Summary
Caenorhabditis elegans is a useful model to explore the functions of polyunsaturated fatty acids in development and physiology. This protocol describes an efficient method of supplementing the C. elegans diet with polyunsaturated fatty acids.
Abstract
Fatty acids are essential for numerous cellular functions. They serve as efficient energy storage molecules, make up the hydrophobic core of membranes, and participate in various signaling pathways. Caenorhabditis elegans synthesizes all of the enzymes necessary to produce a range of omega-6 and omega-3 fatty acids. This, combined with the simple anatomy and range of available genetic tools, make it an attractive model to study fatty acid function. In order to investigate the genetic pathways that mediate the physiological effects of dietary fatty acids, we have developed a method to supplement the C. elegans diet with unsaturated fatty acids. Supplementation is an effective means to alter the fatty acid composition of worms and can also be used to rescue defects in fatty acid-deficient mutants. Our method uses nematode growth medium agar (NGM) supplemented with fatty acidsodium salts. The fatty acids in the supplemented plates become incorporated into the membranes of the bacterial food source, which is then taken up by the C. elegans that feed on the supplemented bacteria. We also describe a gas chromatography protocol to monitor the changes in fatty acid composition that occur in supplemented worms. This is an efficient way to supplement the diets of both large and small populations of C. elegans, allowing for a range of applications for this method.
Introduction
Fatty acids are essential structural components of membranes as well as efficient energy storage molecules. Additionally, fatty acids can be cleaved from cellular membranes by lipases and be enzymatically modified to produce signaling effectors1. Naturally occurring polyunsaturated fatty acids (PUFAs) contain two or more cis double bonds. The omega-3 fatty acids and the omega-6 fatty acids are distinguished from each other based on the positions of double bonds with respect to the methyl end of the fatty acid. Healthy diets require both omega-6 and omega-3 fatty acids. However, Western diets are particularly rich in omega-6 fatty acids and poor in omega-3 fatty acids. A high omega-6 to omega-3 fatty acid ratio is associated with increased risk of cardiovascular and inflammatory diseases, however, the precise beneficial and detrimental functions of specific fatty acids are not well understood2. The roundworm Caenorhabditis elegans is useful in studying fatty acid function because it synthesizes all of the enzymes necessary to produce a range of omega-6 and omega-3 fatty acids, including an omega-3 desaturase, an activity that is absent in most animals3,4 . Mutants lacking fatty acid desaturase enzymes fail to produce specific PUFAs, leading to a range of developmental and neurological defects4-6.
To study the physiological effects of dietary fatty acids, we have developed a biochemical assay compatible with genetic analysis using both mutant and RNAi knock-down techniques in C. elegans. Supplementation with specific PUFAs is achieved by adding a fatty acid sodium salt solution to the agar medium prior to pouring. This results in PUFA uptake by the E. coli food source, where it accumulates in the bacterial membranes. C. elegans ingest the PUFA-containing bacteria, and this dietary supplementation is sufficient to rescue the defects of PUFA-deficient mutants. Supplementation of most fatty acids has no detrimental effects on wild type animals, however, specific omega-6 fatty acids, especially dihomo-gamma linolenic acid (DGLA, 20:3n-6) cause a permanent destruction of C. elegans germ cells7,8 .
Gas chromatography is used to monitor the uptake of the supplemented fatty acid in the bacterial food source (either OP50 or HT115) as well as in the nematodes. The addition of the detergent Tergitol (NP-40) in the media allows for even distribution of fatty acids through the entire plate and more efficient uptake of the fatty acids by the E. coli and the nematodes. We have found that unsaturated fatty acids are readily taken up by bacteria and C. elegans, but the uptake of saturated fatty acids is much less efficient. This article will describe step-by-step how to supplement the agar media with fatty acids, as well as how to monitor fatty acid uptake in the nematode using gas chromatography.
Subscription Required. Please recommend JoVE to your librarian.
Protocol
Polyunsaturated fatty acids are sensitive to heat, light and oxygen. Therefore, care must be taken when preparing fatty acid supplementation plates such that fatty acids are not exposed to excess heat and light. NGM media containing 0.1% Tergitol (NP-40) is autoclaved and partially cooled, after which fatty acid sodium salts are added with constant stirring. The plates are allowed to dry in the dark. Uptake of fatty acids by C. elegans cultured on these plates can then be monitored by gas chromatography.
