BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies
Introduction
Despite improvements in screening and early detection, colorectal cancer (CRC) remains a leading cause of cancer death in the United States. Advances in molecular biology have increased our knowledge of the genetic and epigenetic events involved in tumorigenesis and have led to the development of novel targeted therapeutics. The Ras-Raf-mitogen-activated protein kinase (MAPK) signaling pathway has been implicated as a critical mediator of colorectal carcinogenesis. KRAS and NRAS mutations are present in 50% of CRCs and their therapeutic significance is well defined (1-3). Studies support the hypothesis that KRAS/NRAS mutations result in constitutive activation of the Ras-Raf-MAPK pathway, downstream of epidermal growth factor receptor (EGFR), rendering these tumors resistant to anti-EGFR therapies (4-7). These findings prompted the incorporation of KRAS/NRAS mutation status into the clinical treatment algorithm for CRC, and established the Ras-Raf-MAPK pathway as a principal target for the development of novel molecular therapeutic agents for the treatment of CRC (8,9).
BRAF, another potent modulator of the MAPK pathway, has recently emerged as a prognostic biomarker and promising new target for the treatment of CRC. Oncogenic mutations in BRAF are present in 10% of CRC. Studies demonstrate that carriers of BRAF mutations possess discrete clinical characteristics and oncologic outcomes (10-12). Furthermore, BRAF status is believed to be responsible for the 12-15% of patients who fail anti-EGFR (10,13,14). Because of its increasing significance, the National Comprehensive Cancer Network guidelines now recommend BRAF mutation testing in patients with metastatic disease. In this article, we will review the role of BRAF mutations in the development of CRC and summarize the molecular and clinicopathologic features unique to this genetic subtype.
BRAF carcinogenesis pathway
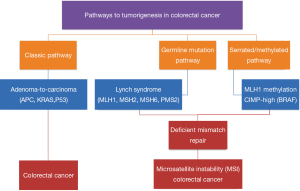
It is widely accepted that there are multiple pathways that lead to the development of CRC (Figure 1). The classic adenoma-to-carcinoma pathway is typically seen with the loss of APC and/or p53 tumor suppressor genes with chromosomal instability (1). A second pathway involves the loss of DNA mismatch repair and is exemplified by the germline mutations seen in Lynch Syndrome (15,16). BRAF appears to act via a third pathway; the serrated/methylator pathway (17-19). These tumors are characterized by the methylation of CpG islands that cause the silencing of critical tumor suppressor genes and are termed CpG Island Methylator Phenotype (CIMP) tumors.
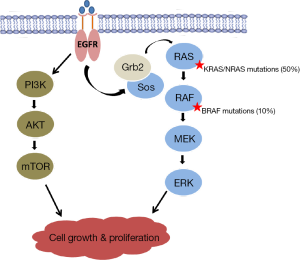
The BRAF oncogene codes for a serine/threonine kinase which acts downstream of KRAS in the MAPK pathway (Figure 2). BRAF mediates its effect by activating mitogen-activated protein kinase kinase (MAPKK or MEK), thus promoting cell proliferation. BRAFV600E is an activating mutation that accounts for approximately 90% of all BRAF mutations seen in CRC (3,20). It results from the transversion of thymidine to adenine at nucleotide 1799 in the kinase domain, causing a valine to glutamate substitution that leads to constitutive activation of MEK and uninhibited EGFR-independent cellular proliferation (10,21). BRAF and KRAS/NRAS mutations are mutually exclusive in CRC (3,13,14). This fact supports the hypothesis that BRAF is the principal effector of KRAS/NRAS in the MAPK pathway and that both mutations have equivalent effects on tumorigenesis.
Clinicopathologic characteristics
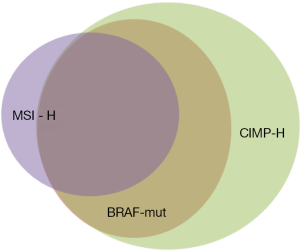
Knowledge about the clinical implications of BRAF mutations in CRC is rapidly increasing, but it is already evident that BRAF mutated tumors comprise a discrete disease subtype with a unique patient population and associated prognosis. As previously discussed, BRAF mutant cancers are highly methylated (CIMP-high) when compared to BRAF wild-type tumors. Additionally, BRAF mutation is strongly associated with microsatellite instability (MSI). Table 1 summarizes the incidence of MSI-high, CIMP-high and BRAF mutated tumors observed in a variety of CRC cohorts. In sporadic CRCs, BRAF mutation is seen in approximately 60% of MSI high tumors and only 5-10% of microsatellite stable (MSS) tumors (3,10,13,14). This is because BRAFV600E mutation results in hypermethylation of the MLH1 gene promoter, resulting in loss of the tumor suppressor function and leading to diminished DNA mismatch repair (29-32). This occurs exclusive of the germline mismatch repair mutations seen in Lynch Syndrome (Figure 3).

