Risk of coronary artery disease in patients with ankylosing spondylitis: a systematic review and meta-analysis
Introduction
Chronic inflammation is increasingly recognized as a non-traditional risk factor for coronary artery disease (CAD) as its association with accelerated atherosclerosis is well-established (1,2). Several studies have illustrated the deleterious effect of oxidative stress and inflammatory cytokines on endothelial function (3-6). Chronic inflammation has also been demonstrated to promote a hypercoagulable state as a result of excessive activation of the coagulation cascade as well as chronic inhibition of the anti-coagulation and fibrinolytic pathway (7,8). These factors may well serve as the elemental pathophysiology for the development of premature CAD. Moreover, an increased incidence of CAD has been observed in several autoimmune inflammatory disorders, such as rheumatoid arthritis, idiopathic inflammatory myositis, systemic sclerosis, primary biliary cirrhosis and systemic vasculitides (9-13).
Ankylosing spondylitis (AS), a form of seronegative spondyloarthritis (SpA), is a chronic systemic arthritis that primarily affects the sacroiliac joint and the axial skeleton. Characteristic clinical manifestation includes back pain and progressive stiffness of the spine though it can also involve the hips, shoulders and peripheral joints. Extra-articular manifestations, including uveitis, may also be seen in patients with AS and other forms of SpA. AS is typically a disease of young males, with a peak age of onset between 20 and 30 years and a male-to-female ratio of about 3 to 1, although there can be considerable geographical and ethnic variation (14,15).
In light of chronic inflammation, patients with AS may be at an increased risk of developing premature CAD. However, the data on CAD risk in these patients remain inconclusive owing to conflicting epidemiological studies. Thus, to further investigate this association, we conducted a systematic review and meta-analysis of observational studies that compared the risk of CAD in patients with AS versus non-AS controls.
Methods
Search strategy
Two investigators (P.U. and I.S.) independently searched published studies indexed in MEDLINE and EMBASE database from inception to July 2014 as well as the American College of Rheumatology annual conference abstract database from 2006-2013, using the search strategy described in Supplementary material. A manual search of references of selected retrieved articles was also performed.
Inclusion criteria
The inclusion criteria were as follows: (I) epidemiological study (cross-sectional, case-control or cohort study) published as original study or conference abstract reporting CAD incidence or prevalence in patients with AS; (II) relative risk (RR), odds ratio (OR), hazard ratio (HR), standardized incidence ratio (SIR) or standardized prevalence ratio (SPR) with 95% confidence intervals (CIs) were provided; (III) non-AS participants and were used as a reference group for cohort study and cross-sectional study while participants without CAD were used for case-control study.
Study eligibility was independently appraised by each investigator noted above. Differing decisions were resolved by consensus with the senior investigator. Quality of the included cohort and case-control studies was independently evaluated by the two investigators using the Newcastle-Ottawa quality assessment scale which assessed each study in three areas including: (I) the selection of the study groups; (II) the comparability of the groups; (III) the ascertainment of the exposure or outcome of interest for case-control or cohort studies respectively (16). Adapted Newcastle-Ottawa quality assessment was used to appraise the quality of cross-sectional studies (17).
Data extraction
A standardized data collection form was used to extract the following information: title of the article, first author’s last name, authors’ affiliation, publication year, country where the study was conducted, year of publication, study size, study population, criteria used for the diagnosis of AS, definition and diagnosis of CAD, average duration of follow up, number of cases, number of controls, percentage of female and adjusted effect estimates with 95% CI. This data extraction was independently performed by the two investigators.
Statistical analysis
Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration. Adjusted point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird (18). We used a random-effect model rather than a fixed-effect model in light of the high likelihood of between study variance. The statistical heterogeneity was assessed by Cochran’s Q test. This test was complemented with the I2 statistic, which quantifies the proportion of total variation across studies that is due to heterogeneity rather than chance. A value of I2 of 0% to 25% indicates insignificant heterogeneity, 26% to 50% low heterogeneity, 51% to 75% moderate heterogeneity, and 76% to 100% high heterogeneity (19).
