Abstract
On Mars, liquid water may form in regolith when perchlorate salts absorb water vapor and dissolve into brine, or when ice-salt mixtures reach their melting temperature and thaw. Brines created in this way can chemically react with minerals, alter the mechanical properties of regolith, mobilize salts in the soil, and potentially create habitable environments. Although Martian brines would exist in contact with regolith, few studies have investigated how regolith alters the formation and stability of brines at Mars-relevant conditions. To fill this gap, we studied magnesium perchlorate brine in a Martian regolith simulant at salt concentrations up to 5.8 wt.%. We measured the water mass fraction and water activity between 3 and 98% relative humidity at 25 °C using the isopiestic method, and monitored salt and ice crystallization between −150 °C and 20 °C with differential scanning calorimetry. Results show that regolith inhibits salt and ice crystallization, allowing water to form and persist at much colder and drier conditions than pure brine. Remarkably, in several samples, neither salt nor ice crystallized at any conditions. These results suggest that brines could exist in regolith for longer periods of the Martian year than previously thought, and could persist indefinitely under certain conditions. By retaining water, inhibiting salt and ice crystallization, and maintaining habitable water activity, briny regolith may be a more favorable environment for life than pure brine alone. These findings indicate the critical importance of brine–regolith interactions for understanding the properties, evolution, and potential habitability of Mars's surface.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Water is a central focus for the exploration of Mars because it affects geochemistry, regolith properties, and habitability. NASA's Curiosity and Perseverance rovers are searching for signs of life where surface water once flowed, while avoiding "Special Regions" that are protected for the presence of potentially habitable liquid water (Rummel et al. 2014). Although it is difficult for liquid water to exist on present-day Mars due to low temperatures and atmospheric pressures, liquid water may form via deliquescence (Gough et al. 2011), adsorption (Möhlmann 2005), and/or eutectic ice melting (Stillman & Grimm 2011). Deliquescence occurs when salts adsorb atmospheric water vapor, dissolve, and form brine. In contrast, adsorption occurs when water vapor adheres onto regolith surfaces and forms thin films. Eutectic ice melting occurs when salty ice melts to form brine at a temperature below 0 °C. Deliquescence, adsorption, and eutectic melting could also occur simultaneously to form brines in Martian regolith. Prior experiments have shown that salty regolith takes up water twice as fast as regolith alone, and found evidence of both deliquescence and adsorption (Slank et al. 2022). Once formed, brines are more stable than pure water because salts reduce the freezing point and evaporation rate of water (Chevrier et al. 2009; Gough et al. 2011).
Of the many salts detected on Mars's surface, perchlorates are notable for their abundance and widespread distribution in the regolith, as well as their low eutectic temperatures and extreme hygroscopicity. Perchlorates were first discovered on Mars by the Phoenix lander's Wet Chemistry Laboratory (WCL), which found 0.4–0.6 wt.% perchlorate in the near-surface regolith (Hecht et al. 2009). Subsequent geochemical modeling of WCL data suggests that Mg(ClO4)2 was the dominant salt in that regolith (Toner et al. 2014b), although other studies suggest that calcium (Kounaves et al. 2014b) or sodium (Stillman & Grimm 2011) were the dominant cations. The discovery of perchlorate by Phoenix spurred a reanalysis of Viking lander data, which concluded that perchlorates in the regolith could explain the anomalous detection of chlorinated methane, a result initially attributed to terrestrial contamination (Navarro-González et al. 2010). Evidence of perchlorates has also been found in Gale Crater, where the Sample Analysis at Mars instrument on the Curiosity rover measured evolved O2 and HCl at a temperature that suggests they were likely produced by thermal decomposition of perchlorate and/or chlorate in the regolith (Archer et al. 2014; Ming et al. 2014; Sutter et al. 2017). Most recently, multiple instruments on the Perseverance rover have independently detected perchlorates in aqueously altered rocks in Jezero Crater (Farley et al. 2022; Scheller et al. 2022; Tice et al. 2022; Wiens et al. 2022). Trace perchlorate has even been detected in Martian meteorites at ∼parts per million to parts per billion levels (Kounaves et al. 2014a; Jaramillo et al. 2019). The confirmed and probable detections of perchlorates at several sites on Mars and in Martian meteorites suggests that perchlorate is likely ubiquitous across Mars's surface (Sutter et al. 2017), and may be produced in the atmosphere and deposited across the globe (Catling et al. 2010).
Perchlorate detections are significant because some perchlorate salts may deliquesce and form brine at Martian surface conditions (Chevrier et al. 2009; Gough et al. 2011; Toner et al. 2014a). Once formed, perchlorate brines can remain liquid far below the freezing point of pure water. For example, magnesium perchlorate solution remains a stable liquid down to the eutectic temperature, which is ∼209 K according to Toner et al. (2015b), although the precise value for the eutectic temperature is debated (see also Chevrier et al. 2009 and Appendix A of Stillman & Grimm 2011). Perchlorate brines can also form metastable liquids at temperatures as low as 150 K due to supercooling (Toner et al. 2014a). The potential existence of perchlorate brines on Mars's surface has motivated extensive research on the properties of pure perchlorate brines at Mars-relevant conditions (Chevrier et al. 2009; Hecht et al. 2009; Gough et al. 2011, 2023; Fischer et al. 2014, 2016; Nuding et al. 2014, 2015; Toner et al. 2015b; Nikolakakos & Whiteway 2015; Toner & Catling 2016).
In the absence of salts, thin films of water can exist in regolith as adsorbed and/or unfrozen water (Anderson 1967; Möhlmann 2004). Adsorbed water forms when water vapor adheres to regolith surfaces and creates thin films, typically just a few monolayers thick. Adsorbed water is distinct from bulk liquid water, yet is interesting because it can enable photoredox reactions of minerals and could hypothetically support life (Möhlmann 2005). Unfrozen water is a similar physiosorbed state in which thin films of water persist in frozen soils at the interface between substrate and ice. The amount of unfrozen water is a function of the soil's temperature, mineralogy, and specific surface area (Anderson 1967). Unfrozen water is also a distinct phase from liquid water, yet is inhabited in terrestrial environments and may also be habitable on Mars (Gilichinsky 2002).
