Abstract
Inhibitory control, the ability to restrain a prepotent but ineffective response in a given context, is thought to be indicative of a species’ cognitive abilities. This ability ranges from “basic” motoric self-regulation to more complex abilities such as self-control. During the current study, we investigated the motoric self-regulatory abilities of 30 pet dogs using four well-established cognitive tasks – the A-not-B Bucket task, the Cylinder task, the Detour task, and the A-not-B Barrier task – administered in a consistent context. One main goal of the study was to determine whether the individual-level performance would correlate across tasks, supporting that these tasks measure similar components of motoric self-regulation. Dogs in our study were quite successful during tasks requiring them to detour around transparent barriers (i.e., the Cylinder and Detour tasks), but were less successful with tasks requiring the production of a new response (i.e., A-not-B Bucket and A-not-B Barrier tasks). However, individual dog performance did not correlate across tasks, suggesting these well-established tasks likely measure different inhibitory control abilities, or are strongly influenced by differential task demands. Our results also suggest other aspects such as perseveration or properties of the apparatus may need to be carefully examined in order to better understand canine motoric self-regulation or inhibitory control more generally.
Similar content being viewed by others
Inhibitory control can be generally defined as the ability to suppress predominant, but ineffective, responses in favor of more effective, delayed responses (Beran, 2015; Diamond, 1990). Complex cognition, a term that has been used to encompasses abilities such as flexibility, imagination, causal reasoning, prospection, or future-planning (see Emery & Clayton, 2004), is thought to depend on inhibitory control. Indeed, self-control, an inhibitory control ability, is deemed an important sub-component of future planning (McCormack & Atance, 2011). Voluntary regulation of an automatic behavioral response is thought to be essential for adaptive responses across different contexts. In this way, inhibitory control may allow animals to improve their ability to respond to problems in a flexible manner. This may be particularly important for rapidly changing environments, in which a once-beneficial behavior can become counterproductive or possibly even harmful to the individual (Marshall-Pescini, Virányi, & Range, 2015). For instance, a subordinate individual may inhibit directly reaching for food if that individual is in the presence of more dominant individuals to avoid conflicts with other members of a social group (Byrne & Bates, 2007).
Recent studies suggest that inhibitory control is multifaceted, such that it encompasses a range of abilities, from the relatively “basic” capability of motoric self-regulation to the complexity of self-control (Beran, 2015). Self-control is often described as a more complex cognitive ability within the umbrella of inhibitory control. For instance, having the ability to exert self-control may allow an individual to decide between two outcomes, a highly desirable one that is costly (e.g., the outcome is delayed or requires more effort) and a less desirable one that is less costly (e.g., the outcome is more immediate or requires less effort) (Beran, 2015). On the contrary, motoric self-regulation, another ability encompassed by inhibitory control, may be exhibited by the ability to restrain a prepotent response. Hence, the cognitive requirements of motoric self-regulation are thought to be lesser than the requirements of abilities such as self-control. Yet, motoric self-regulation is a necessary component of self-control.
Studies investigating inhibitory control by humans have focused primarily on self-control (e.g., Duckworth & Kern, 2011), in comparison studies using non-human animals have mainly focused on motoric self-regulation (review by Kabadayi, Bobrowicz, & Osvath, 2018). Motoric self-regulation, the ability to restrain a motor response, has been investigated in a wide range of birds, insects, fish, and mammals (e.g., Glady, Genty, & Roeder, 2012; Lucon-Xiccato, Gatto, & Bisazza, 2017; MacLean et al., 2014; Vernouillet, Anderson, Clary, & Kelly, 2016), particularly primates (e.g., Amici, Aureli, & Call, 2008) and dogs (e.g., Bray, MacLean, & Hare, 2014; Brucks, Marshall-Pescini, Wallis, Huber, & Range, 2017a). Recent interest in motoric self-regulation in nonhuman animals has shown interspecies variability in this ability (e.g., Amici et al., 2008; Kabadayi et al., 2016; MacLean et al., 2014). Variation in motoric self-regulation ability may contribute to species differences in more complex cognitive abilities. To further understand motoric self-regulation, numerous tasks have been developed (see Kabadayi et al., 2018). For example, a few commonly used tasks include A-not-B tasks (e.g., Bray et al., 2014; MacLean et al., 2014), Cylinder tasks (e.g., MacLean et al., 2014; Vernouillet et al., 2016), and Detour tasks (e.g., Bray, MacLean, & Hare, 2015; Marshall-Pescini et al., 2015). The simplicity and ease of implementation of motoric self-regulation tasks have made them quite popular recently in comparative studies investigating factors underlying inhibitory control across a wide variety of species (e.g., Amici et al., 2008; Kabadayi et al., 2016; MacLean et al., 2014). However, the behavioral data obtained using different tasks do not always correlate, which suggests that inhibitory control might be context-dependent (Bray et al., 2014; Brucks et al., 2017a; Fagnani, Barrera, Carballo, & Bentosela, 2016; Marshall-Pescini et al., 2015). With respect to inhibitory control, context often refers to whether the subject is being asked to inhibit a social or appetitive impulse, and whether the conditions surrounding the administration of the tasks differ (e.g., social contexts, familiar contexts, reward contexts; Bray et al., 2014). Another explanation for this lack of correlation is that inhibitory control tasks vary in the skills an individual needs to possess in order to succeed – often referred to as task demands (Bray et al., 2014). Thus, the lack of correlation across tasks, may be due to the comparison of tasks that evaluate different inhibitory control abilities (e.g., Brucks et al., 2017a). Furthermore, performance during tasks developed to measure inhibitory control may be administered under different contexts (e.g., social and non-social contexts; see Bray et al., 2014). Thus, comparing tasks previously used to evaluate the same motoric self-regulation ability, within a consistent context, would allow us to investigate whether tasks demands, such as other non-inhibitory factors, influence an individual’s performance during each task. Therefore, the purpose of our current study was to investigate whether behavioral measures obtained using four well-established tasks of motoric self-regulation – the A-not-B Bucket task, the Cylinder task, the Detour task, and the A-not-B Barrier task – administered in a similar context, would show individual-level correlation.
Each of the tasks used in our study has been argued to measure an individual’s ability to resist a motoric prepotent response and to instead make a more appropriate behavioral response, but does not require the individual to choose between an immediate but lesser option and a delayed but greater option. The A-not-B Bucket task requires an individual to inhibit searching for a reward in a previously consistent location (location A) after witnessing the reward being moved to an alternative location (location B), and instead modify its behavior to search for the reward at its new location (e.g., Amici et al., 2008; Bray et al., 2014; Fagnani et al., 2016; MacLean et al., 2014; Topál, Gergely, Erdohegyi, Csibra, & Miklósi, 2009). The Cylinder task requires an individual to restrain the response to reach directly for a reward situated behind a transparent cylindrical-shaped barrier, and instead detour around the barrier to retrieve the reward (e.g., Bray et al., 2014; Diamond, 1990; Kabadayi et al., 2016; Marshall-Pescini et al., 2015; Vernouillet et al., 2016; Vlamings, Hare, & Call, 2010). In a similar vein, the Detour task requires an individual to restrain the response to attempt to directly retrieve a reward placed behind a transparent fence, and instead detour around the apparatus to obtain the reward (e.g., Bray et al., 2014; Marshall-Pescini et al., 2015; Pongrácz et al., 2001; Smith & Litchfield, 2010). Finally, the A-not-B Barrier task combines components of the Detour task and A-not-B type tasks (Abramson, Soto, Zapata, & Hernández Lloreda, 2018; Osthaus, Marlow, & Ducat, 2010). During the A-not-B Barrier task, an individual consistently learns to detour around one side of a barrier (side A) to retrieve a reward, but during test trials the barrier is presented in a shifted location, such that the initial detour route is inaccessible, requiring the subject to detour around the other side (side B) of the barrier. Although these motoric self-regulation tasks have been tested on a variety of species, one of the most common test subjects are dogs, which are also the subject of our study.
