Abstract
The origin and evolution of the 14N/15N ratio of Titan's atmosphere has long been a subject of debate. Clearly a better understanding of the N isotopic fractionation mechanism would greatly help resolve this. Photodissociation of N2 by solar radiation has been suggested to either play a negligible role in fractionating the N isotopes in Titan, due to its rather low escape velocity, or to preferentially remove 15N through self-shielding controlled photochemical reactions. Here, we systematically measure the branching ratios of 14N15N between N(4S)+N(2P) and N(4S)+N(2D) channels. We find that many of its absorption states predominantly dissociate into N(4S)+N(2P) with a strong isotope effect between 14N2 and 14N15N. Since N atoms produced from N(4S)+N(2P) acquire velocities close to Titan's escape velocity, these findings provide a new N isotope fractionation mechanism for Titan that has not been considered before, potentially providing important constraints on the origin and evolution of Titan's N2-dominated atmosphere.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Titan, the largest moon of Saturn, has the thickest nitrogen (N2)-dominated (∼98%) atmosphere in the solar system, with a surface pressure of ∼1.5 bars (∼50% higher than the sea-level pressure on Earth; Niemann et al. 2005; Waite et al. 2005). While Triton and Pluto have tenuous N2-dominated atmospheres, only Earth has a comparably thick N2-dominated atmosphere among the solar system planetary bodies. Titan's peculiar nitrogen isotopic composition, however, has been a constant puzzle that has yet to be fully understood since the first flyby by Voyager 1 in 1980. As the 14N/15N ratio of Titan carries the signature of the building blocks from which it originated, and is affected by diverse fractionation processes that shaped the subsequent evolution of its thick N2-dominated atmosphere (Lunine et al. 1999; Lammer et al. 2000; Erkaev et al. 2020), it has attracted numerous studies through direct astronomical observations, laboratory experiments, and theoretical modeling (Lammer et al.2008; Brown et al. 2010; Krasnopolsky 2019; Scherf et al. 2020), including the flagship Cassini-Huygens spacecraft mission (Niemann et al. 2005; Waite et al. 2005).
Prior to the arrival to the Saturn system by Cassini-Huygens in 2004, the 14N/15N ratio in Titan's atmosphere was represented by HC14N/HC15N, which was measured to be ∼60 by several different methods (Krasnopolsky 2016; Marten et al. 2002). Later on the 14N/15N ratio in N2 of Titan's atmosphere was deduced to be 167.7 using the data collected by the Cassini-Huygens probe gas chromatograph mass spectrometer (GCMS) (Niemann et al. 2010). This value is in stark contrast with the protosolar nebular 14N/15N ratio of 440.5 ± 5.8 and solar wind composition of 458.7 ± 4.1 (Marty et al. 2011), Jupiter's 435 ± 65 (Owen et al. 2001), Saturn's >357 estimated by Fletcher et al. (2014), and Earth's atmosphere and primordial mantle compositions of 272 and 283, respectively (Marty & Zimmermann 1999; Cartigny & Marty 2013), which likely originated from chondritic meteorites (Marty 2012; Harries et al. 2015). Previous studies predicted a loss of 10–100 times of present atmospheric mass from Titan (Lunine et al. 1999; Lammer et al. 2000; Niemann et al. 2005; Waite et al. 2005) based on the assumed initial 14N/15N ratio of ∼60. The most recent models by Mandt et al. (Mandt et al. 2009, 2014; Erkaev et al. 2020; Scherf et al. 2020) and Berezhnoi (Berezhnoi 2010) showed that the 14N/15N ratio in Titan's atmosphere cannot have been changed drastically over the timescale of the solar system history, after carefully considering all the currently known nitrogen fractionation processes. The highest initial 14N/15N ratio was proposed to be <190 (Mandt et al. 2014; Erkaev et al. 2020). This has led to the conclusion by Mandt et al. that Titan's nitrogen mainly comes from cometary ammonia ices, which has a 14N/15N ratio of 120–140 (Rousselot et al. 2013; Shinnaka et al. 2014, 2016), with ∼50% contribution also from refractory organics with an average 14N/15N of ∼231 (from 160 to 320; Miller 2019; Erkaev et al. 2020). A most recent model suggested Titan could have lost 0.5–16 times of present atmospheric mass (Erkaev et al. 2020). The total loss of nitrogen during the entire lifetime of Titan predicted by these models greatly depends on the solar activity, and how efficiently nitrogen isotopes can be fractionated in different physico-chemical processes (Erkaev et al. 2020). Evidently, understanding the origin of such an atmosphere is essential for icy worlds in general, and Titan's evolution in particular.
Thus, quantitatively knowing all the possible nitrogen fractionation processes is crucial to correctly understand the origin and evolutionary history of Titan's atmosphere. It is now generally accepted that there are two competing processes that fractionate the nitrogen isotope in Titan's atmosphere. The first one is the photochemical process (photolysis) initiated by the solar vacuum ultraviolet (VUV) photodissociation of N2, followed by incorporation of the dissociated N atoms into nitriles such as HCN, which eventually get deposited on Titan's icy surface. This process is expected to occur deep inside Titan's atmospheric interior, and has been shown to preferentially remove 15N, and hence increase Titan's atmospheric 14N/15N as opposed to reducing it, due to the strongly isotope abundance dependent photodissociation properties of 14N2 and 14N15N via the self-shielding effect (Krasnopolsky 2016; Liang et al. 2007). According to Erkaev et al. (2020), the photochemical process is the most efficient fractionator among the various nonthermal processes (the dominant nitrogen loss mechanism in the present-day atmosphere of Titan). However, to incorporate nitrogen into nitriles, the presence of CH4 in Titan's atmosphere is required to form nitriles from N2 and CH4 dissociation products. The availability of CH4 in Titan's atmosphere is likely to be episodic and mainly limited to recent history of <1 Gyr, thus the significance of this process might be limited in extent over a relatively short, recent period of Titan's atmospheric evolution (Erkaev et al. 2020). Tobie et al. (2006) showed that episodic outgassing of CH4 could have also taken place prior to 1 Gyr, specifically prior to ∼2.5 Gyr and it could have theoretically contributed to the fractionation of 14N/15N, while it should be less significant than at present due to its anticorrelation with the solar VUV flux. Contrary to this view, Krasnopolsky suggested that photodissociation of N2 in the absorption bands between 80–100 nm and the subsequent, irreversible loss of 15N to HCN plays a significant role in shaping Titan's composition from a cometary source with 14N/15N of 127 to the present Titan's atmospheric value of 167.7 (Krasnopolsky 2016).
The second process is the atmospheric escape through various thermal and nonthermal processes over the history of Titan, which preferentially remove 14N from the upper atmosphere (Lunine et al. 1999; Lammer et al. 2000; Erkaev et al. 2020), to help reduce 14N/15N to the observed value in Titan's present atmosphere from an expected higher primordial composition. However, the photodissociation induced escape of N from the upper atmosphere of Titan (Shematovich 1999; Shematovich et al.2003) has long been believed to have no significant contribution to the nitrogen isotope fractionation in Titan's atmosphere, because photodissociation of N2 in the wavelength range 80–100 nm produces N atoms with velocities much higher than the escape velocity of Titan. Consequently, both 14N and 15N could escape from Titan with almost equal efficiency; therefore, it does not contribute to isotopic fractionation processes (Lunine et al. 1999; Lammer et al. 2000; Berezhnoi 2010). However, all of the literature thus far assumes N2 dissociates into the channel N(4S)+N(2D). The next energetically available channel N(4S)+N(2P) is not experimentally explored. This channel is ∼1.2 eV higher in internal energy than that of N(4S)+N(2D), thus the kinetic energy of N atoms generated will be ∼0.6 eV lower than that from the channel N(4S)+N(2D). This would generate N atoms with velocities close to or slightly below the escape velocity of Titan, thus can be a most efficient N isotope fractionator, similar to the dissociative recombination of 14N15N+ that has been shown to play an important role in enriching 15N in the atmosphere of Mars (Fox & Hać 1997).
