Abstract
We report the first detection in the interstellar medium of N-cyanomethanimine (H2CNCN), the stable dimer of HCN of highest energy and the most complex organic molecule identified in space containing the prebiotically relevant NCN backbone. We have identified a plethora of a-type rotational transitions with 3 ≤ Jup ≤ 11 and Ka ≤ 2 that belong to this species toward the Galactic center G+0.693-0.027 molecular cloud, the only interstellar source showing the three cyanomethanimine isomers (including the Z- and E-isomers of C-cyanomethanimine, HNCHCN). We have derived a total column density for H2CNCN of (2.9 ± 0.1) × 1012 cm−2, which translates into a total molecular abundance with respect to H2 of (2.1 ± 0.3) × 10−11. We have also revisited the previous detection of E- and Z-HNCHCN and found a total C/N-cyanomethanimine abundance ratio of 31.8 ± 1.8 and a Z/E-HNCHCN ratio of 4.5 ± 0.2. While the latter can be explained on the basis of thermodynamic equilibrium, chemical kinetics are more likely responsible for the observed C/N-cyanomethanimine abundance ratio, where the gas-phase reaction between methanimine (CH2NH) and the cyanogen radical (CN) arises as the primary formation route.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
How life originated on Earth is one of the key open questions still lingering in the astrobiological community. One of the most accepted hypotheses is the so-called RNA world (Gilbert 1986), which suggests that this macromolecule may have performed both the metabolic and genetic functions that in present-day living organisms are carried out by proteins and DNA, respectively. Although the chemical processes forming the first RNA molecules remain unknown, numerous prebiotic chemistry experiments have shown that RNA building blocks, the ribonucleotides, can be synthesized from much simpler molecules (Powner et al. 2009; Patel et al. 2015; Becker et al. 2016, 2019), with nitriles (organic species with the −C≡N functional group) playing a dominant role (e.g., Balucani 2009; Menor Salván et al. 2020). The clearest example is found on their simplest representative, hydrogen cyanide (HCN), which has been proven to be an essential ingredient in initiating many prebiotic synthesis mechanisms (e.g., Oró & Kimball 1961; Ferris & Hagan 1984; Santalucia et al. 2022; Sandström 2023). Among them, HCN oligomerization reactions are especially relevant, as these are evidenced to be the major routes forming the two purine nucleobases assembling both RNA and DNA chains: adenine (H5C5N5; Chakrabarti & Chakrabarti 2000; Jung & Choe 2013) and guanine (H5C5N5O; Sanchez et al. 1968; Choe 2018). Since HCN is ubiquitous in the interstellar medium (ISM), these processes might similarly occur through interstellar chemistry, making the study of the molecular complexity in the ISM an essential task to gain a deeper insight into this "vital" question.
A crucial step triggering the HCN oligomer cascade concerns the initial formation of HCN dimers (H2C2N2). The formation of such species from two separate HCN molecules did not appear to be efficient under the typical temperatures of the ISM (∼10–100 K) due to the large activation energy barrier involved (∼36,000 K; Smith et al. 2001; Yim & Choe 2012). However, the successful detections in the ISM of the two most stable HCN dimers, the E- and Z-isomers of C-cyanomethanimine (HNCHCN; Zaleski et al. 2013 and Rivilla et al. 2019, respectively), triggered the study of alternative chemical pathways (e.g., Vazart et al. 2015; Shivani & Tandon 2017; Shingledecker et al. 2020; García de la Concepción et al. 2021).
In this regard, the exhaustive characterization of new HCN dimers in the ISM would substantially contribute to a better understanding of how these compounds could be formed in the interstellar environment. From the entire catalog of possible HCN dimers, only a single additional isomer has shown superior stability compared to two isolated HCN molecules (Evans et al. 1991). That is N-cyanomethanimine (H2CNCN), a higher-energy member within this family (3570 ± 130 K above the Z-isomer, which is the global minimum; Puzzarini 2015), whose identification in the ISM has so far remained elusive. This species is of prime astrobiological interest because, besides being a HCN dimer, it contains the NCN backbone, a fundamental structure of purine nucleobases that is only present in a handful of simpler interstellar molecules: cyanamide (NH2CN; Turner et al. 1975), carbodiimide (HNCNH; McGuire et al. 2012), isocyanogen (CNCN; Agúndez 2018), and the cyanomidyl radical (HNCN; Rivilla et al. 2021a). Furthermore, recent experiments conducted by Vasconcelos et al. (2020) have proposed an alternative route for adenine formation within interstellar dust grain ices that relies on H2CNCN as the primary precursor, so its detection in the ISM would increase the chances of adenine being formed under extreme interstellar conditions.
In this work, we present the first detection of several rotational lines of N-cyanomethanimine in the ISM toward the Galactic center G+0.693-0.027 molecular cloud (hereafter G+0.693), the same source that also hosts the unique detection of both C-cyanomethanimine isomers. This cloud, which belongs to the Sgr B2 complex, emerges as one of the most promising candidates for the search for complex organic molecules (Herbst et al. 2020). Its chemistry is believed to be affected by low-velocity shocks likely driven by large-scale cloud–cloud collisions, sputtering molecules that are formed in the surface of dust grains into the gas phase (Martín et al. 2008; Zeng et al. 2020). These particular conditions have endowed it with an unprecedented chemical richness, portrayed in the more than 130 molecular species that have been already identified toward it, many of them of high prebiotic interest (e.g., Rivilla et al. 2019, 2022c, 2023; Zeng et al. 2019, 2021, 2023; Jiménez-Serra et al. 2020, 2022; Rodríguez-Almeida et al. 2021a, 2021b).
This paper is organized as follows. In Section 2, we present the observational data, while in Section 3, we report the detection of N-cyanomethanimine toward G+0.693 and revisit the analysis of the two isomers of C-cyanomethanimine. In Section 4, we discuss the origin of their observed relative isomeric ratios by means of their main formation and destruction chemical routes. Finally, in Section 5, we outline our conclusions.
2. Observations
We have analyzed data from the recently enhanced high-sensitivity spectral survey of G+0.693 (Rivilla et al. 2023; Sanz-Novo et al. 2023). New ultradeep observational runs were performed using the Yebes 40 m (Guadalajara, Spain; project 21A014, PI: Rivilla) and IRAM 30 m (Granada, Spain; project 123-22, PI: Jiménez-Serra) radio telescopes, which have remarkably reduced the rms of the spectra in comparison with previous works (e.g., Rivilla et al. 2020a, 2021a, 2021b, 2022c; Zeng et al. 2020; Rodríguez-Almeida et al. 2021a; Colzi et al. 2022). The observations were centered at αJ2000 = 17h47m22s, 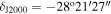 and were conducted using position-switching mode, the off position being located at an offset of Δα = −885'', Δδ = 290''. This particular reference position was selected because it does not show significant emission from abundant molecules such as CS and HC3N. The line intensity of the spectra was directly measured in antenna temperature (
and were conducted using position-switching mode, the off position being located at an offset of Δα = −885'', Δδ = 290''. This particular reference position was selected because it does not show significant emission from abundant molecules such as CS and HC3N. The line intensity of the spectra was directly measured in antenna temperature (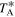 ) units, as the molecular emission toward G+0.693 is extended over the beam (e.g., Brünken 2010; Jones et al. 2012; Li et al. 2020; Zheng et al. 2024).
) units, as the molecular emission toward G+0.693 is extended over the beam (e.g., Brünken 2010; Jones et al. 2012; Li et al. 2020; Zheng et al. 2024).
For the Yebes 40 m observations, the Nanocosmos Q-band (7 mm) HEMT receiver was used to cover the whole Q-band frequency range (31.07–50.42 GHz), providing a raw frequency resolution of ∼38 kHz (Tercero et al. 2021). On the other hand, the new IRAM 30 m observation set was gathered by combining the broadband heterodyne Eight MIxer Receiver and the fast Fourier transform spectrometer FTS200, providing a raw channel width of ∼195 kHz along the three spectral ranges covered: 83.20–115.41, 132.28–140.39, and 142.00–173.81 GHz. In both cases, the spectra were ultimately smoothed to achieve a final resolution of ∼256 kHz (1.5–2.5 km s−1) for the Yebes 40 m data and ∼615 kHz (1.1–2.2 km s−1) for the IRAM 30 m observations, which is more than enough to resolve G+0.693 molecular line profiles that present typical line widths of ∼15–25 km s−1. The rms of the spectra ranges from 0.25 to 0.9 mK across the frequency range observed with the Yebes 40 m radio telescope and between 0.5–2.5 mK and 1.0–1.6 mK for the IRAM 30 m data sets at 3 mm and 2 mm, respectively. The half-power beam width (HPBW) of the Yebes 40 m telescope varies from ∼55'' at 31 GHz down to ∼35'' at 50 GHz, while the HPBW of the IRAM 30 m radio telescope ranges from ∼29'' down to ∼14'' along the frequency range observed with it. We refer to Rivilla et al. (2023) and Sanz-Novo et al. (2023) for further details on both the observations and the data reduction process. Data belonging to the spectral ranges unrelated to this new set of observations come from our previous IRAM 30 m survey (Rivilla et al. 2021a, 2021b, 2022c).
3. Analysis and Results
The identification of the three C2H2N2 isomer molecular lines and their fitting has been performed using the version from 2023 November 15 of the Spectral Line Identification and Modelling (SLIM) tool within the MADCUBA package (Martín et al. 2019). For each of these species, we incorporated the spectroscopic data available within the Cologne Database for Molecular Spectroscopy (CDMS) catalog (Endres et al. 2016). We just considered the rotational transitions without taking into account the complex hyperfine structure arising from the presence of two 14N nuclei, since it is not resolved at the observed spectral resolution (e.g., the maximum separation between the two H2CNCN hyperfine components that reproduce the bulk of the emission of its most intense unblended transition at 41.81 GHz shown in Figure 1 is ∼6.2 kHz or, equivalently, ∼0.04 km s−1, a difference that further collapses for those transitions at higher frequencies). Nonetheless, we did also carry out the analysis of the three isomers including the entries with hyperfine structure lines, not observing substantial modification in the results obtained.
