Abstract
In two experiments, rats received pairings of an almond flavor (Experiments 1 and 2B) or a vanilla flavor (Experiment 2A) with sucrose. In each experiment, half of the rats received prior exposure to the flavor and half were exposed to water. Conditioned preference was then assessed through two-bottle, flavor versus water, choice tests. Latent inhibition (indicated by a weaker preference in pre-exposed subjects) was observed in the experiment using the vanilla flavor. However, facilitation (a stronger preference in pre-exposed subjects) instead of latent inhibition was evident with the almond flavor, both across acquisition trials and in the final choice test. These results indicate that, unlike most other paradigms of Pavlovian conditioning, conditioned stimulus pre-exposure in flavor preference learning may either facilitate or retard the acquisition (or the expression) of a conditioned flavor preference. We explore the proposal that the critical difference between the flavors lies in their hedonic values, with facilitation being more likely in a flavor that is initially disliked.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selection of which foods to eat or reject is one of the most critical behavioral choices required of an animal. Food and fluid selection is influenced by both innate and learned taste and flavor preferences. Most animals (e.g., Ramirez, 1990) have innate predispositions to accept some foods (e.g., sweet tasting) and reject others (e.g., bitter tasting) (Birch, 1999), but they also acquire feeding responses on the basis of experience. A preference for a flavor can be increased, for example, by pairing it with a stimulus with positive properties, such as sucrose. Flavor preference learning may enhance the hedonic value of foods (Myers & Sclafani, 2006; Sclafani & Ackroff, 2006) and it is mediated, in part, by brain neurochemical systems implicated in innate taste preferences and drug reward (Touzani, Bodnar, & Sclafani, 2010).
The acquisition of flavor preferences has been interpreted as an instance of classical conditioning, with the flavor being the conditioned stimulus (CS) and the positive event (e.g., sucrose) with which it is paired being the unconditioned stimulus (US). At least two different associations have been proposed to develop from such pairings: flavor–taste and flavor–nutrient learning (see, e.g., Fedorchak & Bolles, 1987; Myers & Sclafani, 2006; Sclafani & Ackroff, 1994). In flavor–taste learning, the critical association is between the representation of the flavor and the representation of the sensory properties of the taste of the US. Flavor–nutrient learning is based on the motivational properties of the US generated by its nutritive post-oral positive consequences (Sclafani, 2001). Which of these forms of learning controls performance, when the preference for the flavor is tested, seems to depend on motivational factors; flavor–nutrient preference is enhanced in hungry animals, but there is no evidence of this modulation on flavor–taste learning (Fedorchak & Bolles, 1987; Harris, Gorissen, Bailey, & Westbrook, 2000).
It has been suggested that flavor–nutrient learning involves a form of expectancy or predictive learning (e.g., Campbell, Capaldi, Sheffer, & Bradford, 1988; Drucker, Ackroff, & Sclafani, 1994) that depends on standard associative mechanisms, and thus obeys the standard laws of conditioning, being susceptible, for example, to blocking, latent inhibition, extinction, and so on (e.g., Garcia-Burgos & González, 2012; Garcia-Burgos, González, & Hall, 2013; Harris et al., 2000). On the other hand, flavor–taste learning has been thought to involve a mechanism that produces a change in the hedonic properties of the flavor (the flavor comes to “taste better”; Drucker et al., 1994); this mechanism could operate according to different laws (Campbell et al., 1988; De Houwer, Thomas, & Baeyens, 2001; Drucker et al., 1994; Pearce, 2002) making it resistant to blocking, extinction, and latent inhibition (Garcia-Burgos et al., 2013; González, Garcia-Burgos, & Hall, 2014; González, Morillas, & Hall, 2015, 2016; Harris et al., 2000; see also Harris, Shand, Carroll, & Westbrook, 2004). The present series of experiments focuses on the latent inhibition procedure.
Latent inhibition, retarded conditioning after prior exposure to the to-be-conditioned stimulus, can be obtained in flavor preference conditioning, but only in certain circumstances. In particular, its occurrence seems to depend on the motivational state of the rat. Thus, De la Casa, Marquez, and Lubow (2009) found latent inhibition in hungry rats, whereas Delamater (2011) found no effect in non-hungry animals (at least on initial testing – a difference was later observed when rats were tested after they had been given exposure to the US alone). The role of motivational state was explored in a series of experiments by Garcia-Burgos et al. (2013) using rats as the subjects and sucrose as the US. They found that acquisition of latent inhibition was independent of the motivational state of the rats during training, but its expression depended on the animal’s motivational state at the time of test. Latent inhibition was obtained when the rats were food-deprived during the preference test, but it was consistently absent when they were given free access to food before the test. They took this to support the hypothesis that flavor-nutrient learning, which would control performance when animals are hungry (Harris et al., 2000), is susceptible to latent inhibition, whereas flavor-taste learning, which controls performance when they are not, is immune to it.