Despite extensive research on pure perchlorate brines (salt + water) and on adsorbed and unfrozen water in regolith (water + regolith), systems of brines in Martian regolith (salt + regolith + water) are relatively poorly studied. Numerous terrestrial investigations have found that brines freeze at colder temperatures when they exist in the pore space of clays and soils (Banin & Anderson 1974; Bing & Ma 2011; Wang et al. 2021). We might expect Martian regolith to similarly depress the freezing point of Mg(ClO4)2 brine, yet previous studies have treated regolith as inert and neglected any effect it may have on the brine's properties. Some experimental work has explored how Mars-like regolith affects the phase transitions of perchlorate brine, but the salt concentrations were much higher than what has been measured on Mars. Primm et al. (2018) found that regolith does not affect the freezing temperature, deliquescent relative humidity (RH), or efflorescent RH when mixed at a 1:1 wt.% ratio with Mg(ClO4)2 salt. Meanwhile, Toner et al. (2014a) found that ice crystals formed in briny regolith at conditions where the pure brine would remain a supercooled liquid. Understanding how ice and salt form in briny regolith is crucial for assessing the astrobiological potential of putative Martian brines because crystallization damages cells and locks away H2O in a form that is inaccessible to life.
In this work, we show that a Martian regolith simulant dramatically influences the properties of Mg(ClO4)2 brine across a wide range of Mars-relevant temperatures, RHs, and salt concentrations. By comparing experimental brine-regolith mixtures with the modeled behavior of pure brine and ideal brine-regolith mixtures, we explore how regolith affects the water content, water activity, solubility, and phase transitions of Mg(ClO4)2 solutions. Then, we interpret our experimental results to assess how briny regolith would influence the geochemistry and potential habitability of Mars's surface.
2. Materials and Methods
2.1. Salt and Regolith Materials
In these experiments, we studied mixtures of magnesium perchlorate brine in Mojave Mars Simulant (MMS) regolith. We chose Mg(ClO4)2 because magnesium and perchlorate are major components of the water-soluble fraction of Martian regolith (Hecht et al. 2009; Toner et al. 2014b), and such salts may form brine at Mars-relevant conditions (Gough et al. 2011; Toner et al. 2014a, 2015a). Additionally, the properties of pure aqueous Mg(ClO4)2 solutions have been extensively studied (Chevrier et al. 2009; Gough et al. 2011; Toner et al. 2014a), which provides important points of reference which we compare with our new data on brine-regolith mixtures.
For regolith, we used MMS because it physically and chemically resembles actual Martian regolith (Peters et al. 2008). MMS is composed of weathered basalt crushed to a grain size of 0.5–1.27 mm. It was originally developed to test the Phoenix and Curiosity spacecraft (Peters et al. 2008), and has been used previously to investigate mixtures of Mg(ClO4)2 and regolith (Toner et al. 2014a; Primm et al. 2018). While MMS best suits our experimental needs, there are a variety of other Martian regolith simulants with different elemental composition, mineralogy, and grain sizes, which may exhibit different properties. We considered and tested Mars Global Simulant (MGS), a simulant created to match the mineralogy of Martian regolith (Cannon et al. 2019). MGS was ruled out for this work because some of the component minerals (e.g., anhydrite, silica, and anhydrous MgSO4) are extremely hygroscopic agents that created a strong hysteresis effect during adsorption and desorption. Although such active components may occur in Martian regolith, we chose to focus on Mg(ClO4)2 as the main active hygroscopic component in this study.
One potential interaction between regolith and brine is that cations in the regolith can exchange with dissolved salts, altering the composition of the brine (Appelo & Postma 2005) . To focus this study on physical interactions, we chose to control brine composition by replacing any exchangeable cations in the regolith with Mg2+ prior to the experiments. First, we saturated the MMS with Mg(ClO4)2 over three sequential extractions, then repeatedly rinsed the regolith with deionized water to remove any soluble species, draining excess water using filter paper over a vacuum. Finally, we dried the regolith in a vacuum overnight at 60 °C to dehydrate it.
We then created the brine-regolith samples by pipetting the desired amount of Mg(ClO4)2 solution into ∼0.5 g of MMS. Then, we added deionized water in excess (∼0.05 mL), which wetted the entire sample. Upon addition of deionized water, we assume the salt is evenly distributed throughout the sample. We created samples of MMS mixed with Mg(ClO4)2 at 0% (no salt), 0.6%, 1.0%, 2.2%, and 5.8% by weight.
2.2. Measuring Water Content and Water Activity in Briny Regolith
Water activity (aw ) quantifies the availability of water in a solution, and is useful for describing habitability because all known life requires aw ≥ 0.585 to grow (Stevenson et al. 2017). We measured water content and aw of briny regolith at 3%–98% RH and 25 °C using the isopiestic method, which equilibrates samples at a specified RH using a reference solution with a known vapor pressure (Rard & Platford 1991). We prepared the reference solution from a 99.999% pure Sigma Aldrich H2SO4 solution, and the concentration was measured in triplicate by neutralizing the solution with NH3, evaporating to dryness, and weighing the residue. In the experiments, samples of briny regolith and RH reference solutions were placed into an apparatus, which is described in detail by Toner & Catling (2018). The apparatus was then evacuated using a vacuum pump, sealed with a Teflon stopcock, and submerged in a 25 °C bath for 2–3 days. Though temperatures on Mars's surface are frequently much colder, 25 °C is a standard reference temperature and allows us to compare results with data from the literature. In the bath, water vapor exchanged between the sample and the reference solution until the system reached equilibrium. Because the H2SO4 reference solution has a well-defined vapor pressure as a function of concentration, we precisely know the RH of the system. Consequently, the RH also gives the water activity of the sample because the entire system is in equilibrium (RHeq = aw ). By taking the masses of sample and reference both before and after equilibration, we measured the water content, salt concentration, and aw of the brine-regolith mixture. For the isopiestic experiments, we used MMS mixed with Mg(ClO4)2 in the following amounts: 0 wt.% (no salt), 0.57 ± 0.0037 wt.%, 1.0 ± 0.012 wt.%, 2.2 ± 0.027 wt.%, and 5.8 ± 0.062 wt.% (the variations reported here and throughout are the sample standard deviation).