Pet dogs (Canis lupus familiaris) are especially interesting for the examination of motoric self-regulation due to their strong problem-solving skills (e.g., Frank & Frank, 1982; Marshall-Pescini, Valsecchi, Petak, Accorsi, & Previde, 2008) and their ability to succeed at complex inhibitory control tasks involving self-control (e.g., delay of gratification task: Leonardi, Vick, & Dufour, 2011; Müller, Riemer, Virányi, Huber, & Range, 2016; but see Brucks, Soliani, Range, and Marshall-Pescini, 2017b; reversal learning task: Tapp et al., 2003; Wobber & Hare, 2009). The possession of these abilities has been suggested as resulting from the domestication process (Gácsi et al., 2009), as dogs have adapted to live with humans and to rely on them for survival (e.g., Hare & Tomasello, 2005). To coexist with humans, the ability to inhibit undesirable or ineffective responses may be particularly important (Udell & Wynne, 2010). For example, pet dogs routinely need to wait to be given food, water, or the opportunity to exercise. To better understand how domestication may influence inhibitory control, several studies have focused on comparing this ability between wolves and domesticated dogs (Marshall-Pescini et al., 2015), or between diverse groups of dogs (e.g., working and pet dogs, Bray et al., 2015; shelter and pet dogs, Fagnani et al., 2016). Interestingly, domesticated dogs tend to form strong attachments to their owners, resulting in a social dependency on humans that may interfere with their performance on tasks commonly used to assess inhibitory control. For instance, dogs can be sensitive to an experimenter’s cues such as pointing and gazing (e.g., Kis et al., 2012; Topál et al., 2009), demonstrate human-based social learning (e.g., Pongrácz et al., 2001), and will often seek social feedback when faced with an insoluble task (Miklósi et al., 2003). Results of these studies suggest that a dog’s ability to inhibit prepotent responses has evolved to be heavily context-dependent. Therefore, to better understand how the social context can influence complex forms of inhibitory control in dogs, we first need a clear assessment of their basic motoric self-regulatory abilities during situations that do not include social cuing or feedback. For this purpose, we administered all our tasks using a similar socio-communicative context only to attract the subject’s attention to the task, but thereafter ensuring a carefully controlled non-communicative context to avoid cueing or other forms of feedback. This approach was held consistent across all tasks administered. Specifically, the main experimenter only interacted with the dog by calling its name during the baiting phase to initiate the trial, but did not provide any cues once the trial begun. This procedure was similar to that previously used by Bray et al. (2014), who argued the social context during their study was minimal.
During the current study, we first examined whether individual characteristics (e.g., breed, sex, age, and weight) influenced a dog’s performance during four well-established motoric self-regulation tasks. Next, we focused on whether components of the task itself influenced performance. We examined whether perseverance influenced responding during the A-not-B Bucket task by assessing whether individual dogs performed the “A-not-B” error, defined as continuing to choose a previously baited location after witnessing the reward being moved to another location. During the Cylinder task, we focused on whether the visual properties of the cylindrical-barrier during early trials, influenced later self-regulation performance – by providing initial experience of an opaque cylinder to one group of dogs, and a transparent cylinder to another (also see Marshall-Pescini et al., 2015). Finally, we investigated whether dog’s motoric self-regulation responses were consistent across tasks. To do so, we examined whether the number of trials required for an individual dog to successfully inhibit its prepotent motoric response was correlated across tasks at an individual level. If these tasks all measure motoric self-regulation, we would expect behavioral measures to positively correlate across some or all tasks. On the contrary, if demands for each task differ, we would not expect to find significant correlations among tasks.
Methods
Subjects
During this study, 30 pet dogs (17 female (F), 13 male (M); Table 1) were recruited from the Winnipeg area in Manitoba, Canada. Recruitment posters were placed in dog parks, daycare centers, and veterinary offices. Participation was completely voluntary and all pet owners were able to withdraw their dog from the study at any time. To participate, all dogs had to be vaccinated against Rabies, Bordetella, and Canine Distemper/Parvo. All pet dogs were between 8 months and 14 years of age (M ± SE: 5.0 ± 2.7 years), and included a variety of pure and mixed breeds (see Table 1).
Dogs were provided with a food or toy reward during the experimental session. Each dog’s specific reward was chosen based on subject preference and dietary restrictions as indicated by the owner. PureBites® Freeze Dried Beef Liver, Zuke’s® Tiny Naturals Tasty Chicken Recipe, or Rollover® Salmon and Rice, as well as an assortment of various pet toys, were provided as options. Additionally, the owners were allowed to bring their own preferred option for their dog (that option was only chosen twice). Water was freely available throughout the entire experimental session.
General procedures
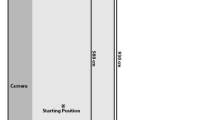
All pet dogs were individually tested in an indoor training center located at the Winnipeg Humane Society, in Winnipeg, Manitoba, Canada. Owners completed a consent form and a questionnaire regarding their dogs’ medical and behavioral histories, as well as dietary restrictions and individual characteristics (e.g., breed, sex, age, and weight), prior to participation. All tasks were conducted in a large room typically used by the facility for training purposes (18.5 m × 15.6 m, see Fig. 1). Prior to beginning the experimental session, each dog was given 10–15 min to habituate to the room and interact with the experimenters, during which they could not see nor interact with the task apparatuses which were within fenced enclosures (Fig. 1). This procedure was conducted to ensure subjects were reasonably comfortable prior to the start of the experimental session. Additionally, each dog was provided with a 5- to 10-min break between each task. Prior to and after each experimental session, the flooring of the room and the experimental apparatuses were thoroughly cleaned with a bleach and water solution to control for potential odor cues. All experimental sessions were coded in real-time and performance during each task was digitally-recorded (FujiFilm Finepix XP60 digital camera) for offline scoring. This study was approved by the Animal Care Committee at the University of Manitoba (protocol #F16-024).
Schematic representation of the experimental room where the four motoric self-regulation tasks were conducted. Each task was situated in a fenced enclosure to visually-occlude the dog from seeing the apparatuses when outside of the enclosure: (a) A-not-B Bucket task, during which a dog has to retrieve a reward in a baited bucket; (b) Cylinder task, during which a dog has to retrieve a reward from within a cylinder; (c) Detour task, during which a dog has to retrieve a reward placed behind a V-shaped fence; (d) A-not-B Barrier task, during which a dog has to retrieve a reward placed behind a barrier. Dotted lines and arrowheads indicate a dog’s hypothetical pathways when performing during each task
Each dog completed four tasks that measured motoric self-regulation during a single one-hour experimental session: the A-not-B Bucket task, the Cylinder task, the Detour task, and the A-not-B Barrier task (see Fig. 1 for room and apparatus configuration). The materials and procedure of these tasks were generally designed after Bray et al. (2014) and Fagnani et al. (2016) for the A-not-B Bucket task, Bray et al. (2014) and Marshall-Pescini et al. (2015) for the Cylinder task, Bray et al. (2015) for the Detour task, and Osthaus et al. (2010) for the A-not-B Barrier task. Each dog completed the tasks sequentially, with task order counter-balanced across dogs. Throughout the session, three individuals [Experimenter 1 (E1), Experimenter 2 (E2), and a dog handler] were present for each task, with each task conducted in a separate testing enclosure within the larger room. E1 was responsible for showing the reward to the dog during each task, interacting with the dog, and baiting the apparatus (the same individual was assigned the role of E1 during the entire study). E2 digitally recorded each task and manually scored the choice of the dog during each trial. The dog handler was responsible for holding the dog’s leash and waiting with the dog at the starting position prior to the start of each trial. The starting position was located 2-m away from the centre of each apparatus, and was indicated by a mark on the floor (Fig. 1). To ensure the dogs were comfortable during the experiment, at least one of the dog’s owners was also present in the testing arena during each task, but to prevent unintentional cuing (e.g., Kis et al., 2012; Topál et al., 2009), they were asked to sit motionless behind the apparatuses and to face away from the dog until the end of the task. Owners were also asked to ignore their dogs if they were approached.