Results
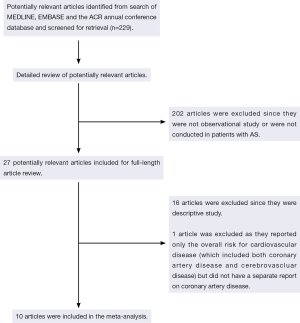
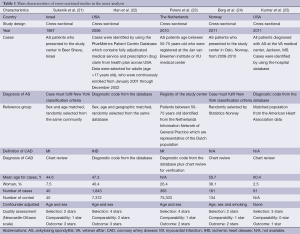
Our search strategy yielded 229 potentially relevant studies. Two hundred and two studies were excluded based on abstract screening as they were clearly not cohort, case-control or cross-sectional studies or were not conducted in patients with AS, leaving 27 studies for full-length article review. Sixteen of them were excluded since they were descriptive studies without a control group and one study was excluded because they reported only the overall risk for cardiovascular disease (which included both CAD and cerebrovascular disease) but did not have a separate report on CAD (20). Ten studies [five retrospective cohort studies and five cross-sectional studies (21-30)] with 25,795 patients with AS met our eligibility criteria and were included in the meta-analysis. Figure 1 outlines our search methodology and literature review process. The detailed description and Newcastle-Ottawa quality assessment scale of the included studies are provided in Tables 1 and 2.
Full table
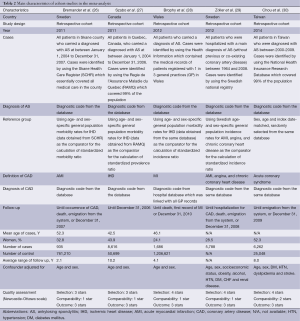
Full table
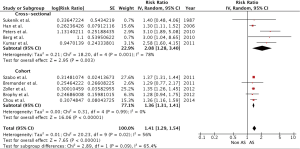
Our meta-analysis demonstrated a statistically significant increased CAD risk among patients with AS with a pooled risk ratio of 1.41 (95% CI: 1.29-1.54). Subgroup analysis revealed a statistically significant increased CAD risk for both types of study with pooled risk ratios of 1.36 (95% CI: 1.31-1.41) and 2.08 (95% CI: 1.28-3.40) for cohort and cross-sectional studies, respectively. The overall statistical heterogeneity was moderate with an I2 of 56%. Most of the statistical heterogeneity came from cross-sectional studies as their I2 was 78% while cohort studies had an I2 of 0%. Figure 2 demonstrates the forest plots of our findings.
Sensitivity analysis
To confirm the robustness of our results, we performed jackknife sensitivity analysis by excluding one single study at a time (31). The results of this sensitivity analysis suggested that our results were robust as the pooled risk ratios remained significantly elevated, ranging from 1.37 to 1.48, with the corresponding 95% CI bounds remained more than one.
We also performed a sensitivity analysis by excluding the studies with lower quality. We excluded the study by Berg et al. (24) and Kumar et al. (25) as their Newcastle-Ottawa scores were only five and four, respectively. The study by Peters et al. (23) was also excluded as the authors included only older patients (>50 years old) and, thus, a possibility of selection bias. The pooled effect was slightly reduced after the exclusion of these studies with a pooled risk ratio of 1.36 (95% CI: 1.31-1.41). Interestingly, the I2 was dramatically reduced to 0% with this sensitivity analysis, suggesting that these lower quality studies were the main source of statistical heterogeneity.
Evaluation for publication bias
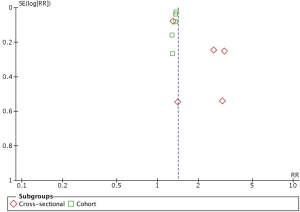
Evaluation for publication bias was performed using funnel plot as shown in Figure 3. The graph is asymmetric, suggesting that publication bias in favor of positive studies may be present.
Discussion
Our study is the first systematic review and meta-analysis of observational studies assessing the risk of CAD among patients with AS. We are able to demonstrate a statistically significant association between AS and CAD with an overall 1.41-fold increased risk compared with non-AS participants.
This finding is not only of important from medical standpoint, but also from socioeconomic perspective as patients with AS, typically young adults in their working age, are already vulnerable for a reduced productivity because of their musculoskeletal symptoms (32-34). Their capability to work might be further jeopardized because of the coronary artery complication, which certainly could cause more economic and psychological consequences.
The pathophysiology behind the association between AS and CAD is not well-described though an increasingly number of evidence are pointing toward the detrimental effect of chronic inflammation to the endothelial cell integrity. It has been demonstrated that endothelial dysfunction and direct endovascular injury from inflammatory cytokine, activated inflammatory cells and oxidative stress can accelerate the progression of atherosclerosis (3-6). Furthermore, chronic inflammation related to autoimmune disease has been linked to a thrombophilic state (7,8), another predisposing factor for the development of CAD.
In addition, other conventional cardiovascular risk factors, particularly metabolic syndrome and dyslipidemia, are more prevalent in patients with AS compared with healthy individuals (35) which, again, might be a direct consequence of the underlying inflammatory process (36) in conjunction with decreased functional capacity and physical activity secondary to their arthritis.