1. Preparation of Fatty Acid Supplemented Media
- Measure out media components into an appropriately sized flask. Per 1 L, add 17 g Bacto-agar, 2.5 g tryptone, 3 g NaCl, 1 ml 5 mg/ml cholesterol dissolved in ethanol, and 10 ml 10% Tergitol dissolved in water.
- Add 80% of final desired volume of Millipore water and autoclave the media along with empty glass bottles (equal to the number of different fatty acid concentrations to be tested), as well as appropriate graduated cylinders.
- Cool media in a water bath set to 55 °C. While the media is cooling prepare the working stock solution of the fatty acid sodium salt by breaking open the glass vial, using safety precautions and ensuring glass particles do not mix in with the fatty acids. Weigh out enough fatty acid to make a 100 mM working stock.
- Bring the fatty acid solution to a final concentration of 100 mM with purified water. Fully dissolving the fatty acid sodium salt typically takes about 20-30 min. Purge the working stock of fatty acid with argon or nitrogen, because contact with an inert gas prevents oxidation. Cap the vial and store the working stock in the dark until media has cooled.
- (Optional) If RNAi media is desired, measure ampicillin and Isopropyl β-D-1-thiogalactopyranoside (IPTG) solutions here.
- When the agar has cooled to 55 °C add per 1 L: 1 ml of 1 M MgSO4, 1 ml of 1 M CaCl2, and 25 ml of phosphate buffer (108.3 g KH2PO4 and 35.6 g K2HPO4 per 1 L autoclaved). Add the filter sterilized ampicillin and IPTG solutions if making RNAi plates. For all types of plates, add sterile water to bring the final media volume to 1 L.
- Near a flame, transfer media to an autoclaved and appropriately sized graduated cylinder and then add warm sterile water to desired volume. Transfer media back to initial flask and mix by stirring.
- Near a flame, transfer media into a number of bottles equal to the number of concentrations to be tested by measuring with an autoclaved and appropriately sized graduated cylinder. Maintain the aliquoted media as liquid using a water bath until the fatty acid working stock has fully dissolved.
- Place one bottle on a stir plate and stir until the media is warm to the touch, but not hot. Make sure to leave yourself enough time to stir in the fatty acid stock solution and pour plates before the media begins to solidify. If stir plate has temperature control, set it to 55 °C.
- Dilute the 100 mM fatty acid working stock into the media for the final concentration desired. Stir media until the white precipitate is in solution (approximately 1 min). Media may still be slightly cloudy afterwards.
- (Optional) If RNAi media is desired add ampicillin to 0.1 mg/ml and IPTG to 2 mM final concentration.
- Pour media using an automated pipette aid and a sterile 25 ml pipette, adding 8 ml of media per 60 mm plate or 25 ml of media per 100 mm plate. Repeat fatty acid addition and plate pouring steps for the remaining bottles.
- After the agar has solidified, cover the fatty acid-supplemented plates with a box or store at room temperature in a well-vented drawer to protect from light oxidation.
- Seed E. coli OP50 onto plates two days after plates have dried. For 60 mm plates, pipette 300 µl of an overnight OP50 culture (grown at 37 °C). If RNAi plates were poured, seed with 300 µl of RNAi bacteria after the plates have dried for four days. Plate drying time may need to be adjusted due to differences in humidity in various lab environments.
- Incubate the plates in a dark environment at room temperature while the bacterial lawn is drying.
2. Inducing Germ Cell Destruction by Supplementation of DGLA
- C. elegans grown on plates containing 0.3 mM DGLA (20:3n-6) become sterile due to the destruction of germ cells in both larval stage and adult nematodes.
- Prepare a synchronized population of L1 larvae by treating gravid hermaphrodites with alkaline hypochlorite solution(for a 10 ml solution: 0.5 ml of 5M NaOH, 2.5 ml of household bleach, and 7 ml H2O). Gently rock the gravid hermaphrodites in this solution until the adult worms dissolve. Eggs will be preserved, and can be pelleted by low speed centrifugation.
- Wash egg preparation 3 times in M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4, H2O to 1 L. Add MgSO4 after autoclaving). To provide enough oxygen, resuspend the eggs in a 15 ml plastic tube to a final volume of 5 ml of M9 buffer and rock on a shaker overnight.
