Abstract
We use a thermodynamic statistical model to evaluate how the composition of Europa's internal ocean may have been affected by clathrate hydrate formation. Assuming an input of the observed O2 and CO2 from the surface into a mildly acidic ocean (pH < 6), and considering the possibility of contributions by reduced (with CH4 and H2S) or oxidized (CO2-bearing) hydrothermal fluids, we calculate the fractional occupancies in clathrate and deduce the effect on the ocean's composition. The structure of the clathrate formed, and therefore its density and composition is influenced by the relative amount of O2 compared to the other compounds present. We also include a mixture of noble gases—argon, krypton, and xenon—based on cometary abundances measured at comet 67P and find that the Ar/Kr ratio can be affected by almost two orders of magnitude. In most cases, the formed clathrate is likely to become part of the icy crust, with guest molecules possibly accessible to future in situ measurements by the Europa Clipper and JUICE missions.
Export citation and abstract BibTeX RIS
1. Introduction
Europa is one of the most promising bodies in the solar system for potentially finding life, because of the presence of a liquid water ocean (Khurana et al. 1998; Kivelson et al. 2000) in contact with the silicate interior. This ocean is likely to host a complex chemistry enabled by exchanges with the silicate interior (Kargel et al. 2000; McKinnon & Zolensky 2003; Vance et al. 2007) and with a surface processed by radiation (Hand et al. 2007; Paranicas et al. 2009; Greenberg 2010). This allows possible chemical disequilibria from which life could prosper without need for sunlight (Chyba 2000; Chyba & Hand 2001; Zolotov & Shock 2003, 2004). In preparation for the future JUICE and Europa Clipper missions, it is useful to quantify the different factors at play in regulating the composition of Europa's ocean.
One process potentially affecting the ocean's composition is the formation of clathrate hydrates (hereafter clathrates). Because the pressure and temperature conditions in Europa's ocean are favorable to clathrate formation and stability, these crystalline structures can alter the ocean's composition by preferentially trapping guests that have a greater ability to stabilize clathrate cages. Additionally, the presence of a large amount of clathrate is a potentially important factor in Europa's evolution: if the clathrate is dense enough to sink, it could cover the seafloor and inhibit water-rock interaction (Hand et al. 2006). Alternatively, if the clathrate is light enough to float, it could be incorporated into the icy crust and alter its thermal and mechanical properties, e.g., contributing to retaining Europa's heat (Kamata et al. 2019).
Here we use a thermodynamic statistical model to assess, in several scenarios, the composition of the mixture both trapped in clathrate and dissolved in Europa's ocean, as well as the resulting clathrate density. We consider species that are both susceptible to be enclathrated and to be added in large enough quantities into the ocean to induce clathrate formation: CO2, H2S, O2, and CH4. Both surface radiolytic processes and oxidized hydrothermal fluids can bring CO2 into the ocean (Kargel et al. 2000; Carlson et al. 2009), while reduced hydrothermal fluids could bring in H2S and CH4 (McCollom 1999; Hand et al. 2007). Molecular oxygen O2 is the most abundant clathrate-forming radiolytic product that may be able to reach the ocean from the surface (Hand et al. 2006). Small quantities of noble gases argon, krypton, and xenon are also included (in ratios consistent with cometary ice) to track the evolution of their relative abundances in the ocean. Our study differs from previous works on this topic (Prieto-Ballesteros et al. 2005; Hand et al. 2006) in that we calculate the clathrate fractional occupancies (and therefore density) and also consider the impact on noble gases.