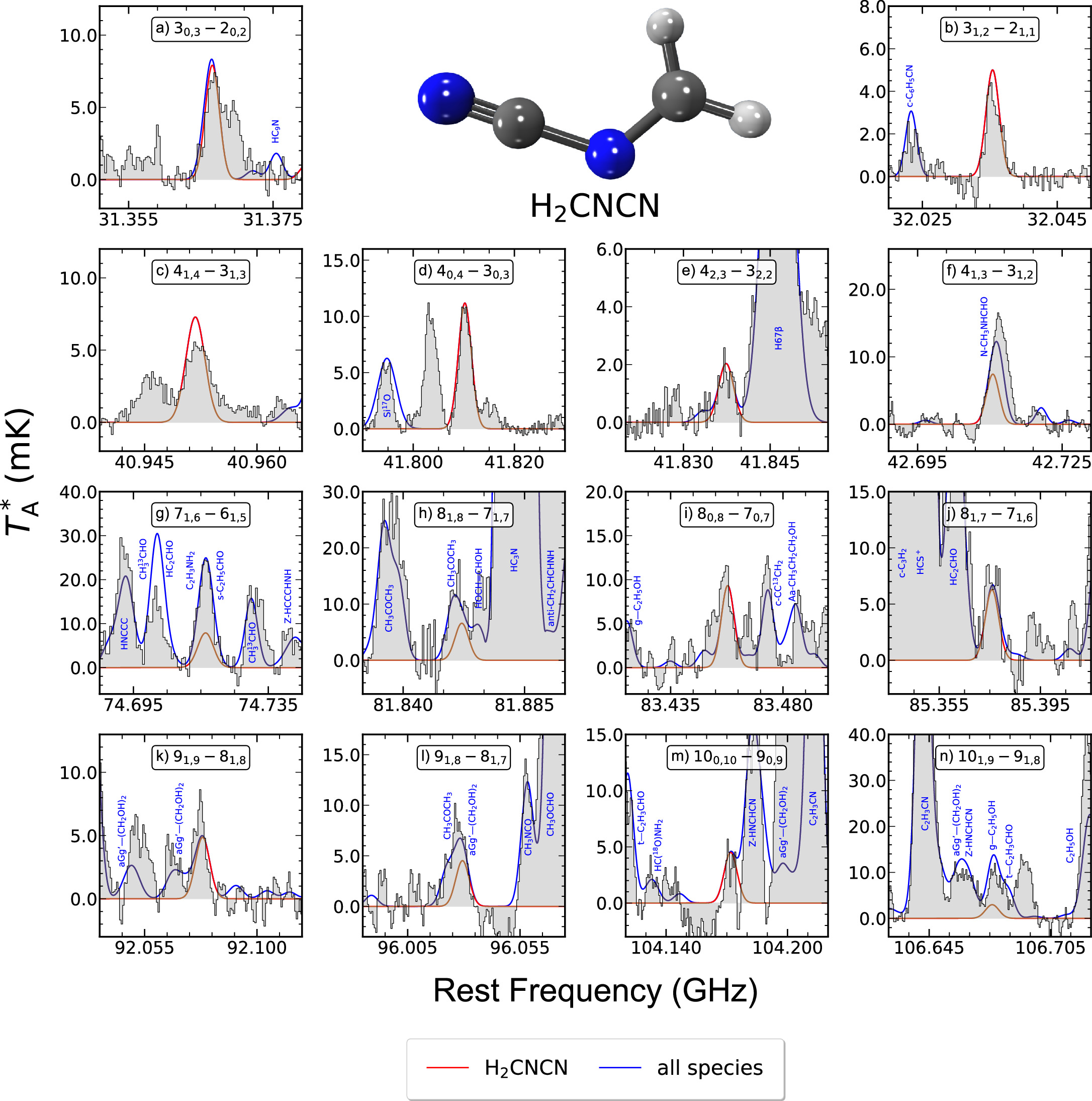
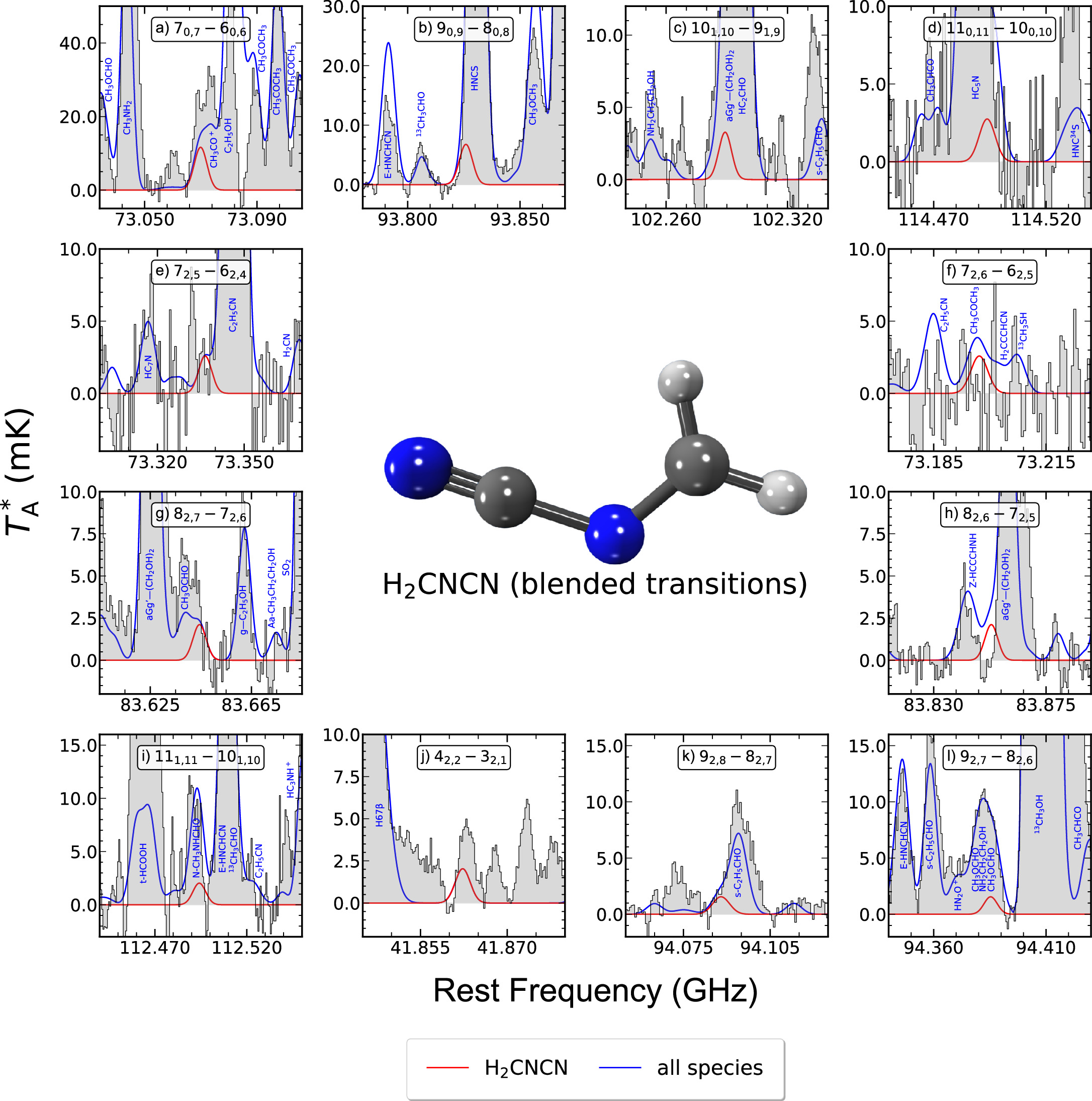
Figure 1. Unblended or partially blended H2CNCN transitions detected toward G+0.693. The black histogram and the gray shaded areas indicate the observed spectrum, while the red and blue solid lines represent the best LTE fit obtained for H2CNCN and the emission of all the species already identified in this cloud (which are indicated by the blue labels), respectively. Panel labels indicate the H2CNCN rotational transition being plotted using the common notation for asymmetric tops: 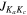 , where J refers to the total angular momentum of the molecule while Ka,c point to its projections along the a and c principal axes. Transitions shown here are those that have been used to perform the LTE line fitting of this molecule, and their spectroscopic information is given in Table 2. The molecular structure of H2CNCN is depicted in the upper middle part (carbon atoms in dark gray, nitrogen atoms in blue, and hydrogen atoms in white colors).
, where J refers to the total angular momentum of the molecule while Ka,c point to its projections along the a and c principal axes. Transitions shown here are those that have been used to perform the LTE line fitting of this molecule, and their spectroscopic information is given in Table 2. The molecular structure of H2CNCN is depicted in the upper middle part (carbon atoms in dark gray, nitrogen atoms in blue, and hydrogen atoms in white colors).
Download figure:
Standard image High-resolution imageThe modeling of the line profiles of the C2H2N2 isomers has been conducted assuming local thermodynamic equilibrium (LTE) conditions, since collisional coefficients for these species have not yet been calculated. The SLIM code operates under this assumption and generates a synthetic spectra to be compared with the observed one. To derive the physical parameters describing the molecular emission, we used the automatic fitting routine SLIM-AUTOFIT, which provides the best nonlinear least-squares LTE fit to the data using the Levenberg–Marquardt algorithm. The free parameters fitting the spectra are the total column density of the molecule (N), the excitation temperature (Tex), the local standard of rest velocity (vLSR), and the full width at half-maximum (FWHM).
We have performed the LTE line fitting of the species of interest in this work by considering the already modeled emission of the more than 130 molecular species previously identified toward G+0.693 (Requena-Torres et al. 2006, 2008; Rivilla et al. 2018, 2019, 2020b, 2021a, 2021b, 2022a, 2022b, 2022c, 2023; Zeng et al. 2018, 2020, 2021, 2023; Bizzocchi et al. 2020; Jiménez-Serra et al. 2020, 2022; Rodríguez-Almeida et al. 2021a, 2021b; Colzi et al. 2022; Alberton et al. 2023b; Fatima et al. 2023; Massalkhi et al. 2023; San Andrés et al. 2023; Sanz-Novo et al. 2023). In the forthcoming sections, we provide the details of the analysis for each cyanomethanimine species, whose results are summarized in Table 1.
Table 1. Derived Physical Parameters of the Best LTE Fit for the Three Cyanomethanimine Species Targeted in This Work
| Molecule | N | Tex | FWHM | vLSR |
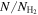 a
a
|
|---|---|---|---|---|---|
| (×1012 cm−2) | (K) | (km s−1) | (km s−1) | (×10−11) | |
| H2CNCN | 2.9 ± 0.1 | 7.9 ± 0.3 | 21.9 ± 1.4 | 68.1 ± 0.5 | 2.1 ± 0.3 |
| Z-HNCHCN | 75 ± 3 | 14.1 ± 0.8 | 22.3 ± 0.8 | 67.0 ± 0.5 | 55 ± 9 |
| E-HNCHCN | 16.8 ± 0.3 | 15.5 ± 0.5 | 22.1 ± 0.6 | 67.8 ± 0.2 | 12 ± 2 |
Notes. The FWHM estimates were first determined by only fitting the most unblended lines of these species, while the remaining three parameters were obtained through the complete LTE modeling in which these FWHM values were fixed (see Sections 3.1 and 3.2). Their uncertainties correspond to 1σ standard deviations.
a Fractional abundances with respect to H2. To compute them, we used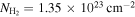 as derived by Martín et al. (2008) while assuming an uncertainty of 15% of its value.
as derived by Martín et al. (2008) while assuming an uncertainty of 15% of its value.Download table as: ASCIITypeset image
3.1. Detection of H2CNCN
N-cyanomethanimine (H2CNCN) is a planar asymmetric-top molecule with a large total dipole moment of 4.84D (Bak et al. 1978; Bak & Svanholt 1980). To perform its search toward G+0.693, we used the spectroscopic entry 054514 (2014 February) from the CDMS catalog, which incorporates the rotational spectroscopy from the Bak & Svanholt (1980), Winnewisser et al. (1984), and Stolze et al. (1989) experimental works. We have identified a plethora of distinct a-type rotational transitions belonging to this species, which cover an energy level range between Jup = 3 and Jup = 11 and correspond to different Ka = 0, 1, 2 ladders. This delineates nearly the entirety of the most prominent rotational spectroscopic features attributed to the R-branch a-type lines, rendering this detection exceptionally robust. Figure 1 shows all of the transitions we have selected to perform the LTE fit for H2CNCN, which are all those among those targeted that are either unblended or present certain blending with other molecular species but in which case the combined profiles closely match the observed spectrum. The rest of the H2CNCN lines covered by the observations are too faint to be detected or exhibit more significant blending with the emission from other molecular species (either unidentified or already modeled toward G+0.693), being the predicted emission for all these other lines also consistent with the observed spectrum (see Figure 8 of Appendix A). The spectroscopic information of the transitions displayed in Figure 1 is given in Table 2. All of them have been identified within a signal-to-noise ratio (S/N) in integrated intensity larger than 6 (see Table 2).