Full table
Phenotypically, BRAF mutated CRCs display different characteristics when compared to BRAF wild-type. Studies have demonstrated that BRAF mutant tumors are more prevalent in women and in patients of advanced age, typically age >70 years (14,36-38). Wild-type cancers are distributed widely throughout the colon and rectum however, BRAF mutated cancers are rarely found in left sided colon and rectal cancers but instead are primarily located in the proximal colon (36-39). Additionally BRAF mutant tumors tend to be MSI-high, mucinous histology, serrated and poorly differentiated (14,22,37,40-42). Tran et al. (39) further defined BRAF mutant colorectal tumors as a distinct subtype when they delineated a unique pattern of metastatic spread in BRAF mutant cancers, when compared to wild-type tumors. In this study of a cohort of 524 patients with metastatic CRC, 57 (11%) patients were found to harbor a BRAF mutation (55 BRAFV600E, 1 BRAFG593D, 1 BRAFQ609X). Again, female gender, right-sided primary tumor and MSI were statistically significant risk factors associated with BRAF mutant cancers. In patients with BRAF mutations, metastatic spread was more commonly via peritoneal disease (46% vs. 24%) or distant lymph node metastasis (53% vs. 38%) when compared to BRAF wild-type tumors, and less likely to result in lung metastasis (35% vs. 49%). This is clinically relevant as these patients are therefore less likely to undergo metastasectomy as their disease is present in sites not amenable to resection. Not surprisingly, BRAF mutation also conferred poorer overall survival with a median of 10.4 vs. 34.7 months. These data suggest that BRAF mutation may serve as a major driver of right-sided tumor biology given the strong association between BRAF mutations and proximal colon cancers and may contribute to the differences in prognosis and metastasis observed between right-sided and left-sided colon cancers.
Prognostic and predictive value of BRAF mutation
Overall survival in metastatic CRC has improved significantly due to the development of new chemotherapy and targeted drugs, as well as more liberal use of curative surgical metastasectomies. Despite these advances however, patients with BRAF mutant CRC have low response rates to conventional therapies and poor overall survival. This is true for patients regardless of their stage at the time of diagnosis. Samowitz et al. (22) evaluated a large cohort of patients (n=911) with stage I through IV colon cancer. BRAF mutation was seen in 9.3% of all tumors and 52% of MSI high tumors. In this series, the 5-year overall survival of patients with BRAF mutant CRC was significantly lower when compared to wild-type tumors at 47.5% vs. 60.7%. The difference was even more pronounced when patients with MSI high tumors were excluded from the analysis, as MSI high tumors are generally associated with a good overall prognosis. They found that in MSS tumors, BRAF mutation was prognostic for poor overall survival on univariate and multivariate analysis with adjustments for clinicopathologic factors including age, stage, and tumor location. MSI tumors conferred excellent prognosis in this study and BRAF mutation status had no significant effect on 5-year overall survival.
Others have reported similar findings in early stage disease. Roth et al. (43) evaluated the prognostic value of KRAS and BRAF mutations in stage II and III colon cancer and found that while BRAF mutation did not predict tumor recurrence, it was prognostic for poor overall survival. This was especially true in MSI-low and MSS tumors (HR =2.2; 95% CI, 1.4-3.4; P=0.0003). Similarly, Fariña-Sarasqueta et al. (44) found in a cohort of stage II and III colon cancer, that BRAF mutation was an independent prognostic factor for decreased overall survival in MSS tumors with no difference in overall survival in MSI-high tumors.