For each task, at the beginning of a trial, E1 stood centered behind the apparatus. The handler led the dog to the starting position and stood behind the dog holding the leash. E1 attracted the dog’s attention by showing the reward and calling the dog’s name. While continuing to call the dog’s name, E1 baited the apparatus by either placing the reward inside a bucket (A-not-B Bucket task), in the centre of a cylinder (Cylinder task), or behind the fence (Detour task) or barrier (A-not-B Barrier task). E1 subsequently backed away from the apparatus and gave the release command, commonly “go,” unless the dog was taught another release command. The handler then released the dog’s leash, which was the cue used to indicate the start of the trial for purposes of measuring latency (see Behavioral Measures sections of each task). During each trial, both experimenters and the handler averted their eyes from the dog by looking toward the ceiling, and remained motionless with their hands crossed, until the end of the trial.
A trial was considered “successful” if the dog correctly inhibited its prepotent response and retrieved the reward with either its paw or mouth without making contact with the apparatus (see Cylinder task, Detour task, and A-not-B Barrier task) or went directly to the baited location (see A-not-B Bucket task). A trial was “unsuccessful” if the dog did not inhibit its prepotent response and made contact with the apparatus or chose a non-baited location first. A trial was aborted if the dog did not attempt to retrieve the reward within 30 s, and a session was terminated after five aborted trials or if the dog was non-responsive (i.e., did not look at the reward, at E1 when called, or avoided the apparatus). At the completion of the trial, E1 handed the dog back to the handler who then brought the dog back to the starting position to prepare for the next trial.
A-Not-B bucket task
Materials
The apparatus for the A-not-B Bucket task consisted of three opaque plastic buckets (35.6 cm height × 17.8 cm diameter) placed in a line with 1.2-m gaps between each bucket (Fig. 1a). The line of buckets was situated perpendicular to the starting position. Rocks were placed in each bucket, covered by a circular piece of cardboard, to eliminate movement. To control for odour cues, each bucket also contained an identical perforated plastic sphere with an inaccessible reward (either a treat or toy). The perforated containers were not visible to the dogs from the starting position.
Procedure
This task was divided into a Training phase followed by a Testing phase.
-
Training phase. During the task, a dog was situated at the starting position during the baiting procedure, facing the middle bucket (M). E1 stood behind Bucket M, and while showing the dog the reward, she moved to one of the three buckets, placing the reward in the bucket. The dog was released and permitted as many choices as necessary to locate the reward (a choice was defined as when the snout of the dog passed over the rim of a bucket). When a dog successfully retrieved the reward from the baited bucket on its first choice, E1 baited a different bucket during the subsequent trial (the order during which each bucket was baited was counterbalanced across dogs). This procedure was repeated until dogs successfully retrieved the reward from each of the three buckets during its first choice, following which the dog immediately progressed to the Testing phase.
-
Testing phase. The Testing phase started with E1 repeatedly baiting one of the two side buckets (herein referred to as Bucket A) consistently (herein referred to as “A-trials”). Once the dog successfully chose the baited bucket on its first choice three times, not necessarily consecutively, the “A-not-B” testing trials were conducted. During testing trials, E1 first baited Bucket A, visibly placing the reward inside it, and then stood behind Bucket A for 1 s before reaching back into the bucket and retrieving the reward. E1 then walked to the bucket on the opposite side of the array (herein referred to as Bucket B), holding the reward in plain view of the dog, and baited Bucket B while calling the dog’s name. E1 then stood behind the Bucket M and started the trial by giving the release command. Testing “A-not-B” trials were repeated until the dog choose Bucket B as its first choice when retrieving the reward. The assignment of Bucket A (either to the dog’s left or right) was counterbalanced across dogs, but remained consistent for each individual dog. Hence, during this task, dogs had to inhibit a learned motoric response to search in a location that had previously been baited after seeing the reward being moved to a new location.
Behavioral measures
The dependent measures evaluated for each dog were: (1) the number of training trials, (2) the number of A-trials a dog required before successfully choosing Bucket A on its first choice three times, (3) A-not-B Bucket Task Score – the number of testing trials a dog required before successfully choosing Bucket B on its first choice, and (4) A-not-B Bucket Latency – the duration between release of the dog’s leash to when its snout passed over the rim of one bucket during testing trials.
Cylinder task
Materials
Two cylinder apparatuses were used for this task. Each consisted of a hollow, open-ended cylindrical tube (20 cm diameter × 22 cm length) secured to a wooden platform (61 cm length × 31 cm width × 3 cm height), which was weighed down with a heavy sand-filled pillowcase to prevent movement. One cylinder was constructed from opaque cardboard (henceforth referred to as the “opaque cylinder”), thus the contents of the cylinder were only visible when viewed from the side openings but not from the starting point. The other was constructed from transparent acrylic material (henceforth referred to as the “transparent cylinder”), thus the contents of the cylinder were visible from all points (Fig. 1b).
Procedure
During the task, a cylinder was positioned perpendicular to the dog’s viewpoint at the starting position (Fig. 1b). A reward was placed in the center of the cylinder. The dog was situated at the starting position during baiting. Once the dog was released, in order for it to access the reward, the dog had to inhibit attempting to directly reach for the reward (which would cause it to contact the cylinder), and instead detour to one of the two open sides of the cylinder.
The dogs were divided into two groups. The Opaque-Transparent group (n = 13 dogs; six females) experienced the first five trials (Block 1: trials 1–5) with the opaque cylinder and the last five trials (Block 2: trials 6–10) with the transparent cylinder. The Transparent group (n = 13 dogs; 9 females) experienced all ten trials (Block 1: trials 1–5 and Block 2: trials 6–10) with the transparent cylinder. The side opening through which the cylinder was baited (left or right) by E1 was counterbalanced across dogs and groups, but remained consistent for an individual dog. Comparison of testing performance between the two groups of dogs allowed us to assess the influence of experiencing an opaque cylinder on subsequent performance when presented with a transparent cylinder.
Behavioral measures
The dependent measures evaluated for each dog were: (1) the number of trials a dog required before successfully detouring through one of the side openings of the transparent cylinder, (2) Cylinder Task Score – the absolute number of successful trials during the transparent cylinder trials 6–10 (Block 2) for both groups, (3) the absolute number of successful trials during the transparent cylinder trials 1–5 (Block 1) for the Transparent group, and (4) Cylinder Latency – the duration between when the handler released the leash to when the dog’s snout passed through either side of the cylinder (successful trial) or when the dog touched the cylinder (unsuccessful trial) during a transparent trial.
Detour task
Materials
The apparatus consisted of a V-shaped fence constructed from two rectangular panels (overall: 152 cm height × 426 cm length) with a small opening at the vertex. The panels were arranged to form a V-shape with an angle of 80°. Two heavy sand-filled pillowcases were placed against each panel to prevent movement. The framework of the panels was constructed from PVC tubes (155 cm length × 71 cm width × 99 cm height) and covered by blue plastic tarpaulins. A transparent vinyl barrier was fixed at the vertex (40 cm width × 99 cm height), joining the two panels. The vertex of the fence pointed towards the starting position. Thus, from the starting position the dog could see “into” the V-shaped fence and hence, when present, the reward was visible to the dog (Fig. 1c). A line was drawn on the floor in front of the fence from the apparatus to the starting point to demarcate a 2-m central dividing line.