Table 2. List of H2CNCN Rotational Transitions Detected toward G+0.693 and Selected for This Molecule LTE Fit
| Frequency | Transition a |
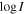
| Eup | rms |
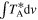
| S/N b | Blending c |
|---|---|---|---|---|---|---|---|
| (GHz) | (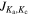 ' '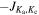 '') '') | (nm2 MHz) | (K) | (mK) | (mK km s−1) | ||
| 31.3664834(10) | 30,3 − 20,2 | −5.469 | 3.0 | 0.5 | 179(15) | 53(5) | Blended with U |
| 32.0353082(10) | 31,2 − 21,1 | −5.505 | 5.9 | 0.5 | 113(13) | 34(4) | Unblended |
| 40.9516386(13) | 41,4 − 31,3 | −5.183 | 7.7 | 0.5 | 165(15) | 56(5) | Unblended |
| 41.8100713(12) | 40,4 − 30,3 | −5.133 | 5.0 | 0.5 | 253(19) | 87(7) | Unblended |
| 41.8372354(12) | 42,3 − 32,2 | −5.444 | 16.2 | 0.5 | 46(11) | 16(4) | Unblended |
| 42.7104885(13) | 41,3 − 31,2 | −5.147 | 7.9 | 0.5 | 168(15) | 58(6) | Blended: N-CH3NHCHO and U |
| 74.7161692(19) | 71,6 − 61,5 | −4.468 | 17.1 | 2.4 | 180(60) | 10(3) | Blended: s-C2H5CHO and C2H3NH2 |
| 81.8614891(21) | 81,8 − 71,7 | −4.344 | 20.5 | 2.4 | 151(60) | 9(3) | Blended: CH3COCH3 |
| 83.4576038(20) | 80,8 − 70,7 | −4.317 | 18.0 | 1.6 | 213(40) | 19(4) | Blended with U |
| 85.3755815(21) | 81,7 − 71,6 | −4.309 | 21.2 | 1.6 | 144(40) | 13(3) | Unblended⋆ |
| 92.0779012(21) | 91,9 − 81,8 | −4.204 | 24.9 | 0.9 | 112(22) | 19(4) | Blended with U |
| 96.0290895(21) | 91,8 − 81,7 | −4.169 | 25.8 | 1.5 | 104(35) | 11(4) | Blended:  -(CH2OH)2 and CH3COCH3 -(CH2OH)2 and CH3COCH3
|
| 104.171562(10) | 100,10 − 90,9 | −4.057 | 27.5 | 1.8 | 104(42) | 9(4) | Slightly blended: Z-HNCHCN |
| 106.675828(10) | 101,9 − 91,8 | −4.045 | 31.0 | 1.8 | 69(41) | 6(3) | Blended: g-C2H5OH |
Notes. For each transition, we provide its rest frequency in units of GHz, its associated quantum numbers, the base 10 logarithm of its integrated intensity at a fixed temperature of 300 K in units of nm2 MHz (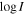 ), the energy in K of the upper level involved in the transition (Eup), the noise measured in mK within line-free spectral ranges close to it (rms), the integrated intensity over the line width as derived from the fit in mK km s−1 (
), the energy in K of the upper level involved in the transition (Eup), the noise measured in mK within line-free spectral ranges close to it (rms), the integrated intensity over the line width as derived from the fit in mK km s−1 (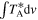 ), and the detection level (in terms of the S/N). The numbers in parentheses represent the uncertainty associated with the last digits. The last column accounts for the possible contamination of other molecular species.
), and the detection level (in terms of the S/N). The numbers in parentheses represent the uncertainty associated with the last digits. The last column accounts for the possible contamination of other molecular species.
 , where J refers to the total angular momentum of the molecule, while Ka,c points to its projections along the a and c principal axes.
b
The S/N is calculated from the integrated intensity over the line width (
, where J refers to the total angular momentum of the molecule, while Ka,c points to its projections along the a and c principal axes.
b
The S/N is calculated from the integrated intensity over the line width ( ) and noise level
) and noise level 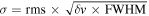 , where δ
v is the spectral resolution of the spectra in velocity units and the FWHM is estimated from the LTE line fitting.
c
The term "unblended" alludes to those transitions that exhibit no contamination of other molecular species, while an asterisk is added when scarce line blending accounting for less than 5% of the targeted line total integrated intensity is present. "U" refers to blending with an unknown (not yet identified) species.
, where δ
v is the spectral resolution of the spectra in velocity units and the FWHM is estimated from the LTE line fitting.
c
The term "unblended" alludes to those transitions that exhibit no contamination of other molecular species, while an asterisk is added when scarce line blending accounting for less than 5% of the targeted line total integrated intensity is present. "U" refers to blending with an unknown (not yet identified) species.Download table as: ASCIITypeset image
To achieve the best LTE modeling for H2CNCN, we followed a two-step methodology. First, we fitted only the most unblended transitions (depicted in panels (b), (d), (e), and (j) of Figure 1), which provided the most accurate estimate for the FWHM. To do so, we ran AUTOFIT leaving the four parameters free and obtained an FWHM of 21.9 ± 1.4 km s−1, which we subsequently kept fixed in the second step. Then, we fitted all of the transitions shown in Figure 1 and ran AUTOFIT again but now leaving only the other three parameters free. We obtained vLSR = 68.1 ± 0.5 km s−1, Tex = 7.9 ± 0.3 K, and N = (2.9 ± 0.1) × 1012 cm−2 (see Table 1). The derived H2CNCN column density translates into a total molecular abundance with respect to H2 of (2.1 ± 0.3) × 10−11, assuming  (Martín et al. 2008) with an associated uncertainty of 15% of its value.
(Martín et al. 2008) with an associated uncertainty of 15% of its value.
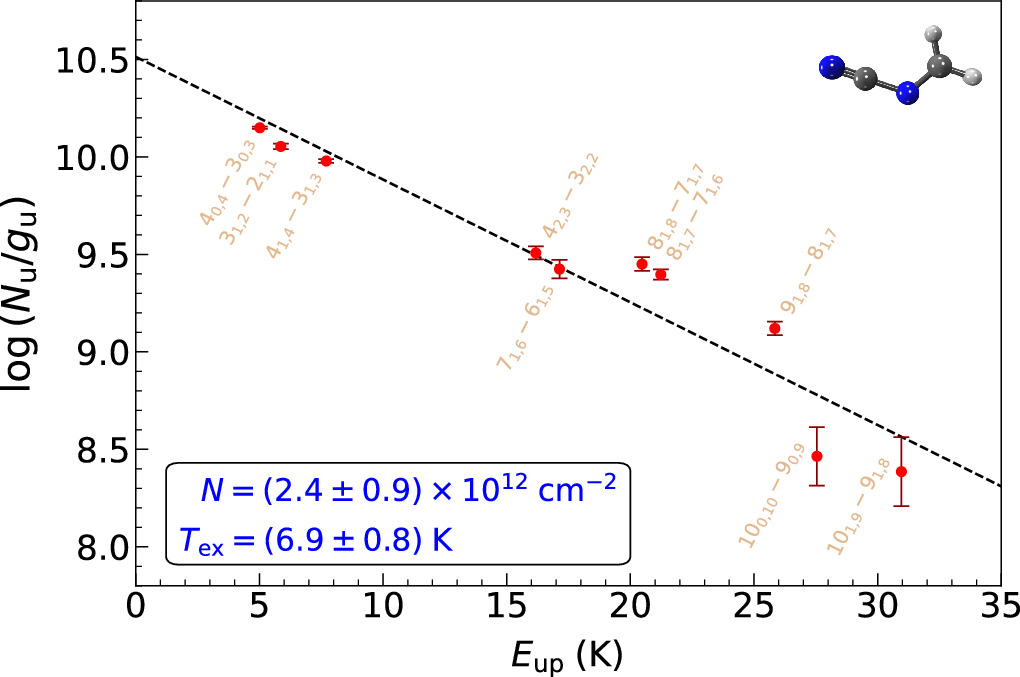
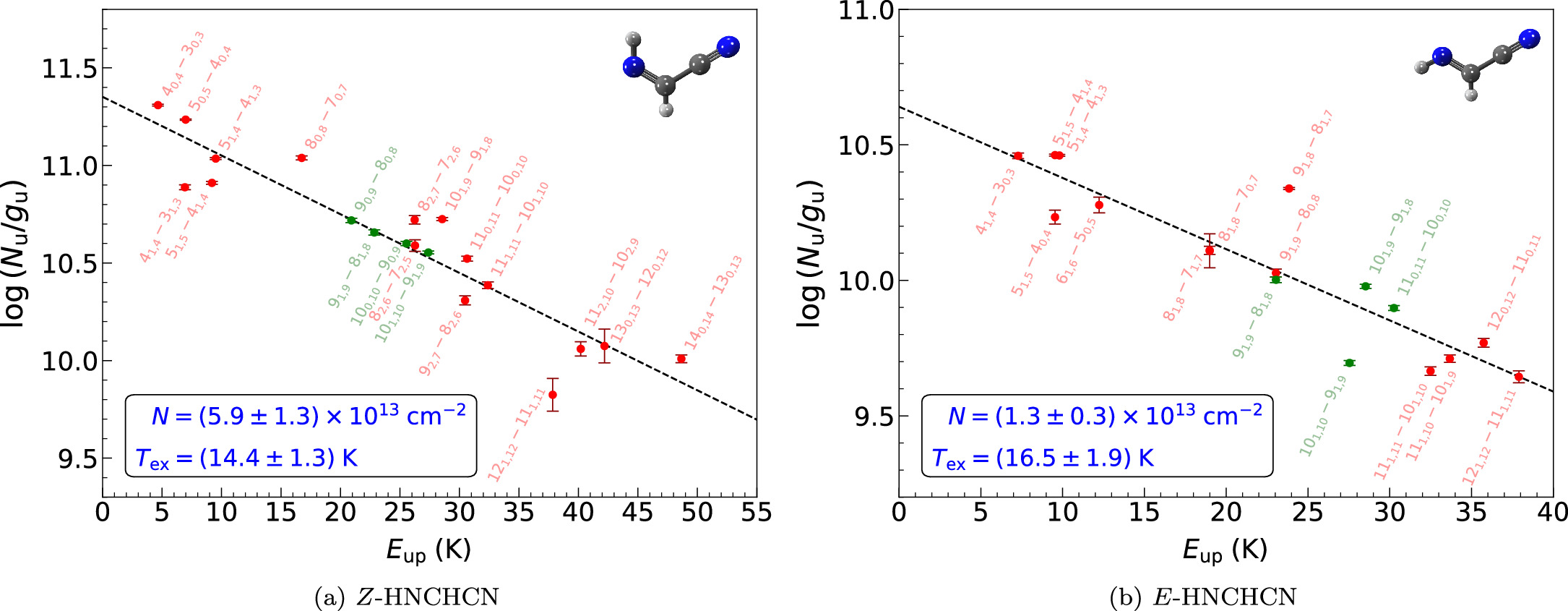
We have also performed a complementary rotational diagram analysis (Goldsmith & Langer 1999) for H2CNCN, which is implemented in MADCUBA and incorporates two distinctive functionalities that provide greater versatility in contrast with the method conventionally used. On one hand, the MADCUBA rotational diagram accounts for opacity effects. Moreover, a very recent update allows one to consider the predicted emission from all of the molecular species previously detected in the source. This is done by subtracting the predicted line profiles of the blending species from the observed data to generate an "unblended" spectral data set, from which the rotational diagram for the species of interest can be derived following the same procedure that would apply for unblended lines. Figure 2 shows the rotational diagram we obtained for H2CNCN. To compute it, we used all of the transitions shown in Figure 1 but excluded those that exhibit blending with yet-unidentified species (see Table 2) and employed their associated velocity integrated intensity over the line width as calculated by MADCUBA. This analysis yields N = (2.4 ± 0.9) × 1012 cm−2 and Tex = 6.9 ± 0.8 K, which are in perfect agreement with SLIM-AUTOFIT estimates.
