Anthocyanin degradation kinetics and thermodynamic analysis of Hibiscus rosa-sinensis L. Clitoria ternatea L. and Hibiscus sabdariffa L.
DOI:
https://doi.org/10.37934/progee.19.1.112Keywords:
Anthocyanins degradation kinetic, Hibiscus sabdariffa L, Clitoria ternatea L, Hibiscus rosa-sinensis L, thermodynamicAbstract
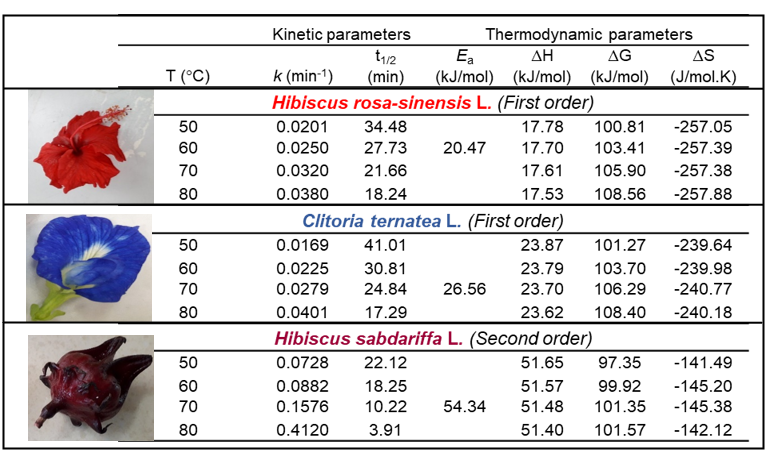
Anthocyanins are natural occurrence red pigments existed in most flowers with high health benefited values. These anthocyanins rich flowers have a short shelf life and fast degradation when in fresh stage. These anthocyanins rich flowers have a short shelf life and fast degradation when in fresh stage. Therefore, drying is a conventional way to preserve them from rotten in order to be reachable for urban consumers who have busy life style and limited space for planting. The present study was conducted to evaluate the anthocyanin degradation kinetics of Hibiscus rosa-sinensis L., Clitoria ternatea L. and Hibiscus sabdariffa L. at drying temperatures of 50, 60, 70 and 80 °C for durations of 10, 20, 30 and 40 min. Anthocyanin degradation kinetic order of Hibiscus rosa-sinensis L., Clitoria ternatea L. and Hibiscus sabdariffa L. were determined by constructing natural logarithm Arrhenius equation plots from k values obtained from zero-, first-, and second-order integrated rate law plots at each temperature levels of 50 °C, 60 °C, 70 °C and 80 °C, based on the highest coefficient of determination (R2). Fresh flower of Clitoria ternatea L. has been revealed possessed the highest amount of TMA followed by Hibiscus sabdariffa L. and the Hibiscus rosa-sinensis L. Results revealed that anthocyanins degradation for Hibiscus rosa-sinensis L. and Clitoria ternatea L. followed first-order kinetic behaviour, while Hibiscus Sabdariffa L. followed the second-order. Anthocyanins of Hibiscus Sabdariffa L. has been discovered having high k values which led to shorter half-life values. However, anthocyanins of Hibiscus Sabdariffa L. is more stable during heat drying treatment as evidenced by higher activation energy and activation enthalpy, but lower free Gibbs energy and absolute value of entropy in comparison to Hibiscus rosa-sinensis L. and Clitoria ternatea L. Therefore, Hibiscus sabdariffa L. is highly recommended to be used as food colorant in food processing industries which involve heating.
References
H.M. Inggrid, Jaka, H. Santoso, Natural red dyes extraction on roselle petals, IOP Conference Series: Materials Science and Engineering. 162 (2016) 012029. https://doi.org/10.1088/1757-899X/162/1/012029.
H.A. Almahy, H.H. Abdel-Razik, Y.A. El-Badry, E.M. Ibrahim, Ultrasound-assisted extraction of anthocyanin pigments from Hibiscus sabdariffa (Rosella) and its phytochemical activity at Kingdom of Saudi Arabia, International Journal of Chemical Sciences. 2 (2015) 168–174. https://www.tsijournals.com/abstract/ultrasoundassisted-extraction-of-anthocyanin-pigments-from-hibiscus-sabdariffa-rosella-and-its-phytochemical-activity-at-13532.html.
Y. Tao, P. Wang, J. Wang, Y. Wu, Y. Han, J. Zhou, Combining various wall materials for encapsulation of blueberry anthocyanin extracts: Optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties, Powder Technology. 311 (2017) 77–87. https://doi.org/10.1016/j.powtec.2017.01.078.
C. Li, J. Qiu, L. Ding, M. Huang, S. Huang, G. Yang, J. Yin, Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals, Plant Physiology and Biochemistry. 112 (2017) 335–345. https://doi.org/10.1016/j.plaphy.2017.01.019.