2. Model and Assumptions
2.1. Clathrate Formation Model
Our model is based on the approach depicted in Mousis et al. (2013) to study the evolution of Lake Vostok on Earth. We refer the reader to that work for a complete description of our method. It has also been previously applied to Enceladus' ocean (Bouquet et al. 2015). The model starts with a user-defined mixture dissolved in a given volume of water, to which a small amount of a user-defined gaseous mixture is added. We calculate the time needed, with our input and starting from a volatile-poor ocean, for the dissolved gas to reach the solubility limit at which any added volume of gas triggers clathrate formation. This point is the initial step of the model. The enclathrated mixture will differ from the one added to the liquid because the guest molecule-water interactions in each cage vary as a function of the parameters describing the shape of the intermolecular potential. From the determination of the amount of each compound removed from water via this trapping, our model computes the evolution of the mixture dissolved in water. Eventually the system reaches a steady state where the mixture trapped in clathrate is the same as the input. Knowing the fractional occupancy of each species in the clathrate allows for the calculation of the clathrate's density (Hand et al. 2006; Sloan & Koh 2007).
We note that, e.g., an O2 fractional occupancy of 75% in large cages means that O2 is found in 75% of the occupied large cages of the clathrate, but how many of these cages are occupied, i.e., the filling rate, is not determined by our model and must be assumed. The volume of dissolved gas that leads to clathrate formation is calculated based on the dissociation pressure of a clathrate hosting one species ("pure" clathrate). We calculate how much of the mixture needs to be added before this species reaches a fugacity sufficient to cause formation of pure clathrate, and assume clathrate formation from this point. The species that first reaches the threshold for clathrate formation is hereafter called the driving species. To calculate this threshold, we assume an ocean 100 km deep, under a 10 km icy crust, which is within the observational constraints (Anderson et al. 1998), resulting in a 10 MPa hydrostatic pressure at the ice-water interface, and 100 MPa at the seafloor. This ocean receives a gas input discussed in Section 2.2.
Clathrate exist in several structures, the two most common in natural environments being structure I and structure II (hereafter sI and sII, respectively). The two structures contain large and small cages, with sII having both the smallest and largest cages. The clathrate structure that forms is a function of the guest molecule's properties and how well it can stabilize a given cage size. The formation of sII may be promoted both by very small guest molecules stabilizing the smallest cages, and bigger molecules that only fit in the sII large cages. Intermediate size guests may promote sI formation. While for a pure clathrate (only one type of guest) the structure is usually known experimentally, the situation becomes more complex with multiple guest clathrate, which is the case in this study. For example a pure CH4 gas would be normally trapped in sI clathrate, but adding a small amount of propane is enough to cause creation of a sII clathrate that traps both species. All this information on clathrate structure and additional discussion can be found in Sloan & Koh (2007). In this study we perform calculations and display results for both sI and sII formation and comment on which is more likely based on what is known of the guests' properties.
2.2. Input of Volatiles
We consider the addition of volatiles to the ocean from the icy crust and from putative hydrothermal activity. The latter can provide different volatiles depending on whether a reducing or oxidizing hydrothermal fluid is assumed. Since the state of oxidation of Europa's silicate interior is unknown, it is useful to consider both end members to determine how each could contribute to the composition of the ocean and clathrate formed.
Molecular oxygen. O2 is present on Europa's surface (Spencer & Calvin 2002) most likely the result of radiolytic processes, along with hydrogen peroxide H2O2 (Carlson et al. 1999a), which can decompose into more O2. Radiolytic products may be brought down into the ocean at a fast rate allowed by ridge building and melt-through processes, allowing for an influx of up to 3 × 1011 moles yr−1 of O2 (Greenberg 2010) to the ocean. We use this value throughout our study.
