. [PubMed]
- Alschuler LN, Gazella KA. eds. The definitive guide to cancer: an integrative approach to prevention, treatment, and healing. Berkeley: Celestial Arts, 2010.
- Jacot W, Pouderoux S, Thezenas S, et al. Increased prevalence of vitamin D insufficiency in patients with breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 2012;134:709-17. [PubMed]
- Fakih MG, Andrews C, Momahon J. at al. A prospective clinical trial of cholecalciferol 2000 iu/day in colorectal cancer patients: evidence of a chemotherapy-response interaction. Anticancer Res 2012;32:1333-8. [PubMed]
- Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab 2013;98:3001-9. [PubMed]
- Tretli S, Schwartz GG, Torjesen PA, et al. Serum levels of 25 hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung and lymphoma: a population based study. Cancer Causes Control 2012;23:363-70. [PubMed]
- Suhail N, Bilal N, Khan HY, et al. Effect of vitamins C and E on antioxidant status of breast cancer patients undergoing chemotherapy. J Clin Pharm Ther 2012;37:22-6. [PubMed]
- Greenlee H, Kwan ML, Kushi LH, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the life after cancer epidemiology (LACE) cohort. Cancer 2012;118:2048-58. [PubMed]
- Kucuktulu E, Guner A, Kahraman I, et al. The protective effects of glutamine on radiation-induced diarrhea. Support Care Cancer 2013;21:1071-5. [PubMed]
- Jahangard-Rafsanjani Z, Gholami K, Hadjibabaie M, et al. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial. Bone Marrow Transplant 2013;48:832-6. [PubMed]
- Babaee N, Moslemi D, Khalilpour M, et al. Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study. Daru 2013;21:18. [PubMed]
- Maiti PK, Ray A, Mitra TN. The effect of honey on mucositis induced by chemoradiation in head and neck cancer. J Indian Med Assoc 2012;110:453-6. [PubMed]
- Abdulrhman M, El Barbary NS, Ahmed Amin D, et al. Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy induced oral mucositis: a randomized controlled trial. Pediatr Hematol Oncol 2012;29:285-92. [PubMed]
- Fallahi B, Beiki D, Abedi SM, et al. Does vitamin E protect salivary glands for I-131 radiation damage in patients with thyroid cancer? Nucl Med Commun 2013;34:777-86. [PubMed]
- Hamad A, Fragkos KC, Forbes A. A systematic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr 2013;32:353-60. [PubMed]
- Liu ZH, Huang MJ, Zhang XW, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double center and double blind randomized controlled trial. Am J Clin Nutr 2013;97:117-26. [PubMed]
- Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth 2000;84:367-71. [PubMed]
- Marchand L. End of life care. In: Rakel D. eds. Integrative Medicine. Philadelphia, PA: Elsevier Saunders, 2012:732-43.
- Ryan JL, Heckler CE, Roscoe JA, et al. Ginger (zingiber officinale) reduces acute chemotherapy induced nausea: a URCC CCOP Study of 576 patients. Support Care Cancer 2012;20:1479-89. [PubMed]
- Ryan JL, Heckler CE, Ling M, et al. Curcumin for radiation dermatitis: a randomized, double blind, placebo controlled clinical trial of thirty breast cancer patients. Radiat Res 2013;180:34-43. [PubMed]
- Kassab S, Cummings M, Berkovitz S, et al. Homeopathic medicines for adverse effects of cancer treatments. Cochrane Database Syst Rev 2009;CD004845. [PubMed]
- Lamers J, Hartman H, Goldschmidt H, et al. Psychosocial support in patients with multiple myeloma at time of diagnosis: who wants what? Psychooncology 2013;2210:2313-20. [PubMed]
- Serfaty M, Wilkinson S, Freeman C, et al. The ToT study: helping with touch or talk (ToT): a pilot randomized controlled trial to examine the clinical effectiveness of aromatherapy massage versus cognitive behavioral therapy for emotional distress in patients with cancer/palliative care. Psychooncology 2012;21:563-9. [PubMed]
- Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320-5. [PubMed]
- Hoffman CJ, Ersser SJ, Hopkinson JB. Mindfulness based stress reduction in breast cancer: a qualitative analysis. Complement Ther Clin Pract 2012;18:221-6. [PubMed]