The adverse effect of non-steroidal anti-inflammatory drugs (NSAIDs), one of the most commonly use medications in patients with AS, on the cardiovascular system is also well-recognized (37-39). Use of NSAIDs might be another contributory cause of elevated CAD risk.
Even though most of the included studies are of high quality, there are some limitations and, thus, the results should be translated with caution.
First, most of the included studies were conducted using medical registry-based database and, thus, a possibility of coding inaccuracy for both AS and CAD. Second, statistical heterogeneity was present in this study, though the heterogeneity was significantly reduced after exclusion of lower quality studies. Third, this is a meta-analysis of observational studies which, at the best, can only demonstrate an association, not causality. Therefore, we cannot make a conclusion that AS itself versus other potential confounders, such as use of NSAIDs, causes the increased CAD risk. Furthermore, these studies were at risk of detection bias as the patients, because of their AS, exposed to more medical examinations and investigations and, thus, more likelihood of CAD detection (40).
Conclusions
In conclusion, our meta-analysis demonstrated a statistically significant increased CAD risk among patients with AS with 41% excess risk. Physicians should be aware of this association, and an appropriate management for conventional cardiovascular risk factor modification should be incorporated to the routine care for these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Supplementary
Database: Ovid MEDLINE
- exp Coronary Disease/
- coronary disease$.mp.
- exp Coronary Artery Disease/
- coronary arter$ disease$.mp.
- exp Coronary Stenosis/
- coronary stenos$.mp.
- coronary atheroscleros$.mp.
- (coronary adj3 disease$).mp.
- (coronary adj3 syndrome$).mp.
- cad.mp.
- coronary arterioscleros$.mp.
- exp Myocardial Infarction/
- myocardial infarct$.mp.
- exp Coronary Thrombosis/
- coronary thrombos$.mp.
- exp Angina, Unstable/
- unstable angina.mp.
- (unstable adj3 angina).mp.
- exp Angina, Stable/
- stable angina.mp.
- exp Angina Pectoris/
- angina pectoris.mp.
- acs.mp.
- ami.mp.
- exp Cardiovascular Diseases/
- cardiovascular disease.mp.
- or/1-26
- Bechterew$ Disease.mp.
- Marie Struempell Disease.mp.
- spondylarthr$.mp.
- exp Spondylarthritis/
- exp Spondylarthropathies/
- exp Spondylitis, Ankylosing/
- ankylosing spondylitis.mp.
- or/28-34
- 27 and 35
Database: EMBASE
- exp Coronary Artery Disease/
- exp Coronary Artery Atherosclerosis/
- coronary arter$ atheroscleros$.mp.
- exp Coronary Artery Obstruction/
- coronary arter$ obstruction$.mp.
- (coronary arter$ adj5 stenos$).mp.
- coronary atheroscleros$.mp.
- coronary arterioscleros$.mp.
- (coronary adj3 disease$).mp.
- (coronary adj3 syndrome$).mp
- coronary arter$ disease$.mp.
- exp Heart Infarction/
- heart infarction.mp
- exp Coronary Artery Thrombosis/
- coronary thrombosis.mp.
- exp Angina Pectoris/
- angina pectoris.mp.
- myocardial infarct$.mp.
- acs.mp.
- ami.mp.
- acute angina.mp.
- (unstable adj3 angina).mp.
- cad.mp.
- or/1-23
- exp Spondylarthropathies/
- exp Spondylitis, Ankylosing/
- ankylosing spondylitis.mp.
- Bechterew$ Disease.mp.
- Marie Struempell Disease.mp.
- spondylarthr$.mp.