Carbon dioxide. CO2 has been detected on Europa's surface (Carlson et al. 2009). If delivery of surface O2 to the ocean is assumed, the same then applies to CO2. We quantify the delivery of CO2 from the surface to the ocean by using the numbers of Hand et al. (2006): we consider intermediate values of their estimates (O2/H2O = 3% and CO2/H2O = 0.655% in moles) along with the above value of 3 × 1011 moles yr−1 of O2 to obtain a CO2 flux of 6.55 × 1010 moles yr−1. In addition, CO2 may result from hydrothermal processes at Europa's seafloor. As an upper limit for a possible hydrothermal CO2 input, we consider numbers derived from Kargel et al. (2000), who drew an analogy to Io to estimate a delivery of SO2 and CO2 to the ocean of 6.9 × 1011 moles yr−1 each. We also consider a more modest input based on the approach of Hand et al. (2007), who scaled Earth's numbers for hydrothermal heat flux and fluid volume to Europa and used measured abundances of various compounds (including CO2 as well as H2S and CH4, discussed below) in Earth hydrothermal fluids to estimate delivery to Europa's ocean. By using the lower end of their fluid flux estimate and abundances from McCollom (1999), we find a CO2 flux of 1.3 × 109 moles yr−1. The accuracy of both estimates is of course debatable, but they represent two qualitatively different possibilities of a flux of CO2 either significantly smaller or larger than the flux of O2. CO2 is also susceptible to undergo speciation into carbonates, a form in which it would not be trapped in clathrates. Calculation of equilibria in conditions relevant to Europa (Zolotov & Kargel 2009) indicates that a mildly acidic pH (<6.5) is required for CO2 to be the dominant form over HCO−3. We consider this to be the case (see Section 2.3 for the justification and Section 4 for the implication of a higher pH).
Sulfur dioxide. SO2 is present on the surface of Europa (Lane et al. 1981; Carlson et al. 2009) and its inclusion in clathrate at Europa has been discussed before (Prieto-Ballesteros et al. 2005; Hand et al. 2006). There is also the possibility of a large input from the silicate interior into the ocean (Kargel et al. 2000). However, we chose to disregard SO2 as a clathrate former in this study. We consider it unlikely for underwater volcanism to produce SO2, as the lower temperature and high activity of water would shift the speciation of sulfur to H2S (Herzig et al. 1998), or, under oxidizing conditions, to sulfates. This substantially limits the possibility of hydrothermal SO2 input. Sulfur dioxide would still be delivered from the icy crust, but speciation calculations with SUPCRT92 (Johnson et al. 1992) in conditions relevant to Europa's ocean via the reaction  show that the bisulfite ion is favored over SO2 (log K = −1.6 and −1.2 at 10 and 100 MPa, respectively, at 0°C) and that any significant SO2 activity is unlikely unless the pH is very low (<2).
show that the bisulfite ion is favored over SO2 (log K = −1.6 and −1.2 at 10 and 100 MPa, respectively, at 0°C) and that any significant SO2 activity is unlikely unless the pH is very low (<2).
Methane. CH4 is present in reduced hydrothermal fluids on Earth (McDermott et al. 2015) and is a hydrothermal input to consider for Europa (McCollom 1999), with the additional possibility of biotic methane. However, methane has not been detected at Europa's surface (Carlson et al. 2009), which could mean it is absent among the species reaching the surface from the ocean, or it is readily destroyed by radiation processing. While speciation under oxidizing conditions (low H2 fugacity) may be detrimental to CH4, metastability is possible in the cold ocean (Zolotov & Kargel 2009) and we consider methane to be stable for our purpose. Similarly to the CO2 case (see above), we consider a large and a small hydrothermal input of CH4. The large input is set to 6.9 × 1011 moles yr−1 (i.e., the same carbon flux as when considering CO2, but under CH4 form), and the small input is based on estimates of heat flux and abundances of CH4 in Earth's hydrothermal fluids (McCollom 1999; Hand et al. 2007), which yields 7.4 × 108 moles yr−1. Again, the main point is to explore the effect of an input significantly larger or smaller than the O2 flux.