- L1 larvae should be added to the supplemented plates two days after seeding with OP50, or one day after plating the RNAi bacteria. Incubate worms at 20 °C for three days or until they reach the adult stage.
- Adult worms can be scored for sterility using a dissecting microscope with high enough magnification to visualize embryos developing in the uterus. Successful germ cell loss will appear as a clear uterus void of eggs.
- Worms can also be scored by fixing and staining with the nucleic acid dye diamindinophenylindole (DAPI), which facilitates the visualization of nuclei in germ cells and developing embryos. A rapid DAPI stain is achieved by picking worms into a drop of M9 buffer on a watchglass9.
- Flood watch-glass with 1 ml of a 0.2 ng/m. DAPI in 95% ethanol solution, let sit for approximately 5 min.
- Pick worms into a drop of VectaShield on a slide, and then cover with a coverslip.
- Seal coverslip and store at 4 °C in the dark overnight for optimum staining, however slides can also be viewed immediately. Score for presence or absence of germ cell nuclei using a fluorescence microscope equipped with a UV lamp and filter.
3. Confirming Fatty Acid Uptake by Gas Chromatography
Overall fatty acid composition of C. elegans can be determined by producing fatty acid methyl esters (FAMEs) which are then separated and quantified using gas chromatography4.
- Collect 500-1,000 adult worms by washing them off of the plates with water and transferring to a silanized 13 mm x 100 mm glass screw top tube.
- Let worms settle by gravity, and then remove as much water as possible with a glass Pasteur pipette.
- Wash worms once with water, and then again remove as much water as possible.
- FAMEs are formed by adding 1 ml of 2.5 % H2SO4 in methanol, and then heating at 70 °C for 1 hr in a water bath.
- Remove the tubes from the water bath and cool for 1 min.
- Extract FAMEs by adding 1.5 ml water and 0.25 ml of hexane.
- Recap tubes and shake vigorously.
- Centrifuge tubes in a tabletop clinical centrifuge for 1 min at maximum speed to separate hexane from aqueous solvent.
- Transfer hexane (top layer) to a GC vial insert within a GC vial, being careful not to transfer any of the aqueous phase. For GC analysis, 1-2 μl of FAMEs in hexane is injected onto a polar capillary gas chromatography column suitable for FAMEs analysis. The Agilent 7890 GC injector is set at 250 °C, with a flow rate of 1.4 ml/min, and the GC oven is programmed for an initial temperature of 130 °C, which is held for 1 min. Subsequently, the temperature is ramped 10 °C/min until 190 °C, and then ramped again at 5 °C/min until 210 °C and held for an additional 1 min.
- To ensure that uptake of DGLA has occurred, analyze FAMEs by flame ionization detection (FID) or mass spectrometry (MS) detection, using authentic standards for the identification of the C. elegans fatty acids.
Subscription Required. Please recommend JoVE to your librarian.
Representative Results
Supplementation of the C. elegans diet is limited by the ability of the bacterial food source to uptake and incorporate fatty acid into the bacterial membrane. To determine the ability of E. coli OP50 to assimilate various fatty acids into its membranes, OP50 was plated onto media with no supplement, 0.1 mM and 0.3 mM concentrations of stearic acid (18:0), sodium oleate (18:1n-9), and sodium DGLA (20:3n-6). Plates were dried at room temperature for 2 days in the dark, and incubated at 20 °C for 3 days. Bacterial lawns were collected by gently scraping the lawn into water with a flame-sterilized spatula. Bacteria were pelleted by centrifugation, and treated with 2.5% H2SO4 in methanol to produce fatty acid methyl esters, which were analyzed by GC/MS following the methods listed in the Procedure, step 3. The results demonstrate that unsaturated fatty acids (oleate and DGLA) incorporate into OP50 in higher amounts than the saturated fatty acid stearic acid (Figure 1A).
Additionally, L1 stage N2 larvae were grown on the same batch of supplemented plates and harvested after three days growth at 20 °C. Worms were washed off of the plates and fatty acids in total worm preps were analyzed by GC/MS. The change in supplemented fatty acids is graphed in Figure 1B. These studies demonstrate that supplementation of saturated fatty acids does not change the relative amount of saturated fatty acids in worm tissues, while supplementation of unsaturated fatty acids increased the relative amounts of unsaturated fatty acids in C. elegans lipids. Taken together, the data shown in Figure 1A and Figure 1B demonstrate that the relative accumulation of supplemented fatty acids in C. elegans correlates directly with the relative accumulation of fatty acids in the dietary E. coli.