Figure 2. Rotational diagram of H2CNCN using the unblended and partially blended transitions shown in Figure 1 (red points and labels) but excluding those blended with species not yet identified (see Table 2). The dashed line depicts the best linear fit to the data, while the resulting values for the column density (N) and excitation temperature (Tex) are displayed in blue within the black box. The molecular structure of H2CNCN is shown on the upper right side.
Download figure:
Standard image High-resolution image3.2. Analysis of E- and Z-HNCHCN
Both the E- and Z-isomers of the C-cyanomethanimine species (HNCHCN) were previously detected toward G+0.693 by Rivilla et al. (2019), albeit on the basis of a less sensitive IRAM 30 m spectral survey with narrower spectral coverage (85–109 GHz). In this regard, the presence of new high-sensitivity Yebes 40 m observations at frequencies of ∼30–50 GHz, along with the significant enhancement of the spectral sensitivity at also higher frequencies, has prompted us to revisit the detection of these isomers toward G+0.693.
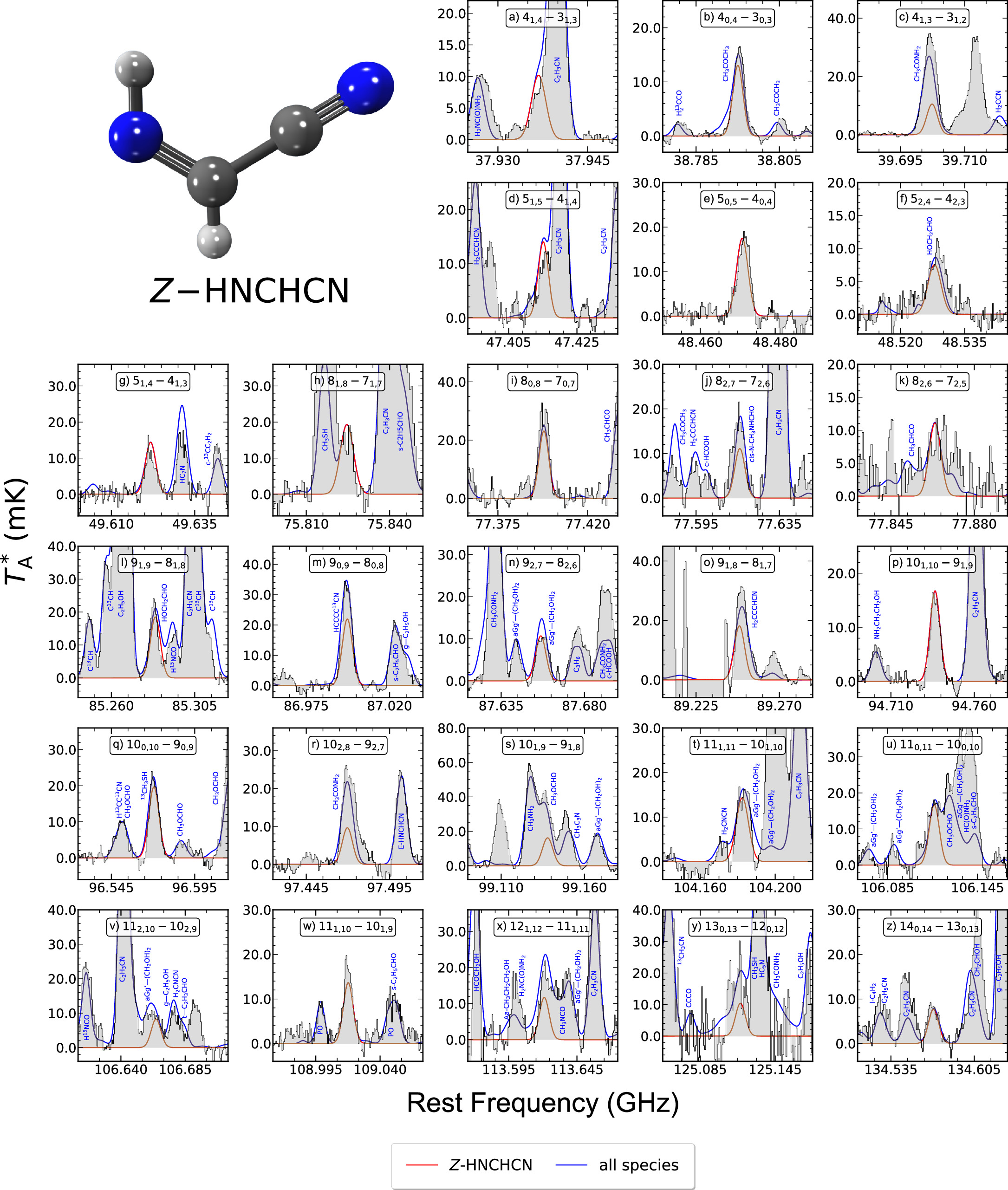
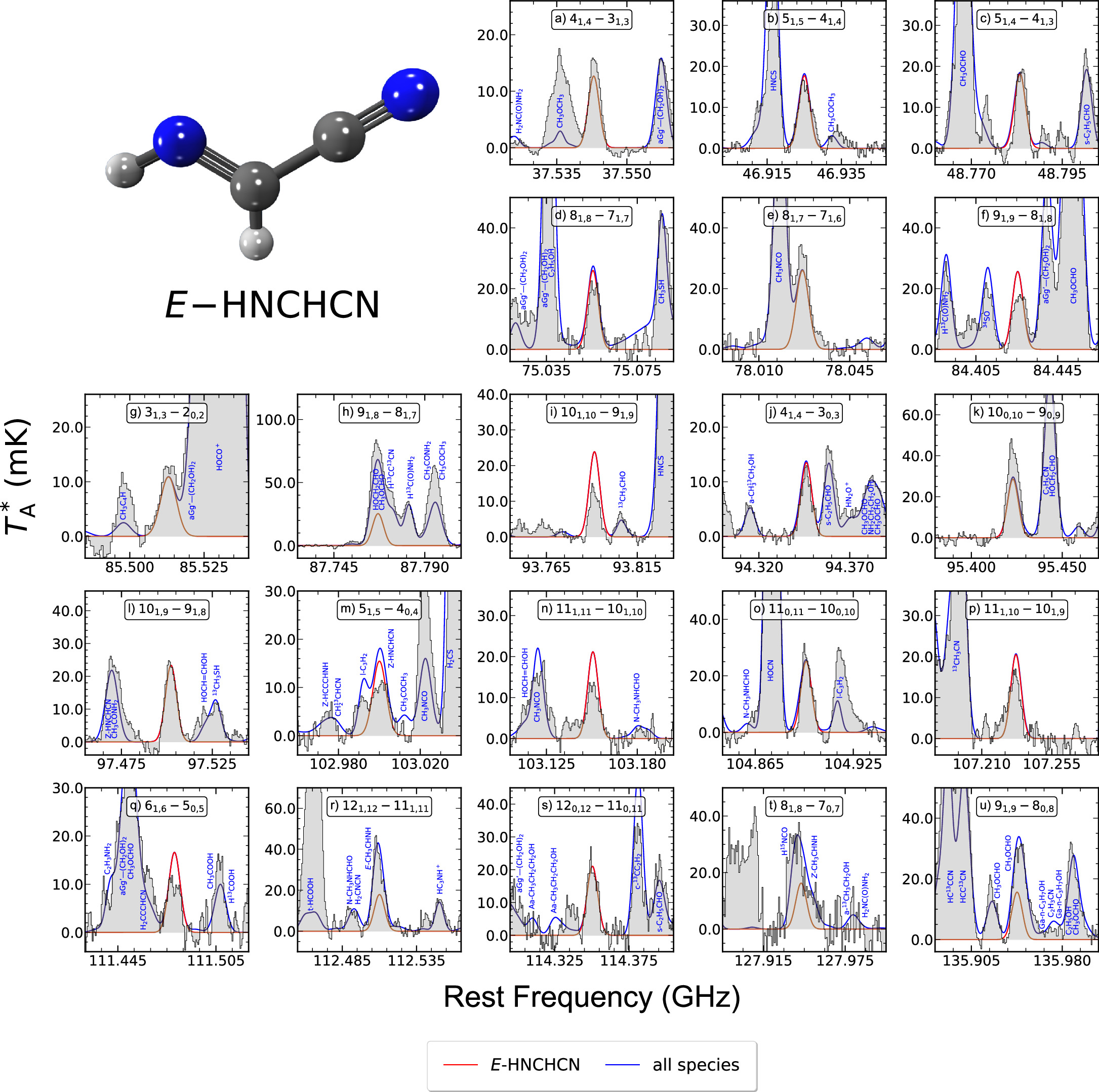
To perform the new analysis of these two species, we employed the same spectroscopic entries from the CDMS catalog that Rivilla et al. (2019) also used: 054512 for Z-HNCHCN and 054513 for E-HNCHCN (both dated 2018 November), which contain the rotational transitions measured by Takano et al. (1990), Zaleski et al. (2013), and Melosso et al. (2018). We have almost tripled the number of Z- and E-HNCHCN unblended or partially blended lines detected toward G+0.693 with an S/N in integrated intensity above 6, which are shown in Figures 3 and 4, respectively. For both isomers, these lines correspond to different Ka = 0, 1, 2 ladders of a-type rotational transitions sweeping a wider range of energy levels (∼5–50 K) than those previously covered by Rivilla et al. (2019; ∼21–34 K) and starting from Jup = 4 up to Jup = 14. Moreover, we have also targeted for the first time some b-type transitions belonging to E-HNCHCN (see panels (g), (j), (m), (q), (t), and (u) in Figure 4), facilitated by the relatively high μb dipole moment component, albeit smaller compared to its μa counterpart (2.51D versus 3.25D, respectively; Takano et al. 1990). The spectroscopic information of all these transitions is gathered in Table 3 within Appendix B, which highlights how some of the lines that Rivilla et al. (2019) targeted as "unblended" actually exhibit slight blending from other species. Furthermore, we have now detected all of them with a nearly 3 times greater S/N in integrated intensity.
Figure 3. Z-HNCHCN unblended or partially blended transitions detected toward G+0.693. The black histogram, gray shaded areas, red and blue solid lines, blue labels, and panel labels represent the same as indicated in Figure 1. Their spectroscopic information is given in Table 3 within Appendix B. The molecular structure of Z-HNCHCN is drawn on the upper left side, following the same color pattern as in Figure 1.
Download figure:
Standard image High-resolution imageFigure 4. Same as Figure 3 but for E-HNCHCN. The spectroscopic information for all of these transitions is given in Table 3 within Appendix B. E-HNCHCN molecular structure is depicted in the upper left corner, colored according to the same palette as in Figures 1 and 3.