Procedure
During the task, a dog was situated at the starting position during the baiting procedure. Once released the dog had to inhibit its prepotent response of attempting to directly access the reward which was blocked by the transparent barrier, and instead detour around the fence. At the completion of each trial, the dog was led through an exit at the back of the testing area by E1, who subsequently relinquished the leash to the handler. E1 positioned herself back behind the V-shaped fence while the dog was led to the starting position. This procedure was conducted to prevent the dog from witnessing the experimenters detouring around the fence. Each session consisted of ten trials.
Behavioral measures
The dependent measures evaluated for each dog were: (1) the number of trails required for a dog before successfully detouring around the fence, (2) Detour Task Score – the absolute number of successful trials (out of ten trials), (3) Detour Latency – the duration from when the handler released the leash to when the dog’s entire body passed over either side of the central line demarcation, and (4) Detour Side – which side of the fence the dog detoured around for at least nine trials (out of ten), indicating a dog’s side preference.
A-not-B barrier task
Materials
The A-not-B Barrier apparatus consisted of a large barrier made from a wooden frame and plastic trellis (200 cm height × 430 cm length) with square gaps (11 cm × 11 cm) in diameter. The barrier was placed within a 5-m wide testing arena, with one side pressed flush against the wall, leaving a 0.7-m opening on the opposite side. A line was drawn on the floor in front of the fence from the apparatus to the starting point to demarcate a 2-m central dividing line (Fig. 1d). The reward location was set at approximately 2-m behind the barrier.
Procedure
This task was divided into a Training phase followed by a Testing phase.
-
Training phase. During training (herein referred to as “A-trials” to parallel those of the A-not-B Bucket task), E1 stood behind the barrier, and showed the dog the reward, which she placed centrally behind the barrier. Once the reward was in place, the dog was released and allowed to retrieve the reward. After the dog successfully retrieved the reward from behind the barrier, it was led outside the testing enclosure by E1 through an exit at the back of the testing arena before being relinquished to the handler. The handler then brought the dog back to the starting position after E1 positioned herself back behind the barrier. This procedure was conducted to prevent the dog from seeing experimenters detouring around the apparatus. Side choice was determined when the dog’s entire body passed over either side of the middle line. A-trials were repeated until the dog successfully chose the open side (herein referred to as “Side A”) on its first side choice for a total of three A-trials, not necessarily in a consecutive manner, following which the dog immediately progressed to the Testing phase. The position of Side A (left or right from the dog’s point of view) was counterbalanced across subjects, but consistent across trials for each individual.
-
Testing phase. During testing ‘A-not-B’ trials, the barrier was shifted so that the opening was on the opposite side from the training phase (herein referred to as “Side B”). The movement of the barrier was conducted out of view of the dog, between the last training trial and the first testing “A-not-B” trial. Testing trials continued until the dog successfully chose Side B as its first side choice. Hence, during this task, a dog had to inhibit its motor response to go to the learned opening and detour around a barrier through the new opening.
Behavioral measures
The dependent measures evaluated for each dog were: (1) the number of A-trials required for a dog to successfully chose Side A as its first choice for three trials during Training, (2) A-not-B Barrier Task Score – the number of testing trials that a dog required before successfully choosing Side B as its first choice, and (3) A-not-B Barrier Latency – the duration between when the handler released the dog’s leash to when the dog’s entire body passed over either side of the midline.
Analyses
For all tasks, E2 recorded the trial outcome in real-time, and all trials were re-coded off-line by E1. Disagreement occurred for 3/550 trials, which were resolved through mutual agreement. Latency was also calculated offline.
Analyses were conducted in R (version 3.3.2, R Core Team) using packages lme4 (Bates, Maechler, Bolker, & Walker, 2015), and lsmeans (Lenth 2016). Alpha value for all analyses was set at < 0.05.
Within-task analyses
To examine whether behavioral measures, collected during tasks that required a separate training phase (A-not-B Bucket and the A-not-B Barrier tasks), were influenced by sex, age, weight, task order (to check whether inhibitory control depleted over time), or side of the apparatus tested (herein referred to as “factors”), we conducted generalized linear models (GLMs). For tasks without a separate training phase (Cylinder task and Detour task), and for the data collected during the testing phases, we again examined these same five factors but included group assignment (for the Cylinder task) and Detour Side (for the Detour task) to conduct: (1) GLMs when examining task scores for each task, (2) logistic regressions when examining first-trial performance for each task, and (3) linear mixed models (LMMs) when examining choice latency for each trial during each task. GLMs were fit with a quasi-Poisson distribution as the data was slightly over-dispersed (Venables & Ripley, 2002). Examination of latency allowed us to detect learning (i.e., if latency decreases over trials) or demotivation and/or task difficulty (i.e., if latency increases over trials). For GLMs, parameter estimation was achieved by comparing nested models using an F-test, whereas for logistic regressions and LMMs, parameter estimation was achieved by comparing nested models using a residual maximum likelihood test. Degrees of freedom were estimated using a Satterthwaite approximation. If a factor was found significant, Tukey HSD post hoc analyses were conducted.
Next, to further examine task specific questions, we compared first-trial performance to chance using a Chi-square test for goodness of fit for the “A-not-B” testing trial of the A-not-B Bucket task, and a binomial test for the “A-not-B” testing trial of the A-not-B Barrier task. We also compared first-trial performance during training trials and during testing trials using a z-test to assess the difficulty of both the A-not-B Bucket and the A-not-B Barrier tasks. For the Cylinder task, we evaluated group differences and performance across trial blocks using Wilcoxon rank-sum tests and Wilcoxon signed-rank tests, as appropriate.
Between-task analyses
To examine whether the four tasks measured motoric self-regulation, we performed Spearman’s rank-order correlations between the number of trials required for a dog to successfully meet our criteria for motor self-regulation for each task. We additionally sought to perform principal component analysis (PCA) to examine the relationships between the different tasks and potentially identify whether tasks measured the same inhibitory control ability (see Results section for limitations of this approach). All the behavioral variables were first z-transformed to allow comparison on the same scale.
Results
Not all dogs completed every task (number of dogs completing each task: A-not-B Bucket: n = 23, Cylinder task: n = 21, Detour task: n = 23, and A-not-B Barrier: n = 27; see Table 1 for information regarding individual dogs). For within-task analyses, we only included data from dogs which completed all trials during a particular task, whereas for correlational analyses across tasks, we only used the data from dogs that completed all tasks (n = 21).
Within-task analyses
The factors of sex, age, and weight were often not significant in our GLMs, logistic regressions, and LMMs analyses. As we had no a priori reasons to predict differences based on these variables, and the likelihood of over-fitting by including these variables in GLMs (due to sample size), we have only reported the results of these tests in the Supplementary Material. However, significant effects of factors of task order and side of apparatus are reported, as these are important for better understanding performance measures during the tasks (see Supplementary Material for all remaining analyses).
A-not-B bucket task
Training phase
Dogs needed on average 4.3 ± 0.2 (M ± SE) training trials to successfully retrieve a reward from each of the three baited buckets on their first choice during the Training phase.
Testing phase
During testing, dogs needed on average 3.7 ± 0.2 (M ± SE) A-trials to successfully meet criteria to move to the A-not-B testing trials.
-
a)
A-not-B bucket task score. On average, dogs needed 2.4 ± 0.4 (M ± SE) testing trials before successfully retrieving the reward from Bucket B as their first choice. The position of Bucket B significantly influenced the A-not-B Bucket Task Score (GLM: F(1,22) = 10.225; p = 0.004), with dogs requiring fewer trials to retrieve the reward from the left side compared to the right side (M ± SE: 1.6 ± 0.5 and 3.7 ± 0.6 trials, respectively; t(22) = -3.15, p = 0.005). On average dogs did not show a preference for choosing between either of the incorrect buckets during unsuccessful trials (M: 46.4% and 53.6%, for Buckets A and M, respectively). However, three dogs consistently choose Bucket A, and five dogs consistently choose Bucket M (with four dogs splitting their choices).