To interpret the experimental results, it is useful to compare the measured behavior of brine-regolith mixtures to the modeled behavior for pure brine. We model the water content, water activity, and freezing point depression of pure Mg(ClO4)2 brine by adapting the revised Pitzer model described in Toner et al. (2015b). It is additionally useful to compare the experimental results to the null hypothesis that brine and regolith act independently from each other. We model the water content of such "ideal" brine-regolith mixtures by summing the water content modeled for pure brine and the water content measured in salt-free regolith.
2.3. Measuring the Melting Point of Briny Regolith
At Martian surface conditions, aqueous Mg(ClO4)2 solutions could transition between phases of liquid brine, crystalline ice and/or salt, and glass, which forms when crystallization is kinetically inhibited during the transition from a viscous liquid to a rigid, amorphous solid (Chevrier et al. 2009; Toner et al. 2014a). These phase transitions are well characterized for pure Mg(ClO4)2 brine, but are complicated by the presence of regolith, which binds water molecules to mineral surfaces and could provide nucleation sites for ice or solid salt to crystallize.
We probed phase transitions in brine-regolith mixtures at low temperatures (−150 °C–20 °C) using a TA Instruments Q2000 differential scanning calorimeter (DSC). A DSC detects phase transitions by measuring heat flow through a sample as temperature changes. When a sample changes phase (e.g., melts, freezes, or forms glass), it will release or absorb heat in a manner characteristic of that specific phase transition. By inspecting the heat flow through the sample as a function of temperature, we identified the various phase transitions and the temperatures at which they occurred.
However, it is particularly difficult to measure the freezing temperature in Mg(ClO4)2 brine because perchlorates are prone to supercooling, a metastable state where solutions remain liquid below their eutectic temperature (Toner et al. 2014a). Supercooling may be responsible for the differing eutectic temperatures of Mg(ClO4)2 reported in the literature (Stillman & Grimm 2011; Toner et al. 2015a). To avoid supercooling, we instead probed each sample for its melting temperature, which is theoretically equivalent to the freezing temperature at thermodynamic equilibrium. However, hysteresis in freezing and thawing soils may cause the melting temperature to be higher (Devoie et al. 2022). First, we rapidly cooled the brine-regolith sample down to −150 °C, held that temperature for 5 minutes, then warmed to 20 °C at a rate of 10 °C minute−1. As the sample warmed, we identified melting points at the peak of endothermic dips in the heat-flow curve. For this analysis, we used TA Instruments Universal Analysis software. We note that the DSC measures the external temperature of the hermetically sealed pan that contains the sample. We assume any difference in temperature between the pan and sample is negligible due to the small sample size (∼30–40 mg), high thermal conductivity of the aluminum pan, and duration that the sample is held at low temperature. In the calorimetry experiments, we used MMS mixed with Mg(ClO4)2 in the following amounts: 0.57 ± 0.0042 wt.%, 1.0 ± 0.0064 wt.%, 2.2 ± 0.029 wt.%, and 5.5 ± 0.14 wt.%.
3. Results
3.1. Water Content and Water Activity at 25 °C
The 25 °C isopiestic experiments measured water content (Figures 1 and 2) and brine concentration (Figure 3) as a function of RH in mixtures of Mg(ClO4)2 brine and MMS regolith. These results address a key knowledge gap in the literature about how much water could exist in perchlorate-rich Martian regolith at various RH and wt.% salt.
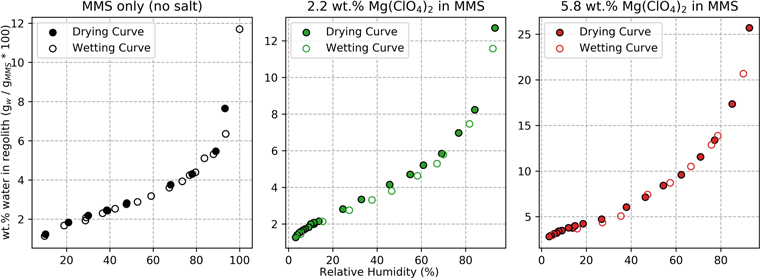
Figure 1. Adsorption isotherms show the minor extent of a wetting-drying hysteresis effect in regolith and brine-regolith mixtures. At any given relative humidity, samples that are drying from a prior wet state retain more water than identical samples that are wetting from a prior dry state (Hillel 1971). The regolith used was Mojave Mars Simulant (MMS) and all experiments were conducted at 25 °C. Data for 0.6 and 1.0 wt.% Mg(ClO4)2 in MMS are excluded here because only drying curves were measured for those samples.