- exp Spondylarthritis/
- or/25-31
- 24 and 32
References
- Frostegård J. Atherosclerosis in patients with autoimmune disorders. Arterioscler Thromb Vasc Biol 2005;25:1776-85. [PubMed]
- Ait-Oufella H, Sage AP, Mallat Z, et al. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res 2014;114:1640-60. [PubMed]
- Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology (Oxford) 2009;48:11-22. [PubMed]
- Rho YH, Chung CP, Oeser A, et al. Interaction between oxidative stress and high-density lipoprotein cholesterol is associated with severity of coronary artery calcification in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:1473-80. [PubMed]
- Niessner A, Sato K, Chaikof EL, et al. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 2006;114:2482-9. [PubMed]
- Hänsel S, Lässig G, Pistrosch F, et al. Endothelial dysfunction in young patients with long-term rheumatoid arthritis and low disease activity. Atherosclerosis 2003;170:177-80. [PubMed]
- Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5-6, 8-9. [PubMed]
- Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 2005;131:417-30. [PubMed]
- Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 2005;52:722-32. [PubMed]
- Ungprasert P, Wijarnpreecha K, Ahuja W. Coronary artery disease in primary biliary cirrhosis: a systematic review and meta-analysis of observational studies. Hepatol Res 2014. [Epub ahead of print]. [PubMed]
- Ungprasert P, Charoenpong P, Ratanasrimetha P, et al. Risk of coronary artery disease in patients with systemic sclerosis: a systematic review and meta-analysis. Clin Rheumatol 2014;33:1099-104. [PubMed]
- Ungprasert P, Suksaranjit P, Spanuchart I, et al. Risk of coronary artery disease in patients with idiopathic inflammatory myopathies: a systematic review and meta-analysis of observational studies. Semin Arthritis Rheum 2014;44:63-7. [PubMed]
- Ungprasert P, Koster MJ, Warrington KJ. Coronary artery disease in giant cell arteritis: A systematic review and meta-analysis. Semin Arthritis Rheum 2014. [Epub ahead of print]. [PubMed]
- Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650-7. [PubMed]
- Raychaudhuri SP, Deodhar A. The classification and diagnostic criteria of ankylosing spondylitis. J Autoimmun 2014;48-49:128-33. [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [PubMed]
- Herzog R, Álvarez-Pasquin MJ, Díaz C, et al. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013;13:154. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Available online: http://www.blackwellpublishing.com/acrmeeting/abstract.asp?MeetingID=799&id=110143
- Sukenik S, Pras A, Buskila D, et al. Cardiovascular manifestations of ankylosing spondylitis. Clin Rheumatol 1987;6:588-92. [PubMed]
- Han C, Robinson DW Jr, Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167-72. [PubMed]
- Peters MJ, Visman I, Nielen MM, et al. Ankylosing spondylitis: a risk factor for myocardial infarction? Ann Rheum Dis 2010;69:579-81. [PubMed]
- Berg IJ, Provan S, van der Heijde DMFM, et al. Increased risk of cardiovascular disease in patients with active analysing spondylitis. Presented at the American College of Rheumatology Annual Scientific Meeting, Chicago, Illinois, 2011:abstract 513.
- Kumar S, Mains T, Majithia V. High prevalence of coronary heart disease and its risk factor in veteran with spondyloarthritides. Presented at the American College of Rheumatology Annual Scientific Meeting, Chicago, Illinois, 2011:abstract 1286.
- Bremander A, Petersson IF, Bergman S, et al. Population-based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res (Hoboken) 2011;63:550-6. [PubMed]
- Szabo SM, Levy AR, Rao SR, et al. Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum 2011;63:3294-304. [PubMed]
- Brophy S, Cooksey R, Atkinson M, et al. No increased rate of acute myocardial infarction or stroke among patients with ankylosing spondylitis-a retrospective cohort study using routine data. Semin Arthritis Rheum 2012;42:140-5. [PubMed]
- Zöller B, Li X, Sundquist J, et al. Risk of subsequent coronary heart disease in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. PLoS One 2012;7:e33442. [PubMed]
- Chou CH, Lin MC, Peng CL, et al. A nationwide population-based retrospective cohort study: increased risk of acute coronary syndrome in patients with ankylosing spondylitis. Scand J Rheumatol 2014;43:132-6. [PubMed]
- Miller RG. The jackknife: a review. Biometrika 1974;61:1-15.
- Gordeev VS, Maksymowych WP, Schachna L, et al. Understanding presenteeism in patients with ankylosing spondylitis: contributing factors and association with sick leave. Arthritis Care Res (Hoboken) 2014;66:916-24. [PubMed]
- Prince DS, McGuigan LE, McGirr EE. Working life and physical activity in ankylosing spondylitis pre and post anti-tumor necrosis factor-alpha therapy. Int J Rheum Dis 2014;17:165-72. [PubMed]
- Dagfinrud H, Kjeken I, Mowinckel P, et al. Impact of functional impairment in ankylosing spondylitis: impairment, activity limitation, and participation restrictions. J Rheumatol 2005;32:516-23. [PubMed]
- Mathieu S, Gossec L, Dougados M, et al. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:557-63. [PubMed]
- Evans J, Goedecke JH, Söderström I, et al. Depot- and ethnic-specific differences in the relationship between adipose tissue inflammation and insulin sensitivity. Clin Endocrinol (Oxf) 2011;74:51-9. [PubMed]
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092-102. [PubMed]
- Ungprasert P, Kittanamongkolchai W, Cheungpasitporn W, et al. What is the “safest” non-steroidal anti-inflammatory drugs. Am Med J 2012;3:115-23.
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006;296:1633-44. [PubMed]
- Ungprasert P, Sanguankeo A, Upala S, et al. Risk of malignancy in patients with giant cell arteritis and polymyalgia rheumatica: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:366-70. [PubMed]