In this study, we systematically measure the photodissociation branching ratios of 14N15N in the energy range below the threshold of the channel N(2D)+N(2D) at 14.523 eV for the first time by using a VUV pump–VUV probe–velocity-map imaging (VUV-VUV-VMI) setup similar to that recently used by Jackson, Ng, and coworkers (Gao et al. 2013; Song et al. 2016; Gao et al. 2020). Many absorption states of 14N15N are found to dissociate predominantly into the channel N(4S)+N(2P), and thus generate N atoms with velocities close to the escape velocity of Titan. A strong isotope effect on the photodissociation branching ratios of 14N2 and 14N15N is observed, similar to that of CO (Shi et al. 2017, 2018; Jiang et al. 2019; Guan et al. 2020; Chi et al. 2020a, 2020b). This can introduce an additional N isotope fractionation effect in the upper atmosphere of Titan.
2. Experimental Method
The branching ratio measurement was performed on a newly constructed time-slice velocity-map imaging (TSVMI) setup equipped with two independently tunable VUV laser beams. A pulsed supersonic N2 molecular beam of natural abundance (the volume percentage of 14N15N is ∼0.8%) was generated by a general valve (Parker, series 9) with a nozzle diameter of 0.5 mm, operating at a repetition rate of 10 Hz and a total stagnation pressure of 30 psi. For several of the weak absorption bands, 15N enriched N2 gas was also used (Global Rare Gases, 14N15N: 50%, 14N2: 25%, 15N2: 25%). The molecular beam passed through two skimmers (Molecular Dynamics, Model 2) with aperture diameters of 2 mm, and entered the photodissociation and photoionization (PD/PI) region of the TSVMI setup. In the PD/PI region, the molecular beam first crossed perpendicularly with the first tunable VUV laser beam (the pump VUV), the 14N15N molecule absorbed a VUV photon and was excited to a specific electronic, vibrational, and rotational quantum state, and then predissociated to produce N atoms in the 4S, 2D, and 2P states. After a delay of ∼10 ns, the second tunable VUV laser beam (the probe VUV) arrived from the opposite direction and crossed with the molecular beam perpendicularly. The probe VUV excited N atoms in the ground 4S state to an intermediate quantum state 2s22p2(3P)4d4P5/2, and N atoms in this intermediate state could absorb a second UV or visible photon contained in the probe VUV laser beam to be ionized (Song et al. 2016; Chang et al. 2019). The N+ ions thus produced were accelerated by the VMI electrostatic lens system, and projected onto an ion imaging detection system, which contains two microchannel plates with diameters of 75 mm, a P47 phosphor screen (Photek, VID275), and a CMOS camera (IDS, UI-3060CP-M-GL Rev.2).
To measure the photodissociation branching ratios of all the quantum states, we need to know the exact positions of these states first. For 14N15N, accurate photoabsorption spectrum has been measured below the photon energy of 109,000 cm−1, while above this energy no spectroscopic measurements have been reported yet (Heays et al. 2011, 2019). To obtain the photofragment excitation (PHOFEX) spectrum of 14N15N and identify all its relevant absorption bands, we first fixed the wavelength of the probe VUV beam for ionizing N(4S) and scanned the wavelength of the pump VUV beam in the range 107,500–117,500 cm−1. The intensity of the N+ ion signal in the time-of-flight (TOF) mass spectrum coupled out from the phosphor screen was monitored as a function of the pump VUV wavelength, which was then the PHOFEX spectrum of 14N15N. The TSVMI setup also acts as a TOF mass spectrometer, which has a mass resolution of ∼150. This enables us to distinguish among 14N2 +, 14N15N+, and 15N2 +, and also between 14N+ and 15N+ ions. After all the peaks in the PHOFEX spectrum were identified, we fixed the wavelength of the pump VUV beam to each of the absorption peaks, and accumulated the image produced by the N+ ion on the phosphor screen. Here, only the central slice of the three-dimensional sphere (Newton sphere) was detected as we did before (Jiang et al. 2019). The wavelength of the probe VUV beam was scanned back and forth to make sure the N atoms with different velocities were probed equally. The images thus obtained were converted to the total kinetic energy release (TKER) spectra, and each of the peaks in the TKER spectra can be easily assigned to the corresponding dissociation channels according to the conservation of the total energy. The branching ratios were obtained by integrating the areas underneath the corresponding peaks in the TKER spectra.
Both of the two tunable VUV beams were generated by using the two-photon resonance-enhanced four-wave mixing method. For the pump VUV beam, a 10 Hz Nd:YAG laser (Quanta-Ray, Pro-230-10E) pumped two dye lasers (Sirah, Cobra-Stretch) at the same time. The first dye laser outputs a UV laser beam at 212.556 nm (ω1), which is resonant with the two-photon transition of Kr at 94,092.86 cm−1: (4p)5( )5p2[1/2](J = 0) ← (4p)6 1S0 The second dye laser outputs a visible laser beam (ω2), which was scanned in the wavelength range 427–746 nm to cover the sum-frequency VUV (2ω1+ω2) energy range 107,500–117,500 cm−1. The exact wavelength of the visible laser (ω2) was monitored by a wavemeter (HighFinesse WS-6). The two laser beams were focused into a T-shaped channel when the Kr gas was pulsed in, where the four-wave mixing process occurred. All wavelength components thus generated, including ω1, ω2, difference-frequency VUV (2ω1–ω2), sum-frequency VUV (2ω1+ω2), and the tripling VUV (3ω1), propagated into the PD/PI region, because no dispersion apparatuses were used between the T-shape channel and the PD/PI region. The side of the probe VUV beam is almost the same as that of the pump VUV beam, except that the Nd:YAG laser and dye lasers were from different manufacturers and the visible laser was only scanned in a narrow wavelength range centered at 617.01 nm to resonantly excite the N(4S) atom to the intermediate quantum state 2s22p2(3P)4d4P5/2 at 110,299.97 cm−1 (Song et al. 2016; Chang et al. 2019). Since all the wavelength components propagated together with the sum-frequency VUV beam, N atoms in the 2s22p2(3P)4d4P5/2 state can be ionized by absorbing another UV (ω1) or visible (ω2) photon in the same laser pulse. Both of the two VUV lasers have a bandwidth of ∼0.3 cm−1, which is narrow enough to resolve each individual rotational transition.
)5p2[1/2](J = 0) ← (4p)6 1S0 The second dye laser outputs a visible laser beam (ω2), which was scanned in the wavelength range 427–746 nm to cover the sum-frequency VUV (2ω1+ω2) energy range 107,500–117,500 cm−1. The exact wavelength of the visible laser (ω2) was monitored by a wavemeter (HighFinesse WS-6). The two laser beams were focused into a T-shaped channel when the Kr gas was pulsed in, where the four-wave mixing process occurred. All wavelength components thus generated, including ω1, ω2, difference-frequency VUV (2ω1–ω2), sum-frequency VUV (2ω1+ω2), and the tripling VUV (3ω1), propagated into the PD/PI region, because no dispersion apparatuses were used between the T-shape channel and the PD/PI region. The side of the probe VUV beam is almost the same as that of the pump VUV beam, except that the Nd:YAG laser and dye lasers were from different manufacturers and the visible laser was only scanned in a narrow wavelength range centered at 617.01 nm to resonantly excite the N(4S) atom to the intermediate quantum state 2s22p2(3P)4d4P5/2 at 110,299.97 cm−1 (Song et al. 2016; Chang et al. 2019). Since all the wavelength components propagated together with the sum-frequency VUV beam, N atoms in the 2s22p2(3P)4d4P5/2 state can be ionized by absorbing another UV (ω1) or visible (ω2) photon in the same laser pulse. Both of the two VUV lasers have a bandwidth of ∼0.3 cm−1, which is narrow enough to resolve each individual rotational transition.
3. Results and Discussions
3.1. Branching Ratios of N(4S)+N(2P) for 14N15N
In the VUV photon energy range below 14.523 eV (the threshold for the channel N(2D)+N(2D)), N2 has the following three energetically available dissociation channels:

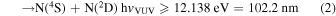

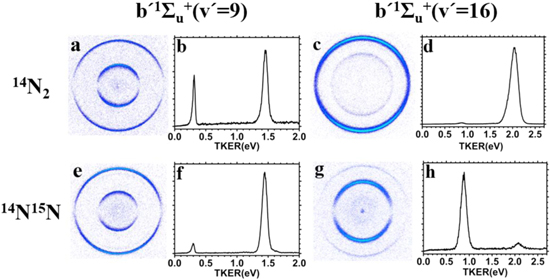
N(4S) is the ground state of the N atom, N(2D) and N(2P) are the first and second excited states, which are 2.384 and 3.576 eV higher than the ground state, respectively. In Figures 1(e), (f), (g), and (h), two typical VMI images and their corresponding TKER spectra for the
b
'1
 (ν' = 9) and
b
'1
(ν' = 9) and
b
'1
 (ν' = 16) vibronic states of 14N15N are presented.
(ν' = 16) vibronic states of 14N15N are presented.
Figure 1. Comparisons of the time-slice velocity-map ion images and the corresponding total kinetic energy release (TKER) spectra between 14N2 (a)–(d) and 14N15N (e)–(h) for the R(0) transition lines of the  (v' = 9) (a), (b), (e), (f) and
(v' = 9) (a), (b), (e), (f) and  (v' = 16) (c), (d), (g), (h) states. The outer and inner rings correspond to the photodissociation channels N(4S)+N(2D) and N(4S)+N(2P), respectively. The underneath areas of the corresponding peaks in the TKER spectra are integrated to obtain the relative branching ratios between the two channels.
(v' = 16) (c), (d), (g), (h) states. The outer and inner rings correspond to the photodissociation channels N(4S)+N(2D) and N(4S)+N(2P), respectively. The underneath areas of the corresponding peaks in the TKER spectra are integrated to obtain the relative branching ratios between the two channels.
Download figure:
Standard image High-resolution imageSimilar to the photodissociation of the main isotopologue 14N2 (Song et al. 2016), no signal into the ground channel N(4S)+N(4S) (Reaction 1) is detected for all the states of 14N15Nobserved in the current study. Two rings of different radii are seen in Figures 1(e) and (g), where the ring of larger size corresponds to the channel N(4S)+N(2D) with a higher velocity, and the ring of smaller size corresponds to the channel N(4S)+N(2P) with a lower velocity, due to the fact that N(2P) has a higher internal energy than N(2D). The images are integrated and converted to the TKER spectra as shown in Figures 1(f) and (h), respectively. The underneath areas of the two peaks are calculated, which are proportional to the relative branching ratios of the corresponding channels. By comparing Figures 1(e) and (f) with Figures 1(g) and (h), it can be seen that the branching ratios strongly depend on the quantum states of 14N15N that are excited. The
b
'1
 (ν' = 9) state predominantly forms N(4S)+N(2D), while the
b
'1
(ν' = 9) state predominantly forms N(4S)+N(2D), while the
b
'1
 (ν' = 16) state mainly dissociates into the channel N(4S)+N(2P). For the purpose of following discussion, all the states that dissociate with substantial amounts into the channel N(4S)+N(2P) (>5%) are listed in Table 1. A full list of all the branching ratio measurements in this study can be found in Tables A1 and A2 in the
(ν' = 16) state mainly dissociates into the channel N(4S)+N(2P). For the purpose of following discussion, all the states that dissociate with substantial amounts into the channel N(4S)+N(2P) (>5%) are listed in Table 1. A full list of all the branching ratio measurements in this study can be found in Tables A1 and A2 in the  and 1
Π
u
states (dots), Rydberg
and 1
Π
u
states (dots), Rydberg  and 1
Π
u
states (triangles).
and 1
Π
u
states (triangles).
Figure 2. Comparison of the photodissociation branching ratios into the channel N(4S)+N(2P) between 14N2 (blue) and 14N15N (red) in the VUV range 107,500–117,500 cm−1: (a) the valence  +(v') state; (b) Rydberg
+(v') state; (b) Rydberg  (v') states; (c) the valence
b
1
Π
u
(
v
'') state (dots) and Rydberg 1
Π
u
(v') states (triangles). Here, the measured values at R(0) transitions are used to represent the branching ratios for each of the absorption states. The statistical uncertainties derived from three to five independent measurements are usually smaller than the sizes of the symbols, and thus not shown in the figure. The branching ratios of 14N2 are adopted from Song et al. (2016).
(v') states; (c) the valence
b
1
Π
u
(
v
'') state (dots) and Rydberg 1
Π
u
(v') states (triangles). Here, the measured values at R(0) transitions are used to represent the branching ratios for each of the absorption states. The statistical uncertainties derived from three to five independent measurements are usually smaller than the sizes of the symbols, and thus not shown in the figure. The branching ratios of 14N2 are adopted from Song et al. (2016).
Download figure:
Standard image High-resolution imageTable 1. Band Origins, Branching Ratios, and Kinetic Energy Releases of 14N and 15N Produced in the Channel N(4S)+N(2P) for Several Relevant Absorption States of 14N2 and 14N15N
| Upper State | 14N15N | 14N2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| νo (eV) | νo (cm−1) | N(2P) (%) a | 14N (eV) | 15N (eV) | νo (eV) | νo (cm−1) b | N(2P) (%) c | 14N (eV) | |
| b'1Σu +(v' = 9) | 13.6517 | 110,110.9 | 5.7 ± 0.2 d | 0.166 | 0.155 | 13.6623 | 110,196.7 | 30.7 ± 0.4 | 0.166 |
| c4'1Σu +(v' = 3) | 13.7081 | 110,565.8 | 3.8 ± 0.6 | 0.196 | 0.183 | 13.7194 | 110,656.7 | 5.7 | 0.195 |
| b'1Σu +(v' = 12) | 13.9004 | 112,116.7 | 16.2 ± 0.9 | 0.295 | 0.275 | 13.9155 | 112,238.5 | 21.0 ± 1.5 | 0.293 |
| c4'1Σu +(v' = 4) | 13.9670 | 112,654.3 | 25.8 ± 0.2 | 0.329 | 0.308 | 13.9811 | 112,768.1 | 57.4 ± 3.7 | 0.326 |
| b'1Σu +(v' = 13) | 13.9811 | 112,767.7 | 96.2 ± 1.0 | 0.337 | 0.314 | 13.9984 | 112,907.6 | 81.8 ± 1.6 | 0.334 |
| b1Πu (v' = 17) | 14.0061 | 112,969.4 | 83.9 ± 0.6 | 0.350 | 0.326 | 14.0256 | 113,127.0 | 88.1 ± 0.9 | 0.348 |
| b'1Σu +(v' = 14) | 14.0594 | 113,399.1 | 89.0 ± 1.0 | 0.377 | 0.352 | 14.0767 | 113,539.2 | 94.3 ± 1.9 | 0.373 |
| b1Πu (v' = 18) | 14.0778 | 113,547.9 | 56.9 ± 0.8 | 0.387 | 0.361 | 14.0975 | 113,707.0 | 78.6 ± 0.9 | 0.384 |
| b'1Σu +(v' = 15) | 14.1367 | 114,022.8 | 93.1 ± 0.7 | 0.417 | 0.389 | 14.1549 | 114,169.6 | 83.8 ± 1.0 | 0.412 |
| b1Πu (v' = 19) | 14.1458 | 114,096.6 | 0.4 ± 0.4 | 0.422 | 0.394 | 14.1655 | 114,255.0 | 10.4 ± 2.3 | 0.418 |
| b'1Σu +(v' = 16) | 14.2109 | 114,621.0 | 90.0 ± 0.8 | 0.456 | 0.425 | 14.2274 | 114,754.2 | 1.3 ± 0.1 | 0.449 |
| c4'1Σu +(v' = 5) | 14.2154 | 114,657.5 | 74.7 ± 2.2 | 0.458 | 0.427 | 14.2371 | 114,833.0 e | 22.6 ± 2.4 f | 0.454 |
| o3 1Πu (v' = 5) | 14.2719 | 115,113.1 | 33.2 ± 0.4 | 0.487 | 0.455 | 14.2899 | 115,258.8 e | 13.4 ± 0.7 | 0.480 |
| b'1Σu +(v' = 17) | 14.2847 | 115,216.8 | 96.6 ± 0.3 | 0.494 | 0.461 | 14.3036 | 115,369.3 | 46.8 ± 0.7 | 0.487 |
| c4 1Πu (v' = 0) | 14.3282 | 115,567.1 | 5.6 ± 0.6 | 0.516 | 0.482 | 14.3280 | 115,565.8 | 1.7 ± 2.1 | 0.499 |
| c5'1Σu +(v' = 0) | 14.3488 | 115,733.4 | 93.0 ± 0.2 | 0.527 | 0.492 | 14.3632 | 115,849.8 | 63.3 ± 0.2 | 0.517 |
| b'1Σu +(v' = 18) | 14.3937 | 116,095.6 | 61.3 ± 2.2 | 0.550 | 0.514 | 14.4074 | 116,206.6 | 79.3 ± 2.8 | 0.539 |
| b'1Σu +(v' = 19) | 14.4453 | 116,511.6 | 96.6 ± 1.7 | 0.577 | 0.538 | 14.4664 | 116,682.3 | 94.7 ± 0.4 | 0.568 |
| c4'1Σu +(v' = 6) | 14.4618 | 116,645.0 | 87.9 ± 4.0 | 0.585 | 0.546 | 14.4819 | 116,806.8 | 27.5 ± 2.8 | 0.576 |
| b'1Σu +(v' = 20) | 14.5105 | 117,037.8 | 73.2 ± 1.4 | 0.611 | 0.570 | 14.5312 | 117,204.6 | 92.5 ± 0.5 | 0.601 |
Notes.
a For each absorption band, the value measured at the R(0) transition line is used for representing the branching ratio of the whole band. b Band origins (νo) of 14N2 are from the Harvard CfA N2 database: https://lweb.cfa.harvard.edu/amp/ampdata/N2ARCHIVE/n2term.html. c The branching ratios for 14N2 are adopted from Song et al. (2016). d The statistical uncertainties are the standard deviations of three to five independent measurements. e Band origins (νo) of 14N2 are from Heays et al. (2009). f For the absorption band (v' = 5) of 14N2, the branching ratio measured at the P(1) transition is used as adopted from Song et al. (2016).
(v' = 5) of 14N2, the branching ratio measured at the P(1) transition is used as adopted from Song et al. (2016).Download table as: ASCIITypeset image
3.2. Isotope-dependent Photodissociation Mechanisms