Download figure:
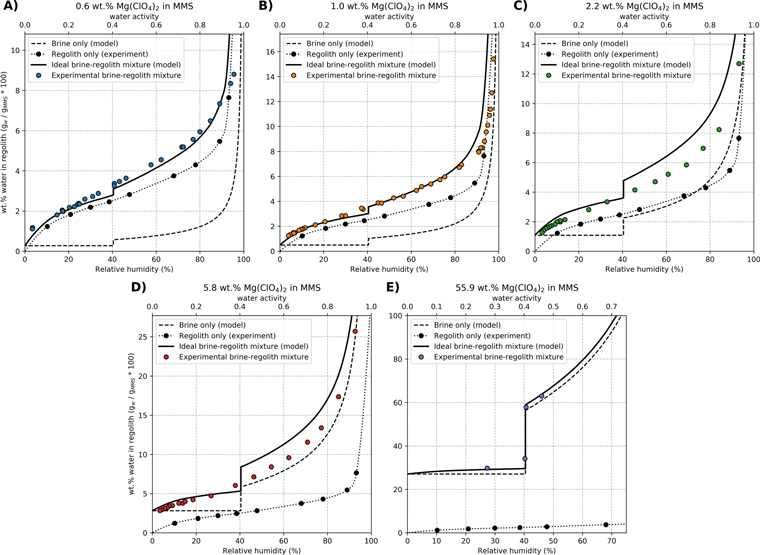
Standard image High-resolution imageFigure 2. The water content in regolith varies depending on the RH and the amount of salt for several systems: modeled pure brine (dashed curves), regolith without salt (black circles and dotted curves), an ideal mixture of brine and regolith (solid curves), and experimental brine-regolith mixtures (colored circles). The curves for the ideal brine-regolith mixtures sum the behavior of pure brine and salt-free regolith, assuming each component behaves independently. However, experimental brine-regolith mixtures deviate greatly from ideal behavior for 0.6–5.8 wt.% salt (A–D). The step changes that occur in some systems at ∼40.4% RH are the result of salt precipitation. Notably, salt phase transitions are absent in the experimental data for 0.6–5.8 wt.% salt (A–D). For comparison, a positive example of salt crystallization in regolith is shown (panel E). All data represent samples at 25 °C that have lost water from a prior wet state (i.e., "Drying Curve" in Figure 1).
Download figure:
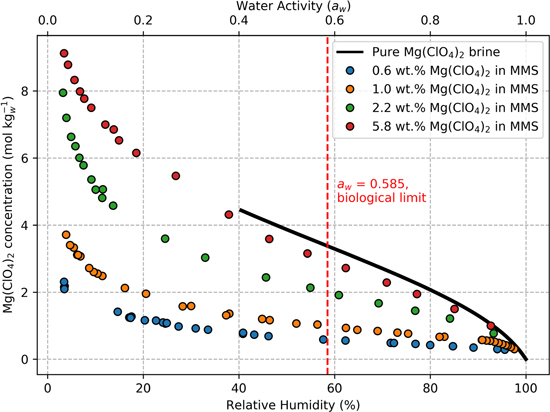
Standard image High-resolution imageFigure 3. At any specified relative humidity, experimental brine-regolith mixtures (circles) are less concentrated than pure brine (black curve). The trend of pure Mg(ClO4)2 brine (black curve) was calculated using a revised Pitzer model for perchlorates described in Toner et al. (2015b), and terminates at the saturation point (4.44 molal, RH = 40.4%). Also shown is the known lower aw limit for life on Earth (aw = 0.585, dashed red line; Stevenson et al. 2017). All data represent samples at 25 °C that have lost water from a prior wet state (i.e., "Drying Curve" in Figure 1).
Download figure:
Standard image High-resolution imageWhen soils adsorb or desorb water, they experience a hysteresis effect where the water content depends on the soil's hydration history (Hillel 1971). At identical temperature and RH conditions, soils drying from a wet state will retain more water than soils wetting from a dry state. To measure this hysteresis effect, we collected data for the wetting and drying curves for regolith and some brine-regolith samples (Figure 1). We measure that the hysteresis effect in our experiments is relatively small (at most, a 0.4 wt.% difference between the two curves), so, for simplicity, we will focus on the drying curves for the remainder of this paper.
Our experiments reveal that brine-regolith mixtures do not behave like ideal mixtures of the brine and regolith end-members, but instead exhibit unique properties (Figure 2). To model the pure brine component (dashed curves in Figure 2), we adapted the revised Pitzer model described in Toner et al. (2015b) and assumed a fixed amount of salt that exists within, but behaves independently from, the regolith. Note that this is an implicit assumption made by any study that neglects the effect of regolith on brine properties. For salt-free regolith systems, we experimentally measured adsorbed water content using the isopiestic method (black circles in Figure 2), then interpolated between experimental data points with a spline curve (dotted curve in Figure 2). Mixtures of brine and regolith have water associated with both salt and mineral phases, and so retain more water than the pure brine or regolith-only end-members. We predicted the behavior of an ideal brine-regolith mixture (solid curves in Figure 2) by summing the water contents of the pure brine model and regolith-only experiments at every RH. However, the measured behavior of experimental brine-regolith mixtures (colored circles in Figure 2) deviates greatly from ideal predictions. For samples with 0.6 wt.% salt in regolith, the actual mixture tended to be wetter than the ideal case, except when RH exceeded ∼90% (Figure 2(A)). Meanwhile, samples with 1 wt.% salt closely matched the ideal case for RH 0%–80%, but contained less water than predicted for RH > 80% (Figure 2(B)). Samples with 2.2 and 5.8 wt.% salt tended to be drier than the ideal mixture over all RH (Figures 2(C) and (D)). Potential explanations for these results are discussed in Section 4.3.
One major deviation from ideal behavior is that salt crystallization is not observed in experimental brine-regolith mixtures with low wt.% salt (Figures 2(A)–(D)). Salt can precipitate from a pure brine when it is saturated, which occurs at RH = 40.4% for Mg(ClO4)2 at 25 °C (Toner et al. 2015b). If water evaporates from the saturated solution, RH and brine concentration remain constant while any excess salt precipitates out of solution. Eventually, only solid Mg(ClO4)2 hexahydrate would remain at 0.021% RH (Besley & Bottomley 1969), with 6 moles of water per mole of salt. We note that Mg(ClO4)2 solution effloresces at 19% RH (Gough et al. 2011), but in this work salt forms according to the phase diagram (i.e., at 40.4% RH) because the experiment strongly promotes equilibrium conditions.