This pattern of results is complicated by the recent finding of González et al. (2015, Experiment 2) that in some circumstances pre-exposure to a flavor can produce the opposite effect to latent inhibition – that is, an apparent facilitation of the acquisition of a conditioned preference. Given the normal robustness of the latent inhibition effect (see Lubow & Weiner, 2010), any instance of a reverse effect is surprising, and requires investigation. The procedure used in the experiment by González et al. differed in a number of ways from those just described. The animals were not food-deprived at any stage, and the substances used as USs were unusual (fructose for some animals and maltodextrin for others). In addition, the almond flavoring used as the CS came from a different supplier from that used in the experiments by Garcia-Burgos et al. (2013), and it produced a solution that the rats appeared to dislike, in that, given a free choice between this and water, they preferred water. (González et al., 2016, using this type of almond in a study of preference acquisition, noted that even after four pairings of this flavor and a sucrose US, the preference for water over the flavor was reduced but not actually reversed.) In explaining their finding of an apparent reversal of the latent inhibition effect, González et al. (2015) speculated that the use of a disliked flavor might be the critical factor. Latent inhibition training involves extensive exposure to the flavor and thus might be expected to allow habituation of an initial aversive response. This factor, which would increase the likelihood of consumption on the test, could be enough to outweigh the retardation of conditioning that such exposure also produces.
Examination of this suggestion requires further work in which factors other than the nature of the CS are controlled. In the present series of experiments, we used just a single reinforcer the effectiveness of which has proved to be high in previous experiments (i.e., sucrose), and we arranged in all the experiments for the rats to be hungry at the time of the test (the motivational state in which a standard latent inhibition effect is most likely to be obtained). This allowed us to focus on the interaction between CS pre-exposure and the nature of the CS flavor. In Experiment 1 we used the same almond flavor as that used by González et al. (2015) in order to determine if the reversal of the latent inhibition effect could be obtained in these conditions. Experiments 2A and 2B allowed a comparison between the effects obtained with this flavor as the CS and another, a vanilla flavor that was known to be hedonically neutral prior to conditioning. In each experiment, half of the animals received prior exposure to the CS, and half received no CS pre-exposure (they were exposed to water during this phase). Subsequently, all animals received pairings of the CS with sucrose. Preference was then assessed through two-bottle, flavor versus water, choice tests.
Experiment 1
Rats were divided in two groups, one receiving pre-exposure to the flavor to be used as the CS (group Pre, for pre-exposed to the flavor), and the other given pre-exposure only to water (group NPre, for not pre-exposed to the flavor). The flavor (almond) was the same as that used in the previous studies (González et al., 2015, 2016) in which an initial aversion was observed. All subjects then received a phase of training in which the flavor was presented mixed with a sucrose solution. Afterwards, preference was assessed through a two-bottle choice test pitting the CS flavor against unflavored water (see Table 1). In order to ensure that the rats drank the flavored solution during the pre-exposure phase, they were maintained on a schedule of restricted access to water, but were given free access to food, as food-deprived rats tend to drink rather little. They were, however, deprived of food prior to the test phase, as latent inhibition is more likely to be obtained when the rats are hungry (Garcia-Burgos et al., 2013).
Method
Subjects and apparatus
The subjects were 16 naïve male Wistar rats (Janvier, France) with a mean body weight of 336 g at the start of the experiment. They were housed in individual home cages and maintained in a temperature-controlled room (21 °C) on a 12:12 h light-dark cycle (lights on at 8:00 a.m.) at the Biomedical Research Center of the University of Granada. Experimental procedures took place with the rats in their home cages during the light period of the cycle. The drinking solutions were presented using inverted 50-ml plastic tubes equipped with stainless steel ball-bearing-tipped spouts. Consumption was estimated by weighing the tubes before and after fluid presentation to the nearest 0.1 g. The solutions used were made up with tap water at room temperature and consisted of a 1% (v/v) solution of almond essence (Shepcote Distributors Ltd, Yorkshire, UK) during the pre-exposure and test phases, and a compound of 1% almond and 10% (w/v) sucrose (AB Azucarera Iberia S.L., Madrid, Spain) during conditioning.
Procedure
To initiate the deprivation schedule, water bottles were removed 24 h before the start of the experiment. The rats were then given 3 days to accommodate to a schedule in which they had daily access to water for 30 min at 10:00 a.m. and 2:00 p.m. Food was removed during experimental sessions but was otherwise freely available, with the exception of the test sessions (see below). The rats were randomly allocated to two weight-matched groups: Group Pre (n = 8) and group NPre (n = 8), for the flavor pre-exposure phase. This phase consisted of one single daily trial (at 10:00 a.m.) over 8 days. Each trial consisted of 10-min access to 10 ml of the flavor (for animals in the Pre condition) or water (for animals in the NPre condition) followed by free access to water for 30 min.
Following the procedure of Garcia-Burgos et al. (2013), the conditioning and test phases were run as a series of 3-day cycles. In each of these cycles, there were two conditioning trials, at 10:00 a.m. on each of two days, on which animals had 10-min access to 10 ml of the 1% almond + 10% sucrose compound. Food was removed during this 10-min period to avoid any pairing of the flavor with the standard diet. After the second conditioning session (the day before the test) the rats had 30-min access to water at 5:30 p.m., instead of at 2:00 p.m., and food was then removed from the cages at 6:00 p.m. Therefore, they were conditioned while thirsty but tested both thirsty and hungry. The test phase consisted of 15-min access to two bottles at 10:00 a.m. the next day, one containing 20 ml of almond and the other 20 ml of water. This conditioning-test cycle was conducted three times in total. After this phase (see Table 1), the preference was assessed over the course of extinction through three additional two-bottle tests (one per day), again with the animals hungry. The positions of the bottles during tests were counterbalanced across subjects and cycles. All procedures were approved by the University of Granada Ethics Committee for Animal Research and were carried out in accordance with the EU Directive 2010/63/EU for animal experiments.