The isotope-dependent photodissociation mechanism of N2 has been the subject of numerous experimental and theoretical studies, due to its prime importance in understanding the N isotope fractionation process among different solar system objects and planetary atmospheres (Lewis et al. 2005; Liang et al. 2007; Muskatel et al. 2011; Chakraborty et al. 2014; Heays et al. 2014; Ajay et al. 2018). Isotope substitution has been shown to alter the photoabsorption peak positions, oscillator strengths, and photodissociation rates of 14N2, 14N15N, and 15N2 (Lewis et al. 2005; Muskatel et al. 2011), while how the branching ratios into different dissociation channels of 14N15N and 15N2 are different from those of 14N2 has never been studied before. Our previous studies have shown that for CO, which is isoelectronic to N2 (Lefebvre-Brion & Lewis 2007), isotope substitution strongly alters its photodissociation branching ratios (Jiang et al. 2019; Guan et al. 2020; Chi et al. 2020a, 2020b). To demonstrate the strong isotope effect of the photodissociation branching ratios for N2, we present the VMI images and their corresponding TKER spectra for the
b
'1
 (ν' = 9) and
b
'1
(ν' = 9) and
b
'1
 (ν' = 16) vibronic states of 14N2 in Figures 1(a), (b) and (c), (d), respectively. For the
b
'1
(ν' = 16) vibronic states of 14N2 in Figures 1(a), (b) and (c), (d), respectively. For the
b
'1
 (ν' = 9) state, a previous study by Song et al. (2016) observed substantial photodissociation into the channel N(4S)+N(2P) (∼30%) for 14N2. Our own data as shown in Figures 1(a) and (b) are consistent with Song et al. (2016). For 14N15N, the branching ratio into the channel N(4S)+N(2P) is much smaller (∼6%), as shown in Figures 1(e) and (f). For the
b
'1
(ν' = 9) state, a previous study by Song et al. (2016) observed substantial photodissociation into the channel N(4S)+N(2P) (∼30%) for 14N2. Our own data as shown in Figures 1(a) and (b) are consistent with Song et al. (2016). For 14N15N, the branching ratio into the channel N(4S)+N(2P) is much smaller (∼6%), as shown in Figures 1(e) and (f). For the
b
'1
 (ν' = 16) state, the branching ratio of the main isotopologue 14N2 into the channel N(4S)+N(2P) measured in the present study is ∼2% as shown in Figures 1(c) and (d), which is in accordance with the study of Song et al. (2016); in the case of 14N15N, the channel N(4S)+N(2P) completely dominates the photodissociation process with a branching ratio of ∼90%, as shown in Figures 1(g) and (h). Thus, a strong and quantum state selective isotope effect on the photodissociation branching ratio similar to that of CO has been observed for N2 for the first time.
(ν' = 16) state, the branching ratio of the main isotopologue 14N2 into the channel N(4S)+N(2P) measured in the present study is ∼2% as shown in Figures 1(c) and (d), which is in accordance with the study of Song et al. (2016); in the case of 14N15N, the channel N(4S)+N(2P) completely dominates the photodissociation process with a branching ratio of ∼90%, as shown in Figures 1(g) and (h). Thus, a strong and quantum state selective isotope effect on the photodissociation branching ratio similar to that of CO has been observed for N2 for the first time.
To compare how differently the photodissociation branching ratio depends on the VUV photon energy (or quantum state) between 14N2 and 14N15N, the branching ratios of the channel N(4S)+N(2P) for 14N2 as obtained by Song et al. (2016) are also plotted in Figure 2 in blue. Similar to CO (Jiang et al. 2019; Guan et al. 2020; Chi et al. 2020a, 2020b), isotope substitution changes the absolute values of the branching ratios for many common upper states of 14N2 and 14N15N, as shown in Figures 1 and 2, and in Table 1. Isotope substitution shifts the relative energy positions of the mutually interacting quantum states, and thus can change the coupling strengths between the directly excited singlet states and the 3 Π u states correlating to each of the dissociation limits. This finally determines the relative quantum yields for each of the channels (Muskatel et al. 2011; Jiang et al. 2019).
Despite the changes of the absolute values of branching ratios due to isotope substitution, the dependences of the branching ratios on the VUV photoexcitation energy for 14N2 and 14N15N show many similarities below the threshold of the channel N(2D)+N(2D), as shown in Figure 2. For 14N2, although the asymptotic limit of the channel N(4S)+N(2P) is located at ∼107,510 cm−1, prominent dissociation into this channel does not occur until a distinct onset at ∼112,000 cm−1 is reached. This is caused by the potential barrier in the adiabatic
C
'3
Π
u
state correlating to the N(4S)+N(2P) channel, which is formed through an avoided crossing with the higher III3
Π
u
state (Walter et al. 1993; Song et al. 2016). Below this onset, only the
b
'1
 (ν' = 9) state at ∼110,200 cm−1 dissociates substantially into the channel N(4S)+N(2P). This has been attributed to the sudden crossing between the
C
'3
Π
u
state and the 25
Π
u
state, which is a repulsive quintet state correlating to the N(4S)+N(2P) channel (Song et al. 2016). For 14N15N, the onset at ∼112,000 cm−1 has also been observed as shown by the red curves in Figure 2; and also, only the
b
'1
(ν' = 9) state at ∼110,200 cm−1 dissociates substantially into the channel N(4S)+N(2P). This has been attributed to the sudden crossing between the
C
'3
Π
u
state and the 25
Π
u
state, which is a repulsive quintet state correlating to the N(4S)+N(2P) channel (Song et al. 2016). For 14N15N, the onset at ∼112,000 cm−1 has also been observed as shown by the red curves in Figure 2; and also, only the
b
'1
 (ν' = 9) state dissociates substantially into the channel N(4S)+N(2P) below the onset with a branching ratio of ∼6%, which is much smaller than that of 14N2 (∼30%). This might indicate that the coupling strength of the
b
'1
(ν' = 9) state dissociates substantially into the channel N(4S)+N(2P) below the onset with a branching ratio of ∼6%, which is much smaller than that of 14N2 (∼30%). This might indicate that the coupling strength of the
b
'1
 (ν' = 9) level to the
C
'3
Π
u
state, and finally to the 25
Π
u
state, is much weaker for 14N15N than that for 14N2.
(ν' = 9) level to the
C
'3
Π
u
state, and finally to the 25
Π
u
state, is much weaker for 14N15N than that for 14N2.
The most prominent difference between 14N2 and 14N15N occurs at photon energies above the aforementioned onset. For 14N2, previous studies by Song et al. (2016) and Helm & Cosby (1989; Walter et al. 1993) revealed two peaks centered at ∼113,550 and ∼116,700 cm−1, where formation of the channel N(4S)+N(2P) dominates the photodissociation process, as shown by the blue curves in Figures 2(a) and (b). In between the two peaks is a prominent valley centered at ∼114,800 cm−1, where the 14N2 molecules predominantly dissociate into the N(4S)+N(2D) channel. For 14N15N, the N(4S)+N(2P) channel dominates the photodissociation process from the aforementioned onset all the way up to the threshold of the N(2D)+N(2D) channel, and no obvious valley structure can be noticed, especially for the valence and Rydberg  states, as shown by the red curves in Figures 2(a) and (b). This may imply very different photodissociation mechanisms between 14N2 and 14N15N in this energy range.
states, as shown by the red curves in Figures 2(a) and (b). This may imply very different photodissociation mechanisms between 14N2 and 14N15N in this energy range.
Since no singlet states correlate with N(4S)+N(2D) and N(4S)+N(2P), the branching ratios into the above two channels are mainly determined by the potential curves of valence 3
Π
u
states (Walter et al. 1993; Lewis et al. 2005; Song et al. 2016). The peak at ∼113,550 cm−1 is caused by the avoided crossing between the
C
'3
Π
u
and III3
Π
u
states, which has been mentioned above (Walter et al. 1993; Song et al. 2016). The mechanism for the peak at ∼116,700 cm−1 is still not clear. Song et al. ascribed this to the higher excitation efficiency to the Rydberg
c
'1
 state in this energy range, which subsequently couples to the
C
'3
Π
u
state to form the N(4S)+N(2P) channel (Song et al. 2016). However, the coupling between the
c
''1
state in this energy range, which subsequently couples to the
C
'3
Π
u
state to form the N(4S)+N(2P) channel (Song et al. 2016). However, the coupling between the
c
''1
 and
C
'3
Π
u
state should mainly occur in the Franck–Condon region (inner part of the
C
'3
Π
u
potential curve), then the avoided crossing between the
