Abstract
Human children and domesticated dogs learn from communicative cues, such as pointing, in highly similar ways. In two experiments, we investigate whether dogs are biased to defer to these cues in the same way as human children. We tested dogs on a cueing task similar to one previously conducted in human children. Dogs received conflicting information about the location of a treat from a Guesser and a Knower, who either used communicative cues (i.e., pointing; Experiments 1 and 2), non-communicative physical cues (i.e., a wooden marker; Experiment 1), or goal-directed actions (i.e., grasping; Experiment 2). Although human children tested previously struggled to override inaccurate information provided by the Guesser when she used communicative cues, in contrast to physical cues or goal-directed actions, dogs were more likely to override the Guesser’s information when she used communicative cues or goal-directed actions than when she used non-communicative physical cues. Given that dogs did not show the same selective bias towards the Guesser’s information in communicative contexts, these findings provide clear evidence that dogs do not demonstrate a human-like bias to defer to communicative cues. Instead, dogs may be more likely to critically evaluate information presented via communicative cues than either physical or non-communicative cues.
Similar content being viewed by others
When human infants enter the world, they are greeted with a “blooming, buzzing confusion” (James, 1890). Although infants in every species are faced with the challenge of navigating a complex sensory environment, human infants face a particularly daunting learning task. In addition to making sense of a general flood of sensory information, human infants face the unique challenge of learning to navigate a complex cultural environment characterized by intricate tools, rituals, and language. Fortunately for human infants, they do not learn to navigate this complex cultural environment on their own. Instead, infants receive help from adults in their community who are often highly motivated to teach information about this complex culture (e.g., Brand, Baldwin, & Ashburn, 2002; Csibra & Gergely, 2011). Human adults even use a special set of communicative cues, such as high-pitched infant-directed speech and eye contact, to signal their intention to teach information to young infants and children (e.g., Grieser & Kuhl, 1988; Kuhl et al., 1997). Infants appear to be sensitive to these communicative cues early in development (e.g., Cooper, Abraham, Berman, & Staska, 1997; Csibra, 2010; Farroni et al., 2002, 2003), and begin modifying their behavior to follow these cues as early as 12 months of age (e.g., Behne, Carpenter, & Tomasello, 2005; Behne, Liszkowski, Carpenter, & Tomasello, 2012).
Some scholars have argued that this early sensitivity to communicative cues may crucially support our complex human culture (e.g., Csibra & Gergely, 2011; Tomasello, 2008; Tomasello, Carpenter, Call, Behne, & Moll, 2005). Rather than needing to independently discover information about their cultural environment, young infants are able to quickly gather relevant information from other people’s communicative cues. Our human ability to transmit communicative information so easily contrasts starkly with that of our closest primate relatives who are much less sensitive to the communicative cues of others (e.g., Itakura, Agnetta, Hare, & Tomasello, 1999; Povinelli, Reaux, Bierschwale, Allain, & Simon, 1997; Tomasello, Call, & Gluckman, 1997) and less likely to use others’ communicative cues when they have the opportunity to rely on their own individual observation (e.g., Call, Carpenter, & Tomasello, 2005; Horner & Whiten, 2005). The fact that our closest primate relatives do not demonstrate a human-like sensitivity to communicative cues raises the possibility that our sensitivity to communicative cues may underlie our unique ability to maintain complex cultural tools and customs across many generations.
However, it remains unclear exactly which aspects of our human sensitivity to communicative cues support our uniquely complex human culture. To address this question, researchers have compared humans to other non-human species that show a greater sensitivity to communicative cues than non-human primates. Such research has shown that one notable species – the domesticated dog (Canis familiaris) – demonstrates a strikingly human-like sensitivity to human communicative cues (e.g., Hare & Tomasello, 2005; Johnston, McAuliffe, & Santos, 2015; Topál, Kis, & Oláh, 2014).