Results Footnote 1 and discussion
Although the almond flavoring used in this study is less preferred than water in a choice test, our thirsty rats drank it as readily as water in the conditions of the pre-exposure phase, in which just a single bottle was available. Over the course of the pre-exposure phase (see Table 2), mean consumption (g) of almond for the Pre group was 8.5; for the NPre group the mean water consumption was 8.9. A 2 (Pre-exposure) × 8 (Trial) mixed ANOVAFootnote 2 yielded no significant main effect of group, F(1, 14) = 1.73, p = .209, or trial, F(3.28, 45.98) = 1.12, p = .352, nor of the interaction, F < 1.
For the three two-trial conditioning cycles, mean consumption (g) of the almond + sucrose compound over the six conditioning trials was 9.6, 9.7, 9.8, 9.8, 9.8, and 9.8, for group Pre; and 6.4, 8.9, 9.0, 9.6, 9.8, and 9.8, for group NPre. A 2 (group) × 6 (trial) ANOVA yielded a main effect of trial, F(2.05, 28.68) = 8.19, p = .001, ηp2 = .369, and a Group × Trial interaction , F(2.05, 28.68) = 6.19, p < .01, ηp2 = .307. The main effect of group fell short of the conventional level of significance, F(1, 14) = 4.48, p = .053, ηp2 = .242. The source of the interaction lies in the neophobic response to the flavor + sucrose compound in group NPre on the first conditioning trial. On this trial the groups differed significantly, t(14) = 3.43, p = .004, d = 1.72; no other between-group differences were found on conditioning trials (lowest p = .304).
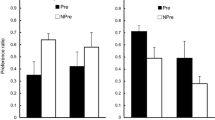
Preference during the tests was expressed as a ratio (consumption of the flavor/total consumption; see Table 3 for raw consumption scores). Ratio scores for the three tests given during the acquisition cycles are presented in the left panel of Fig. 1. It is evident that both groups showed an increase in consumption of the flavor over these trials, but that only the pre-exposed group developed a substantial preference. A 2 (group) × 3 (test trial) mixed ANOVA showed a significant main effect of group, F(1, 14) = 6.20, p = .026, ηp2 = .307, and of trial, F(1.81, 25.29) = 3.53, p = .048, ηp2 = .202. The Holm-Bonferroni post hoc test found no differences among tests, lowest p = .086, and the Group × Trial interaction was not significant, F < 1. The difference between groups was maintained over the three extinction tests as shown in the right panel of Fig. 1. A 2 (group) × 3 (trial) mixed ANOVA showed a significant main effect of group, F(1, 14) = 11.18, p = .005, ηp2 = .444. The main effect of trial and the interaction with group were not significant, Fs < 1.
Experiment 1 . Mean preference ratios (±SEM) for the flavor (almond) vs. water choice tests shown separately for the Pre-exposed (Pre) and Non-pre-exposed (NPre) groups for the acquisition (Tests 1, 2, and 3) and extinction (Ext 1, 2, and 3) tests
To an extent, these results accord with those of González et al. (2015) who, in their Experiment 2, found evidence of facilitation after pre-exposure to this almond flavor in rats trained with fructose or with maltodextrin as the US. That experiment differed from the present experiment not only in the nature of the reinforcer, but also in the fact the rats were not food-deprived at any stage; when the rats were hungry on test, latent inhibition was obtained. Evidently the effects of pre-exposure depend not just on the nature of the CS but on interactions involving motivational state and the nature of the US. For the time being, however we restrict our analysis to the case in which the US is sucrose and the animals are hungry on test.
Experiments 2A and 2B
The purpose of these experiments was twofold. The first was to replicate the facilitation effect seen in Experiment 1, and to extend its generality. Specifically, the procedure used in Experiment 1 involved a change in motivational state from pre-exposure (and conditioning) to the test. Although latent inhibition has been obtained in these conditions (Garcia-Burgos et al., 2013), it is well established that the effect is sensitive to changes in context (e.g., Hall & Channell, 1986) and a change of motivational state might act as such. Holland (2017), who has also reported a case of facilitated learning after pre-exposure to the CS (see General discussion ), has suggested that changing the context may contribute to the effect observed in his experiments. In this experiment, therefore, we avoided this complication by maintaining the rats on a schedule of food deprivation throughout the entire experiment.
Second, we need to confirm that the effect seen in Experiment 1 is indeed a consequence of the nature of the flavor used as the CS. González et al. (2016), having noted that this particular almond flavor was initially disliked by rats, sought alternatives, and found weaker solutions of flavorings from a different supplier that were initially neutral but that supported flavor preference conditioning. Accordingly, we included groups in this experiment that used a neutral flavor (vanilla) as the CS. We expected to find a standard latent inhibition effect in these subjects alongside the facilitation expected for the subjects trained with the almond flavor used in Experiment 1.