C
'3
Π
u
state and the lower
C
3
Π
u
state will be reached first, which leads N2 to the N(4S)+N(2D) channel (Lewis et al. 2005), before it can reach the avoided crossing between the
C
'3
Π
u
and III3
Π
u
states at a larger internuclear distance. Here, we propose a more plausible mechanism, which relies on the coupling to the III3
Π
u
state. The III3
Π
u
state has an equilibrium bond length close to that of the
b
'1
and
C
'3
Π
u
state should mainly occur in the Franck–Condon region (inner part of the
C
'3
Π
u
potential curve), then the avoided crossing between the
C
'3
Π
u
state and the lower
C
3
Π
u
state will be reached first, which leads N2 to the N(4S)+N(2D) channel (Lewis et al. 2005), before it can reach the avoided crossing between the
C
'3
Π
u
and III3
Π
u
states at a larger internuclear distance. Here, we propose a more plausible mechanism, which relies on the coupling to the III3
Π
u
state. The III3
Π
u
state has an equilibrium bond length close to that of the
b
'1
 state (Guberman 2012; Song et al. 2016), and thus the vibrational integrals between them may be large at the high vibrational levels of the
b
'1
state (Guberman 2012; Song et al. 2016), and thus the vibrational integrals between them may be large at the high vibrational levels of the
b
'1
 state, and the III3
Π
u
state could couple with the
C
'3
Π
u
state through their avoided crossing to go into the N(4S)+N(2P) channel. The highest barrier in the III3
Π
u
state caused by the avoided crossing with a higher 3
Π
u
state locates in the range between ∼115,800 and ∼117,000 cm−1, which is coincident with the peak position at ∼116,700 cm−1 (Guberman 2012; Song et al. 2016). This process has also been previously pointed out by Helm et al. as a possible mechanism for the sudden increase of the N(2P) quantum yield at ∼115,500 cm−1 (Helm & Cosby 1989).
state, and the III3
Π
u
state could couple with the
C
'3
Π
u
state through their avoided crossing to go into the N(4S)+N(2P) channel. The highest barrier in the III3
Π
u
state caused by the avoided crossing with a higher 3
Π
u
state locates in the range between ∼115,800 and ∼117,000 cm−1, which is coincident with the peak position at ∼116,700 cm−1 (Guberman 2012; Song et al. 2016). This process has also been previously pointed out by Helm et al. as a possible mechanism for the sudden increase of the N(2P) quantum yield at ∼115,500 cm−1 (Helm & Cosby 1989).
Since both 14N2 and 14N15N photodissociate predominantly into the N(4S)+N(2P) channel in the two peak areas of 14N2 (Figure 2(a)), they should share similar mechanisms as discussed above. However, in contrast to 14N2 with deep valley (dominated by N(4S)+N(2D) channel) at ∼114,800 cm−1, 14N15N still photodissociates predominantly into the N(4S)+N(2P) channel between the two peaks. This cannot be explained by the avoided crossings among the various 3
Π
u
states. One possible reason for this could be due to the crossing of the 25
Π
u
state with the III3
Π
u
state, which should locate in between the two peak positions (see Figure 4 in Song et al. 2016), leading the 14N15N molecules in the
b
'1
 (ν' = 16, 17) levels to predominantly dissociate into the N(4S)+N(2P) channel. This mechanism can be strongly isotope dependent, similar to the case of the
b
'1
(ν' = 16, 17) levels to predominantly dissociate into the N(4S)+N(2P) channel. This mechanism can be strongly isotope dependent, similar to the case of the
b
'1
 (ν' = 9) level as discussed above. Detailed theoretical calculations on the accurate potential curve of the III3
Π
u
state and the couplings with nearby states are needed to quantitatively explain all the similarities and dissimilarities between 14N2 and 14N15N as observed in the current study.
(ν' = 9) level as discussed above. Detailed theoretical calculations on the accurate potential curve of the III3
Π
u
state and the couplings with nearby states are needed to quantitatively explain all the similarities and dissimilarities between 14N2 and 14N15N as observed in the current study.
3.3. Implications for N Isotope Fractionation in Titan's Atmosphere
Proper considerations of all the N isotope fractionation mechanisms are crucial for reconstructing the average initial 14N/15N ratio of Titan's building blocks, and thus for determining the specific reservoir(s) out of which Titan's N2 originated. In this study, we have systematically measured the branching ratios into the channels of producing N(2D) and N(2P) for 14N15N, below the energy threshold of the N(2D)+N(2D) channel. The most important finding is that a strong and state-selective isotope effect on photodissociation branching ratios exists between 14N2 and 14N15N, and most of the absorption states of 14N15N above the onset at ∼112,000 cm−1 predominantly dissociate into the N(4S)+N(2P) channel, especially for the valence and Rydberg  states, as shown in Figures 2(a) and (b). Since N(2P) is 1.192 eV higher in internal energy than N(2D), the N atoms generated from the N(4S)+N(2P) channel have much lower kinetic energies than that of the N(4S)+N(2D) channel. The finding in this study can have a profound effect on the VUV photodissociation induced N escape process in the upper atmosphere of Titan.
states, as shown in Figures 2(a) and (b). Since N(2P) is 1.192 eV higher in internal energy than N(2D), the N atoms generated from the N(4S)+N(2P) channel have much lower kinetic energies than that of the N(4S)+N(2D) channel. The finding in this study can have a profound effect on the VUV photodissociation induced N escape process in the upper atmosphere of Titan.
We have calculated the kinetic energies of 14N and 15N from the N(4S)+N(2P) channel for the absorption states of both 14N2 and 14N15N, as listed in Table 1. The escape energy of 14N at Titan's exobase (above which the collision effect is negligible, thus atoms and molecules can escape if they have enough kinetic energies) is ∼0.314 eV, and that for 15N is ∼0.336 eV (Lammer et al. 2000). As seen in Table 1, the kinetic energies of 14N and 15N are in the range of 0.15–0.62 eV, which is close to the escape energy to have a significant impact on the N fractionation process in Titan's atmosphere (Lammer et al. 2000). The absorption states in Table 1 can be divided into three categories based on their kinetic energy releases.
The first category refers to absorption states that produce both 14N and 15N atoms with kinetic energies lower than their corresponding escape energies for the N(4S)+N(2P) channel, while all N atoms generated in the N(4S)+N(2D) channel have enough kinetic energies to escape from Titan. Therefore, which isotope is enriched is determined by the relative branching ratios of the N(4S)+N(2P) channel between 14N2 and 14N15N. The following three states,
b
'1
 (v' = 9),
(v' = 9),  (v' = 3), and
b
'1
(v' = 3), and
b
'1
 (v' = 12) belong to this category. The branching ratios into the N(4S)+N(2P) channel of the above three states for 14N15N are 5.7%, 3.8%, and 16.2%, respectively; while those for 14N2 are 30.7%, 5.7%, and 21.0%, respectively, which are all larger than the corresponding values of 14N15N, as shown in Table 1. This means all these three states preferentially retain 14N in Titan's atmosphere, because 14N2 generates proportionally more 14N atoms in the N(4S)+N(2P) channel, which stay in Titan's atmosphere, than 14N15N does, if the same photodissociation cross sections are assumed for the two N2 isotopologues.
(v' = 12) belong to this category. The branching ratios into the N(4S)+N(2P) channel of the above three states for 14N15N are 5.7%, 3.8%, and 16.2%, respectively; while those for 14N2 are 30.7%, 5.7%, and 21.0%, respectively, which are all larger than the corresponding values of 14N15N, as shown in Table 1. This means all these three states preferentially retain 14N in Titan's atmosphere, because 14N2 generates proportionally more 14N atoms in the N(4S)+N(2P) channel, which stay in Titan's atmosphere, than 14N15N does, if the same photodissociation cross sections are assumed for the two N2 isotopologues.
The second category refers to all absorption states that produce 14N atoms with enough kinetic energies to escape from Titan's exobase, while producing 15N atoms without enough kinetic energies to escape, for the N(4S)+N(2P) channel. The absorption states  (v' = 4),