Hydrogen sulfide. H2S, just like methane, is a likely product of hydrothermal reactions under reducing conditions (McCollom 1999) but it has not been detected on the surface of Europa, and no limit has been derived (Carlson et al. 2009). It could easily escape from the surface (Moore et al. 2007), and/or be destroyed on Europa's oxidizing surface, with radiolytic processes only producing a small abundance (Carlson et al. 2002). If generated by hydrothermal processes, H2S could coexist metastably with sulfates in the ocean as long as the pH is low enough (<7) to limit speciation to HS− (Zolotov & Kargel 2009). However, coexistence with O2 is unlikely. Experimental evidence in conditions comparable to our study (T down to 278 K, 4 < pH < 8), ionic strength covering several natural bodies of water including Earth seawater points to rapid kinetics of oxidation of H2S by O2, with half-lives on the order of hours (Millero et al. 1987; Zhang & Millero 1994). The products include sulfates, sulfites, and thiosulfate (Zhang & Millero 1993). We consider two subcases of a reduced hydrothermal input including CH4 and H2S. The first is a large input (6.9 × 1011 moles yr−1 of H2S, i.e., the same sulfur amount as estimated in Kargel et al. 2000) and a small one derived from Earth's hydrothermal fluids, yielding 4.95 × 109 moles yr−1 (McCollom 1999; Hand et al. 2007). The amount of CH4 is considered similar. In each case, we then consider that oxidation of H2S by O2 effectively eliminates the limiting reactant. The large hydrothermal input effectively turns Europa's ocean into an anoxic environment as all the O2 reacts with the more abundant H2S. The small hydrothermal input effectively only brings in CH4, the H2S being converted into (mostly) sulfates due to the much larger amount of O2 available.
Noble gases. Argon, krypton, and xenon are important tracers of formation and evolution processes. Their ratios in the ocean may be altered if they are trapped in clathrates; therefore, we include them in the study to assess this effect. We consider only an input from the icy crust, assuming an Ar/H2O ratio in the middle of the 0.1–2.3 × 10−5 range found on comet 67P/Churyumov-Gerasimenko by the Rosetta mission (Balsiger et al. 2015). We then adopt Kr/Ar = 0.089 and Xe/Ar = 0.02, consistent with values measured at comet 67P/Churyumov-Gerasimenko (Rubin et al. 2018). We are mostly concerned about the evolution of the noble gases ratios (Ar/Kr, Ar/Xe, Kr/Xe); therefore, the wide range on the Ar/H2O value is not of great importance here. The goal of the calculation is to assess what happens to a small quantity of these noble gases in an environment where other, more abundant volatiles initiate the formation of clathrate; therefore, we do not consider any initial quantity of these noble gases in the ocean as it would not affect the eventual result (the steady state of Ar/Kr, Ar/Xe, and Kr/Xe ratios).
We consider the possibility of potassium leaching from the silicate interior adding some amount of 40Ar to the ocean through decay of 40K. Using the estimates of Zolotov & Shock (2001) for the present abundance of potassium in the ocean, and adopting a present 40K/39K of 1.17 × 10−2, a half-life of 1.25 Gyr and that 10.7% of decaying 40K is converted to 40Ar we find,4 over 4.5 Gyr, an addition of 7.8 × 1016 moles. This averages to a yearly input one order of magnitude below the one we introduce from the icy crust; we therefore neglect this contribution.
2.3. Geochemical Assumptions
Throughout the study, we assume a mildly acidic pH, enough for CO2 to be dominant over (bi)carbonate, but not so low as to allow for significant SO2 activity (1 < pH < 6). With our assumption of constant delivery of radiolytic surface products, a large amount of sulfuric acid (Carlson et al. 1999b, 2009) and/or sulfate salts (McCord et al. 1999) would be injected into the ocean. Hydrated sulfuric acid is one of the main products obtained during implantation of energetic sulfur ions into water ice (Ding et al. 2013), and is therefore very likely to be present in quantities comparable to other radiolytic products on the surface of Europa, due to its radiation environment (Paranicas et al. 2009). Sulfates and sulfuric acid, along with SO2 and CO2 would be added to the ocean and contribute to an environment rich in sulfate with low equilibrium H2 fugacity. A pH below 7 is therefore possible in Europa's ocean, by contrast with the likely alkaline ocean of Enceladus (Glein et al. 2015, 2018), which does not receive as much oxidant input due to the lack of a robust exogenic source of sulfuric acid. We make this assumption through the study and discuss the effect of a higher pH in Section 4.