We have previously shown that dietary DGLA causes sterility in C. elegans10. Figure 2 illustrates the dose response of DGLA induction of sterility in C. elegans. The concentration of DGLA in worm lipids in which 50% of the population will be sterile is approximately 12%. Interestingly, the response to DGLA can be altered by genetic mutations in C. elegans. A recent finding is that the insulin growth factor-dependent stress pathways can suppress the DGLA-induced germ cell destruction8. Supplementing the diet of worms containing deleterious mutations in either the daf-2 insulin/IGF receptor, daf-2(e1370), or the daf-16/FOXO transcription factor, daf-16(mu86), illustrates the usefulness of this method to unravel genetic pathways that influence the physiological effects of dietary fats. Synchronized L1 larvae were pipetted onto DGLA supplemented media. After 3-4 days of growth, worms were scored for sterility, as determined by the absence of eggs in the uterus of adult worms. DGLA supplemented daf-2(e1370)mutants were fertile, with little to no induced germ cell loss compared to wild type (N2) worms at both 0.15 mM and 0.3 mM supplementations (Figure 3).In contrast, DGLA supplemented worms with inactive FOXO (daf-16(mu86)) displayed a higher percentage of sterile worms compared to wild type when fed on plates containing 0.15 mM DGLA (Figure 3).

Figure 1. Uptake and incorporation of supplemented fatty acids by E. coli OP50 and C. elegans. A. E. coli OP50 was grown on plates containing 0.1 mM or 0.3 mM stearic acid, sodium oleate, or sodium DGLA as well as un-supplemented plates. After five days of growth on plates at 20 °C, E. coli were harvested and fatty acid methyl esters were generated for analysis by GC/MS. Because OP50 does not produce oleic acid or DGLA, and produces only trace amounts of stearic acid, the percentage of each supplemented fatty acid in the E. coli lipids reveals the ability of OP50 to incorporate the supplemented fatty acid. Error bars are SD. B. Change in C. elegans fatty acids in young adults grown for three days, starting at L1 stage, on E. coli plates containing 0.1 mM or 0.3 mM stearic acid, sodium oleate, or DGLA. The values for change in stearic acid and DGLA were obtained by subtracting the relative amount of 18:0 or 20:3 in worms grown on supplemented plates from those of worms grown on unsupplmented plates. To monitor uptake of oleic acid, the sum of oleic acid plus downstream C20 PUFAs (20:3, 20:4n-6, 20:4n-3, and 20:5) were calculated in supplemented and unsupplemented plates, because incorporated oleic acid is further desaturated and elongated. Error bars are SD. Click here to view larger image.

Figure 2. Increasing concentrations of DGLA in worm lipids correlate with increasing sterility in C. elegans. Wild type (N2) worms were treated with various concentrations of DGLA. The % DGLA in total worm lipids and the % of the population that is sterile is plotted for is plotted for 17 data points from five independent feeding experiments using dietary DGLA concentrations ranging from 0-0.3 mM DGLA. Click here to view larger image.
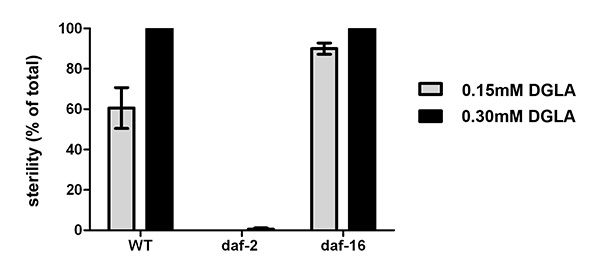
Figure 3. Physiological effects of supplementing C. elegans with DGLA. Starved L1 larval wild type, daf-2(e1370), or daf-16(mu86) were plated onto un-supplemented, 0.15 mM or 0.3 mM DGLA supplemented media and grown to the adult stage. At least 150 individual worms were then scored for sterility. Thedaf-2(e1370) mutants were almost completely fertile, even at 0.3 mM DGLA, while thedaf-16(mu86) mutants display an increased number of sterile worms compared to wild type at 0.15 mM DGLA. Error bars are SEM. Click here to view larger image.