b
'1
(v' = 4),
b
'1
 (v' = 13), and
b
1
Π
u
(v' = 17) belong to this category. If 14N15N dissociates into the N(4S)+N(2P) channel, they produce 14N atoms with kinetic energies of 0.329 eV, 0.337 eV, and 0.350 eV, respectively, all higher than the escape energy of 0.314 eV for 14N, and thus can escape from Titan's exobase; on the other hand, the corresponding kinetic energies of 15N atoms are 0.308 eV, 0.314 eV, and 0.326 eV, respectively, all lower than the escape energy of 0.336 eV for 15N, and thus cannot escape from Titan's exobase. The 14N atoms generated from the above three states of 14N2 in the N(4S)+N(2P) channel all have enough kinetic energies to escape. This is similar to the dissociative recombination process of the 14N15N+ ions in Mars' atmosphere (Fox & Hać 1997), thus the above three states will enrich the heavier 15N isotope in the atmosphere of Titan.
(v' = 13), and
b
1
Π
u
(v' = 17) belong to this category. If 14N15N dissociates into the N(4S)+N(2P) channel, they produce 14N atoms with kinetic energies of 0.329 eV, 0.337 eV, and 0.350 eV, respectively, all higher than the escape energy of 0.314 eV for 14N, and thus can escape from Titan's exobase; on the other hand, the corresponding kinetic energies of 15N atoms are 0.308 eV, 0.314 eV, and 0.326 eV, respectively, all lower than the escape energy of 0.336 eV for 15N, and thus cannot escape from Titan's exobase. The 14N atoms generated from the above three states of 14N2 in the N(4S)+N(2P) channel all have enough kinetic energies to escape. This is similar to the dissociative recombination process of the 14N15N+ ions in Mars' atmosphere (Fox & Hać 1997), thus the above three states will enrich the heavier 15N isotope in the atmosphere of Titan.
The third category refers to all absorption states that produce both 14N and 15N atoms with high enough kinetic energies to escape from Titan's exobase. All the rest of the states listed in Table 1 belong to this category, except for the six states discussed above. As shown in Table 1, the initial kinetic energy obtained by the 15N isotope is always slightly smaller than that obtained by 14N, and furthermore the 15N isotope has a higher escape energy than that of 14N, thus all the absorption states of the third category prefer the enrichment of the heavier 15N isotopes, consistent with Lammer et al. (2000). Lammer et al. (Lammer et al. 2000) further argued that 15N isotopes need fewer collisions to go below the corresponding escape energy than 14N does, and thus has a larger chance to stay in Titan. This is because the kinetic energy of N atoms from photodissociation is higher than the average kinetic energy of Brownian motion of Titan's atmospheric molecules; therefore, collisions between the two would tend to reduce the kinetic energy of the N fragments of photodissociation.
It is obvious that the enrichment of 15N would be further enhanced if 14N15N has a larger branching ratio into the N(4S)+N(2P) channel than 14N2 does, because the N(4S)+N(2P) channel has much smaller kinetic energy release than that of N(4S)+N(2D); however, if 14N15N has a smaller branching ratio into the N(4S)+N(2P) channel than 14N2 does, the 15N enrichment effect would be diminished or even reversed, that is, the 14N is enriched. Which isotope is enriched is determined by the relative photodissociation cross sections and branching ratios between 14N2 and 14N15N.
The atomic N escape induced by the solar VUV photodissociation of N2 is an important process in the upper atmosphere of Titan (Shematovich 1999; Shematovich et al. 2003). To the best of our knowledge, the potentially important N isotope fractionation effect corresponding to photodissociation with associated kinetic energies and branching ratios into N(4S)+N(2P) has not been included yet in any models for predicting the initial 14N/15N ratio of Titan's atmosphere. The above N isotope fractionation mechanism does not rely on the presence of CH4 in Titan's atmosphere, as the photochemical effect did (Erkaev et al. 2020), thus they can fractionate the N isotopes continuously for the entire lifetime of Titan's atmosphere. More importantly, the solar VUV photon flux was up to several hundred times the present value in the early solar system (Mandt et al. 2009; Tu et al. 2015; Erkaev et al. 2020), thus the photodissociation rate of N2 should be much higher, consequently the fractionation mechanism as found in this study could be more efficient in the early history of Titan than that at the present, if the exobase level remained unchanged during the Titan's lifetime. Future modeling work would need to consider such an effect and its contribution to the 14N/15N evolution in Titan's atmosphere. The exobase level could be higher in the past due to the higher solar flux (Erkaev et al. 2020), which could decrease the escape velocities of 14N and 15N, and thus will diminish the fractionation effect as found in this study. Such an effect should also be considered in future modeling work.
Besides the VUV energy range as described in the current study, there are two other ranges that could also contribute to the above N isotope fractionation mechanism. The first one is at ∼103,000 cm−1, where only the N(4S)+N(2D) channel is available. There is not enough energy to dissociate into the N(4S)+N(2P) channel at 103,000 cm−1, as the threshold of this channel is at ∼107,500 cm−1. The absorption states in this energy range also produce N atoms with kinetic energies close to the escape energy. For example, the band origins of the b 1 Π u (v' = 3, 4) states of 14N15N were measured by Heays et al. to be at 102,841 cm−1 (12.750 eV) and 10,3517 cm−1 (12.834 eV), respectively (Heays et al. 2011); they dissociate to produce the 14N isotope with high enough kinetic energy to escape from Titan's exobase, while the 15N isotope does not get enough energy to escape. They are similar to the states of the second category as discussed above. The second one is the energy range higher than the threshold of the channel N(2D)+N(2D). Song et al. have shown that 14N2 can still dissociate substantially into the channels N(4S)+N(2D) and N(4S)+N(2P) above the energy threshold of the channel N(2D)+N(2D), which produce N atoms with high enough kinetic energy to escape from Titan (Song et al. 2016); however, when N2 dissociates into the channel N(2D)+N(2D), the N atoms produced will not get enough energy to escape. If the photodissociation branching ratios into the N(2D)+N(2D) channel are also isotope dependent in the high energy range, then it can also contribute significantly to the N isotope fractionation in Titan's atmosphere.
4. Summary
In summary, we have systematically measured the branching ratios into the two channels N(4S)+N(2D) and N(4S)+N(2P) for 14N15N in the energy range below N(2D)+N(2D). A strong and state selective isotope effect on the branching ratios for 14N2 and 14N15N has been observed. New N isotope fractionation mechanisms related to this isotope effect have been identified for the first time, which may provide additional constraints on the origin and evolution history of Titan's thick N2-dominated atmosphere. Besides Titan, our findings may also find important applications in predicting the origins and evolution histories of the tenuous N2 atmospheres of Pluto and Triton (Scherf et al. 2020).
This work is supported by the National Natural Science Foundation of China (grant No. 21973100), the Program for Young Outstanding Scientists of Institute of Chemistry, Chinese Academy of Science (ICCAS), and Beijing National Laboratory for Molecular Sciences (BNLMS). H.G. is also supported by the K. C. Wong Education Foundation. Pan Jiang is supported by the China Postdoctoral Science Foundation.
Appendix
A full list of all the branching ratio measurements in this study can be found in Tables A1 and A2.
Table A1. Photodissociation Branching Ratios into the Channel N(4S)+N(2P) for 1Σu + States of 14N15N Measured in the Current Study
| Term | ν' | J'' | VUV (cm−1) | N(4S)+N(2P) (%) a |
|---|---|---|---|---|
b
'(1
 ) ) | ν' = 6 | P(2) | 107,927.7 | 0.00 |
| P(1) | 107,933.2 | 0.00 | ||
| R(0,2) | 107,939.3 | 0.00 | ||
| R (1) | 107,940.1 | 0.00 | ||