The study was run in as two separate sub-experiments that differed only in that Experiment 2A used the neutral, vanilla, flavor, and Experiment 2B the almond flavor. Therefore, there were four groups of rats, two of which were given pre-exposure to the flavor to be used as the CS (the Pre groups) and two given no pre-exposure (the NPre groups). One pair of groups (the A groups) received the almond solution as the CS; the other pair (the V groups) received a vanilla solution (see Table 1). All were hungry (as well as thirsty) during the whole procedure. As in the previous experiments, the final test phase consisted of a choice test between the flavor and unflavored water.
Method
The subjects were 32 male naïve Wistar rats (Janvier, France). The mean body weight at the start of the experiment was 341 g for rats in the V groups (Experiment 2A) and 413 g for rats in the A groups (Experiment 2B). The housing, apparatus, and general maintenance procedures for both experiments were the same as described for Experiment 1. The sucrose and almond solutions were the same as were used in Experiment 1. The flavor used in the V condition was 0.035% (v/v) vanilla concentrate essence (supplied by Manuel Riesgo, S.A., Spain).
To initiate the deprivation schedule, food and water were removed 24 h before the start of the experiment. The rats were then given 3 days to accommodate to a schedule in which they had daily access to water for 30 min at 10:00 a.m. and access to water and food for 90 min at 1:30 p.m.; this additional access to water and food during the afternoon was maintained throughout the experiment.
The rats were then allocated to two weight-matched groups: Group Pre and group NPre (n = 8 in each sub-experiment), for the flavor pre-exposure phase. This phase consisted of one single daily 10-min trial across eight days in which the animals in the Pre/V condition were exposed to the vanilla flavor, whereas those in the Pre/A condition received the almond solution used in Experiment 1. Animals in the NPre conditions were exposed to water.
Conditioning occurred over 2 days (one trial a day at 10:00 a.m.). In each trial, animals had 10-min access to 10 ml of either vanilla + sucrose (groups V) or almond + sucrose (groups A). Testing took place over 3 days, with one test each day at 10:00 a.m., consisting of 15-min access to two bottles, one containing 20 ml of the flavor used as the CS and the other 20 ml of water. The positions of the bottles were counterbalanced across subjects and tests. In details not specified here, the procedure described for Experiment 1 was followed.
Results
Experiment 2A
V groups. Mean consumption (g) of water across pre-exposure days in the Npre group was 2.6; that of the vanilla solution for the Pre group was 3.1 (see Table 2, for more details). A 2 (group) × 8 (trial) mixed ANOVA did not yield any significant effect; largest F(4.06, 56.92) = 1.35, p = .261. Both groups showed signs of neophobia to the vanilla + sucrose solution on the first conditioning trial, but drank it readily on the second. Mean consumption (g) of the vanilla + sucrose compound in group Pre was 4.5 on the first trial and 9.1 on the second; equivalent scores for group Npre were 4.1 and 9.3. A 2 (group) × 2 (trial) ANOVA yielded a main effect of trial, F(1, 14) = 104.49, p < .001, ηp2 = .882. The main effect of group and the interaction were not significant, Fs < 1.
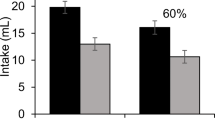
Absolute amounts consumed during the three test sessions are shown in Table 3. These scores generate the mean preference ratios (consumption of the flavor/total consumption) shown on the left in Fig. 2. It is evident that both groups showed a preference for the flavor, but that the Npre group showed a stronger preference than the Pre group, a difference that was maintained over the three test trials. Thus, a latent inhibition effect was observed. A mixed ANOVA, with group as the between-subject factor and trial as the within-subject factor, yielded a significant main effect of group, F(1, 14) = 7.52, p < .05, ηp2 = .350. The effects of trial and the interaction were not significant, largest F(1.91, 26.72) = 1.34, p = .277.
Experiment 2B
A groups. Mean consumption (g) of water across pre-exposure days for the Npre group was 4.1; that of almond for group Pre was 4.2 (see Table 2, for more details). A 2 (group) × 8 (trial) mixed ANOVA yielded no significant effects, all Fs < 1. Again, as for the V groups, there was evidence of neophobia on the first conditioning trial, although the effect was sizeable only in group NPre. Mean consumption (g) of the almond + sucrose compound for group Pre was 7.9 on the first trial and 8.5 on the second trial; the scores for group Npre were 6.0 and 8.9. A 2 (group) × 2 (trial) ANOVA yielded a main effect of trial, F(1, 14) = 24.30, p < .001, ηp2 = .634, and a Group × Trial interaction, F(1, 14) = 10.68, p < .01, ηp2 = .433. The main effect of group was not significant, F < 1. We explored the interaction by performing pairwise comparisons using t-tests for paired samples. Consumption during conditioning increased from the first to the second trial significantly in group NPre, t(7) = - 4.34, p < .01, d = 1.53, but not in group Pre t(7) = - 2.57, uncorrected p = .038.
Preference scores for the three test trials (see Table 3 for direct consumption measures) are presented in Fig. 2 (right section). Group Pre showed a strong and roughly maintained preference. Group Npre showed evidence of a preference only on the first test trial, but this seemed to be lost on subsequent trials, presumably as a consequence of extinction produced by testing with the flavor alone in the absence of the US. Thus pre-exposure facilitated the acquisition (or display) of a preference for almond in these subjects; that is a reverse of the latent inhibition effect was obtained. A mixed ANOVA, with group as the between-subject factor and trial as a within-subject factor, yielded significant main effects of group, F(1, 14) = 4.84, p < .05, ηp2 = .257, and of trial, F(1.79, 25.01) = 6.18, p < .01, ηp2 = .306; the Holm-Bonferroni post hoc test found differences between the first and the second trials. The interaction was not significant, F < 1.