Subscription Required. Please recommend JoVE to your librarian.
Discussion
Here we describe a method of supplementation of C. elegans with dietary unsaturated fatty acids. As mentioned above, care must be taken in the preparation of PUFA supplemented plates because the reactive nature of the double bonds in PUFAs causes these fatty acids to be sensitive to oxidation through heat and light11. To avoid oxidation, it is important to add the PUFA to the liquid agar medium after the media has cooled to 55 °C and stores plates in a dark environment.
Others have introduced free fatty acids to C. elegans using a DMSO or ethanol carrier, or else by directly adding fatty acids by microinjection into the vulva12-14. Partial rescue of mutant phenotypes was achieved by fatty acid supplementation of growth plates without the use of Tergitol(NP40)14-15. There are apparently a variety of effective ways to introduce fatty acid into C. elegans, although it is difficult to compare the efficiency of various methods because, in most experiments, the uptake of the fatty acids in the nematodes was not monitored. We find that our method allows consistent and efficient supplementation of unsaturated fatty acid to population sizes of a few nematodes to tens of thousands.
We have found it essential to monitor the uptake of supplemented fatty acids in C. elegans to aid in the interpretation of experimental outcomes. For example, the uptake of fatty acid by the bacteria food depends on the E. coli strain used as a food source for the nematodes. We have found that OP50 takes up greater amounts of unsaturated fatty acids than E. coli strains HT115, HB101, or NA22, strains commonly used for feeding RNAi experiments (HT115) or used for high density nematode growth (HB101 and NA22). Because of the variation in uptake of certain fatty acids, it is important to test several concentrations of fatty acid and to monitor uptake using gas chromatography. Most labs have achieved rescue of mutant phenotypes using fatty acid concentrations in the range of 0.08-0.2 mM5,6,15-18, however, effects on lifespan have been reported with supplementation of arachidonic acid at a concentration as low as 0.01 mM14, and others have used doses as high as 0.6 mM for rescue of a phenotype induced by feeding RNAi using the HT115 E. coli strain19. In our hands, supplementation of E. coli OP50 with very high concentrations of PUFAs, greater than 0.4 mM, result in excessive dietary PUFA accumulation in worm lipids, up to 50% of total fatty acids. This leads to nonspecific slow growth and abnormalities of the vulva.
A limitation of the technique is the inability to efficiently supplement with saturated fatty acids. Solubility issues at the time of adding the supplement to the media as well as the inefficient uptake and integration of saturated fatty acids in E. coli leave this method more suitable for supplementing unsaturated fatty acids (Figure 1).
Our method provides reproducible supplementation of mono- and polyunsaturated fatty acids, including fatty acids not normally produced by C. elegans, such as docosahexaenoic acid (DHA, 22:6n-3). Growth and neurological defects in PUFA-deficient mutants such as fat-3 can be rescued by relatively low levels of dietary PUFA5. Fatty acid supplementation can be used by researchers who wish to alter the fatty acid composition of C. elegans by dietary means in order to study a range of physiological processes.
Subscription Required. Please recommend JoVE to your librarian.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We thank Chris Webster for performing the preliminary experiments shown in Figure 3 of the representative results and Jason Watts and Chris Webster for helpful comments on the manuscript. Funding for this study was provided by a grant from the National Institutes of Health (USA) (R01DK074114) to JLW. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Materials
| Name | Company | Catalog Number | Comments |
| Bacto-Agar | Difco | 214010 | |
| Tryptone | Difco | 211705 | |
| NaCl | J.T. Baker | 3624-05 | |
| Tergitol | Sigma | NP40S-500mL | |
| Cholesterol | Sigma | C8667-25G | (5 mg/mL in ethanol) |
| MgSO4 | J.T. Baker | 2504-01 | |
| CaCl2 | J.T. Baker | 1311-01 | |