In Figure 2, salt crystallization appears as a sharp transition at RH = 40.4% in the pure brine and ideal brine-regolith mixture models (dashed and solid curves, respectively), as well as in the experimental data for 56 wt.% salt in regolith (Figure 2(E)). However, this salt crystallization phase change is notably absent from all other experimental brine-regolith data, even at RH well below 40.4% (Figures 2(A)–(D)). There is similarly no evidence of salt crystallization in the wetting curves (Figure 1). The smooth trend of the data suggests that salt never precipitated in the experiments with 0.6–5.8 wt.% salt in regolith (Figures 2(A)–(D)). This conclusion is further supported by visual evidence, as salt crystals were not observed in any samples with 0.6–5.8 wt.% salt (Figures 2(A)–(D)), but were seen in samples with an arbitrarily high salt fraction (56 wt.%) at RH ≤ 40.4% (Figure 2(E)).
While we observe no evidence of salt formation in samples with <5.8 wt.% salt, it is possible that crystallization could have occurred undetected in the 0.6 and 1.0 wt.% samples (Figures 2(A)–(B)). In these samples, salt crystallization would be indicated by a relatively small loss of water (0.30 and 0.54 wt.% H2O, respectively), which might be hidden in the gaps between measured data. By comparison, any salt crystallization in the 2.2 and 5.8 wt.% samples would have been detected because the relatively large loss of water (1.2 and 3.1 wt.% H2O, respectively) would be apparent in the experimental data (Figures 2(C)–(D)). Because salt crystallization was plainly observed in the 56 wt.% samples (Figure 2(E)), but did not occur in the 5.8 or 2.2 wt.% samples, we infer that salt also did not crystallize in the 1.0 and 0.6 wt.% samples.
We find that brines in regolith are less concentrated than pure brine at the same RH and temperature (Figure 3). The magnitude of the decrease in salt concentration varies based on the wt.% salt in regolith, with low wt.% salt samples (e.g., blue circles) deviating most from the behavior of the pure brine (black curve). Meanwhile, samples with a higher wt.% salt (e.g., red circles) behaved more like a pure brine.
Although brines on Mars will change concentration in response to environmental RH and temperature conditions, it is informative to consider the inverse relationship to interpret how regolith affects brines in laboratory studies on Earth. In Figure 3, we see that for a given salt concentration in solution, water activity is much lower in brine-regolith mixtures (circles) compared to the pure brine (black line). Regolith reduces aw in brine because mineral surfaces bind water molecules, which lowers the availability of water in solution (see Section 4).
3.2. Depressed Melting Point in Briny Regolith
The depressed aw we measure in brine-regolith mixtures (Figure 3) implies a corresponding depression in the freezing point relative to the pure brine (Marion & Kargel 2008; Toner et al. 2015b). However, freezing points of perchlorate brines are notoriously difficult to measure due to supercooling; instead, we measured the melting point, which is theoretically equivalent at thermodynamic equilibrium. However, it is likely that the measured melting points are higher than the samples' freezing points, because soils cause a hysteresis effect where thawing occurs at a higher temperature than freezing (Devoie et al. 2022).
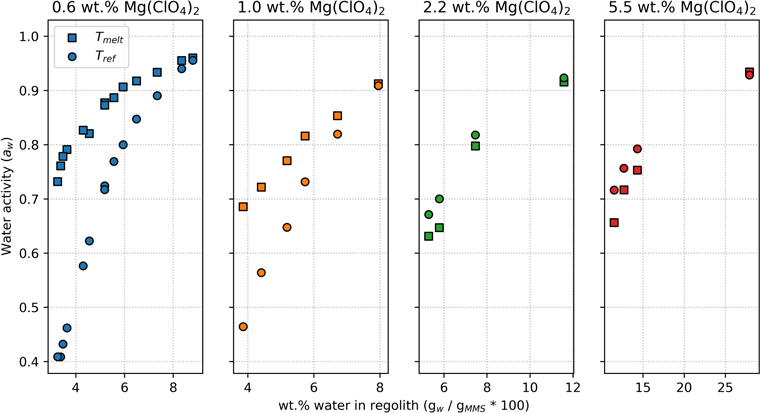
Calorimetry measurements show that melting points in brine-regolith samples are depressed relative to pure brine at all salt concentrations (Figure 4). The most significant melting point depressions occurred in samples with the lowest wt.% of salt in regolith (blue squares, upper panel); meanwhile, samples with higher wt.% salt behaved more like the pure brine (red squares, upper panel). We did not measure any melting points lower than the eutectic temperature of Mg(ClO4)2, which is the coldest temperature that a stable liquid brine can reach (209.3 K, according to Toner et al. 2015b).
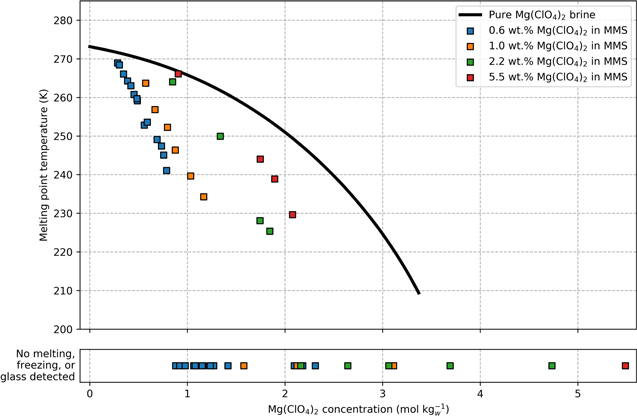
Figure 4. Measured melting points of brine-regolith samples (squares) are depressed compared to pure brine (black line) at a given salt concentration (mol kgw −1). The freezing point of pure Mg(ClO4)2 brine was calculated using a revised Pitzer model described in Toner et al. (2015b). At all wt.% salt measured, some relatively high-concentration samples showed no signs of freezing, melting, or forming glass whatsoever, over the full range of temperatures (−150 °C–20 °C). The concentrations of these unfrozen samples are shown in the lower panel. All data represent samples that have lost water from a prior wet state (i.e., "Drying Curve" in Figure 1).