Discussion
These test results confirm the findings of Experiment 1 showing a stronger preference on test in pre-exposed subjects than in non-pre-exposed subjects when the CS flavor was almond. In fact, in neither experiment was there good evidence of preference acquisition in the Npre group trained with this flavor. With exception of the first test trial of Experiment 2B, all preference scores for Npre subjects were close to or below .5. For subjects given the vanilla flavor, on the other hand, preferences (mean ratio scores above .5) were obtained in both Pre and NPre groups, with the exception of the third test in group Pre; but in these a standard latent inhibition effect was obtained, with the pre-exposed subjects showing a lesser preference than the non-pre-exposed subjects.
General discussion
The results reported here demonstrate that the effects of prior exposure to the flavor to be used as the CS in flavor preference conditioning depend on the nature of the flavor used. Specifically, with our vanilla solution, a standard latent inhibition effect was obtained; but with our almond solution (one that rats initially dislike) subjects given prior exposure show a facilitation on the test of the conditioning produced by pairing the almond with sucrose.
The results from Experiment 2A, rats trained with the vanilla flavor, accord with previous work on the effects of CS pre-exposure in this conditioning paradigm. Some, in which the rats have not been hungry on test, have failed to find a latent inhibition effect (Delamater, 2011, in the initial test; Garcia-Burgos et al., 2013, Experiments 1, 2, and 3; González et al., 2015, Experiment 2). But the effect has been found in experiments using flavors thought to be neutral and rats that were hungry (and thirsty) throughout all experimental phases (De la Casa et al., 2009; Garcia-Burgos et al., 2013, Exp. 1), or during the test phase (Garcia-Burgos et al., 2013, Exp. 2). Thus, our results using the vanilla flavor are consistent with previous studies. These results have been taken to support the hypothesis that, when animals are hungry on test, flavor preference is based on a flavor-nutrient association, and that this kind of learning is susceptible to latent inhibition.
The striking finding from our present study, however, is that pre-exposure with the almond flavor as the CS not only did not produce latent inhibition, it induced the opposite effect – an apparent facilitation of flavor preference conditioning. The only other example of such facilitation in flavor preference conditioning that we are aware of is found in Experiment 2 of González et al. (2015), in which the same almond solution was used along with fructose and maltodextrin as USs. In that experiment the rats were not food deprived – and latent inhibition was, in fact, found in González et al.’s Experiment 1, when they were. The role of motivational state and its interaction with the nature of the reinforcer is unclear at this stage. However, the fact that facilitation of flavor preference conditioning after flavor pre-exposure has been found both in thirsty (González et al., 2015; Experiment 2) as well as in hungry (and thirsty) animals (the present experiments) suggests that, whatever the underlying facilitation mechanism may be, it does not seem to depend on the motivational state at the time of testing, unlike that controlling the expression of latent inhibition. Facilitation of conditioning by prior exposure of the CS flavor may be a wider and more general effect in which acceptance of an initially less preferred flavor increases with repeated experience, provided that the stimulus has no potentially aversive consequences.
In orthodox studies of associative learning, a facilitating effect of CS pre-exposure has previously been observed only under very specific conditions. Thus Holland (2017), using an appetitive conditioning procedure with rats as subjects, has found that pre-exposure to an auditory or a visual cue can produce facilitation, but only when the response measure during conditioning was time spent in the food cup (other measures showing no effects or standard latent inhibition). The effect was found only when two cues (one pre-exposed, one not) were presented during conditioning, or, in the case of the auditory cue, when pre-exposure and conditioning occurred in a very quiet context. Holland offered a partial explanation for these effects in terms of the interaction between the unconditioned and conditioned responses controlled by the stimuli (see below), although he acknowledged that these considerations could not accommodate all features of his results.
Examples of facilitation have been found with flavor stimuli, but again, only in restricted conditions. Pre-exposure has been found to facilitate subsequent olfactory (Hoffmann & Spear, 1989) and taste aversion conditioning (Chotro & Alonso, 2001; Gaztañaga, Aranda-Fernández, Díaz-Cenzano, & Chotro, 2015) in infant rats. This effect has usually been explained in terms of a deficiency in processing the stimulus, due to the immaturity of the sensory system of the infant rat; previous experience with the CS would allow a better processing of the stimulus. This has not been supposed to operate with adult animals. Facilitation of conditioned taste aversion after CS pre-exposure has been found in adult rats when only a small amount of the flavor solution was offered during a single-trial conditioning procedure using a complex flavor compound as CS (Bennett, Tremain, & Mackintosh, 1996). This effect was attributed to the occurrence of stimulus unitization (McLaren, Kaye, & Mackintosh, 1989) during pre-exposure, rendering the flavor more effective as a CS (see also Fanselow, 1990, for a similar analysis of a facilitation effect obtained in fear conditioning to contextual cues). This seems unlikely to be the source of the effect seen in our experiments. Both of our flavor stimuli were similar in (presumably, rather low) complexity. Further, they were presented to the subjects under identical conditions during training, using standard procedures in terms of number of trials and the amount of solution provided. It is difficult to see why one should be sensitive to a unitization process and the other not.