| K2HPO4 | J.T. Baker | 3254-05 | |
| KH2PO4 | J.T. Baker | 3246-05 | |
| Sodium dihomogamma linolenate | NuCHEK | S-1143 | |
| Warm sterile Millipore water | |||
| Sterile water for collecting worms | |||
| Nuclease-free Water for DGLA stock solution | Ambion | AM9932 | |
| Ampicillin | Fisher Scientific | BP1760-25 | 100 mg/ml in water (for RNAi plates) |
| Isopropyl-beta-D-thiogalactopyranoside (IPTG) | Gold Biotechnology | 12481C100 | 1 M in water (for RNAi plates) |
| HSO4 | J.T. Baker | 9681-03 | |
| Methanol | Fisher Scientific | A452-4 | |
| Hexane | Fisher Scientific | H302-4 | |
| diamindinophenylindole (DAPI) | Sigma | D9542 | |
| VectaShield | Vector Laboratories | H-1000 | |
| Glass Flask | Corning | 4980-2L | |
| Autoclaveable Glass bottles with stirbars | Fisherbrand | FB-800 | |
| Autoclaveable Glass Graduated Cylinder | Fisherbrand | 08-557 | |
| Stir Plate | VWR | 97042-642 | |
| Waterbath at 55+ °C | Precision Scientific Inc. | 66551 | |
| Screwcap Brown Glass Vial | Sun SRI | 200 494 | |
| Argon gas tank | |||
| Automated Pipette aid | Pipette-Aid | P-90297 | |
| Sterile Serological Pipettes (25 ml) | Corning | 4489 | |
| Bunsen Burner | VWR | 89038-534 | |
| Dissection microscope | Leica | TLB3000 | |
| Silanized glass tube | Thermo Scientific | STT-13100-S | for FAMEs derivitization |
| PTFE Screw caps | Kimble-Chase | 1493015D | |
| Clinical tabletop centrifuge | IEC | ||
| GC Crimp Vial | SUN SRi | 200 000 | |
| GC Vial Insert | SUN SRi | 200 232 | |
| GC Vial cap | SUN SRi | 200 100 | |
| Gas Chromatograph | Agilent | 7890A | |
| Mass Spectrometry Detector | Agilent | 5975C | |
| Column for gas chromatography | Suppelco | SP 2380 | 30 m x 0.25 mm fused silica capillary column |
References
- Haeggstrom, J. Z., Funk, C. D. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 111, 5866-5898 (2011).
- de Lorgeril, M., Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 10, 50 (2012).
- Spychalla, J. P., Kinney, A. J., Browse, J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 94, 1142-1147 (1997).
- Watts, J. L., Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 99, 5854-5859 (2002).
- Watts, J. L., Phillips, E., Griffing, K. R., Browse, J. Deficiencies in C20 Polyunsaturated Fatty Acids Cause Behavioral and Developmental Defects in Caenorhabditis elegans fat-3 Mutants. Genetics. 163, 581-589 (2003).
- Kahn-Kirby, A. H., et al. Specific Polyunsaturated Fatty Acids Drive TRPV-Dependent Sensory Signaling In Vivo. Cell. 119, 889-900 (2004).
- Brock, T. J., Browse, J., Watts, J. L. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2, e108 (2006).
- Webster, C. M., Deline, M. L., Watts, J. L. Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev. Biol. 373, 14-25 (2013).
- Kadyk, L. C., Lambie, E. J., Kimble, J. glp-3 is required for mitosis and meiosis in the Caenorhabditis elegans germ line. Genetics. 145, 111-121 (1997).
- Watts, J. L., Browse, J. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Dev. Biol. 292, 381-392 (2006).
- Pryor, W. A., Stanley, J. P., Blair, E. Autoxidation of polyunsaturated fatty acids: II. A suggested mechanism for the formation of TBA-reactive materials from prostaglandin-like endoperoxides. Lipids. 11, 370-379 (1976).
- Kniazeva, M., Shen, H., Euler, T., Wang, C., Han, M. Regulation of maternal phospholipid composition and IP(3)-dependent embryonic membrane dynamics by a specific fatty acid metabolic event in C. elegans. Genes Dev. 26, 554-566 (2012).
- Edmonds, J. W., et al. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell. 19, 858-871 (2010).
- O'Rourke, E. J., Kuballa, P., Xavier, R., Ruvkun, G. omega-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 27, 429-440 (2013).
- Taubert, S., Van Gilst, M. R., Hansen, M., Yamamoto, K. R. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 20, 1137-1149 (2006).
- Lesa, G. M., et al. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 116, 4965-4975 (2003).
- Brock, T. J., Browse, J., Watts, J. L. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 176, 865-875 (2007).
- Goudeau, J., et al. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol. 9, e1000599 (2011).
- Yang, F., et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 442, 700-704 (2006).