Several other studies have demonstrated that the adverse prognosis seen in BRAF mutated CRC is not limited to MSS tumors. Lochhead et al. (28) examined the implication of BRAF mutation on overall survival in 1,253 patients with colon and rectal cancers. BRAF mutation was present in 182 (14%) patients, with 55% of those tumors exhibiting MSI-high phenotypes. In this study MSS/BRAF mutant tumors were associated with the highest CRC-specific mortality (HR =2.10; 95% CI, 1.5-2.9; P<0.001). Patients with MSI-high tumors fared better than MSS tumors in general, however MSI-high/BRAF mutant tumors did worse than their MSI-high/BRAF wild-type counterparts with a HR of 0.44 (95% CI, 0.26-0.75; P=0.003) when compared to a HR of 0.26 (95% CI, 0.13-0.52; P<0.001). Likewise, Sinicrope et al. (45) found BRAF mutation conferred a worse overall survival in patients with stage III colon cancer regardless of mismatch repair proficiency. However, given the drastic difference in prognosis between MSS/BRAF-mutant and MSI/BRAF-mutant tumors, molecular subtyping alone is an insufficient prognosticator and further underscores that evaluation of mismatch repair proficiency remains critical in the subtyping of CRCs.
Studies in the setting of metastatic disease have further validated these findings. The MRC FOCUS Trial sought to evaluate KRAS and BRAF mutation as prognostic factors in advanced disease. In this study of stage IV CRC, BRAFV600E mutation was found to be a negative prognostic marker for overall survival (HR =1.82; 95% CI, 1.36-2.43; P<0.0001), however there was no significant difference in progression free survival (46). To date, BRAF mutation remains the only oncogenic mutation that predicts poor prognosis in metastatic CRC. Tran et al. (39) elucidated the impact of BRAF mutation and MSSI in stage IV CRC. In this cohort, BRAF mutation was again found to be a negative prognostic marker for all patients. More importantly, they reported a strong association between MSI and BRAF mutation and demonstrated poorer overall survival in MSI metastatic CRC suggesting that unlike early stage disease, MSI is a negative prognostic factor in advanced disease and is likely driven by BRAF mutation (39). The poor response to therapy and shorter overall survival of BRAF mutant tumors observed in the studies outline above, are independent of the therapy used and persist over consecutive lines of systemic therapy (47-49).
The role of BRAF mutation status as a predictive molecular marker is less clear. Perhaps the most investigated predictive role of BRAF mutation is as a biomarker of anti-EGFR antibody resistance. KRAS mutation is an established predictive biomarker for anti-EGFR therapy resistance. The KRAS oncogene renders colorectal tumors resistant to anti-EGFR therapies by activating the Ras-Raf-MAPK pathway downstream of EGFR. Similarly, several studies have suggested that BRAF mutation also confers poor outcomes with anti-EGFR therapy through a similar mechanism. Di Nicolantonio et al. (50) and Loupakis et al. (51), in their small cohorts, studied response to anti-EGFR therapy in combination with other chemotherapeutic agent but failed to identify patients with BRAF mutant tumors who responded to anti-EGFR monoclonal antibodies. While other studies have failed studies have failed to show a negative relationship between BRAF mutation and anti-EGFR response, it does appear that BRAF mutation may have a strong predictive role for poor response to cetuximab and should be considered in individualized treatment plans. Richman et al. (46) sought to investigate the predictive implication of BRAF mutation in tumor response to conventional chemotherapeutic agents (irinotecan and oxaliplatin) independent of anti-EGFR therapy in metastatic CRC. BRAF mutation was not found to be a predictive biomarker for irinotecan or oxaliplatin. Patients benefited from the addition of either drug to Fluorouracil in first-line treatment with a slight improvement in progression-free survival but no benefit in overall survival.
Recent data have also suggested that BRAF mutation predicts poor outcomes after metastasectomy. Yaeger et al. (52) described their experience with complete resection of patients with metastatic CRC and noted that patients with BRAF mutations were less likely to undergo metastasectomy (26% vs. 41% at 2 years from diagnosis) due to increased peritoneal spread and decreased liver involvement. Patients with BRAF mutated tumors that were able to undergo complete resection had a trend towards shorter relapse-free survival (7 vs. 11 months) and had a statistically significant shorter overall survival (61% vs. 86% at 2 years) when compared to BRAF wild-type patients undergoing R0 resection for metastatic CRC.