In considering explanations for his results, Holland (2017) explored the notion that pre-exposure to a CS might have two opposed effects, one that produces latent inhibition and one that facilitates learning, and that the outcome of an experiment would depend on factors that determine the balance of the two. One possibility for his procedure (although he acknowledged that it could not supply an explanation for the entire pattern of results) was that pre-exposure might allow habituation of a competing response evoked initially by the stimulus, and that the elimination of this competition could be enough to overcome the retardation of learning that was also occurring. In Holland’s case the competing response was taken to be an orienting response. In our experiments the obvious candidate would be an unconditioned aversive response that tended to suppress consumption of an unpreferred flavor. Pre-exposure would allow habituation of this response: it would also produce latent inhibition, but the consumption of the flavor shown by non-pre-exposed subjects, which did not undergo habituation, might still be less than that of pre-exposed subjects. It should be acknowledged, however, that in the present experiments there was rather little evidence for a difference between the flavors in measures of consumption taken before the final test. It is true that only with the almond flavor did non-pre-exposed animals drink less of the flavor + sucrose compound than pre-exposed animals during the first conditioning trial, indicating, albeit indirectly, that acceptance for this flavor may increase as a consequence of pre-exposure; no such difference was found when the flavor was neutral. But other measures failed to reveal any difference between the flavors. In particular, initial consumption of an unpreferred flavor might be expected to be low at the start of the pre-exposure phase and increase over the pre-exposure trials; but in none of our studies did we find that the amount consumed (of either of the flavors) varied across pre-exposure trials. We acknowledge that this null result might simply reflect the insensitivity of a test in which a single bottle of fluid is offered to a thirsty rat.
Although consumption did not increase during pre-exposure, it remains possible that pre-exposure to an unpreferred CS flavor may increase its palatability (consumption by thirsty animals being an insensitive measure of this). Even though no effects of pre-exposure have been found on palatability using the taste reactivity test procedure (Neath, Limebeer, Reilly, & Parker, 2010), studies using microstructural analysis of the licking response have produced positive results. In a recent study, Lin, Amodeo, Arthurs, and Reilly (2012) found that the cluster size of licks for tastants such as saccharin and quinine became larger as familiarity with the flavors increased with exposure to them. Lin et al. concluded that the pleasure of drinking increased (or the dislike decreased) as the novel, and potentially dangerous, tastant was repeatedly presented without consequences, and became accepted as safe. This proposal prompts the suggestion that it is the change from “potentially dangerous” to “safe” that is responsible for the facilitation effect seen with an initially unpreferred flavor. We can only speculate as to why this should occur, but the following possibilities deserve further consideration.
First, it has been shown in studies of latent inhibition that the effect is reduced in the presence of a negative affective state. Thus, exposing human participants to a negative affect video clip (Lazar, Kaplan, Sternberg, & Lubow, 2012), or inducing in rats emotional stress (e.g., Smith, Fieser, Jones, & Schachtman, 2008) or negative affect using a consummatory successive negative contrast procedure (De la Casa, Mena, Ruiz-Salas, Quintero, & Papini, 2017) all impair latent inhibition. Consuming an unpleasant flavor may be, in itself, a source of negative affect, which could interfere with the normal development of latent inhibition (although it is less clear that the effect would be reversed). A second possibility emerges if we assume that there are constraints on learning (see, e.g., Garcia, Brett, & Rusiniak, 1989) such that an association between a dangerous event and a sweet and nutritive consequence is difficult to form. If so, then pre-exposure to such an event, rendering it safe, would allow learning to occur when otherwise it would not. Even if the processes responsible for the latent inhibition effect begin to operate during exposure to an unpreferred flavor, conditioning with this flavor as the CS can be expected to be superior to that shown to a novel CS, which will be barely able to support any conditioning at all. It will require further evidence to test the value of these suggestions. We turn, therefore, in conclusion, to the more general picture.
Latent inhibition is a ubiquitous effect in associative learning, found in a variety of experimental preparations with different stimuli (Lubow & Weiner, 2010). That exposure to a stimulus should reduce its effectiveness as a CS is readily accepted. Equally, however, it is unsurprising that exposure of an unpreferred flavor (at least, one that turns out to be safe) should promote its acceptance and enhance preference for it. This effect of mere exposure has also been found consistently in human eating behavior, both in adults (Pliner, 1982), and, especially, among young children (e.g., Birch & Marlin, 1982; Hausner, Olsen, & Møller, 2012; see Cooke, 2007, for a review), although the factors that moderate the effect are not yet well understood (see Montoya, Horton, Vevea, Citkowicz, & Lauber, 2017). The results presented here thus highlight the importance of taking into account the hedonic value of a flavor in order to understand the effect of pre-exposure on later conditioning. Unpreferred flavors will benefit from pre-exposure in the acquisition of a preference through subsequent conditioning. However, no such benefit, rather impairment, may be predicted for a neutral flavor, for which a latent inhibition effect is more likely to be found.