Download figure:
Standard image High-resolution imageHowever, not all samples had their melting point depressed by regolith. Instead, many samples showed no signs of melting, freezing, or forming glass whatsoever (Figure 4, lower panel). The brine in these samples remained unfrozen, despite being held at −150 °C for 5 minutes, then warmed by 10 °C per minute to 20 °C. This behavior was observed in samples at each wt.% salt, but only occurred in samples with very low water content.
3.3. Inferring Water Activity at Lower Temperature
Melting point measurements can also be used to determine the water activity at low temperatures. During melting, ice fixes the vapor pressure of the system and therefore the sample's water activity follows Equation (1) (Marion & Kargel 2008):
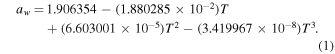
We used Equation (1) to calculate the aw of each sample at its measured melting temperature (Tmelt), then compared to the measured aw at 25 °C (Tref) (Figure 5). We note that Equation (1) has previously been shown to provide reliable predictions for the water activity of perchlorate brines at their melting points (Toner et al. 2015b; Toner & Catling 2016). Even for a brine in regolith, Equation (1) remains a reliable method for inferring aw whenever ice is present, because ice controls the vapor pressure of the system.
Figure 5. To probe how the aw of briny regolith changes with temperature, we predicted the aw at each sample's melting temperature (Tmelt, squares) via Equation (1), then compared to the measured aw of the same sample at 25 °C (Tref, circles). Note that the melting temperature varies for each sample (see Figure 4), but for all samples Tmelt < Tref = 25 °C. All data represent samples that have lost water from a prior wet state (i.e., "Drying Curve" in Figure 1).
Download figure:
Standard image High-resolution imageThe direction and magnitude of the change in aw with temperature depends on the amount of salt and water with respect to regolith (Figure 5). All samples with 2.2 wt.% salt and all but one sample with 5.5 wt.% salt were projected to have lower aw at the melting temperature than at 25 °C, i.e., water in solution was less available at colder temperatures. Conversely, samples with more Mars-like wt.% salt (0.6 and 1.0 wt.%) had a much higher aw at the melting point, which suggests that water was more available at the colder temperature. Notably, we project that every sample had aw exceeding the lower limit for habitability (aw > 0.585) at the melting temperature.
The extreme changes in aw with temperature predicted here are atypical for most materials and are likely due to hysteresis in the freezing and thawing process. Previous work shows that wet soils thaw at a higher temperature than they freeze at (Devoie et al. 2022). This hysteresis occurs because freezing concentrates water in ice grains and within larger pore spaces, which reduces the melting point depression caused by interactions with the regolith surface (known as the Gibbs–Thomson effect).
4. Discussion and Implications for Mars
4.1. Regolith Inhibits Salt Precipitation
Our results demonstrate that salt formation is suppressed in Mars-like brine-regolith mixtures, which indicates that water can persist in regolith at drier conditions than in pure brine. In Figure 2, salt formation appears as an abrupt transition at RH = 40.4%, where the saturated brine begins precipitating salt while the RH and dissolved ion concentration remain constant. This phase change is apparent in the models for pure brine and the ideal brine-regolith mixtures, as well as the experimental data for 56 wt.% salt (Figure 2(E)). However, it is absent from all other experimental brine-regolith data (Figures 2(A)–(D)). In fact, we see no evidence of salt formation at any RH for MMS with 0.6–5.8 wt.% salt, which suggests that regolith completely suppresses salt formation in these samples. At lower temperatures, salt formation was similarly suppressed, and we never observed salt crystallization while probing phase transitions with the DSC between −150 °C and 20 °C.
Salt formation may have been suppressed because brines in regolith are more dilute than pure brine at a given RH (Figure 3). Salt precipitates from pure Mg(ClO4)2 brine at the saturation concentration (4.44 molal at 25 °C). At lower concentrations, all ions remain dissolved in solution. Brines in regolith are more dilute and can remain below the saturation concentration even at RH < 40.4%, where a pure brine would precipitate salt. Notably, the samples with the most Mars-like salt contents (0.6 and 1 wt.%) maintained a concentration below 4.44 molal at every RH measured (3%–98%), which could explain why no salt formed in these samples. Meanwhile, saltier samples (2.2 and 5.8 wt.%) exceeded the saturation concentration at low RH, yet still showed no signs of precipitating salt.
The equations that describe the precipitation of Mg(ClO4)2 hexahydrate provide another plausible explanation for why salt crystallization was suppressed in brine-regolith mixtures:
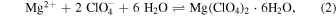

Here, Ksp is the solubility constant that describes salt precipitation, ai is the activity of each ion or water, γi is the ion activity coefficient, and m is the molal saturation concentration of brine. In our experiments, we find that aw tends to be lower in brine-regolith mixtures than in a pure brine at the same concentration (Figure 3). Because Ksp remains constant regardless of whether the reaction occurs in regolith or not, if regolith reduces the aw of brine, then it must proportionally increase the saturation concentration (m) and/or activity coefficient (γ). Considering the respective powers of each term in Equation (3), even a small decrease in aw could cause an exponential increase in the concentration required to precipitate salt (m), which may explain the observed increase in solubility.