Targeting BRAF in colorectal cancer (CRC)
The BRAFV600E mutation has been widely studied in melanoma. In 2011 vemurafenib, a protein kinase inhibitor of BRAFV600E was approved by the FDA for the treatment of metastatic melanoma after promising results in phase 3 studies with a reasonable safety profile. This made BRAF mutation in CRC an attractive therapeutic target. Unfortunately, the clinical response of single agent BRAFV600E inhibition in early phase studies of metastatic CRC is not as robust as that seen in melanoma (53,54). Efforts have now been focused on identifying mechanisms of early resistance to BRAFV600E inhibition in CRC. Prahallad et al. (55) reported decreased sensitivity to BRAF inhibition in BRAFV600E mutated CRC lines when compared to melanoma cells. Treatment with PLX4032 kinase inhibitor in CRC cells resulted in an increase in EGFR activation due to ERK mediated feedback leading to cellular proliferation. Prahallad and colleagues further elucidated this feedback loop. ERK is phosphorylated and activated in BRAF mutant tumors and negatively regulates EGFR receptor signaling. However, when BRAF mutant CRC cells are treated with vemurafenib, pERK is inhibited resulting in increased EGFR signaling and ultimately cellular proliferation. These findings were later confirmed by Corcoran et al. (56). Notably, this EGFR feedback activation after vemurafenib treatment is not seen in melanoma confirming that BRAFV600E differs functionally between these two cancer types.
Mao et al. (57) have also identified the PI3K/AKT pathway activation as an alternative means of resistance in BRAF mutant CRC. Their study demonstrated that BRAF mutant CRC cell lines had higher levels of PI3K/AKT activation when compared to their melanoma counterparts. Furthermore, the group demonstrated that cell lines with mutations in PTEN or PI3CA showed less growth inhibition when treated with BRAFV600E inhibitor PLX4720 and combination treatment with PI3K inhibitors and PLX 4720 resulted in the growth inhibition and BRAF mutant colorectal cells.
These results have provided a rationale for the use of combination therapy strategies in treating this subset of patients with poor outcomes and no effective treatment modalities. Early phase studies are ongoing to explore the synergistic effect of BRAF inhibition, anti-EGFR therapy, and PIK3CA inhibition. Corcoran et al. (58) reported their phase I/II experience with dabrafenib (D) in combination with MEK inhibitor trametinib (T) in BRAF mutant metastatic CRC. Forty-three patients received combination D+T therapy with 1 patient achieving a prolonged complete response (>22 months), 5 patients (12%) with a partial response, and 22 patients (51%) with stable disease. These data suggest that suppression of the MAPK signaling pathway with combination BRAF and MEK inhibition may be beneficial in a subset of BRAF mutated patients with metastatic CRC and may result in a durable response. Other trials are aimed at investigating “triple combination” therapy. Bendell et al. (59) demonstrated that combination therapy with dabrafenib (D), trametinib (T) plus or minus panitumumab (P) anti-EGFR antibody was well tolerated in patients with BRAF mutant metastatic CRC. In the triple therapy arm (D+P+T), 4/6 patients (67%) achieved partial tumor responses, with the remaining 2 patients exhibiting stable disease. In the doublet arm (D+P), 7/8 patients had stable disease, again demonstrating that combination therapy with two or three agents could be administered with acceptable toxicity and showed early evidence of good clinical activity. Given these findings, combination therapy using novel targeted therapeutics and/or traditional cytotoxic agents may lead to better and more durable clinical responses in patients with BRAF mutant colorectal tumors when compared to monotherapy with BRAFV600 inhibition.
Conclusions
BRAF mutant tumors represent a discrete subset of CRC characterized by poor overall survival, unique patterns of metastatic spread, and limited response to current chemotherapy and targeted therapies. BRAF has emerged as a key prognostic and predictive biomarker and represents a promising molecular target in the treatment of CRC. To date, monotherapy with vemurafenib and other single agent BRAFV600 inhibitors have not produced the desired antitumoral activity and clinical efficacy observed in melanoma due to ERK and/or PIK3CA mediated resistance. Further understanding of the mechanisms of resistance to BRAF inhibition is necessary to develop combination therapeutic strategies which offer the best hope of long term survival in this subset of patients with limited viable therapeutic options.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525-32. [PubMed]
- Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature 1987;327:293-7. [PubMed]
- Rajagopalan H, Bardelli A, Lengauer C, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002;418:934. [PubMed]
- Chang DZ, Kumar V, Ma Y, et al. Individualized therapies in colorectal cancer: KRAS as a marker for response to EGFR-targeted therapy. J Hematol Oncol 2009;2:18. [PubMed]
- Soulières D, Greer W, Magliocco AM, et al. KRAS mutation testing in the treatment of metastatic colorectal cancer with anti-EGFR therapies. Curr Oncol 2010;17:S31-40. [PubMed]
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34. [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [PubMed]
- Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol 2005;6:322-7. [PubMed]