- Hoffman CJ, Erssner SJ, Hopkinson JB, et al. Effectiveness of mindfulness based stress reduction in mood, breast and endocrine related quality of life, and well being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol 2012;30:1335-42. [PubMed]
- Cramer H, Lauche R, Paul A, et al. Mindfulness based stress reduction for breast cancer- a systematic review and meta analysis. Curr Oncol 2012;19:e343-52. [PubMed]
- Siddons HM, Wooten AC, Costello AJ. A randomized, wait-list controlled trial: evaluation of a cognitive-behavioral group intervention on psycho-sexual adjustment for men with localized prostate cancer. Psychooncology 2013;2210:2186-92. [PubMed]
- Greer JA, Traeger L, Bemis H, et al. A pilot randomized controlled trial of brief cognitive behavioral therapy for anxiety in patients with terminal cancer. Oncologist 2012;17:1337-45. [PubMed]
- Traeger L, Penedo FJ, Benedict C, et al. Identifying how and for whom cognitive behavioral stress management improves emotional well being among recent prostate cancer survivors. Psychooncology 2013;22:250-9. [PubMed]
- Stephen J, Rojubally A, MacGregor K, et al. Evaluation of cancerchatcanada: a program of online support for Canadians affected by cancer. Curr Oncol 2013;20:39-47. [PubMed]
- Durant KT, McCray AT, Safran C. Identifying gender preferred communication styles within online cancer communities: a retrospective, longitudinal analysis. PLos One 2012;7:e49169. [PubMed]
- Chochinov HM, Hack T, Hassard T, et al. Dignity in the terminally ill: a cross-sectional cohort study. Lancet 2002;360:2026-30. [PubMed]
- Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol 2005;23:5520-5. [PubMed]
- Chochinov HM, Hack T, McClement S, et al. Dignity in the terminally ill: a developing empirical model. Soc Sci Med 2002;54:433-43. [PubMed]
- Chochinov HM, Kristjanson LJ, Breibart W, et al. Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: a randomized controlled trial. Lancet Oncol 2011;12:753-62. [PubMed]
- Hall S, Goddard C, Opio D, et al. A novel approach to enhancing hope in patients with advanced cancer: a randomized phase II trial of dignity therapy. BMJ Support Palliat Care 2011;1:315-21. [PubMed]
- Wise M, Marchand L, Aeschlimann E, et al. Integrating a narrative medicine telephone interview with online life review education for cancer patients: lessons learned and future directions. J Soc Integr Oncol 2009;7:19-25. [PubMed]
- Wise M, Marchand L, Roberts L, et al. Effects of a dignity enhancing life review intervention for advanced cancer patients. Not published.
- Tayarani-Najaran Z, Talasaz-Firoozi E, Nasiri R, et al. Antiemetic activity of volatile oils from menthe spicata and menthe x piperita in chemotherapy induced nausea and vomiting. Ecancermedicalscience 2013;7:290. [PubMed]
- Fellowes D, Barnes K, Wilkinson S. Aromatherapy and massage for symptom relief in cancer patients. Cochrane Database Syst Rev 2008;CD002287. [PubMed]
- Kozak L, Vig E, Simons C, et al. A feasibility study of caregiver-provided massage as supportive care of Veterans with cancer. J Support Oncol 2013;11:133-43. [PubMed]
- Salmon J. Evaluation of an acupuncture service in oncology. Acupunct Med 2013;121:39-55.
- Cassileth BR, Van Zee KJ, Yeung KS, et al. Acupuncture in the treatment of upper limb lymphedema: results of a pilot study. Cancer 2013;119:2455-61. [PubMed]
- Bokmand S, Flyger H. Acupuncture relieves menopausal discomfort in breast cancer patients: a prospective, double blinded, randomized study. Breast 2013;22:320-3. [PubMed]
- Hou XB, Yu S. Thirty six cases of postsurgical gastroparesis of abdominal cancer treated by comprehensive therapy of acupuncture-moxibustion. World J Acupuncture-Moxibustion 2013;23:53-5.
- Meng Z, Garcia MK, Hu C, et al. Randomized controlled trial of acupuncture for prevention of radiation induced xerostomia among patients with nasopharyngeal carcinoma. Cancer 2012;118:3337-44. [PubMed]
- Bradt J, Dileo C, Grocke D, et al. Music interventions for improving psychological and physical symptoms in cancer patients. Cochrane Database Syst Rev 2011;CD006911. [PubMed]