T. Mizuno, A. Uehara, D. Mizuta, T. Yabuya, T. Iwashina, Contribution of anthocyanin–flavone copigmentation to grayed violet flower color of Dutch iris cultivar ‘Tiger’s Eye’ under the presence of carotenoids, Scientia Horticulturae. 186 (2015) 201–206. https://doi.org/10.1016/j.scienta.2015.01.037.
A.E.-M.M.R. Afify, H.M.M. Hassan, Free radical scavenging activity of three different flowers-Hibiscus rosa-sinensis, Quisqualis indica and Senna surattensis, Asian Pacific Journal of Tropical Biomedicine. 6 (2016) 771–777. https://doi.org/10.1016/j.apjtb.2016.07.006.
Y.W. Mak, L.O. Chuah, R. Ahmad, R. Bhat, Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts, Journal of King Saud University - Science. 25 (2013) 275–282. https://doi.org/10.1016/j.jksus.2012.12.003.
A.M. Siti Azima, A. Noriham, N. Manshoor, Phenolics, antioxidants and color properties of aqueous pigmented plant extracts: Ardisia colorata var. elliptica, Clitoria ternatea, Garcinia mangostana and Syzygium cumini, Journal of Functional Foods. 38 (2017) 232–241. https://doi.org/10.1016/j.jff.2017.09.018.
W. Phrueksanan, S. Yibchok-anun, S. Adisakwattana, Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes, Research in Veterinary Science. 97 (2014) 357–363. https://doi.org/10.1016/j.rvsc.2014.08.010.
S. Sukkhaeng, S. Promdang, U. Doung-ngern, Fruit characters and physico-chemical properties of roselle (Hibiscus sabdariffa L.) in Thailand—A screening of 13 new genotypes, Journal of Applied Research on Medicinal and Aromatic Plants. 11 (2018) 47–53. https://doi.org/10.1016/j.jarmap.2018.10.001.
E. Jung, Y. Kim, N. Joo, Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.), Journal of the Science of Food and Agriculture. 93 (2013) 3769–3776. https://doi.org/10.1002/jsfa.6256.
J.-R. Cheng, X.-M. Liu, Z.-Y. Chen, Y.-S. Zhang, Y.-H. Zhang, Mulberry anthocyanin biotransformation by intestinal probiotics, Food Chemistry. 213 (2016) 721–727. https://doi.org/10.1016/j.foodchem.2016.07.032.
C. Cui, S. Zhang, L. You, J. Ren, W. Luo, W. Chen, M. Zhao, Antioxidant capacity of anthocyanins from Rhodomyrtus tomentosa (Ait.) and identification of the major anthocyanins, Food Chemistry. 139 (2013) 1–8. https://doi.org/10.1016/j.foodchem.2013.01.107.
F.A. Sulaiman, M.O. Kazeem, A.M. Waheed, S.O. Temowo, I.O. Azeez, F.I. Zubair, T.A. Adeyemi, A. Nyang, O.S. Adeyemi, Antimicrobial and toxic potential of aqueous extracts of Allium sativum, Hibiscus sabdariffa and Zingiber officinale in Wistar rats, Journal of Taibah University for Science. 8 (2014) 315–322. https://doi.org/10.1016/j.jtusci.2014.05.004.
K. Vasisht, M. Dhobi, S. Khullar, S.K. Mandal, M. Karan, Norneolignans from the roots of Clitoria ternatea L., Tetrahedron Letters. 57 (2016) 1758–1762. https://doi.org/10.1016/j.tetlet.2016.03.024.
F. Tavakolifar, M.H. Givianrad, M. Saber-Tehrani, Extraction of anthocyanins from Hibiscus Sabdariffa and assessment of its antioxidant properties in extra virgin olive oil, Fresenius Environmental Bulletin. 25 (2016) 3709–3713.
P.-J. Tsai, H.-P. Huang, Effect of polymerization on the antioxidant capacity of anthocyanins in Roselle, Food Research International. 37 (2004) 313–318. https://doi.org/10.1016/j.foodres.2003.12.007.
C. Carrillo, C. Buvé, A. Panozzo, T. Grauwet, M. Hendrickx, Role of structural barriers in the in vitro bioaccessibility of anthocyanins in comparison with carotenoids, Food Chemistry. 227 (2017) 271–279. https://doi.org/10.1016/j.foodchem.2017.01.062.
A. Aboonabi, I. Singh, Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2–ARE as an indicator and modulator of redox, Biomedicine & Pharmacotherapy. 72 (2015) 30–36. https://doi.org/10.1016/j.biopha.2015.03.008.
C. Mazewski, K. Liang, E. Gonzalez de Mejia, Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro, Journal of Functional Foods. 34 (2017) 254–265. https://doi.org/10.1016/j.jff.2017.04.038.
T.H. Kouakou, N.G. Konkon, K. Ayolié, A.P. Obouayeba, Z.H. Abeda, M. Koné, Anthocyanin production in calyx and callus of Roselle (Hibiscus sabdariffa L.) and its impact on antioxidant activity, Journal of Pharmacognosy and Phytochemistry. 4 (2015) 9–15. https://www.phytojournal.com/archives/2015.v4.i3.618/anthocyanin-production-in-calyx-and-callus-of-roselle-hibiscus-sabdariffa-l-and-its-impact-on-antioxidant-activity.