-
b)
First trial performance. Bucket choice was not significantly different from random (chance = 7.6, n = 11, 8, and 4 for Buckets B, M and A, respectively; Goodness of fit: χ2(2) = 3.22, p = 0.199). First trial performance during B-trials was significantly lower than during A-trials (first-trial performance: 87% and 48% for A-trials and B-trials respectively; z-score: 2.83, p = 0.005). The location of Bucket B was not significant (Logistic regression: χ2(1) = 1.262, p = 0.261).
-
c)
A-not-B bucket latency. Once in the Testing phase, dogs were quick to make a choice (M ± SE: 3.6 ± 0.5 s), but we found a significant increase in latency, although likely not biologically important, with each successive testing trial (0.6 ± 0.2 s; LMM: χ2(1) = 7.66, p = 0.006).
Cylinder task
-
a)
Cylinder task score. On average, dogs needed 1.8 ± 0.3 (Mean ± SE) trials before successfully detouring through one of the side openings of the transparent cylinder.
When investigating performance when first presented with a cylinder (trials 1–5), dogs in the Opaque-Transparent group had more successful trials with the opaque cylinder than dogs in the Transparent group with the transparent cylinder (M ± SE, 4.8 ± 0.1 and 2.4 ± 0.7, respectively; Wilcoxon rank-sum: W = 93, p = 0.001; Fig. 2). When comparing similar levels of experience with the transparent cylinder, dogs in the Opaque-Transparent group had more successful trials (Block 2) compared to dogs in the Transparent group (Block 1) (M ± SE, 4.1 ± 0.3 for the Opaque-Transparent group; Wilcoxon rank-sum: W = 80.5, p = 0.036; Fig. 2). Performance by the Opaque-Transparent group did not change between Blocks (Wilcoxon signed-rank: W = 115, p = 0.075; Fig. 2), which might suggest dogs had successfully transferred the correct detour response acquired during Block 1 to the transparent cylinder. However, when we compared performance during trial 5 to trial 6, we found that the Opaque-Transparent group showed a significant decline in performance (Fig. 3; Z = -2.17, p = 0.030). For the Transparent group, performance significantly improved between Blocks 1 and 2 (M ± SE, 4.3 ± 0.3 successful trials for Block 2; Wilcoxon signed-rank: W = 0, p = 0.035; Fig. 2), and did not show any decline between trials 5 and 6 (Fig. 3; Z = 0.32, p = 0.749). Together these results suggest these dogs were still learning to make the correct detour response partway through the task. Finally, there was no difference in performance between the two groups during Block 2 (Wilcoxon rank-sum: W = 50, p = 0.907; Fig. 2), supporting that by the end of testing dogs in both groups were performing similarly.
-
b)
First trial performance. Group assignment did not significantly influence detour success during the first transparent trial (M: 53.8% for both groups; Logistic regression: χ2(1) = 0, p = 1).
-
c)
Cylinder latency. During transparent trials, dogs were quick to make a choice (M ± SE: 3.9 ± 0.4 s), and we found a significant decrease in latency, although again, likely not biologically important, with each successive trial (-0.3 ± 0.1 s; LMM: χ2(1) = 2.45, p = 0.022).
Number of successful trials during the first block (Trials 1–5) and last block (Trials 6–10) of trials for the group of dogs who had experience with an opaque cylinder (Opaque-Transparent group, n = 13) and the group of dogs who only experienced the transparent cylinder (Transparent group, n = 13). Bars indicate Standard error of the mean. *p<0.05
Proportion of dogs successfully detouring through one of the openings of the cylinder during each trial of the Cylinder task for the group of dogs who had experience with an opaque cylinder (Opaque-Transparent group, n = 13) and the group of dogs who only experienced the transparent cylinder (Transparent group, n = 13). There was a significant decrease in performance between Trial 5 (last trial with the opaque cylinder) and Trial 6 (first trial with the transparent cylinder) for the Opaque-Transparent group (p < 0.05)
Detour task
-
a)
Detour task score. On average, dogs needed 1.2 ± 0.1 (M ± SE) trials before successfully detouring around the apparatus. Interestingly, we observed a Detour Side preference (n = 11, 7, and 5 for left, right, and none, respectively), during which individuals detoured around the fence using the same side for at least nine trials (chance = 5, Binomial: p = 0.010).
-
b)
First trial performance. Most dogs (n = 19; 82.6%) successfully detoured around the fence during the first trial.
-
c)
Detour latency. Throughout the task, dogs were quick to make a choice (M ± SE: 2.1 ± 0.2 s), and latency did not change significantly with each successive trial (-0.1 ± 0.1 s; LMM: χ2(1) = 3.37, p = 0.066).
A-not-B barrier task
Training
Dogs needed on average 3.2 ± 0.1 (M ± SE) A-trials to successfully meet criteria to proceed to testing.
Testing
-
a)
A-not-B barrier task score. On average, dogs required 1.8 ± 0.1 (M ± SE) testing trials before successfully choosing Side B as their first choice.
-
b)
First trial performance. Side choice during testing was not different from chance (chance = 14, n = 9 and 19 for the correct detouring Side B and incorrect detouring Side A, respectively; Binomial: p = 0.087). First trial performance during B-trials was significantly lower than during A-trials (first-trial performance: 93% and 32% for A-trials and B-trials respectively; z-score: 4.77, p < 0.001).
-
c)
A-not-B barrier latency. Once in the testing phase, dogs were quick to make a choice (M ± SE: 4.1 ± 0.8 s), and latency did not change significantly with each successive trial (Estimate: 0.93 ± 0.85; χ2(1) = 1.2, p = 0.266).
Correlations between tasks
The number of trials a dog required before performing its first successful trial was not correlated across tasks (Table 2), suggesting the four tasks did not have the same task demands.
We were unable to use a PCA approach, as the Kaiser-Meyer-Olkin index (KMO) performed on our dataset indicated low correlation between variables (KMO = 0.5), and as such, the PCA cannot efficiently group the original behavioral variables into relevant components.
Discussion
One main goal of our study was to evaluate whether an individual dog’s motoric self-regulation was consistent across four well-established tasks. Our results showed that at the individual-level, behavioral measures were not correlated across tasks. Supporting that even when focusing on the most basic inhibitory control ability, motoric self-regulation, results are inconsistent. This variability may indicate that motoric self-regulation is context-dependent, with each task measuring a different ability required for self-regulation, or that task demands, such as other non-inhibitory factors, were influencing an individual’s response during each task (Bray et al., 2014), a topic we will return to in the Discussion below. We will, however, first discuss how our results extend current knowledge of the individual tasks, and offer suggestions for future studies. Then, we will address potential explanations for the lack of consistency across tasks.
Task-Specific results
A-not-B bucket task
The A-not-B Bucket task requires individuals to resist searching in a previously baited location after witnessing the reward being moved to a new location. During our study, dogs had no difficulty choosing Bucket A during A-trials, as evidenced by the high performance during the first A-trial. However, once in the Testing phase fewer than half of the dogs successfully located the reward during the first trial when the reward was moved to Bucket B. This differs from previous studies that have typically reported high success rates (e.g., Bray et al., 2014: 83%; MacLean et al., 2014: 89%). Our failure to replicate the first-trial success reported by previous studies is perplexing as our methodology was developed to closely replicate those used by Bray et al. (2014). However, this drop in performance between the training trials and the first testing trial suggests that testing protocol was difficult for our subjects, and supports that we were measuring the ability of dogs to engage in inhibitory control.