We also assume that the ocean acts mostly as an accumulator of volatiles, without any major outgassing, clathrate formation being the only way to subtract volatiles from it (whether the clathrate sinks or becomes part of the crust is discussed in Section 4). The only exception is our consideration of the oxidation of H2S by O2 eliminating the limiting reactant (see Section 2.2, paragraph on H2S). We do not introduce any reaction involving species other than those in the model. We discuss other possible reactions that could act as sinks in Section 4.
Moreover, we consider that whether volatiles from the icy crust reach the ocean as clathrate or not is irrelevant to our study: in the liquid phase, there is ample opportunity for the clathrate to "re-equilibrate" with the surrounding mixture, including change of structure if it is thermodynamically favorable (Dec 2012; Vu & Choukroun 2015).
We divide our calculations into four cases. In each case, the icy crust brings the amount of O2 and CO2 discussed above (3 × 1011 moles yr−1 and 6.55 × 1010 moles yr−1, respectively) along with noble gases, but the hydrothermal input is different (oxidized or reduced, large or small). These cases are summarized in Table 1. In Case 1, it appeared that enough CO2 accumulated in the ocean to drive clathrate formation (in addition to the already forming clathrate due to O2). To explore the consequence of this situation we ran a Case 1 bis, starting from the timestep in Case 1 when enough CO2 has accumulated to drive clathrate formation.
Table 1. Cases Considered in this Study
| Hydrothermal | Flux (1010 moles yr−1) | Time before Clathrate | Notes | ||||
|---|---|---|---|---|---|---|---|
| Input | O2 | CO2 | H2S | CH4 | Formation Starts | ||
| Case 1 | Large, oxidized | 30* | 76 | ⋯ | ⋯ | 470 Myr | ⋯ |
| Case 1bis | Large, oxidized | 30 | 76* | ⋯ | ⋯ | 350 Myr (after Case 1 starts) | CO2 driven formation starting during Case 1 |
| Case 2 | Small, oxidized | 30* | 6.68 | ⋯ | ⋯ | 770 Myr | ⋯ |
| Case 3 | Large, reduced | 0 | 6.55 | 54.4 | 69.4* | 90 Myr | O2 consumed by H2S oxidation |
| Case 4 | Small, reduced | 30* | 6.55 | 0 | 0.495 | 772 Myr | H2S consumed by O2 reduction |
Note. The asterisks marks the compound that first reaches a partial pressure sufficient to trigger clathrate formation.
Download table as: ASCIITypeset image
3. Results
The results of our calculations are displayed in Figures 1–4. For all cases, we have calculated how long it would take for the driving species to reach a partial pressure triggering clathration (result in Table 1; the method of calculation is detailed in Mousis et al. 2013 and was applied to all compounds in the input mixture to determine which one first reaches a partial pressure sufficient to trigger clathration). Since this time is found to be shorter than 1 Gyr in all cases, we run the model for a duration equivalent to 4 Gyr (at each timestep the equivalent of 10 Myr of gas input is added into the ocean, the calculation runs for 400 timesteps). This result is obtained at 10 MPa. We also performed this calculation at 100 MPa, and found the partial pressure that triggers clathration to be decreased by 12%–14% for all species considered, except CO2. The time to reach clathrate formation would therefore be shortened by this amount. The particular case of CO2 is discussed in the description of Case 1.