- Cohen SJ, Cohen RB, Meropol NJ. Targeting signal transduction pathways in colorectal cancer--more than skin deep. J Clin Oncol 2005;23:5374-85. [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [PubMed]
- Michaloglou C, Vredeveld LC, Mooi WJ, et al. BRAF(E600) in benign and malignant human tumours. Oncogene 2008;27:877-95. [PubMed]
- Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med (Berl) 1997;75:587-93. [PubMed]
- Fransén K, Klintenäs M, Osterström A, et al. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis 2004;25:527-33. [PubMed]
- Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer 2011;128:2075-84. [PubMed]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816-9. [PubMed]
- Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res 1998;58:1713-8. [PubMed]
- Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer 2004;4:988-93. [PubMed]
- Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999;96:8681-6. [PubMed]
- Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38:787-93. [PubMed]
- Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol 2005;23:6771-90. [PubMed]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291-310. [PubMed]
- Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res 2005;65:6063-9. [PubMed]
- Barault L, Charon-Barra C, Jooste V, et al. Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res 2008;68:8541-6. [PubMed]
- Lee S, Cho NY, Choi M, et al. Clinicopathological features of CpG island methylator phenotype-positive colorectal cancer and its adverse prognosis in relation to KRAS/BRAF mutation. Pathol Int 2008;58:104-13. [PubMed]
- Dahlin AM, Palmqvist R, Henriksson ML, et al. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res 2010;16:1845-55. [PubMed]
- Min BH, Bae JM, Lee EJ, et al. The CpG island methylator phenotype may confer a survival benefit in patients with stage II or III colorectal carcinomas receiving fluoropyrimidine-based adjuvant chemotherapy. BMC Cancer 2011;11:344. [PubMed]
- Jover R, Nguyen TP, Pérez-Carbonell L, et al. 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology 2011;140:1174-81. [PubMed]
- Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151-6. [PubMed]
- Wang L, Cunningham JM, Winters JL, et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res 2003;63:5209-12. [PubMed]
- Oliveira C, Pinto M, Duval A, et al. BRAF mutations characterize colon but not gastric cancer with mismatch repair deficiency. Oncogene 2003;22:9192-6. [PubMed]
- French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res 2008;14:3408-15. [PubMed]
- Veigl ML, Kasturi L, Olechnowicz J, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci USA 1998;95:8698-702. [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [PubMed]
- Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998;58:3455-60. [PubMed]
- Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 2009;58:90-6. [PubMed]
- Kalady MF, Dejulius KL, Sanchez JA, et al. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum 2012;55:128-33. [PubMed]
- Li WQ, Kawakami K, Ruszkiewicz A, et al. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer 2006;5:2. [PubMed]
- Gonsalves WI, Mahoney MR, Sargent DJ, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst 2014;106:dju106. [PubMed]
- Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623-32. [PubMed]
- Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 2004;53:1137-44. [PubMed]
- Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol 2006;19:59-68. [PubMed]
- Naguib A, Mitrou PN, Gay LJ, et al. Dietary, lifestyle and clinicopathological factors associated with BRAF and K-ras mutations arising in distinct subsets of colorectal cancers in the EPIC Norfolk study. BMC Cancer 2010;10:99. [PubMed]
- Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466-74. [PubMed]
- Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol 2010;21:2396-402. [PubMed]
- Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 2015;148:88-99. [PubMed]
- Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol 2009;27:5931-7. [PubMed]
- Morris V, Overman MJ, Jiang ZQ, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer 2014;13:164-71. [PubMed]
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98-9. [PubMed]
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75. [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [PubMed]
- Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 2009;101:715-21. [PubMed]
- Yaeger R, Cercek A, Chou JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316-24. [PubMed]
- Yang H, Higgins B, Kolinsky K, et al. Antitumor activity of BRAF inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Cancer Res 2012;72:779-89. [PubMed]
- Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol 2010;28:abstr 3534.
- Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483:100-3. [PubMed]
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227-35. [PubMed]
- Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013;19:657-67. [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Phase 1-2 trial of the BRAF inhibitor dabrafenib (D) plus MEK inhibitor trametinib (T) in BRAF V600 mutant colorectal cancer (CRC): Updated efficacy and biomarker analysis. J Clin Oncol 2014;32:abstr 3517.
- Bendell JC, Atreya CE, Andre T, et al. Efficacy and tolerability in an open-label phase I/II study of MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in combination in patients (pts) with BRAF V600E mutated colorectal cancer (CRC). J Clin Oncol 2014;32:abstr 3515.