During our study, we required dogs to continue to make choices until the testing criteria was met, which allowed us to examine whether dogs would perseverate in searching the initially rewarded bucket during testing. As many previous studies focused only on first-test trial (and in many cases, the only trial) performance, we cannot evaluate whether our sample of dogs showed more or less perseveration compared to previous studies (but see Fagnani et al., 2016). Therefore, it would be informative for future studies to provide dogs with multiple testing trials as we have, to more carefully evaluate the role of perseveration during this task.
Although our dogs showed poorer first-trial testing performance, when we examined the unsuccessful testing trials, on average dogs split their choices between the middle bucket (Bucket M) and the previously baited bucket (Bucket A). Hence, similarly to what was found by Bray et al. (2014), on average our dogs did not commit the “A-not-B error” (although three of 12 certainly did), in which a subject continues to choose the originally baited bucket after the reward was visibly moved. Thus, although our dogs did not show strong first-trial performance, they also did not show strong perseveration errors (A-not-B errors). Surprisingly, even through the side placement of the A and B buckets were counterbalanced across dogs, we found that the side on which Bucket B was located affected testing performance, but not training or A-trial performance. At this time, we cannot explain this result.
In order to successfully perform during the A-not-B Bucket task, dogs had to: (1) learn a response pattern (find a reward hidden in a bucket), (2) possess object permanence (the reward is still in the bucket despite not being visible), and (3) follow a displaced object (the reward moves from one bucket to another). Overall, our dogs readily learned the response pattern to retrieve the reward from Bucket A, and needed few A-trials to meet testing criteria. They also showed object permanence in that they were able to accurately retrieve the reward from Bucket A during training trials and A-trials. However, the difficulty with the testing aspect of the task seemed to stem from a dog’s inability to follow the reward displacement procedure, as some dogs needed up to seven trials to correctly choose Bucket B. Difficulty with visible reward displacement has also been shown in dogs using similar tasks in social contexts (Kis et al., 2012; Topál et al., 2009).
Cylinder task
The Cylinder task requires dogs to resist reaching directly for a reward contained within a, typically, transparent cylinder and instead detour to one of the side openings. Detouring performance during our study was quite high, similar to what was observed in previous studies using the Cylinder task with pet dogs (e.g., Bray et al., 2014; Fagnani et al., 2016; MacLean et al., 2014; Marshall-Pescini et al., 2015). However, to better understand how the properties of the cylinder affects task success, we examined whether initial experience with an opaque cylinder would affect performance when presented with a transparent cylinder using a similar approach to the one Marshall-Pescini et al. (2015) followed in their study.
Dogs in the Opaque-Transparent group were highly successful at detouring around the opaque cylinder, showing no difference in performance when subsequently presented with a transparent cylinder. However, dogs in the Transparent group had low detour performance during the first five trials, and only with additional experience did their performance improve. This result suggests that learning with an opaque cylinder allowed the dogs in the Opaque-Transparent group to quickly acquire the correct detouring response, and transfer this response when presented with the transparent cylinder. In contrast, dogs in the Transparent group likely needed to acquire the correct detouring response, while also attempting to inhibit retrieving the reward. Together these results suggest that experience with an opaque cylinder facilitates later task performance by allowing the animals to learn the necessary detouring response without the need to self-regulate, as suggested by previous studies (e.g., tamarins, Santos et al., 1999). Thus, facilitation when “trained” with an opaque cylinder likely has more to do with learning the required detouring response, and not motor self-regulation itself. Indeed, when first experiencing the transparent cylinder (the sixth trial), the proportion of dogs in the Opaque-Transparent group showing successful detour performance dropped (from 100% to 69%), suggesting many dogs were disrupted by the introduction of the transparent cylinder – likely due to the need to self-regulate. Meanwhile, dogs in the Transparent group learned the appropriate detouring response rather steadily over trials (as has been reported in previous studies, e.g., Kabadayi et al., 2017; Vernouillet et al., 2016). Learning the appropriate response with just a few trials might explain why there were no differences in performance over ten trials with a transparent cylinder between a group of pet dogs that received training with an opaque cylinder and a group of pet dogs that only experienced the transparent cylinder (Marshall-Pescini et al., 2015). Hence, the properties of the cylinder when initially performing the Cylinder task may influence task difficulty, by breaking the task into two sequentially presented components (when the initial cylinder is opaque) compared to concurrent components.
Detour task
The Detour task requires dogs to inhibit going through a transparent barrier in a fence to retrieve a reward, and instead to detour around the apparatus. During our study, most dogs were successful, even during the first trial, with many beginning to detour upon release by the handler (as supported by low Detour Latency values). This fast and successful performance suggests the dogs understood the necessary detour response. Contrary to a similar study which reported that dogs spent time at the fence in proximity to the food (Marshall-Pescini et al., 2015), our dogs did not spend much, if any, time in front of the fence or the transparent barrier. However, methodological differences may explain these discrepancies. During our study, the transparent barrier was only present at the vertex of the fence, with the remainder of the fence being opaque, whereas Marshall-Pescini and colleagues used a wire mesh fence, allowing the reward to be visible from multiple positions. Likely, the greater visibility of the reward provided a greater challenge for the dogs to self-regulate in the previous study compared to ours (e.g., Vallortigara & Regolin, 2002). Likewise, Bray et al. (2015) also used a fully transparent apparatus, and again reported poorer detouring performance in dogs compared to our study. Thus, visibility of the reward might explain why pet dogs in our study had better performance on average compared to previous studies.
We also observed that dogs had a significant side preference when detouring, and this side preference was not accounted for by task order or the side of baiting during the previous tasks. This side preference could be due to behavioral or motoric lateralization (Tomkins, Thomson, & McGreevy, 2010), or may simply be due to the tendency for a dog to replicate a previously rewarded response. Similar detouring side preferences have been reported for this task by dogs (Pongrácz et al., 2001), mice (Juszczak & Miller, 2016), and quokkas (Wynne & Leguet, 2004).
A-not-B barrier task
The A-not-B Barrier task requires dogs to inhibit a previously rewarded path around a barrier, and instead detour to a novel opening. Dogs needed few trials to initially learn the task, as most of the dogs performed successfully during the first training trial. However, the overall performance dropped during the first testing trial, with only a third of the dogs switching to the novel side opening during testing. Once again, this result suggests that testing presented some difficulty, and required inhibitory control. However, this spatial perseveration did not last long, as by the second testing trial most dogs were successfully detouring. These results are similar to what has been previously reported using a similar apparatus in dogs (Osthaus et al., 2010). One potential explanation for the dogs’ low success when first experiencing the shifted barrier, is that dogs may have little experience with such large barriers or walls shifting position in their everyday lives, this is especially likely given the barrier was moved when the dog was not present.
Correlation between tasks
One of our main goals for this study was to investigate the consistency of motoric self-regulation among tasks, at an individual level, by pet dogs. Overall, the dogs performed successfully in the four tasks, as most of them required only a few trials to successfully inhibit their prepotent motoric response (Supplementary Material, Fig. S1). High performance was also reported in previous studies with similar tasks and suggests that dogs easily exhibit motoric self-regulation. However, our results showed that individual performance was not consistent across tasks, as we found no significant correlation for the number of trials a dog needed to successfully self-regulate their motoric response for the first time on each task. This result was also supported by the lack of correlations between the behavioral variables collected during the four tasks that prevented us to perform a PCA. Previous studies have also reported a lack of correlations among measures of inhibitory control tasks performed by dogs (Bray et al., 2014; Brucks et al., 2017a; Fagnani et al., 2016; Marshall-Pescini et al., 2015), with one explanation being that inhibitory control is context-dependent (Bray et al., 2014). However, even when minimizing contextual differences, by focusing on reward-based tasks, and limiting social influences, we still found that individual performance was not correlated among tasks. Therefore, we will now evaluate how each task may have different requirements, also referred to as task demands, which may influence performance differently.