| ν' = 2 | R(0) | 108,472.4 | 0.00 |
| R (1) | 108,474.0 | 0.00 | ||
| ν' = 7 | R(0, 3) | 108,880.4 | 0.00 |
| R(1,2) | 108,881.7 | 0.00 | ||
| ν' = 8 | R (3) | 109,464.1 | 0.66 ± 0.05 |
| R (2) | 109,466.8 | 0.71 ± 0.14 | ||
| R(0) | 109,467.3 | 0.59 ± 0.08 | ||
| R (1) | 109,467.9 | 0.59 ± 0.02 | ||

| ν' = 9 | P(2) | 110,101.6 | 5.63 ± 0.23 |
| P(1) | 110,107.0 | 5.03 ± 0.12 | ||
| R (3) | 110,109.6 | 4.73 ± 0.15 | ||
| R (2) | 110,112.5 | 5.83 ± 0.15 | ||
| R(0) | 110,113.2 | 5.68 ± 0.22 | ||
| R (1) | 110,113.8 | 5.43 ± 0.55 | ||

| ν' = 3 | P(2) | 110,557.4 | 2.97 ± 0.50 |
| P(1) | 110,562.0 | 3.67 ± 0.64 | ||
| R(0) | 110,568.9 | 3.83 ± 0.58 | ||
| R (1) | 110,571.3 | 3.27 ± 0.42 | ||
| R (2) | 110,573.1 | 2.77 ± 0.67 | ||
| ν' = 10 | P(1) | 110,827.1 | 0.83 ± 0.25 |
| R(0,2) | 110,833.4 | 0.60 ± 0.03 | ||
| R (1) | 110,834.2 | 0.55 ± 0.05 | ||

| ν' = 11 | P(1) | 111,462.3 | 0.28 ± 0.02 |
| R (3) | 111,464.4 | 0.32 ± 0.07 | ||
| R (2) | 111,467.5 | 0.29 ± 0.03 | ||
| R(0) | 111,468.4 | 0.31 ± 0.01 | ||
| R (1) | 111,468.8 | 0.26 ± 0.04 | ||

| ν' = 12 | P(2) | 112,107.2 | 16.87 ± 0.65 |
| P(1) | 112,112.9 | 16.93 ± 0.80 | ||
| R (3) | 112,114.4 | 17.97 ± 0.45 | ||
| R (2) | 112,117.8 | 16.93 ± 0.57 | ||
| R(0,1) | 112,119.0 | 16.20 ± 0.87 | ||

| ν' = 4 | P(2) | 112645.9 | 26.13 ± 0.21 |
| P(1) | 112,650.4 | 27.47 ± 0.84 | ||
| R(0) | 112,657.4 | 25.83 ± 0.21 | ||
| R (1) | 112,660.2 | 24.33 ± 0.46 | ||
| R (2) | 112,662.1 | 22.50 ± 0.79 | ||

| ν' = 13 | P(4) | 112,742.6 | 96.23 ± 0.15 |
| P(3) | 112,751.3 | 96.27 ± 0.21 | ||
| P(2) | 112,758.4 | 96.17 ± 0.12 | ||
| R (3) | 112,766.8 | 95.63 ± 0.38 | ||
| R (2) | 112,769.3 | 96.60 ± 0.17 | ||
| R(0) | 112,769.9 | 96.20 ± 0.98 | ||
| R (1) | 112,770.6 | 96.67 ± 0.12 | ||

| ν' = 14 | P(2) | 113,389.7 | 88.13 ± 0.35 |
| R (2) | 113,399.8 | 88.97 ± 0.35 | ||
| R(0,1) | 113,401.4 | 89.03 ± 1.00 | ||

| ν' = 15 | P(3) | 114,005.7 | 91.35 ± 0.35 |
| P(2) | 114,013.2 | 92.47 ± 0.91 | ||
| R(4) | 114,014.5 | 94.15 ± 0.92 | ||
| P(1) | 114,018.8 | 91.97 ± 0.91 | ||
| R (3) | 114,019.9 | 93.00 ± 0.50 | ||
| R (2) | 114,023.2 | 93.27 ± 0.55 | ||
| R(0,1) | 114,025.0 | 93.07 ± 0.65 | ||

| ν' = 16 | P(6) | 114,574.7 | 89.70 ± 1.84 |
| P(5) | 114,586.6 | 89.35 ± 3.04 | ||
| R(7) | 114,592.5 | 90.20 ± 0.57 | ||
| P(4) | 114,596.8 | 91.50 ± 0.14 | ||
| R(6) | 114,602.8 | 92.30 ± 0.14 | ||
| P(3) | 114,605.1 | 91.15 ± 0.35 | ||
| R(5) | 114,610.8 | 90.85 ± 0.35 | ||
| P(2) | 114,612.4 | 90.25 ± 2.81 | ||
| P(1)/R(4) | 114,617.6 | 91.30 ± 0.17 | ||
| R(3) | 114,621.8 | 90.47 ± 2.92 | ||
| R(0,2) | 114,623.8 | 90.03 ± 0.84 | ||
| R(1) | 114,624.6 | 92.40 ± 0.80 | ||
| ν' = 5 | P(5) | 114,630.4 | 54.47 ± 1.37 |
| P(4) | 114,637.4 | 55.83 ± 3.00 | ||
| P(3) | 114,643.5 | 65.00 ± 1.40 | ||
| P(2) | 114,649.0 | 72.87 ± 2.02 | ||
| R(0,7) | 114,660.5 | 74.73 ± 2.18 | ||
| R(1,6) | 114,662.7 | 71.07 ± 1.46 | ||
| R(2,5) | 114,664.1 | 52.20 ± 1.65 | ||
| R(3,4) | 114,665.0 | 23.53 ± 4.08 | ||

| ν' = 17 | P(4) | 115,190.8 | 96.57 ± 0.35 |
| P(3) | 115,200.2 | 96.53 ± 0.21 | ||
| P(2) | 115,207.3 | 96.53 ± 0.32 | ||
| R(4) | 115,209.7 | 97.30 ± 0.17 | ||
| P(1) | 115,212.9 | 96.17 ± 0.25 | ||
| R (3) | 115,214.6 | 97.23 ± 0.23 | ||
| R (2) | 115,217.9 | 96.70 ± 0.10 | ||
| R(0,1) | 115,219.2 | 96.60 ± 0.26 | ||
| ν' = 0 | P(5) | 115,700.2 | 93.60 ± 0.50 |
| P(4) | 115,709.7 | 93.37 ± 0.15 | ||
| P(3) | 115,717.6 | 93.34 ± 0.23 | ||
| P(2) | 115,724.5 | 93.50 ± 0.30 | ||
| P(1) | 115,729.6 | 93.63 ± 0.15 | ||
| R(4) | 115,731.4 | 93.27 ± 0.51 | ||
| R(3) | 115,734.7 | 93.77 ± 0.21 | ||
| R(0) | 115,735.9 | 92.97 ± 0.15 | ||
| R (1) | 115,736.6 | 93.37 ± 0.23 | ||
| R (2) | 115,737.0 | 92.90 ± 0.10 | ||