C.-L. Zhao, Y.-Q. Yu, Z.-J. Chen, G.-S. Wen, F.-G. Wei, Q. Zheng, C.-D. Wang, X.-L. Xiao, Stability-increasing effects of anthocyanin glycosyl acylation, Food Chemistry. 214 (2017) 119–128. https://doi.org/10.1016/j.foodchem.2016.07.073.
G.A. Garzón, R.E. Wrolstad, Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus), Food Chemistry. 114 (2009) 44–49. https://doi.org/10.1016/j.foodchem.2008.09.013.
I. Tontul, A. Topuz, Effects of different drying methods on the physicochemical properties of pomegranate leather (pestil), LWT. 80 (2017) 294–303. https://doi.org/10.1016/j.lwt.2017.02.035.
A.D. Joseph, G.M. Adogbo, Processing and packaging of Hibiscus sabdariffa for preservation of nutritional constituents, International Journal of Scientific & Engineering Research. 6 (2015) 532–536. https://www.ijser.org/researchpaper/Processing-and-Packaging-of-Hibiscus-Sabdariffa-for-Preservation-of-Nutritional-Constituents.pdf.
L. Szalóki-Dorkó, G. Végvári, M. Ladányi, G. Ficzek, M. Stéger-Máté, Degradation of Anthocyanin Content in Sour Cherry Juice During Heat Treatment, Food Technology and Biotechnology. 53 (2015). https://doi.org/10.17113/ftb.53.03.15.3931.
P. Atkins, J. Paula, Chapter 22 – The rates of chemical reactions, in: Atkins’ Physical Chemistry, 8th Ed., WH Freeman and Company, 2006: pp. 807–809.
L. Fan, Y. Wang, P. Xie, L. Zhang, Y. Li, J. Zhou, Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation, Food Chemistry. 275 (2019) 299–308. https://doi.org/10.1016/j.foodchem.2018.09.103.
G. Qiu, D. Wang, X. Song, Y. Deng, Y. Zhao, Degradation kinetics and antioxidant capacity of anthocyanins in air-impingement jet dried purple potato slices, Food Research International. 105 (2018) 121–128. https://doi.org/10.1016/j.foodres.2017.10.050.
J.N. Coupland, Chapter 1 – Basic thermodynamics, in: An Introduction to the Physical Chemistry of Food, Springer, New York, 2014: pp. 1–16.
M.K. Bolade, I.B. Oluwalana, O. Ojo, Commercial practice of Roselle (Hibiscus sabdariffa L.) beverage production: Optimization of hot water extraction and sweetness level, World Journal of Agricultural Sciences. 5 (2009) 126–131.
R.C. Chisté, A.S. Lopes, L.J.G. de Faria, Original article: Thermal and light degradation kinetics of anthocyanin extracts from mangosteen peel (Garcinia mangostana L.), International Journal of Food Science & Technology. 45 (2010) 1902–1908. https://doi.org/10.1111/j.1365-2621.2010.02351.x.
H.C.B. Costa, D.O. Silva, L.G.M. Vieira, Physical properties of açai-berry pulp and kinetics study of its anthocyanin thermal degradation, Journal of Food Engineering. 239 (2018) 104–113. https://doi.org/10.1016/j.jfoodeng.2018.07.007.
K.L. Ng, C.Y. Cheok, Evaluation of thermal degradation kinetic order of anthocyanins extracted from Garcinia Mangostana L. rind., Progress in Energy and Environment. 13 (2020) 16–25. https://www.akademiabaru.com/submit/index.php/progee/article/view/1064.
G.B. Escher, M. Wen, L. Zhang, N.D. Rosso, D. Granato, Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L. (butterfly pea) blue petals, Food Chemistry. 331 (2020) 127341. https://doi.org/10.1016/j.foodchem.2020.127341.
I.F. Pérez-Ramírez, E. Castaño-Tostado, J.A. Ramírez-de León, N.E. Rocha-Guzmán, R. Reynoso-Camacho, Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage, Food Chemistry. 172 (2015) 885–892. https://doi.org/10.1016/j.foodchem.2014.09.126.
L.G. Maciel, M.A.V. do Carmo, L. Azevedo, H. Daguer, L. Molognoni, M.M. de Almeida, D. Granato, N.D. Rosso, Hibiscus sabdariffa anthocyanins-rich extract: Chemical stability, in vitro antioxidant and antiproliferative activities, Food and Chemical Toxicology. 113 (2018) 187–197. https://doi.org/10.1016/j.fct.2018.01.053.
A. Sinela, N. Rawat, C. Mertz, N. Achir, H. Fulcrand, M. Dornier, Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products, Food Chemistry. 214 (2017) 234–241. https://doi.org/10.1016/j.foodchem.2016.07.071.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.