Another explanation for the lack of salt formation is that brines in regolith may become supersaturated. Supersaturation is a metastable state where the concentration of dissolved ions exceeds what could ordinarily be dissolved at equilibrium conditions. Perchlorates are particularly prone to the similar metastable state of supercooling. Similar mechanisms could allow metastable states where both salt and ice formation are inhibited for perchlorate brines in regolith. Although regolith may be expected to decrease supercooling by providing additional nucleation sites, prior work that added regolith to Mg- and Ca-perchlorate brine did not observe a decrease in supercooling (Toner et al. 2014a). Further experiments probing the phase transitions during supercooling and supersaturation are needed to understand the mechanisms responsible for both processes.
4.2. Regolith Inhibits Ice Crystallization
We also found that regolith inhibits ice crystallization in Mg(ClO4)2 brine. These results indicate that brines can persist at lower temperatures when in regolith. Previously, the phase boundary between liquid and ice in Mg(ClO4)2 solution was only well understood for pure brine (Chevrier et al. 2009; Toner et al. 2015b). The ice line for pure Mg(ClO4)2 brine is shown as a solid black line in Figure 4. However, when brine is in regolith, the melting temperature is much lower than pure brine or is eliminated completely (Figure 4). These findings greatly expand the potential physical extent and the annual duration of unfrozen water on Mars's surface.
The magnitude of the melting point depression decreases with increasing wt.% of salt in regolith (Figure 4). We hypothesize this trend exists because high salt content absorbs more water, increasing the overall water content of the brine-regolith mixture (Figure 2). Since the regolith only strongly interacts with the solution that is closest to the brine-regolith interface, the overall effect of regolith is diminished when more brine is present. Consequently, saltier, wetter samples behave more like pure brine. These results are consistent with previous studies, which found that 1:1 mixtures by weight of Mg(ClO4)2 salt in MMS had a similar freezing temperature to pure Mg(ClO4)2 brine (Primm et al. 2018). However, the measured perchlorate content in Martian regolith is much lower (∼0.4–0.6 wt.%; Hecht et al. 2009; Toner et al. 2014b). We find that at Mars-relevant wt.% salt, regolith does strongly depress the melting point of Mg(ClO4)2 brine. These results are also consistent with numerous terrestrial studies, which demonstrate that soils reduce the freezing point of various salt solutions (Banin & Anderson 1974; Bing & Ma 2011; Wang et al. 2021).
Some relatively dry samples showed no signs of melting or freezing whatsoever, despite being cooled to −150 °C. When wet soils freeze, a layer of unfrozen water can persist as thin, mobile films between soil surfaces and ice (Anderson 1967; Wang et al. 2021). In our experiments, the samples that remained entirely unfrozen had very low water content and high salt concentration. We hypothesize that all the water in these samples existed as unfrozen brine, tightly bound to regolith surfaces and ions. The strong bonds to regolith and ions inhibit freezing completely, allowing unfrozen water to persist to at least −150 °C, which is colder than the lowest temperatures on Mars's surface. These results suggest that at Mars-relevant wt.% salt thin films of highly concentrated, adsorbed brines could persist throughout diurnal and seasonal cycles, potentially persisting over multiple Mars years.
4.3. Structure of Brines in Regolith
Brine-regolith mixtures exist in a phase between two end-members: adsorbed water (water + regolith) and pure brine (water + salt). The behavior of the mixture as a whole is highly dependent on the relative amount of salt in the regolith. Samples with low wt.% salt absorb less water, so the limited water adheres tightly to regolith surfaces. As a result, the system as a whole behaves more like an adsorbed fluid. Conversely, samples with high wt.% salt absorb more water, so proportionally more water exists away from the regolith–solution interface. As a result, the regolith has less of an effect on the brine's properties, and the system approaches the behavior of a pure brine. Brines in regolith follow this trend in terms of water content (Figure 2), water activity (Figure 3), brine concentration (Figure 3), and melting point (Figure 4).
One peculiar observation is that saltier brine-regolith samples tend to contain less water than the sum of the brine-only and regolith-only end-member states (i.e., experimental data versus ideal model in Figures 2(C)–(D)). We hypothesize that this may be due to large, weakly charged ClO4 − anions, which are attracted to exchangeable cations adsorbed on the regolith and subsequently block H2O molecules from adhering to regolith surfaces. As a result, saltier samples (Figures 2(C)–(D)) contain less water in the adsorbed phase compared to regolith samples with little salt (Figures 2(A)–(B)) or no salt (Figure 2, black circles). Future work would be needed to test this hypothesis and explore the effect in more detail.
Regolith disrupts crystallization via the Gibbs–Thomson effect, where the interfacial tension of a fluid on a curved surface increases the energy required to form crystals (Devoie et al. 2022). This effect is strongest very near regolith surfaces and diminishes farther from the interface. When briny regolith freezes, thin films of unfrozen brine can persist tightly bound to regolith surfaces. We hypothesize that some samples in this study contain so little water that the entirety of the brine remains in an unfrozen state at the regolith–solution interface (Figure 4, lower panel). It is unclear from these experiments how thick the unfrozen layer is, or at what water content the free brine regime begins, though we anticipate a region of transition between the two. Prior investigations of unfrozen water found between one and 25 layers of unfrozen water in salt-free clays, depending on temperature (Anderson 1967). We hypothesize that the addition of salts would further disrupt crystallization and increase the thickness of the unfrozen layer, but future work would be needed to quantify the thickness of this physiosorbed layer for various surface areas and ion concentrations. Future studies could also employ other Martian regolith simulants to explore how brine properties vary in regolith of differing elemental composition, mineralogy, grain size, and specific surface area.
Although adsorbed and unfrozen brines are distinct phases from liquid water, they have several key properties relevant to geochemistry and astrobiology. Adsorbed water can enable geochemical weathering of the surface, and could hypothetically support life (Möhlmann 2005). Meanwhile, unfrozen water in soil is mobile and can transport ions throughout the matrix (Anderson 1967; Hoekstra & Miller 1967; Stillman et al. 2010). On Earth, unfrozen brines support diverse populations of microbes in the permafrost (Gilichinsky et al. 1993), and could feasibly also support life and/or preserve biosignatures on Mars (Gilichinsky 2002). Future experiments with live microbes would lead to a more robust understanding of the habitability of this unique phase of water in briny Martian regolith.