Based on the training requirements, the four tasks used in our study may be categorized into two different groups: detouring tasks (i.e. Cylinder task and Detour task) and response switching tasks (i.e., A-not-B Bucket task and A-not-B Barrier task). Detouring tasks require individuals to withhold a prepotent response to a visible reward, and instead to detour around a barrier to retrieve the reward. In comparison, response switching tasks require dogs to learn one response pattern, but quickly switch to producing a new response when task conditions are changed. Detouring tasks can hence be thought to be measuring more “spontaneous” responses compared to response switching tasks, which may be measuring learning a response pattern.
Detouring tasks and response switching tasks also differ with regards to whether perseveration is rewarded. During detouring tasks, perseveration is rewarded but not required; an individual may continue to perform the previously successful response and will retrieve a reward. During response switching tasks, perseveration is not rewarded; an individual must change their previously successful response to a new response in order to retrieve a reward. Indeed, perseveration has been shown to be an important factor in explaining variation during tasks measuring inhibitory control by dogs (Brucks et al., 2017a; Osthaus et al., 2010; Pongrácz et al., 2003).
However, even an attempt to categorize motor self-regulation tasks in this simple way comes with problems. For instance, during the response switching tasks, an incorrect choice during the A-not-B Bucket was simply unrewarded, whereas during the A-not-B Barrier an incorrect choice results in the dog encountering the barrier. For this reason, the A-not-B Barrier task has also been categorized as a detouring task (Kabadayi et al., 2018). Indeed, during both the Cylinder and the Detour tasks, failure to inhibit the prepotent response also resulted in encountering a barrier.
Another difference between the detouring tasks is perceptually-based – during our tasks the visual characteristics of the barriers. Transparency, for instance, is an important aspect of the Cylinder and Detour tasks, as the reward is typically visible behind a transparent barrier. As briefly discussed above, visibility of the reward has been shown to influence an individual’s detour performance (Kabadayi et al., 2018). Individuals typically showing less self-regulation behavior when rewards are fully visible (Vallortigara & Regolin, 2002), compared to when they are behind partially occluded or semi-transparent barriers (dogs: Brucks et al., 2017a; mice: Juszczak & Miller, 2016). The high success of our dogs during the Detour task, where most of the apparatus was opaque, support these previous studies. One potential avenue for future studies would be to modify the tasks to increase the difficulty or to systematically vary physical parameters such as transparency or size of the apparatus.
Alternatively, the lack of correlations between tasks might be due to the high performance of the dogs. Indeed, ceiling and floor effects tend to reduce the strength of correlations. A similar situation could have arisen in previous studies examining the correlations between motoric self-regulation tasks (Bray et al., 2014; Fagnani et al., 2016; Marshall-Pescini et al., 2015). However, in our case, although most dogs performed well, and were usually successful by the first two trials, we still observed individual differences (Supplementary Materials, Fig. S1), with some dogs showing fairly consistent performance across the tasks, and others showing considerable variability. Better understanding the mechanisms underlying these individual differences may help to shed light on which task demands most strongly influence the variability seen when attempting to compare performance during motoric self-regulation tasks, and perhaps tasks of inhibitory control more generally.
Conclusions
We examined motor self-regulation using four well-established tasks. Our results highlight some important within-task considerations for future studies, such as providing additional testing trials during A-not-B Bucket task to evaluate perseverance, or to examine performance on the Cylinder task on a trial-by-trial basis to better understand the mechanisms driving performance when initially experiencing the task with an opaque or transparent cylinder. Our results also highlight some important considerations when attempting to understand why individual performance measures do not correlate across tasks attempting to measure a “basic” component of inhibitory control, motoric self-regulation. Future studies designed to evaluate issues such as task demands, as well as more carefully evaluate individual differences, will certainly advance our understanding of inhibitory control in pet dogs.
References
Abramson, J.Z., Soto, D.P., Zapata, S.B., & Lloreda, M.V.H. (2018). Spatial perseveration error by alpacas (Vicugna pacos) in an A-not-B detour task. Animal Cognition, 21(3), 433–439.
Amici, F., Aureli, F., & Call, J. (2008). Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology, 18, 1415–1419. https://doi.org/10.1016/j.cub.2008.08.020
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
Beran, M. (2015). The comparative science of ‘self-control’: What are we talking about? Frontiers in Psychology, 6(51), 1–4. https://doi.org/10.3389/fpsyg.2015.00051
Bray, E. E., MacLean, E. L., & Hare, B. A. (2014). Context specificity of inhibitory control in dogs. Animal Cognition, 17, 15–31. https://doi.org/10.1007/s10071-013-0633-z
Bray, E. E., MacLean, E. L., & Hare, B. A. (2015). Increasing arousal enhances inhibitory control in calm but not excitable dogs. Animal Cognition, 18(6), 1317–1329. https://doi.org/10.1007/s10071-015-0901-1
Brucks, D., Marshall-Pescini, S., Wallis, L. J., Huber, L., & Range, F. (2017a). Measures of dogs’ inhibitory control abilities do not correlate across tasks. Frontiers in Psychology, 8(849), 1–17. https://doi.org/10.3389/fpsyg.2017.00849
Brucks, D., Soliani, M., Range, F., & Marshall-Pescini, S. (2017b). Reward type and behavioural patterns predict dogs’ success in a delay of gratification paradigm. Scientific Reports, 7, 1–10. https://doi.org/10.1038/srep42459
Byrne, R.W., & Bates, L.A. (2007). Sociality, evolution, cognition. Current Biology, 17, R714–R723. https://doi.org/10.1016/j.cub.2007.05.069
Diamond, A. (1990). Developmental time course in human infants and infant monkeys, and the neural bases of inhibitory control in reaching. Annals of the New York Academy of Science, 608(1), 637–676. https://doi.org/10.1111/j.1749-6632.1990.tb48913.x
Duckworth, A. L., & Kern, M. L. (2011). A Meta-Analysis of the Convergent Validity of Self-Control Measures. Journal of Research in Personality, 45, 259–268. https://doi.org/10.1016/j.jrp.2011.02.004
Fagnani, J., Barrera, G., Carballo, F., & Bentosela, M. (2016). Is previous experience important for inhibitory control? A comparison between shelter and pet dogs in A-not-B and cylinder tasks. Animal Cognition, 19(6), 1165–1172. https://doi.org/10.1007/s10071-016-1024-z
Frank, H., & Frank, M.G. (1982). Comparison of problem-solving performance in six-week-old wolves and dogs. Animal Behaviour, 30, 95–98. https://doi.org/10.1016/s0003-3472(82)80241-8
Gácsi, M., Gyoöri, B., Virányi, Z., Kubinyi, E., Range, F., Belényi, B., & Miklósi, A. (2009). Explaining Dog Wolf Differences in Utilizing Human Pointing Gestures: Selection for Synergistic Shifts in the Development of Some Social Skills. PLoS One, 4(9), 1–6. https://doi.org/10.1371/journal.pone.0006584
Glady, Y., Genty, E., & Roeder, J. (2012). Brown Lemurs (Eulemur fulvus) can master the qualitative version of the reverse-reward contingency. Plos One, 7(10), 1–7. https://doi.org/10.1371/journal.pone.0048378
Hare, B., & Tomasello, M. (2005). Human-like social skills in dogs? Trends in Cognitive Sciences, 9(9), 439–444. https://doi.org/10.1016/j.tics.2005.07.003
Juszczak, G. R., & Miller, M. (2016). Detour behavior of mice trained with transparent, semitransparent and opaque barriers. PLoS One, 11(9), 1–23. https://doi.org/10.1371/journal.pone.0162018
Kabadayi, C., Bobrowicz, K., & Osvath, M. (2018). The detour paradigm in animal cognition. Animal Cognition, 21(1), 21–35. https://doi.org/10.1007/s10071-017-1152-0
Kabadayi, C., Krasheninnikova, A., O’Neill, L., van de Weijer, J., Osvath, M., & von Bayern A. M. (2017). Are parrots poor at motor self-regulation or is the cylinder task poor at measuring it? Animal Cognition, 20(6), 1137–1146. https://doi.org/10.1007/s10071-017-1131-5
Kabadayi, C., Taylor, L. A., von Bayern, A. M., & Osvath, M. (2016). Ravens, New Caledonian crows and jackdaws parallel great apes in motor self-regulation despite smaller brains. Royal Society Open Science, 3(160104), 1–7. https://doi.org/10.1098/rsos.160104
Kis, A., Topál, J., Gácsi, M., Range, F., Huber, L., Miklósi, A., & Virányi, Z. (2012). Does the A-not-B error in adult pet dogs indicate sensitivity to human communication? Animal Cognition, 15(4), 737–743. https://doi.org/10.1007/s10071-012-0481-2
Lenth, R. V. (2016). Least-Squares Means: The R Package lsmeans. Journal of Statistical Software, 69(1), 1–33. https://doi.org/10.18637/jss.v069.i01
Leonardi, R., Vick, S., & Dufour, V. (2011). Waiting for more: the performance of domestic dogs (Canis familiaris) on exchange tasks. Animal Cognition, 15, 107–120.