Download figure:
Standard image High-resolution imageWe have carried out the LTE line fitting for the Z- and E-HNCHCN isomers by using all of the transitions depicted in Figures 3 and 4, respectively. As already explained for H2CNCN (see Section 3.1), we followed a similar two-step approach in both cases, whose results are summarized in Table 1. In the case of Z-HNCHCN, we first constrained its FWHM by fitting the unblended lines shown in panels (e), (g), (p), and (z) of Figure 3 while leaving the four parameters unfixed, obtaining an FWHM of 22.3 ± 0.8 km s−1. As for E-HNCHCN, we derived a similar FWHM of 22.1 ± 0.6 km s−1 by fitting the seven unblended transitions displayed in panels (b)–(d), (j), (l), (o), and (s) in Figure 4. In the second step, we repeated the fit but including the rest of the transitions shown in Figure 3 for Z-HNCHCN and Figure 4 for the E-isomer while keeping the FWHM fixed to the former values, respectively. We obtained N = (7.5 ± 0.3) × 1013 cm−2, Tex = 14.1 ± 0.8 K, and vLSR = 67.0 ± 0.5 km s−1 for Z-HNCHCN and N = (1.68 ± 0.03) × 1013 cm−2, Tex = 15.5 ± 0.5 K, and vLSR = 67.8 ± 0.2 km s−1 for E-HNCHCN. The column density derived for each isomer results in a molecular abundance with respect to H2 of (5.5 ± 0.9) × 10−10 for Z-HNCHCN and (1.2 ± 0.2) × 10−10 for E-HNCHCN, which were calculated following the same approach as explained in Section 3.1.
As for N-cyanomethanimine, we have also performed a rotational diagram analysis for both C-cyanomethanimine isomers, which we show in Figure 5. In line with the methodology we followed for H2CNCN (see Section 3.1), we constructed this diagram by using all of the unblended and partially blended rotational transitions, which are shown in Figures 3 and 4 for Z- and E-HNCHCN, respectively, while incorporating their associated velocity integrated intensity. As done for H2CNCN, we did not include those transitions that are blended with species that have not yet been identified (see Table 3 within Appendix B). The results derived from this complementary analysis are N = (5.9 ± 1.3) × 1013 cm−2 and Tex = 14.4 ± 1.3 K for Z-HNCHCN and N = (1.3 ± 0.3) × 1013 cm−2 and Tex = 16.5 ± 1.9 K for E-HNCHCN, being in both cases consistent with SLIM-AUTOFIT outcomes.
Figure 5. Rotational diagrams of Z-HNCHCN (left) and E-HNCHCN (right) computed using these species' unblended and partially blended transitions detected toward G+0.693 (colored points and labels; see Figures 3 and 4 for each species, respectively), excluding those contaminated with yet-unknown species (see Table 3 within Appendix B). Transitions in green correspond to those previously targeted by Rivilla et al. (2019) that these authors used to perform the LTE fit of these species, whereas the rest of the lines we have identified are displayed in red. The dashed line delineates the best linear fit to the data achieved. The resulting column density (N) and excitation temperature (Tex) estimates are presented in blue framed within the black box. The molecular structure of each isomer is plotted on the upper right side.
Download figure:
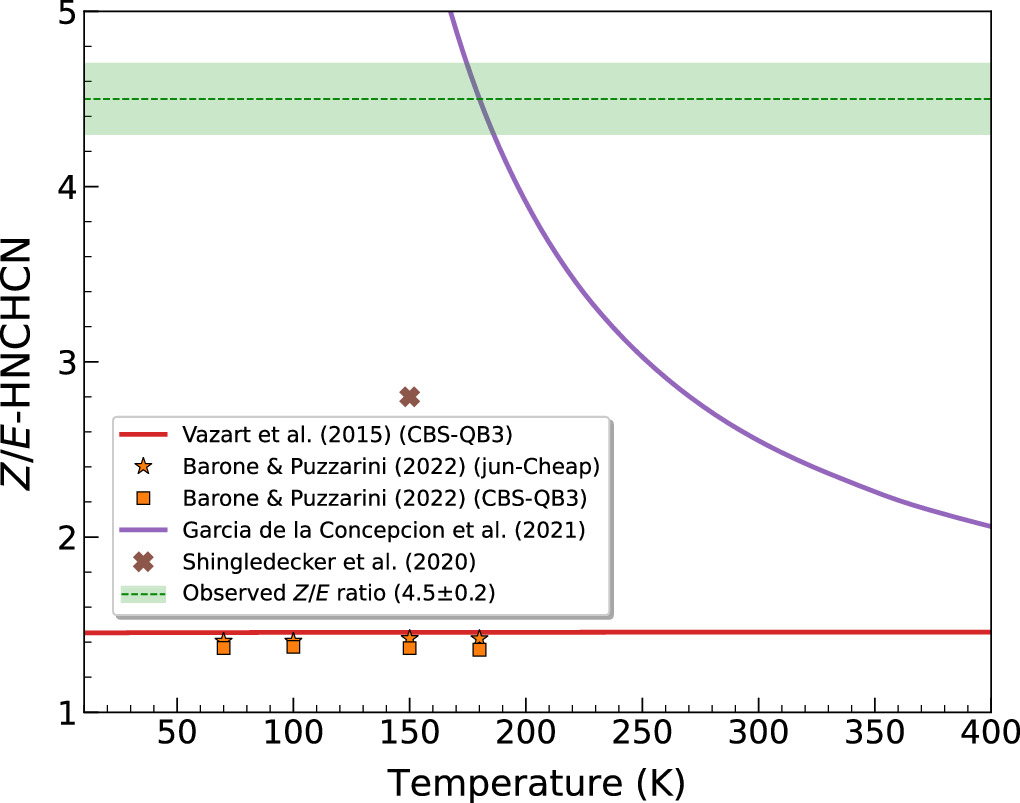
Standard image High-resolution imageThe substantial increase in the number of detected lines, along with their improved S/N and broader energy distribution, has resulted into a more accurate characterization of the physical parameters describing the emission from these two isomers. Our enhanced fit now sets a higher but consistently similar Tex for both isomers of almost twice the value derived by Rivilla et al. (2019) for Z-HNCHCN (8 ± 2 K), which was the only one they could explicitly obtain from the lower-sensitivity data. The notable difference in the Tex, linked to the more precise scrutiny of the molecular line blending from other species, leaves its imprint in the column density estimates as well, which are now set to be ∼2–3 times lower in comparison with Rivilla et al.'s (2019) findings. Similarly, we have also computed a rather lower Z/E-HNCHCN abundance ratio toward G+0.693 of 4.5 ± 0.2, which remains consistent within the uncertainty with the former estimate (6.1 ± 2.4). Nonetheless, we emphasize the comparably refined accuracy concerning this new result, which highlights the direct impact that full spectroscopic coverage exerts on determining the physical parameters.
3.3. The C/N-cyanomethanimine Abundance Ratio toward G+0.693
Based on the new column density estimates derived for both the Z- and E-HNCHCN isomers (see Table 1), we have computed a total column density for C-cyanomethanimine toward G+0.693 of (9.2 ± 0.3) × 1013 cm−2, which results in a total molecular abundance with respect to H2 of (6.8 ± 1.0) × 10−10. This value, although ∼3 times lower than that previously derived by Rivilla et al. (2019), is of the same order as the abundances of other complex nitriles detected toward this cloud, such as CH3CN, C2H3CN, or C2H5CN (Zeng et al. 2018). This indicates that C-cyanomethanimine might indeed be a relatively abundant species in the ISM, which could favor its detection toward other astronomical sources.
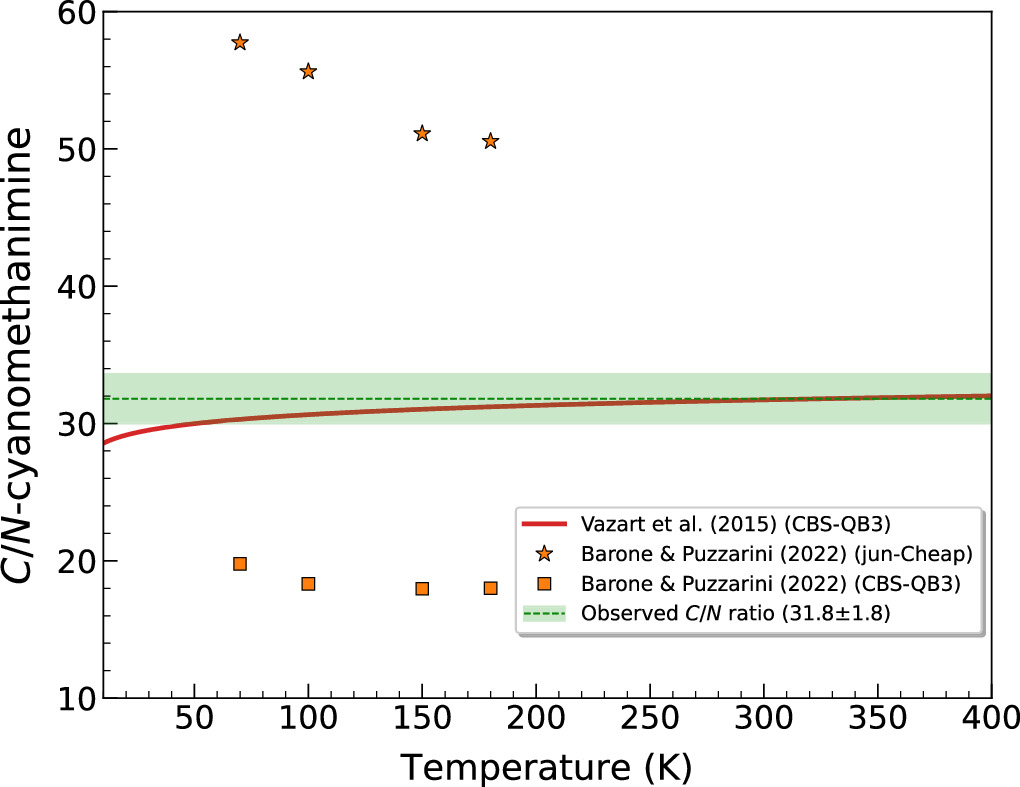
Nevertheless, this scenario significantly shifts for the N-cyanomethanimine species (H2CNCN), which exhibits a remarkably lower molecular abundance with respect to H2 of (2.1 ± 0.3) × 10−11. In fact, H2CNCN emerges as one of the least abundant species detected thus far toward G+0.693, being ∼2–3 times lower in abundance in comparison to other even more complex nitrogen-bearing species already identified in this cloud (Rivilla et al. 2022c). This disparity becomes even sharper with respect to both C-cyanomethanimine isomers, for which we have computed Z, E-HNCHCN/H2CNCN isomeric ratios of 25.9 ± 1.6 and 5.8 ± 0.3, respectively. Consequently, we have derived a C/N-cyanomethanimine abundance ratio of 31.8 ± 1.8 toward G+0.693.
At this point, it is worth mentioning that while the Z- and E-HNCHCN isomers exhibit quite similar Tex of ∼14 K, we have derived a much lower value of ∼8 K for H2CNCN (see Table 1). Although we have already demonstrated the reliability of our results regarding the LTE fitting of these species, we have also evaluated the possibility of a Tex of ∼14 K for H2CNCN. In this scenario, we have obtained a poorer fit and a reduction of ∼25% in its estimated column density, which results into an even lower molecular abundance and hence a greater C/N-cyanomethanimine ratio. In any case, our observational results set the N-isomer as over 1 order of magnitude less abundant compared to the more stable C-cyanomethanimine species, which suggests that its detection toward other sources could become a much more challenging task.
4. Discussion
4.1. High-energy Isomers in the ISM
In astrochemistry, there is still a prevalent belief that the search for high-energy isomers in the ISM is bound to fail, since these isomers are expected to exhibit molecular abundances that could be several orders of magnitude lower than their most stable analogs. This idea is rooted in the so-called minimum energy principle (MEP), which states a strong correlation between the observed abundances of the different members that compose a given isomeric family and their relative energies, with the most stable member expected to exhibit the highest abundance (Lattelais et al. 2009). In this respect, the cyanomethanimine family analyzed in this work would seem to adhere to this principle. However, the detection of N-cyanomethanimine toward G+0.693 cast doubts on the reliability of the MEP as a predictive tool for assessing high-energy isomer traceability in the ISM, as discussed below.