4.4. Implications for Habitability, Planetary Protection, and Exploration of Mars
By inhibiting salt and ice formation, regolith stabilizes brines and may improve their habitability. Crystallization damages cells and locks away H2O molecules in forms inaccessible to life (i.e., ice or hydrated salt), but our experiments reveal that regolith prevents crystallization over a wide range of Mars-relevant conditions (between 25 °C and −150 °C and at RH as low as 3%). Accounting for the salt- and ice-inhibiting effects of regolith, potential brines on Mars could contain more bioavailable water and have fewer hazards for life than previously thought. These effects are astrobiologically significant because they expand the range of conditions where we could search for life in potential Martian brines. They are also relevant for planetary protection because they expand the regions where terrestrial microbes delivered by spacecraft may be able to survive.
A crucial parameter for assessing habitability is water activity, which measures the biological availability of water in a brine and sets a lower limit for life (life requires aw ≥ 0.585 to grow; Stevenson et al. 2017). Planetary protection also relies on aw to designate "Special Regions," areas that merit additional safeguards due to their potential habitability (Rummel et al. 2014). Previously, the habitability of potential Martian brines has been assessed using the well-documented aw of pure brine, while neglecting any effect of regolith. Our results demonstrate that regolith strongly reduces the aw of a brine at a given concentration at 25 °C, and frequently even lowers aw below the limits for habitability and Special Region status (Figure 3). In Figure 3, any point that falls to the left of the red dashed line is not habitable. However, regolith itself does not necessarily make brines less habitable, because any actual brines on Mars would adjust aw according to environmental conditions (i.e., temperature and RH), while molal salt concentration varies as water exchanges with the atmosphere. Nevertheless, these findings demonstrate that predicting habitability on Mars based on the aw of pure brines is not a sufficient approach, and future studies should account for how strongly regolith depresses aw .
Despite having lower aw at 25 °C, brines in regolith may—perhaps counterintuitively—have more habitable aw at the colder melting temperature. We calculate that every sample in our study had aw permissible for life (aw > 0.585) at its melting temperature (Figure 5). However, the aw at the freezing temperature is likely lower because hysteresis causes freezing to occur at a lower temperature than thawing in soils (Devoie et al. 2022). Regardless of whether aw is permissible for life, low temperatures remain a barrier for habitability. The measured melting temperatures were frequently below the known limits for cell division (−18 °C) and metabolism (−33 °C; Rummel et al. 2014). Future experiments measuring aw below 0 °C are necessary to better understand the low-temperature habitability of perchlorate brines in regolith.
While low-aw brines like those we measure at 25 °C are generally less habitable, low aw itself is not necessarily biocidal (Hallsworth 2021). Some terrestrial organisms can survive wet–dry cycles, even when aw drops below their minimum required value for metabolism or reproduction (Meisner et al. 2021). Brines that experience changing aw conditions could still support life that is dormant during low-aw periods, then active when aw conditions permit (Möhlmann 2005). On the other hand, chaotropicity (i.e., the disruption of intermolecular bonding and structure) may be the most limiting factor for habitability in potential Martian brines, regardless of whether aw is permissible (Hallsworth 2021).
In addition to the implications for geochemistry and habitability, these results are also important for understanding ion mobilization and the mechanical properties of Martian regolith. The Phoenix lander observed perchlorate ions distributed throughout the sampled soil and locally concentrated in patches, which suggests mobilization and could be evidence of thin brine films (Cull et al. 2010). Our results help explain how perchlorate could migrate in the cold and dry polar conditions: regolith could keep brines mobile by inhibiting salt and ice crystallization. Phoenix and several other missions also encountered unusually cohesive regolith (Moore et al. 1999; Arvidson et al. 2004a, 2004b, 2009; Minitti et al. 2013). In the example of the Phoenix lander, the regolith was so unexpectedly cohesive that operators had to improvise a "sprinkle" delivery method to collect some samples (Arvidson et al. 2009). Perchlorate brine films in the regolith could explain the strong cohesion observed. Our results are also relevant to the ongoing Perseverance mission, which is equipped to collect regolith samples in Jezero Crater (Moeller et al. 2021), where perchlorate salts have been detected (Farley et al. 2022; Scheller et al. 2022; Tice et al. 2022; Wiens et al. 2022).
5. Conclusion
Interactions with regolith are critical for assessing the abundance, persistence, and habitability of putative brines on Mars. Experiments show that regolith can inhibit freezing and salt precipitation, allowing water to persist at much colder and drier conditions than are possible for pure brine. Regolith may also make potential surface brines more habitable by inhibiting crystallization of salt and ice, which damages cellular structures. Some relatively dry samples never crystallized over the full range of Martian surface temperatures, suggesting that thin films of unfrozen brine could persist in regolith over diurnal and seasonal cycles. The increased persistence of brines in regolith greatly expands the spatial and temporal extent of potentially habitable conditions on Mars.
Overall, we demonstrate a novel approach for investigating brines as they would likely exist on Mars's surface, i.e., adsorbed to and in the pore space of regolith. Brines in regolith do not behave like ideal mixtures of pure brine and adsorbed water, but are instead a unique system complicated by interactions between all three components (water, salt, and regolith). Therefore, the distinct properties of briny regolith systems must be considered to accurately explore how brines would affect the geochemistry, habitability, and mechanical properties of Mars's surface. This new approach to studying brine-regolith systems holistically will improve astrobiological and geochemical investigations, mission planning, and planetary protection on Mars.
Acknowledgments
This work was supported by NASA Solar Systems Workings grant No. 80NSSC21K0149.