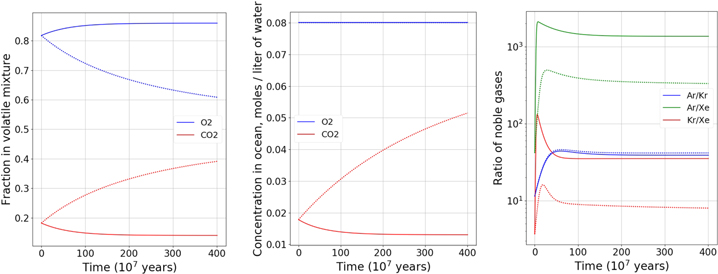
Figure 1. Top: case 1, assuming sI formation (full lines) or sII (dotted lines). Left: evolution of the fractions in the mixture of volatile compounds in the ocean. Center: evolution of the concentrations of these compounds in the ocean. Right: evolution of noble gas ratios in the ocean. Bottom: case 1bis, assuming sI formation (full lines) or sII (dotted lines). Left: evolution of the fractions in the mixture of volatile compounds in the ocean. Right: evolution of the concentrations of these compounds in the ocean.
Download figure:
Standard image High-resolution imageAll calculations of ocean composition evolution have been run and are displayed at 10 MPa. We also performed the same calculations at 100 MPa and found that the resulting evolution is similar and we do not display these in this paper.
Case 1 (Figure 1). The calculation shows O2 is the driving compound for clathrate formation, and pure O2 clathrate is structure II (Sloan & Koh 2007). Figure 1, left and center, shows that steady state is not achieved with the formation of sII clathrate, and the abundance of CO2 keeps rising and quickly (<500 Myr) reaches the point at which CO2 can become a driving compound for clathrate formation. Given the high availability of water, sI clathrate dominated by CO2 will likely form concurrently with O2-dominated sII clathrate. This sI clathrate would also trap a nonnegligible amount of O2, slowing down sII formation.
We ran an additional calculation (Case 1 bis) where CO2 is the driving compound, and O2 abundance starts at the same amount as before, to reflect how sII formation has limited its abundance in the ocean. The results, displayed in Figure 1 (bottom line), show that sI formation is not enough to fully compensate for the flux of O2, likely causing the formation of a smaller quantity of sII clathrate to trap it. However, sI would be the most abundant form, due to the larger flux of CO2. We note, however, that our calculations are run at 10 MPa, corresponding to the ice-ocean interface under 10 km of ice, but at the bottom of a 100 km deep ocean the 100 MPa pressure would greatly decrease the CO2 fugacity coefficient, raising the threshold for CO2 driven clathrate formation. We calculate that 2.7 times as much CO2 could be added into water at 100 MPa before it reaches a partial pressure enabling CO2 driven sI clathrate formation. Therefore, the CO2-dominated sI clathrate formation would occur later (but still within the timeframe of our study) than suggested by the limit shown in Figure 1. Over the whole ocean, the O2/CO2 ratio would be lower than calculated at 10 MPa. We calculate the density of the sI clathrate formed at the last timestep of case 1 bis to be 1.077 g cm−3 assuming 90% of the cages occupied. We also calculate the density of the sII clathrate formed at step 35 of the run (before CO2 exceeds the threshold for clathrate formation) and find 1.037 g cm−3.
The effect of sI formation on noble gas ratios is a significant increase in Ar/Xe (×20), Kr/Xe (×5), and Ar/Kr (×4) in the ocean.
Case 2 (Figure 2). Here again, O2 is the driving compound and sII is favored. However, the CO2 input is insufficient to cause the formation of sI clathrate within the given timeframe. The O2/CO2 ratio goes down and steady state is not reached. We calculate a clathrate density of 1.02 g cm−3 at the last step of the run. The formation of sII clathrate causes a more modest increase in Ar/Xe and Kr/Xe ratio than in Case 1.
Figure 2. Same as Figure 1, but for Case 2.