According to the original formulation of the MEP by Lattelais et al. (2009), the abundance ratios between structural isomers (denoting those molecular species sharing the same generic formula but whose constituent atoms are arranged differently) must be governed by thermodynamic equilibrium, scaling with  ,
11
where ΔE is their electronic energy difference and Tkin indicates the kinetic temperature of the gas. This points to their relative abundances being determined by isomerization mechanisms, which would prevail over other processes. Nevertheless, this is far from being the case of N-cyanomethanimine. With an estimated ΔE of 3570 ± 130 K (Puzzarini 2015) with respect to the most stable Z-HNCHCN isomer, its abundance based on thermodynamical considerations is predicted to be more than 10 orders of magnitude lower in comparison to C-cyanomethanimine at the Tkin of G+0.693 (∼70–140; Zeng et al. 2018), nearly 9 orders of magnitude below what has actually been observed. Therefore, it ought to be explained in terms of kinetics instead. The most plausible explanation for the C/N-cyanomethanimne ratio markedly deviating from the thermodynamic equilibrium prediction is probably related to the very distinct molecular structures of the N- and C-cyanomethanimine species, which makes their unimolecular isomerization impossible under ISM conditions since it must involve a reaction intermediate far superior in energy to both species.
,
11
where ΔE is their electronic energy difference and Tkin indicates the kinetic temperature of the gas. This points to their relative abundances being determined by isomerization mechanisms, which would prevail over other processes. Nevertheless, this is far from being the case of N-cyanomethanimine. With an estimated ΔE of 3570 ± 130 K (Puzzarini 2015) with respect to the most stable Z-HNCHCN isomer, its abundance based on thermodynamical considerations is predicted to be more than 10 orders of magnitude lower in comparison to C-cyanomethanimine at the Tkin of G+0.693 (∼70–140; Zeng et al. 2018), nearly 9 orders of magnitude below what has actually been observed. Therefore, it ought to be explained in terms of kinetics instead. The most plausible explanation for the C/N-cyanomethanimne ratio markedly deviating from the thermodynamic equilibrium prediction is probably related to the very distinct molecular structures of the N- and C-cyanomethanimine species, which makes their unimolecular isomerization impossible under ISM conditions since it must involve a reaction intermediate far superior in energy to both species.
On top of that, the N- and C-cyanomethanimine isomers do not emerge as the unique exception to the MEP, since recent detections in the ISM of many other isomeric families have also raised serious doubts about its general applicability regarding structural isomers. Some of these findings have demonstrated that high-energy structural isomers can share similar abundances to their more stable counterparts, as is the case for the H2CN and H2NC isomers (Cabezas et al. 2021; Agúndez et al. 2023a; San Andrés et al. 2023) and the two most stable isomers within the C3H4O family (trans-propenal, CH2CHCHO, and methyl ketene, CH3CHCO; Bermúdez et al. 2018; Fuentetaja et al. 2023), or even higher, as noted for the C3H2O, C2H4O2, and C2H5N2O isomeric families (see, e.g., Loomis et al. 2015 and Shingledecker et al. 2019; Mininni et al. 2020; Rivilla et al. 2023, respectively). Therefore, it is clear that the MEP cannot be taken as a strict rule to argue about the actual detectability of high-energy structural isomers in the ISM.
In this regard, the MEP only appears to primarily apply for some stereoisomers (also referred as spatial isomers, i.e., those species that possess identical constitutions but differ in the three-dimensional orientation of their bonding atoms), where the Z- and E-HNCHCN isomeric pair of C-cyanomethanimine emerges as the nearest example. In fact, the energy difference of these two isomers is 309 ± 72 K (Takano et al. 1990), which, based on the gas kinetic temperatures of the G+0.693 cloud of ∼70–140 K (Zeng et al. 2018), results in an abundance ratio fairly consistent with that observed by Rivilla et al. (2019) and reported in this work. Although the energy barrier associated with their isomerization is huge (∼15.95 kK; Takano et al. 1990), García de la Concepción et al. (2021) demonstrated that this process can take place, even at the low temperatures of G+0.693, when quantum tunneling is considered using a small curvature approximation (Skodje et al. 1981) and predicted a Z/E-HNCHCN abundance ratio at 150 K that mimicked Rivilla et al.'s (2019) observational value (6.1 ± 2.4). Our revised estimate for this ratio of 4.5 ± 0.2 would correspond to a Tkin of ∼180 K (see Figure 6), slightly higher than those typically associated with this source but still in good agreement with the thermodynamic equilibrium prediction. Furthermore, as Figure 6 shows, this mechanism emerges as the only plausible explanation for this ratio, as none of the formerly suggested chemical pathways are capable of describing the observations (Vazart et al. 2015; Shingledecker et al. 2020; Barone & Puzzarini 2022).
Figure 6. The Z/E-HNCHCN abundance ratio dependence on temperature as predicted by Vazart et al. (2015) and Barone & Puzzarini (2022) chemical models for the CN + CH2NH gas-phase reaction (red line and orange symbols, respectively), Shingledecker et al. (2020) astrochemical simulations (brown cross), and thermodynamics (purple line; García de la Concepción et al. 2021). The green dashed line and shaded area delineate the observed value encompassed by its 1σ uncertainty (4.5 ± 0.2). Barone & Puzzarini (2022) estimates are retrieved for certain temperatures relevant to those derived for G+0.693 (∼70–140 K; Zeng et al. 2018), as no analytical expression for the rate constant is provided.
Download figure:
Standard image High-resolution imageBesides both C-cyanomethanimine isomers, the list of stereoisomers that also support the MEP rule is rather scarce, where the Ga and Aa conformers of n-propanol (n-C3H7OH; Jiménez-Serra et al. 2020), the anti and gauche conformers of ethyl formate (CH3CH2(O)CHO; Rivilla et al. 2017), and the cis and trans conformers of thioformic acid (HC(O)SH; García de la Concepción et al. 2022) are some of the clearest examples. However, there is also growing evidence that thermodynamics cannot account for the observed abundance ratios of many other stereoisomers, such as the cis-cis and cis-trans conformers of carbonic acid (HOCOOH; Sanz-Novo et al. 2023), the cis and trans conformers of methyl formate (CH3OCHO; Neill et al. 2012), and the cis and trans conformers of formic acid (HCOOH; García de la Concepción et al. 2022). We note that all of the stereoisomers so far detected in the ISM whose abundance ratios match the thermodynamic prediction exhibit energy differences ≲500 K, which appears to be a doable energetic boundary for quantum tunneling to efficiently enable their isomerization reactions at the low temperatures of the ISM, as García de la Concepción et al. (2021) demonstrated for several imines. On the other hand, the stereoisomers mentioned above that do not follow the thermodynamic ratio exhibit much higher energy differences, which surely prevents their direct isomerization and hence causes their abundance ratios to be shaped by chemical kinetics instead.
All in all, observational evidence increasingly indicates that the MEP lacks predictive capability regarding which species within a specific isomeric family are more readily detectable and, consequently, cannot be generally used as a reliable indicator of isomeric abundances. In this regard, the detection of N-cyanomethanimine toward G+0.693 provides robust evidence that high-energy isomers can also be found in the ISM. Therefore, although it is possible that in some cases the detection of such species would deserve deeper integrations, we note that it is equally important and necessary to obtaining their spectroscopy to enable their interstellar identification, significantly contributing to providing a full inventory of the molecular complexity of the ISM.
The aforementioned results have also continued to support the prominent role of chemical kinetics, rather than thermodynamics, in establishing the observed abundance ratios between structural isomers. For this reason, we discuss in the following section the possible chemical pathways leading to H2CNCN.
4.2. H2CNCN Interstellar Chemistry
Over the last decade, several authors have investigated the chemistry of cyanomethanimines under interstellar conditions (Vazart et al. 2015; Shivani & Tandon 2017; Shingledecker et al. 2020; Zhang et al. 2020). However, biased by the exclusive detection in the ISM of the Z- and E-HNCHCN isomers (Zaleski et al. 2013; Rivilla et al. 2019), many of these works mainly focused on the chemical processes involving these two species. Consequently, the potential chemical pathways yielding to H2CNCN have often been overlooked, resulting in a limited characterization of the chemistry linked to this isomer.
To date, there is just one formation mechanism leading to H2CNCN that has been specifically studied. This route occurs through the gas-phase reaction between the cyanogen radical (CN) and methanimine (CH2NH), which Vazart et al. (2015) initially examined by performing quantum chemical calculations using a composite CBS-QB3 methodology. This reaction leads to the three cyanomethanimine isomers as the main products,
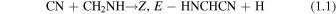
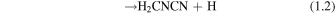
being the C-cyanomethanimine isomers formed when the CN radical attacks the carbon atom of methanimine (route 1.1), while N-cyanomethanimine is produced from the attack on its N-side (route 1.2). All these processes are exothermic and proceed without an entrance barrier, making this reaction a feasible mechanism forming cyanomethanimines under interstellar conditions. Among the two possible pathways, the one leading to the Z, E-HNCHCN isomers is much more thermodynamically favored, hence arising as the predominant route. Although the kinetic calculations performed by these authors establish a rather low Z/E-HNCHCN abundance ratio of ∼1.5 from this reaction (which is far below the 4.5 ± 0.2 ratio we observed; see Figure 6), the prediction for the C/N-cyanomethanimine ratio fits extremely well to the observational value (see Figure 7). Given that this ratio ought to be described in terms of chemical kinetics (see Section 4.1), this result would point to the CN + CH2NH reaction being mainly responsible for C- and N-cyanomethanimine chemistry in G+0.693. Moreover, its viability is also supported by the high abundance of the parent species (CH2NH and CN) found in this region (see Zeng et al. 2018 and Rivilla et al. 2019, respectively). On top of that, this reaction can also proceed at a very fast pace. With associated rate constants at 150 K of ∼10−11–10−10 cm−3 s−1 as derived by Vazart et al. (2015), the observed abundances of the cyanomethanimines would be reproduced through this reaction at timescales smaller than those related to depletion on dust grains due to the cooling of the gas (≳105 yr; Requena-Torres et al. 2006), even in spite of the low H2 densities of this cloud (∼104–105 cm−3; Zeng et al. 2020).
Figure 7. The C/N-cyanomethanimine abundance ratio dependence on temperature as predicted by Vazart et al. (2015) and Barone & Puzzarini (2022) chemical models for the CN + CH2NH reaction (red line and orange symbols, respectively). The green dashed line and shaded area delineate the observed value encompassed by its 1σ uncertainty (31.8 ± 1.8). Barone & Puzzarini (2022) estimates are retrieved for certain temperatures relevant to those derived for G+0.693 (∼70–140 K; Zeng et al. 2018), as no analytical expression for the rate constant is provided.