| ν' = 18 | P(2)/R(6) | 116,086.6 | 60.80 ± 0.17 |
| P(1)/R(5) | 116,091.5 | 62.43 ± 0.96 | ||
| R(0,3) | 116,098.1 | 61.27 ± 2.17 | ||
| R(1,2) | 116,099.5 | 60.57 ± 1.91 | ||

| ν' = 19 | R(0,2) | 116,513.7 | 96.57 ± 1.67 |
| R (1) | 116,514.5 | 97.97 ± 1.88 | ||

| ν' = 6 | P(1) | 116,641.0 | 87.70 ± 3.72 |
| R(0) | 116,648.3 | 87.87 ± 3.98 | ||
| R (1) | 116,650.8 | 87.33 ± 3.76 | ||
| R (2) | 116,653.1 | 86.57 ± 3.59 | ||

| ν' = 20 | P(1) | 117,033.9 | 73.50 ± 2.01 |
| R (3) | 117,035.4 | 64.70 ± 1.98 | ||
| R (2) | 117,038.6 | 65.57 ± 1.01 | ||
| R(0,1) | 117039.9 | 73.20 ± 1.37 |
Note.
a The statistical uncertainties are the standard deviations of three to five independent measurements.Table A2. Photodissociation Branching Ratios into the Channel N(4S)+N(2P) for 1Πu States of 14N15N Measured in the Current Study
| Term | ν' | J'' | VUV (cm−1) | N(4S)+N(2P) (%) a |
|---|---|---|---|---|
| b(1Πu ) | ν' = 9 | Q(2) | 107,540.7 | 0.00 |
| Q(1) | 107,543.5 | 0.00 | ||
| R(0) | 107,547.4 | 0.00 | ||
| R (1) | 107,548.4 | 0.00 | ||
| o3(1Πu ) | ν' = 1 | Q(1) | 107,606.4 | 0.00 |
| R(0) | 107,610.0 | 0.00 | ||
| R (1) | 107,612.8 | 0.00 | ||
| R (2) | 107,615.1 | 0.00 | ||
| R (3) | 107,616.9 | 0.00 | ||
| b(1Πu ) | ν' = 10 | Q(2) | 108,258.1 | 0.00 |
| Q(1) | 108,261.1 | 0.00 | ||
| R(0,2) | 108,265.0 | 0.00 | ||
| R (1) | 108,265.8 | 0.00 | ||
| b(1Πu ) | ν' = 11 | Q(2) | 108,992.9 | 0.00 |
| Q(1) | 108,995.9 | 0.00 | ||
| R (3) | 108,997.6 | 0.00 | ||
| R (2) | 108,999.7 | 0.00 | ||
| R(0) | 109,000.4 | 0.00 | ||
| R (1) | 109,000.6 | 0.00 | ||
| o3(1Πu ) | ν' = 2 | Q(2) | 109,502.9 | 0.50 ± 0.00 |
| Q(1) | 109,503.9 | 1.13 ± 0.12 | ||
| R(0) | 109,507.7 | 1.20 ± 0.10 | ||
| R (1) | 109,510.5 | 0.57 ± 0.09 | ||
| R (2) | 109,512.6 | 0.25 ± 0.06 | ||
| b(1Πu ) | ν' = 12 | Q(2)/R(4) | 109,695.3 | 0.04 ± 0.06 |
| Q(1) | 109,698.6 | 0.07 ± 0.06 | ||
| R(0) | 109,702.4 | 0.10 ± 0.04 | ||
| R (1) | 109,703.0 | 0.00 | ||
| b(1Πu ) | ν' = 13 | Q(2) | 110,384.7 | 0.00 |
| Q(1) | 110,387.9 | 0.00 | ||
| R (2) | 110,391.2 | 0.00 | ||
| R(0) | 110,391.8 | 0.00 | ||
| R (1) | 110,392.2 | 0.00 | ||
| b(1Πu ) | ν' = 14 | Q(4) | 111,038.1 | 0.00 |
| P(3) | 111,042.3 | 0.00 | ||
| R(4)/Q(3) | 111,047.0 | 0.00 | ||
| R (3)/Q(2) | 111,053.3 | 0.00 | ||
| R (2)/Q(1) | 111,058.5 | 0.00 | ||
| R(0,1) | 111,062.0 | 0.00 | ||
| o3(1Πu ) | ν' = 3 | Q(2) | 111,358.4 | 0.00 |
| Q(1) | 111,359.4 | 0.00 | ||
| R(0) | 111,363.2 | 0.00 | ||
| R (1) | 111,365.9 | 0.00 | ||
| R(2) | 111,368.1 | 0.00 | ||
| R (3) | 111,369.8 | 0.00 | ||
| b(1Πu ) | ν' = 15 | Q(3) | 111,709.4 | 0.07 ± 0.12 |
| Q(2) | 111,714.8 | 0.16 ± 0.04 | ||
| Q(1) | 111,718.3 | 0.16 ± 0.04 | ||
| R(0,1) | 111,722.5 | 0.16 ± 0.02 | ||
| b(1Πu ) | ν' = 16 | Q(2) | 112,346.2 | 0.00 |
| Q(1) | 112,350.0 | 0.37 ± 0.29 | ||
| R (2) | 112,352.3 | 0.00 | ||
| R(0,1) | 112,354.0 | 0.15 ± 0.17 | ||
| b(1Πu ) | ν' = 17 | Q(2) | 112,963.9 | 80.07 ± 4.92 |
| R (3) | 112,966.2 | 83.85 ± 1.91 | ||
| Q(1) | 112,967.6 | 83.40 ± 0.17 | ||
| R (2) | 112,969.8 | 83.57 ± 1.17 | ||
| R(0,1) | 112,971.5 | 83.90 ± 0.62 | ||
| o3(1Πu ) | ν' = 4 | Q(2) | 113,184.1 | 0.56 ± 0.20 |
| Q(1) | 113,185.0 | 0.38 ± 0.11 | ||
| R(0) | 113,189.0 | 0.36 ± 0.03 | ||
| R (1) | 113,191.7 | 0.38 ± 0.03 | ||
| R (2) | 113,194.1 | 0.60 ± 0.00 | ||
| b(1Πu ) | ν' = 18 | Q(1) | 113,546.0 | 55.80 ± 2.70 |
| R (2) | 113,547.8 | 56.13 ± 0.51 | ||
| R(0,1) | 113,549.6 | 56.93 ± 0.76 | ||
| b(1Πu ) | ν' = 19 | R(0,1) | 114098.2 | 0.43 ± 0.40 |
| c3(1Πu ) | ν' = 5 | tentative b | 114,693.4 | 3.33 ± 0.75 |
| o3(1Πu ) | ν' = 5 | Q(2) | 115,107.7 | 33.73 ± 1.37 |
| R (3) | 115,110.3 | 33.83 ± 0.25 | ||
| Q(1) | 115,111.5 | 32.43 ± 0.12 | ||
| R (2) | 115,113.6 | 32.63 ± 1.12 | ||
| R(0,1) | 115,115.4 | 33.17 ± 0.35 | ||
| c4(1Πu ) | ν' = 0 | P(2) | 115,559.3 | 6.23 ± 0.42 |
| Q(1,2) | 115,567.3 | 5.03 ± 0.06 | ||
| R(0) | 115,570.9 | 5.63 ± 0.59 | ||
| R (1) | 115,574.5 | 10.07 ± 0.12 | ||
| R (2) | 115,577.7 | 16.20 ± 1.91 | ||
| R (3) | 115,580.4 | 7.70 ± 2.00 |
Notes.
a The statistical uncertainties are the standard deviations of three to five independent measurements. b The signal is weak for this absorption band, and the assignment is only tentative.