Download figure:
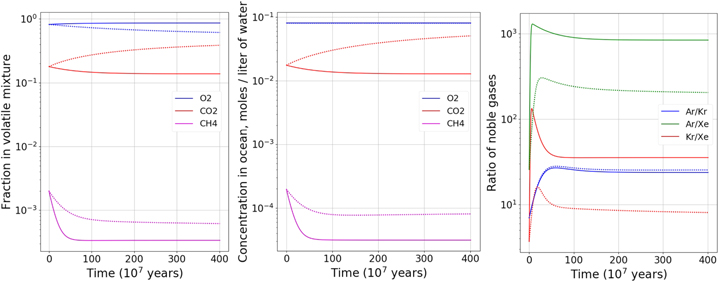
Standard image High-resolution imageCase 3 (Figure 3). Our calculations show that in this case CH4 is the driving compound, and because pure methane clathrate is structure I (Sloan & Koh 2007) we focus on the results associated with this structure. H2S becomes the most abundant compound in the mixture, while the fraction of methane is decreased. While H2S concentration rises we calculate it does not exceed the threshold for pure H2S clathrate formation. The effect on noble gas ratios is comparable to what is seen in Case 1. We calculate a clathrate density of 0.97 g cm−3 at the last step of the run.
Figure 3. Same as Figure 1, but for Case 3.
Download figure:
Standard image High-resolution imageCase 4 (Figure 4). Due to the lower hydrothermal input, O2 is the driving compound for this case, and we focus on sII results. The main effect is that O2/CO2 decreases from 4.6 to 1.6, and methane is slightly depleted in the mixture. The effect on noble gases is very similar to Case 2. We calculate a clathrate density of 1.02 g cm−3 at the last step of the run.
Figure 4. Same as Figure 1, but for Case 4. Mole fractions and abundances for this case are displayed on a log scale.
Download figure:
Standard image High-resolution image4. Discussion
We see that clathrate formation can significantly alter the volatile mixture present in Europa's ocean in a relatively short timeframe with the inputs to the ocean considered in this study. The exact effect is dependent on the amount and content of the input; in particular, the amount of O2 compared to other volatiles, by promoting formation of sII structure while other volatiles tend to form sI, is a deciding factor. We note that a previous work about clathrate in the icy crust of Europa (Hand et al. 2006) offers double occupancy of sI large cages by O2 as an explanation for some spectroscopic observations at Europa's surface. We have not included a double occupancy scenario in any of our calculations.
Whether clathrate that forms sinks or rises up to the icy crust depends on its density and the ocean's. Previous work (Prieto-Ballesteros et al. 2005) has considered low-salinity water (1 g cm−3) or eutectic brines (1.19 g cm−3 for a magnesium sulfate brine) to bound the possible ocean density. In the case of the low-salinity water, only the CH4 rich clathrate produced in Case 3 would ascend to the icy crust. In the case of a eutectic brine, clathrate would ascend to the icy crust in every case considered. We also introduce for discussion an intermediate value, based on Earth seawater at 10 MPa, of 1.028 g cm−3 (Millero et al. 1980). In that case only the clathrate produced in Case 1 (rich in CO2) is susceptible to sinking. We note that at the bottom of a 100 km ocean the pressure of 100 MPa would increase the density of seawater up to 1.07 g cm−3; this opens the possibility of clathrate of intermediate density staying in suspension in the ocean. In summary, in most of the cases envisioned, the clathrate is expected to ascend to the icy crust, making it potentially accessible to future observations, accounting for the rapid ocean-surface exchange that is our work's starting assumption. It sinks in the case of a low-salinity ocean or if the ocean receives a large (compared to radiolytic products from the surface) CO2 input. If contrary to our assumption, SO2 is abundant in the ocean (i.e., it does not speciate into ionic species), it would participate in the formation of denser clathrate (Prieto-Ballesteros et al. 2005; Hand et al. 2006).
Salinity is a factor potentially affecting clathrate formation. Calculations by Prieto-Ballesteros et al. (2005) show a fairly minor effect on the temperature of dissociation (2 K) at eutectic proportions of magnesium sulfate. The effect of chloride salts has been found to be more pronounced (10 K at the eutectic, 5 K for concentrations of 10% by weight. The seawater value of salinity we have considered in our density calculation is 3.5% by weight).