Download figure:
Standard image High-resolution imageNonetheless, a new theoretical study recently carried out by Puzzarini & Barone (2020) has revealed that the energy of the transition states along the reaction profile calculated by Vazart et al. (2015) could be slightly underestimated, causing their computed branching ratios not to be entirely accurate. In a subsequent study, Barone & Puzzarini (2022) performed improved kinetic calculations using both the CBS-QB3 model as well as the more optimized and recently developed jun-Cheap scheme, outlining a slightly divergent C/N-cyanomethanimine abundance ratio as a function of the temperature with respect to the Vazart et al. (2015) results, as shown in Figure 7. The prediction for the Z/E-HNCHCN ratio remains unchanged (see Figure 6). Nevertheless, the differences encountered in the predicted C/N-cyanomethanimine ratio are merely within a factor of ∼2 with respect to our observations, so that the gas-phase CN + CH2NH reaction is still considered as the main mechanism likely producing cyanomethanimines in G+0.693. Moreover, its rate constant peaks at ∼150 K as derived by Barone & Puzzarini (2022), in line with the kinetic temperature of this cloud (∼70–140 K; Zeng et al. 2018). Nonetheless, a more refined study of this process is needed to clarify the small discrepancies detected between the quantum chemical calculations and the astronomical observations.
Besides gas-phase processes, chemical reactions occurring on the icy mantles of interstellar dust grains could also be an important source producing H2CNCN in G+0.693, where grain surface chemistry is believed to play a key role (Rivilla et al. 2020a, 2021b, 2022b, 2023; Molpeceres 2021; San Andrés et al. 2023). However, little research has been done on these process for cyanomethanimine isomers, especially for H2CNCN. According to recent astrochemical models run by Zhang et al. (2020), the surface analog of the already-characterized gas-phase CN + CH2NH reaction could be a second relevant route forming the three cyanomethanimine isomers. However, while it is well established that methanimine (CH2NH) is mainly produced on grains, making this reaction feasible (Theule et al. 2011; Suzuki et al. 2016), the hinted extraordinary reactivity that the CN radical exhibits upon contact with H2O molecules (Rimola et al. 2018), one of the major constituents of dust grain ices, would pose a significant barrier to its occurrence. Indeed, as is also shown in the models of Zhang et al. (2020), the contribution from this reaction to the total abundances of cyanomethanimines seems not to be as significant as that of its gas-phase counterpart, which can easily proceed once CH2NH is desorbed into the gas. Furthermore, the observed abundances for the three cyanomethanimine isomers are reproduced within their models at timescales of <105 yr attending to the CN + CH2NH gas-phase reaction, which strongly supports it as the primary and most efficient formation mechanism. However, the C/N-cyanomethanimine abundance ratio is not consistently explained according to their chemical network, indicating that other chemical processes might also need to be invoked. Moreover, the absence of comprehensive theoretical or experimental investigations examining this particular mechanism on grains forces us to assume the same branching ratio toward the formation of the three isomers in chemical models. Nonetheless, in spite of these shortcomings, it appears more likely that the role of the CN + CH2NH reaction in producing cyanomethanimines is only relevant in the gas phase.
Another possible surface pathway has been proposed by Vasconcelos et al. (2020), whose experiments irradiating a N2-CH4 icy mixture with cosmic rays produced the three isomers of cyanomethanimine. However, it remains unclear whether the presence of additional molecules in the ice, such as H2O, CO, or CO2, can alter the chemistry observed in these irradiation experiments. Intuitively, and because H2O, CO, and CO2 are the main constituents of interstellar ices (see, e.g., Boogert et al. 2015; McClure et al. 2023), the free path of the radicals derived from CH4 and N2 radiolysis is likely to encounter reaction partners of this ternary, reducing the amount of cyanomethanimines being produced and hence rendering this specific route probably not dominant.
Shivani & Tandon (2017) also proposed that both the Z- and E-HNCHCN isomers could be formed in the grains through two consecutive hydrogenations of the cyanogen species (NCCN), which is expected to be abundant in G+0.693 (Rivilla et al. 2019). However, the astrochemical model performed later by Shingledecker et al. (2020) showed that this route has a minor impact on the formation of C-cyanomethanimine in G+0.693. By analogy, it could be proposed that the hydrogenation of the high-energy metastable isomer of NCCN, the isocyanogen species (CNCN), could be another possible route remaining to be added in the H2CNCN solid-phase chemical network. Nonetheless, this alternative route is currently hampered by the poor knowledge of how this precursor could be synthesized through interstellar chemistry, for which the only pathway so far studied (CN + HNC → CNCN + H) has been shown to have high energy barriers when leading to CNCN (Petrie & Osamura 2004). Furthermore, this species exhibits a low abundance in the ISM (<10−10 with respect to H2), with only one reported detection so far (Agúndez 2018). Indeed, using our spectral survey and assuming a Tex of 10 K and an FWHM of 22.0 km s−1, we have just derived an upper limit for its molecular abundance toward G+0.693 of ≤2 × 10−11, which is lower than the abundance of H2CNCN itself. Consequently, all evidence points to this route not being a promising alternative for boosting H2CNCN abundance, as happened for the Z- and E-HNCHCN isomers through the analog reaction triggered by NCCN.
In summary, none of the chemical pathways proposed to date for grain surface chemistry seem capable of substantially increasing the abundance of the three cyanomethanimine isomers to adequately match the observational values. In this context, the gas-phase CN + CH2NH reaction stands out as the primary formation route for these species, while the role of surface chemistry would primarily point to enhancing the abundance of CH2NH in the gas after ejection, thereby enabling the occurrence of this reaction.
5. Summary and Conclusions
We have presented the first detection in the ISM of N-cyanomethanimine (H2CNCN), a highly significant species in the prebiotic context as a potential precursor of adenine, one of the fundamental nucleobases constituting RNA and DNA. We have been able to univocally identify more than a dozen different a-type rotational transitions belonging to this species toward G+0.693 through the recently improved ultrahigh-sensitivity spectral survey of this source using the Yebes 40 m and IRAM 30 m radio telescopes. We have performed an LTE fit to the observed data, deriving a total column density of (2.9 ± 0.1) × 1012 cm−2, which translates into a molecular abundance with respect to H2 of (2.1 ± 0.3) × 10−11. This makes it one of the least abundant complex organic molecules so far detected toward this cloud, which demonstrates how the growing efforts in achieving a greater sensitivity on the observational data are pushing the limits of molecular species detectability in space.
The identification of N-cyanomethanimine in G+0.693 adds to the previous detection of its two more stable isomers in this same region: the Z- and E-isomers of C-cyanomethanimine (HNCHCN). The expanded spectral coverage provided by the new observations to frequencies below 50 GHz allowed us to reevaluate the identification of these two stereoisomers as well. In both cases, we have almost tripled the number of transitions detected, encompassing a significantly broader range of energy levels. This has led to a more accurate characterization of the physical parameters tracing both isomers' emission, which has resulted in a 3 times lower total column density for C-cyanomethanimine of (6.8 ± 1.0) × 10−10 in comparison to that previously inferred. We have found the C/N-cyanomethanimine abundance ratio to be 31.8 ± 1.8, which points to N-cyanomethanimine being over 1 order of magnitude less abundant compared to the more stable C-cyanomethanimine species. We have computed a Z/E-HNCHCN isomeric ratio of 4.5 ± 0.2 (consistent within the uncertainty with the previous estimate of 6.1 ± 2.4) and derived Z, E-HNCHCN/H2CNCN ratios of 25.9 ± 1.6 and 5.8 ± 0.3, respectively. The relative abundance between the Z- and E-HNCHCN isomers closely aligns with the prediction based on thermodynamic equilibrium at the kinetic temperature of G+0.693 (∼70–140 K). However, it should also be noted that thermodynamic control between both C-cyanomethanimine stereoisomers would only be established if their interconversion timescales are shorter than other competitive mechanisms (such as destruction processes), a task pending to be carefully checked in the following mechanistic studies related to these species. On the other hand, the C/N-cyanomethanimine ratio should be described in terms of the chemical reactions involving these species, since the isomerization of N-cyanomethanimine from C-cyanomethanimine seems to be unattainable at low temperatures. The three cyanomethanimine isomers are mainly formed in the gas through the reaction between methanimine (CH2NH) and the cyanogen radical (CN), which seems to be responsible for the observed C/N-cyanomethanimine abundance ratio.
Acknowledgments
We are very grateful to the Yebes 40 m and IRAM 30 m telescope staff for their precious help during the different observing runs. The 40 m radio telescope at Yebes Observatory is operated by the Spanish Geographic Institute (IGN; Ministerio de Transportes, Movilidad y Agenda Urbana). IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain). D.S.A., V.M.R., and A.L.-G. acknowledge the funds provided by the Consejo Superior de Investigaciones Científicas (CSIC) and the Centro de Astrobiología (CAB) through the project 20225AT015 (Proyectos intramurales especiales del CSIC). D.S.A. also extends his gratitude for the financial support provided by the Comunidad de Madrid through the grant PIPF-2022/TEC-25475. V.M.R. has also received support from the project RYC2020-029387-I funded by the Spanish Ministry of Science, Innovation and Universities/State Agency of Research MICIU/AEI/10.13039/501100011033 and by "ESF, Investing in your future." I.J.-S., J.M.-P., L.C., A.M., and A.M.-H. acknowledge financial support through the Spanish grant PID2019-105552RB-C41 funded by MICIU/AEI/10.13039/501100011033. I.J.-S., J.M.-P., L.C., V.M.R., A.M., and A.M.-H. also acknowledge financial support through the Spanish grant PID2022-136814NB-I00 funded by MICIU/AEI/10.13039/501100011033 and by ERDF, UE. A.M. has received support from grant PRE2019-091471 under project MDM-2017-0737-19-2 funded by MICIU/AEI/10.13039/501100011033 and by "ERDF A way of making Europe." A.M.-H. acknowledges funds from grant MDM-2017-0737 Unidad de Excelencia "María de Maeztu" Centro de Astrobiología (CAB, INTA-CSIC). M.S.N. acknowledges a Juan de la Cierva Postdoctoral Fellow proyect JDC2022-048934-I, funded by MICIU/AEI/10.13039/501100011033 and by the European Union "NextGenerationEU/PRTR". B.T. and P.d.V. are thankful for the support from the Spanish MICIU through the project PID2019-107115GB-C21. B.T. also thanks the Spanish MICIU for funding support from grant PID2022-137980NB-I00. G.M. thanks the Japan Society for the Promotion of Science (JSPS International fellow P22013 and KAKENHI grant No. JP22F22013).
Software: Madrid Data Cube Analysis (MADCUBA) on ImageJ is a software developed at the Centre of Astrobiology (CAB) in Madrid, version 10.1.10 (2023 November 15), at https://cab.inta-csic.es/madcuba (Martín et al. 2019).
Appendix A: H2CNCN Transitions Excluded from the LTE Fit
Besides the H2CNCN transitions used to generate the LTE model of this molecule shown in Figure 1, our observational data also encompass many other a-type rotational lines belonging to this species within the almost entirely targeted Jup = 3 to Jup = 11 progressions of the Ka = 0, 1, 2 ladders. These remaining transitions are shown in Figure 8, which just includes the most intense ones among them (with peak intensities down to 1.5 mK and integrated S/N > 3). As can be seen, most of these lines are heavily blended with the emission from other species (either unknown or already detected toward G+0.693), while a few others lie in certain segments of the spectrum where the noise level is too high to provide a clear identification. Since it is evident that these transitions do not qualify for the direct detection of this molecule, they were excluded in our analysis. Nevertheless, it is worth emphasizing that the predicted emissions for all of them are coherent with the observed spectrum, reaffirming the consistency of the LTE model derived for H2CNCN as presented in Section 3.1.
Figure 8. H2CNCN rotational transitions targeted toward G+0.693 but excluded when performing the LTE fit of this molecule, which predicts the emission profiles delineated by the red solid lines. The black histogram and gray shaded areas indicate the observed spectrum, while the blue solid lines represent the emission of all the species already identified in the cloud (whose names are indicated by the blue labels). Transitions shown here are sorted by decreasing peak intensity, which goes down to 1 mK. Panel labels indicate the main rotational transition being targeted using the  common notation.
common notation.