References
Bennett, C. H., Tremain, M., & Mackintosh, N. J. (1996). Facilitation and retardation of flavour aversion conditioning following prior exposure to the CS. The Quarterly Journal of Experimental Psychology Section B, 49, 220–230. https://doi.org/10.1080/713932632
Birch, L. L. (1999). Development of food preferences. Annual Review of Nutrition, 19, 41–62. https://doi.org/10.1146/annurev.nutr.19.1.41
Birch, L. L., & Marlin, D. W. (1982). I don’t like it; I never tried it: Effects of exposure on two-year-old children’s food preferences. Appetite, 3, 353–360. https://doi.org/10.1016/S0195-6663(82)80053-6
Campbell, D. H., Capaldi, E. D., Sheffer, J. D., & Bradford, J. P. (1988). An examination of the relationship between expectancy learning and preference conditioning. Learning and Motivation, 19, 162–182. https://doi.org/10.1016/0023-9690(88)90011-2
Chotro, M. G., & Alonso, G. (2001). Some parameters of stimulus pre-exposure that affect conditioning and generalization of taste aversions in infant rats. International Journal of Comparative Psychology, 14, 25–43. https://escholarship.org/uc/item/8g65x823
Cooke, L. (2007). The importance of exposure for healthy eating in childhood: A review. Journal of Human Nutrition and Dietetics, 20, 297–301. https://doi.org/10.1111/j.1365-277X.2007.00804.x
De Houwer, J., Thomas, S., & Baeyens, F. (2001). Associative learning of likes and dislikes: A review of 25 years of research on human evaluative conditioning. Psychological Bulletin, 127, 853–869. https://doi.org/10.1037//D033-29O9.127.6.853
De la Casa, L. G., Marquez, R., & Lubow, R. E. (2009). Super-latent inhibition of conditioned taste preference with a long retention interval. Learning and Motivation, 40, 329–342. https://doi.org/10.1016/j.lmot.2009.03.001
De la Casa, L. G., Mena, A., Ruiz-Salas, J. C., Quintero, E., & Papini, M. R. (2017). Reward devaluation disrupts latent inhibition in fear conditioning. Learning & Behavior, 46, 49–59. https://doi.org/10.3758/s13420-017-0282-1
Delamater, A. R. (2011). Partial reinforcement and latent inhibition effects on stimulus-outcome associations in flavor preference conditioning. Learning & Behavior, 39, 259–270. https://doi.org/10.3758/s13420-011-0026-6
Drucker, D. B., Ackroff, K., & Sclafani, A. (1994). Nutrient-conditioned flavor preference and acceptance in rats: Effects of deprivation state and non-reinforcement. Physiology & Behavior, 56, 701–707. https://doi.org/10.1016/0031-9384(94)90230-5
Fanselow, M. S. (1990). Factors governing one-trial contextual conditioning. Animal Learning & Behavior, 18, 264–270. https://doi.org/10.3758/BF03205285
Fedorchak, P. M., & Bolles, R. C. (1987). Hunger enhances the expression of calorie- but not taste-mediated conditioned flavor preferences. Journal of Experimental Psychology: Animal Behavior Processes, 13, 73–79. https://doi.org/10.1037/0097-7403.13.1.73
Garcia, J., Brett, L. P., & Rusiniak, K. W. (1989). Limits of Darwinian conditioning. In S. B. Klein & R. R. Mowrer (Eds.), Contemporary learning theories: Instrumental conditioning theory and the impact of biological constraints on learning. (pp. 181–203). Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc.
Garcia-Burgos, D., & González, F. (2012). Posttraining flavor exposure in hungry rats after simultaneous conditioning with a nutrient converts the CS into a conditioned inhibitor. Learning & Behavior, 40, 98–114. https://doi.org/10.3758/s13420-011-0048-0
Garcia-Burgos, D., González, F., & Hall, G. (2013). Motivational control of latent inhibition in flavor preference conditioning. Behavioural Processes, 98, 9–17. https://doi.org/10.1016/j.beproc.2013.04.010
Gaztañaga, M., Aranda-Fernández, P. E., Díaz-Cenzano, E., & Chotro, M. G. (2015). Latent inhibition and facilitation of conditioned taste aversion in preweanling rats. Developmental Psychobiology, 57, 96–104. https://doi.org/10.1002/dev.21263
González, F., Garcia-Burgos, D., & Hall, G. (2014). Analysis of blocking of flavor-preference conditioning based on nutrients and palatable tastes in rats. Appetite, 80, 161–167. https://doi.org/10.1016/j.appet.2014.05.010
González, F., Morillas, E., & Hall, G. (2015). Latent inhibition in flavor-preference conditioning: Effects of motivational state and the nature of the reinforcer. Learning & Behavior, 43, 376–383. https://doi.org/10.3758/s13420-015-0185-y
González, F., Morillas, E., & Hall, G. (2016). The extinction procedure modifies a conditioned flavor preference in nonhungry rats only after revaluation of the unconditioned stimulus. Journal of Experimental Psychology: Animal Learning and Cognition, 42, 380–390. https://doi.org/10.1037/xan0000108
Hall, G., & Channell, S. (1986). Context specificity of latent inhibition in taste aversion learning. The Quarterly Journal of Experimental Psychology Section B, 38, 121–139. https://doi.org/10.1080/14640748608402224