The effect of clathrate formation on noble gas ratios is noticeably different depending on whether structure I or II forms. Ar/Xe and Kr/Xe increase is more pronounced when sI is formed. In future observations of volatiles emanating from Europa, noble gases could help diagnose which clathrate structure has affected the evolution of the mixture; conversely, understanding the abundances of noble gases may require taking trapping into clathrate into account.
If the ocean's pH is higher than assumed in our study, as could be caused by extensive interaction with an ultramafic silicate interior, several of the compounds we considered would predominantly speciate into ionic species, which do not form clathrate. This would be the case for H2S and CO2 (Zolotov & Kargel 2009). However, both CH4 and O2 would be unaffected, and they are the driving compounds for clathrate formation in our calculations. As a result, cases 1 and 2 would result in pure O2 clathrate, and cases 3 and 4 in CH4 + O2 clathrate.
With the fluxes we have chosen, the evolution of the ocean is relatively fast, attaining steady state (for the cases that do) within 1.5 Gyr. Our model does not include the time taken for the whole icy crust to be enriched in radiolytic material, evaluated by Greenberg (2010) at 1 Gyr. This factor would delay the start of the process, but would not significantly affect our conclusions. We also have calculated the time before clathration (Table 1) by counting only the input to the ocean, without any initial dissolved gas, which is unlikely, making this time an upper bound. We also used the highest O2 flux to the ocean estimated by Greenberg (2010) throughout the study. A lower flux would increase the time needed to start clathrate formation (Table 1), and generally increase the timescales of O2 evolution.
We note the model does not take into account the possibility of chemical reactions with, e.g., material of the seafloor. We did take into account the fast oxidation of H2S by O2 but other sinks may be present. Oxidation of iron could act as an additional O2 sink. Formation of metal sulfide (iron being likely) is another possible sink of H2S. At pH = 6, formation of carbonate minerals with iron, magnesium, or calcium is still possible (Zolotov & Kargel 2009), representing a sink of CO2. These last three processes require elements that can be provided by a relatively fresh igneous seafloor. The importance of these sinks depends on the rate of delivery of fresh igneous rocks to the permeable seafloor environment by volcanic processes. Metabolic reactions are also possible sinks or sources of the species we include in our study (McCollom & Shock 1997) but can have a wide range of effects depending on which reaction(s) these hypothetical lifeforms rely on, which itself is a function of the exact conditions in their habitat.
5. Conclusion
We have investigated the content and density of clathrate that would form in Europa's ocean in cases considering different inputs to the ocean (Table 1), in the case of fast exchanges between the ocean and the surface and a mildly acidic ocean (2 < pH < 6). In addition to an O2 and CO2 flux from the icy surface we included the possibility of a reduced (CH4 and H2S) or oxidized (CO2) flux from hydrothermal fluids. We ignored SO2 due to its speciation into sulfites in conditions likely to be met in Europa's ocean. We infer that the relative importance of the O2 flux compared to other compounds is a key factor in deciding the clathrate structure formed and its effect on relative abundances. The most drastic alteration of the original mixture in the ocean is seen when a large input of methane prompts sI clathrate formation. The change in concentration of the compounds subject to clathrate formation could affect which chemical reactions would become energetically favorable in Europa's ocean, e.g., methane oxidation versus methanogenesis (McCollom & Shock 1997). We also find that the effect of clathrate formation on noble gas ratios is especially dependent on which structure is predominant, potentially making noble gas measurements a diagnostic of the clathrate formation process at work. We find that in most cases it can be expected that clathrate will float and be integrated into the icy crust. Material coming up from the ocean would then likely include clathrate, so future investigations could be capable to analyze its content, thus helping to constrain the composition of the ocean.
A.B. and O.M. acknowledge support by CNES. J.H.W. and C.R.G. were supported by the Europa Clipper MASPEX investigation.