Download figure:
Standard image High-resolution imageAppendix B: Z-HNCHCN and E-HNCHCN Spectroscopy
Table 3 gathers the spectroscopic information of the lines belonging to Z-HNCHCN and E-HNCHCN we have detected toward G+0.693 that are unblended or partially blended with the emission of other molecular species. These transitions are shown in Figures 3 and 4, respectively, and are those we used to perform the LTE line fitting of these two isomers. We provide the most relevant spectroscopic parameters for each transition, which are the rest frequency, the quantum numbers, the base 10 logarithm of the integrated intensity at 300 K (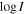 ), the energy of the upper level (Eup), the noise level of the spectra evaluated over line-free spectral ranges close to it (rms), the integrated intensity (
), the energy of the upper level (Eup), the noise level of the spectra evaluated over line-free spectral ranges close to it (rms), the integrated intensity ( ) as calculated from the fit, the detection level (in terms of the integrated S/N), and information regarding line blending. The spectroscopic information has been obtained from the CDMS entries of these isomers, which come from Takano et al. (1990), Zaleski et al. (2013), and Melosso et al. (2018).
) as calculated from the fit, the detection level (in terms of the integrated S/N), and information regarding line blending. The spectroscopic information has been obtained from the CDMS entries of these isomers, which come from Takano et al. (1990), Zaleski et al. (2013), and Melosso et al. (2018).
Table 3. Spectroscopic Information of Z- and E-HNCHCN Unblended and Partially Blended Transitions Detected in G+0.693
| Frequency a | Transition b |

| Eup | rms |
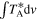
| S/N c | Blending d |
|---|---|---|---|---|---|---|---|
| (GHz) | (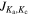 ' ' '') '') | (nm2 MHz) | (K) | (mK) | (mK km s−1) | ||
| Z-HNCHCN | |||||||
| 37.93654(7) | 41,4 − 31,3 | −6.3795 | 6.9 | 0.5 | 236(14) | 78(5) | Blended: C2H3CN |
| 38.7947810(4) | 40,4 − 30,3 | −6.3287 | 4.7 | 0.5 | 302(15) | 100(5) | Slightly blended: CH3COCH3 |
| 39.70212(7) | 41,3 − 31,2 | −6.3402 | 7.1 | 0.5 | 244(14) | 82(5) | Blended: CH3CONH2 and U |
| 47.4148090(8) | 51,5 − 41,4 | −6.1065 | 9.2 | 0.5 | 325(16) | 119(6) | Blended: C2H3CN |
| 48.47090(7) | 50,5 − 40,4 | −6.0665 | 7.0 | 0.5 | 405(18) | 151(7) | Unblended |
| 48.5276164(5) | 52,4 − 42,3 | −6.1548 | 16.5 | 0.5 | 174(12) | 65(5) | Slightly blended: HOCH2CHO and U |
| 49.62143(7) | 51,4 − 41,3 | −6.0674 | 9.5 | 0.5 | 334(16) | 125(6) | Unblended |
| 75.8241226(12) | 81,8 − 71,7 | −5.5395 | 18.7 | 2.4 | 447(60) | 26(3) | Blended: CH3SH and U |
| 77.3975188(8) | 80,8 − 70,7 | −5.5120 | 16.7 | 2.4 | 536(60) | 31(3) | Unblended⋆ |
| 77.61551(7) | 82,7 − 72,6 | −5.5512 | 26.2 | 2.4 | 257(60) | 15(3) | Blended: cis-N-CH3NHCHO |
| 77.86298(7) | 82,6 − 72,5 | −5.5485 | 26.3 | 2.4 | 257(60) | 15(3) | Unblended⋆ |
| 85.2831815(13) | 91,9 − 81,8* | −5.3992 | 22.8 | 1.6 | 429(40) | 39(4) | Slightly blended: HOCH2CHO and H15NCO |
| 86.99658(10) | 90,9 − 80,8* | −5.3738 | 20.9 | 1.6 | 508(41) | 47(4) | Blended: HCCCC13CN |
| 87.6558177(9) | 92,7 − 82,6 | −5.4029 | 30.5 | 1.3 | 247(31) | 28(4) | Blended: 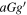 -(CH2OH)2 -(CH2OH)2
|
| 89.24785(10) | 91,8 − 81,7* | −5.3610 | 23.8 | 1.3 | 421(34) | 49(4) | Blended: H2CCCHCN and U |
| 94.73583(10) | 101,10 − 91,9* | −5.2746 | 27.4 | 0.9 | 388(25) | 66(4) | Unblended |
| 96.56986(10) | 100,10 − 90,9* | −5.2510 | 25.5 | 1.5 | 455(40) | 47(4) | Slightly blended: 13CH3SH |
| 97.46902(10) | 102,8 − 92,7 | −5.2744 | 35.1 | 1.5 | 223(35) | 23(4) | Blended: CH3CONH2 and U |
| 99.13721(10) | 101,9 − 91,8 | −5.2367 | 28.6 | 1.5 | 375(40) | 39(4) | Blended: CH3NH2 and CH3OCHO |
| 104.181786(20) | 111,11 − 101,10 | −5.1629 | 32.4 | 1.8 | 332(43) | 30(4) | Slightly blended: 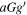 -(CH2OH)2 -(CH2OH)2
|
| 106.115423(20) | 110,11 − 100,10 | −5.1409 | 30.6 | 1.8 | 387(44) | 35(4) | Blended: CH3OCHO |
| 106.663856(20) | 112,10 − 102,9 | −5.1647 | 40.2 | 1.8 | 191(42) | 17(4) | Blended:  -(CH2OH)2 -(CH2OH)2
|
| 109.016976(20) | 111,10 − 101,9* | −5.1254 | 33.8 | 2.9 | 316(70) | 18(4) | Slightly blended with U |
| 113.620315(20) | 121,12 − 111,11 | −5.0620 | 37.8 | 2.8 | 271(70) | 16(4) | Blended: Aa-CH3CH2CH2OH and g-C2H5SH |
| 125.1163734(14) | 130,13 − 120,12 | −4.9511 | 42.2 | 6.6 | 241(153) | 7(4) | Blended: CH3SH |
| 134.5699312(15) | 140,14 − 130,13 | −4.8686 | 48.7 | 1.5 | 177(35) | 22(4) | Unblended⋆ |
| E-HNCHCN | |||||||
| 37.54275(10) | 41,4 − 31,3 | −5.6004 | 7.3 | 0.5 | 297(13) | 97(5) | Slightly blended with U |
| 46.9248305(11) | 51,5 − 41,4 | −5.3274 | 9.5 | 0.5 | 415(16) | 152(6) | Unblended⋆ |
| 48.78317(10) | 51,4 − 41,3 | −5.2940 | 9.8 | 0.5 | 425(16) | 159(6) | Unblended⋆ |
| 75.05519(10) | 81,8 − 71,7 | −4.7600 | 19.0 | 2.4 | 606(60) | 35(3) | Unblended⋆ |
| 78.02648(10) | 81,7 − 71,6 | −4.7272 | 19.6 | 2.4 | 606(60) | 36(3) | Blended: CH3NCO and U |
| 84.42511(10) | 91,9 − 81,8* | −4.6196 | 23.0 | 1.6 | 596(41) | 54(4) | Unblended |
| 85.512670(16) | 31,3 − 20,2 | −5.3412 | 5.5 | 1.6 | 256(40) | 23(3) | Blended:  -(CH2OH)2 and U -(CH2OH)2 and U |
| 87.76650(10) | 91,8 − 81,7 | −4.5869 | 23.8 | 1.3 | 589(34) | 67(4) | Blended: HOCH2CHO and CH3OCHO |
| 93.79107(10) | 101,10 − 91,9* | −4.4949 | 27.5 | 0.9 | 554(26) | 94(5) | Unblended |
| 94.345774(16) | 41,4 − 30,3 | −5.1964 | 7.3 | 0.9 | 317(23) | 54(4) | Unblended⋆ |
| 95.42252(10) | 100,10 − 90,9* | −4.4722 | 25.2 | 1.5 | 663(40) | 68(4) | Blended with U |
| 97.50186(10) | 101,9 − 91,8* | −4.4625 | 28.5 | 1.5 | 542(40) | 57(4) | Unblended |
| 102.999619(16) | 51,5 − 40,4 | −5.0669 | 9.5 | 1.8 | 362(43) | 32(4) | Blended: l-C3H2 and CH3COCH3 |
| 103.1527434(22) | 111,11 − 101,10 | −4.3830 | 32.5 | 1.8 | 489(44) | 44(4) | Unblended |
| 104.895725(20) | 110,11 − 100,10* | −4.3617 | 30.3 | 1.8 | 581(45) | 52(4) | Unblended⋆ |
| 107.231510(20) | 111,10 − 101,9 | −4.3509 | 33.7 | 1.8 | 473(44) | 43(4) | Unblended⋆ |
| 111.479187(16) | 61,6 − 50,5 | −4.9503 | 12.2 | 2.8 | 389(70) | 23(4) | Unblended⋆ |
| 112.509636(20) | 121,12 − 111,11 | −4.2819 | 37.9 | 2.8 | 413(70) | 25(4) | Blended: E-CH3CHNH |
| 114.349931(20) | 120,12 − 110,11 | −4.2617 | 35.7 | 2.8 | 486(70) | 29(4) | Unblended |
| 127.942187(15) | 81,8 − 70,7 | −3.8856 | 19.0 | 6.6 | 383(160) | 10(4) | Blended: H15NCO and Z-CH3CHNH |
| 135.942373(15) | 91,9 − 80,8 | −3.7881 | 23.0 | 1.5 | 356(40) | 44(5) | Blended: CH3OCHO |
Notes.
a The numbers in parentheses represent the experimental uncertainty associated with the last digits, as measured by Takano et al. (1990), Zaleski et al. (2013), and Melosso et al. (2018). b Rotational transitions marked with an asterisk refer to those previously targeted by Rivilla et al. (2019) and that these authors used to perform the LTE modeling of these species. All of the transitions are also labeled following the same notation indicated in Table 2. c The S/N is calculated from the integrated intensity over the line width ( ) and noise level
) and noise level  , where δ
v is the spectral resolution of the spectra in velocity units and the FWHM is estimated from the LTE line fitting. The numbers in parentheses represent the combined standard uncertainty associated with the last digits.
d
The term "unblended" denotes those transitions that exhibit no contamination from other molecular species, while a star symbol is added when scarce line blending accounting for less than 5% of the line total integrated intensity is present. "U" hints at blending with an unknown (not yet identified) species.
, where δ
v is the spectral resolution of the spectra in velocity units and the FWHM is estimated from the LTE line fitting. The numbers in parentheses represent the combined standard uncertainty associated with the last digits.
d
The term "unblended" denotes those transitions that exhibit no contamination from other molecular species, while a star symbol is added when scarce line blending accounting for less than 5% of the line total integrated intensity is present. "U" hints at blending with an unknown (not yet identified) species.Download table as: ASCIITypeset image
Footnotes
- 11
Rigorously, the relative population under thermodynamic equilibrium should be described in terms of the Gibbs free energy associated with their isomerization reaction (ΔG). However, the thermal correction of the free energy is not usually provided for many interstellar isomers, in particular for N-cyanomethanimine. Therefore, to compare it with the isomeric ratios derived from the observations, we used the expression using their relative energies instead (ΔE), which was demonstrated to also be reliable for the C-cyanomethanimine stereoisomers (García de la Concepción et al. 2021).