Lucon-Xiccato, T., Gatto, E., & Bisazza, A. (2017). Fish perform like mammals and birds in inhibitory motor control tasks. bioRxiv 188359.
MacLean, E. L., Hare, B., Nunn, C. L., Addessi, E., Amici, F., Anderson, R. C., et al. (2014). The evolution of self-control. Proceedings of the National Academy of Science of the United States of America, 111, E2140–E2148. https://doi.org/10.1073/pnas.1323533111
Marshall-Pescini, S., Valsecchi, P., Petak, I., Accorsi, P. A., & Previde, E. P. (2008). Does training make you smarter? The effects of training on dogs’ performance (Canis familiaris) in a problem solving task. Behavioural Processes, 78(3), 449–454. https://doi.org/10.1016/j.beproc.2008.02.022
Marshall-Pescini, S., Virányi, Z., & Range, F. (2015). The effect of domestication on inhibitory control: wolves and dogs compared. PLoS One, 10(2), e0118469. https://doi.org/10.1371/journal.pone.0118469
McCormack, T., & Atance, C.M. (2011). Planning in young children: A review and synthesis. Developmental Review, 31, 1–31. https://doi.org/10.1016/j.dr.2011.02.002
Miklósi, A., Kubinyi, E., Topál, J., Gácsi, M., Virányi, Z., & Csányi, V. (2003). A simple reason for a big difference: wolves do not look back at humans, but dogs do. Current Biology, 13, 763-766.Osthaus, B., Marlow, D., & Ducat, P. (2010). Minding the gap: spatial perseveration error in dogs. Animal Cognition, 13, 881–885. https://doi.org/10.1007/s10071-010-0331-z
Müller, C.A., Riemer, S., Virányi, Z., Huber, L., & Range, F. (2016). Inhibitory control, but not prolonged object-related experience appears to affect physical problem-solving performance of pet dogs. PLoS ONE, 11, e0147753.
Osthaus, B., Marlow, D., & Ducat, P. (2010). Minding the gap: spatial perseveration error in dogs. Animal Cognition, 13(6), 881–885
Pongrácz, P., Miklósi, A., Kubinyi, E., Gurobi, K., Topál, J., & Csányi, V. (2001). Social learning in dogs: the effect of a human demonstrator on the performance of dogs in a detour task. Animal Behaviour, 62(6), 1109–1117. https://doi.org/10.1006/anbe.2001.1866
Pongrácz, P., Miklósi A., Kubinyi, E., Topál, J., & Csányi, V. (2003). Interaction between individual experience and social learning in dogs. Animal Behaviour, 65(3), 595–603. https://doi.org/10.1006/anbe.2003.2079
Santos, L. R., Ericson, B. N., & Hauser, M. D. (1999). Constraints on problem solving and inhibition: object retrieval in cotton-top tamarins (Saguinus oedipus oedipus). Journal of Comparative Psychology, 113(2), 186–193. https://doi.org/10.1037/0735-7036.113.2.186
Smith, B. P., & Litchfield, C. A. (2010). How well do dingoes, Canis dingo, perform on the detour task? Animal Behaviour, 80(1), 155–162. https://doi.org/10.1016/j.anbehav.2010.04.017
Tapp, P.D., Siwak, C.T., Estrada, J., Head, E., Muggenburg, B.A., Cotman, C. W., et al. 2003. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learning & Memory, 10, 64–73.
Tomkins, L. M., Thomson, P. C., & McGreevy, P. D. (2010). First-Stepping Test as a measure of motor laterality in dogs (Canis familiaris). Journal of Veterinary Behavior, 5(5), 247–255. https://doi.org/10.1016/j.jveb.2010.03.001
Topál, J., Gergely, G., Erdőhegyi, Á., Csibra, G., & Miklósi, Á. (2009). Differential sensitivity to human communication in dogs, wolves, and human infants. Science , 325(5945), 1269–1272. https://doi.org/10.1126/science.1176960
Udell, M. A. R., & Wynne, C. D. L. (2010). Ontogeny and phylogeny: both are essential to human-sensitive behavior in the genus Canis. Animal Behaviour, 79(2). https://doi.org/10.1016/j.anbehav.2009.11.033
Vallortigara, G., & Regolin, L. (2002). Facing an obstacle: lateralization of object and spatial cognition. In: Rogers LJ (ed) Comparative vertebrate lateralization. Cambridge University Press, Cambridge, pp 383–444. https://doi.org/10.1017/cbo9780511546372.013
Venables, W.N., & Ripley, B.D. (2002). Modern applied statistics with S. 4th. New York: Springer-Verlag.
Vernouillet, A., Anderson, J., Clary, D., & Kelly, D. M. (2016). Inhibition in Clark’s nutcrackers (Nucifraga columbiana): Results of a detour-reaching test. Animal Cognition, 19(3), 661–665. https://doi.org/10.1007/s10071-016-0952-y
Vlamings, P. H., Hare, B., Call, J. (2010). Reaching around barriers: the performance of the great apes and 3–5-year-old children. Animal Cognition, 13(2), 273–285. https://doi.org/10.1007/s10071-009-0265-5
Wobber, V., & Hare, B. (2009). Testing the social dog hypothesis: are dogs also more skilled than chimpanzees in non-communicative social tasks? Behavioural Processes, 81, 423–428.
Wynne, C., & Leguet, B. (2004). Detour behavior in the Quokka (Setonix brachyurus). Behavioural Processes, 67(2), 281–286. https://doi.org/10.1016/j.beproc.2004.04.007
Acknowledgements
Support for this study was provided through a Natural Science and Engineering Research Council Discovery grant to DMK (#312379-2009). We would very much like to thank the Winnipeg Humane Society for lending us the facilities to perform our experiments. We are thankful to the dogs and the dog owners for their participation in the study, as well as to Miriam Christensen and Meara Stow for their help in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
AAAV, LRS, JAM, and DMK designed the study; AAAV, LRS, and JAM conducted the experiments; AAAV, LRS, and DMK analysed the data and wrote the manuscript.
Electronic supplementary material
ESM 1
(PDF 118 kb)
Rights and permissions
About this article
Cite this article
Vernouillet, A.A.A., Stiles, L.R., Andrew McCausland, J. et al. Individual performance across motoric self-regulation tasks are not correlated for pet dogs. Learn Behav 46, 522–536 (2018). https://doi.org/10.3758/s13420-018-0354-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-018-0354-x