Harris, J. A., Gorissen, M. C., Bailey, G. K., & Westbrook, R. F. (2000). Motivational state regulates the content of learned flavor preferences. Journal of Experimental Psychology: Animal Behavior Processes, 26, 15–30. https://doi.org/10.1037/0097-7403.26.1.15
Harris, J. A., Shand, F. L., Carroll, L. Q., & Westbrook, R. F. (2004). Persistence of preference for a flavor presented in simultaneous compound with sucrose. Journal of Experimental Psychology: Animal Behavior Processes, 30, 177–189. https://doi.org/10.1037/0097-7403.30.3.177
Hausner, H., Olsen, A., & Møller, P. (2012). Mere exposure and flavour-flavour learning increase 2-3year-old children’s acceptance of a novel vegetable. Appetite, 58, 1152–1159. https://doi.org/10.1016/j.appet.2012.03.009
Hoffmann, H., & Spear, N. E. (1989). Facilitation and impairment of conditioning in the preweanling rat after prior exposure to the conditioned stimulus. Animal Learning & Behavior, 17, 63–69. https://doi.org/10.3758/BF03205213
Holland, P.C. (2017). Stimulus pre-exposure speeds or slows subsequent acquisition of associative learning depending on learning test procedures and response measure. Learning & Behavior https://doi.org/10.3758/s13420-017-0297-7
Lazar, J., Kaplan, O., Sternberg, T., & Lubow, R. E. (2012). Positive and negative affect produce opposing task-irrelevant stimulus pre-exposure effects. Emotion, 12, 591–604. https://doi.org/10.1037/a0024867
Lin, J. Y., Amodeo, L. R., Arthurs, J., & Reilly, S. (2012). Taste neophobia and palatability: The pleasure of drinking. Physiology & Behavior, 106, 515–519. https://doi.org/10.1016/j.physbeh.2012.03.029
Lubow, R. E., & Weiner, I. (2010). Issues in latent inhibition research and theory: An overview. In R. E. Lubow and I. Weiner (Eds.), Latent Inhibition: Cognition, neuroscience and applications to schizophrenia (pp. 531–557). Cambridge, UK. Cambridge University Press. https://doi.org/10.1017/CBO9780511730184.023
McLaren, I. P. L., Kaye, H., & Mackintosh, N. J. (1989). An associative theory of the representation of stimuli: Applications to perceptual learning and latent inhibition. In R. G. M. Morris (Ed.), Parallel distributed processing: Implications for psychology and neurobiology (pp. 102–130). Oxford: Oxford University Press.
Montoya, R. M., Horton, R. S., Vevea, J. L., Citkowicz, M., & Lauber, E. A. (2017). A re-examination of the mere exposure effect: The influence of repeated exposure on recognition, familiarity, and liking. Psychological Bulletin, 14, 459–498. https://doi.org/10.1037/bul0000085
Myers, K. P., & Sclafani, A. (2006). Development of learned flavor preferences. Develpmental Psychobiology, 48, 380–388. https://doi.org/10.1002/dev.20147
Neath, K. N., Limebeer, C. L., Reilly, S., & Parker, L. A. (2010). Increased liking for a solution is not necessary for the attenuation of neophobia in rats. Behavioral Neuroscience, 124, 398–404. https://doi.org/10.1037/a0019505
Pearce, J. M. (2002). Evaluation and development of a connectionist theory of configural learning. Animal Learning & Behavior, 30, 73–95. https://doi.org/10.3758/BF03192911
Pliner, P. (1982). The effects of mere exposure on liking for edible substances. Appetite, 3, 283–290. https://doi.org/10.1016/S0195-6663(82)80026-3
Ramirez, I. (1990). What do we mean when we say “palatable food”? Appetite, 14, 159–161. https://doi.org/10.1016/0195-6663(90)90085-M
Sclafani, A. (2001). Post-ingestive positive controls of ingestive behavior. Appetite, 36, 79–83. https://doi.org/10.1006/appe.2000.0370
Sclafani, A., & Ackroff, K. (1994). Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiology & Behavior, 56, 399–405. https://doi.org/10.1016/0031-9384(94)90213-5
Sclafani, A., & Ackroff, K. (2006). Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiology & Behavior, 88, 88–94. https://doi.org/10.1016/j.physbeh.2006.03.009
Smith, S., Fieser, S., Jones, J., & Schachtman, T. R. (2008). Effects of swim stress on latent inhibition using a conditioned taste aversion procedure. Physiology & Behavior, 95, 539–541. https://doi.org/10.1016/j.physbeh.2008.06.014
Touzani, K., Bodnar, R. J., & Sclafani, A. (2010). Neuropharmacology of learned flavor preferences. Pharmacology Biochemistry and Behavior, 97, 55–62. https://doi.org/10.1016/j.pbb.2010.06.001
Acknowledgements
This research was supported by grants #PSI2012-33552 from the Ministerio de Economía y Competitividad (MINECO, Spain) and #PSI2015-64345-R (MINECO-FEDER, Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictionalclaims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morillas, E., González, F. & Hall, G. Facilitation and retardation of flavor preference conditioning following prior exposure to the flavor conditioned stimulus. Learn Behav 47, 177–186 (2019). https://doi.org/10.3758/s13420-018-0368-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-018-0368-4