Through the process of domestication and extensive experience with humans, dogs have learned to interpret and rely on human communicative cues, including eye contact (e.g., Kaminski, Schulz, & Tomasello, 2012; Téglás, Gergely, Kupán, Miklósi, & Topál, 2012), high-pitched infant-directed speech (e.g., Ben-Aderet, Gallego-Abenza, Reby, & Mathevon, 2017; Rossano, Nitzschner, & Tomasello, 2014; Scheider, Grassmann, Kaminski, & Tomasello, 2011), and pointing (e.g., Hare, Brown, Williamson, & Tomasello, 2002; Lakatos, Soproni, Dóka, & Miklósi, 2009; Riedel, Schumann, Kaminski, Call, & Tomasello, 2008). Although it is difficult to fully disentangle the root causes of dogs’ sensitivity to human communicative cues, it is clear that at least some of this sensitivity to human communicative cues arises from dogs’ evolutionary history. Most notably, dogs are able to follow human communicative cues from an early age, before they have had the opportunity to learn the importance of these cues from experience (Hare et al., 2002; Riedel et al., 2008; Rossano et al., 2014). Crucially, this early ability to follow communicative cues is not shared by dogs’ close, non-domesticated relatives – gray wolves (Canis lupus; e.g., Gácsi et al., 2009; Hare et al., 2010; Virányi et al., 2008). Even wolf cubs hand-raised by humans do not follow human communicative cues as young dogs do (e.g., Gácsi et al., 2009; Virányi et al., 2008). Thus, dogs seem to have developed the ability to follow human communicative cues across domestication in a way that’s not shared in non-domesticated canids.
That said, dogs are not unique in their ability to follow human communicative cues; other domesticated animals – such as domesticated foxes (Hare et al., 2005), horses (Proops, Walton & McComb, 2010), and goats (Kaminski, Riedel, Call & Tomasello, 2005) – are sometimes able to follow human communicative cues. Thus, it seems that domestication as a general process facilitates animals’ understanding of human communicative cues. However, dogs are unique in the extent to which they share a close evolutionary history with humans; dogs have served as hunting partners, guard dogs, and household companions in a way that no other domesticated species has. Although more research is needed to fully establish the impact of this close evolutionary history, it is clear that dogs have developed strikingly human-like interpretations of human communicative cues across domestication. Not only do dogs follow human communicative cues from an early age (Hare et al., 2002; Riedel et al., 2008; Rossano et al., 2014), but they also seem to expect this information will be referential in the same way as human children (Duranton, Range, & Virányi, 2017; Miklósi et al., 1998; Soproni, Miklósi, Topál, & Csányi, 2001; Téglás et al., 2012). Given dogs’ close evolutionary history with humans and their human-like sensitivity to communicative cues, they provide an ideal comparison species for pinpointing which particular aspects of human sensitivity to communicative cues uniquely support human culture.
Although dogs are sensitive to communicative cues in much the same way as human children, it is not yet clear whether they are motivated to follow these cues to the same degree as human children. Young children follow information presented via communicative cues so readily that they often defer to this information even when it is inaccurate or unhelpful (e.g., Jaswal, Croft, Setia, & Cole, 2010; Palmquist, Burns, & Jaswal, 2012; Palmquist & Jaswal, 2012). In one recent study, 3-year-old children played a sticker-finding game where an experimenter hid a sticker under one of two cups and then indicated where the sticker was hidden by either communicating directly to the child or placing an arrow marker on one of the two hiding locations (Jaswal et al., 2010). In both conditions, the experimenter indicated the incorrect cup that was empty. Although children in both conditions followed the experimenter’s inaccurate cue on the first trial, children in the marker condition quickly learned to reject this cue and chose the opposite cup from the one the experimenter indicated. This successful performance in the marker condition starkly contrasted with children’s performance in the direct communication condition, in which children continued to follow the experimenter’s inaccurate communication, even across repeated trials. These findings suggest that although children are able to avoid inaccurate information provided via physical cues (i.e., a physical marker), they struggle to override inaccurate information when it is provided via direct communication. Thus, it seems that children have a bias to defer to others’ direct communication even if there is reason to believe they are providing inaccurate information.
Prior work with dogs suggests that dogs may also have trouble overriding inaccurate communication provided by humans. Although dogs can learn to avoid inaccurate communication after a large number of trials (i.e., 160–200 trials; Petter, Musolino, Roberts, & Cole, 2009), they struggle to override inaccurate communication on the short time scales in which children are typically tested (e.g., ten trials or less; Kundey et al., 2010; Pongrácz, Hegedüs, Sanjurjo, Kővári, & Miklósi, 2013; Szetei, Miklósi, Topál, & Csányi, 2003). For example, when an experimenter surreptitiously hides a treat under one of two cups and then consistently points to the incorrect location across ten trials, dogs will continue to follow the inaccurate pointing, even though they do not get a reward (Kundey et al., 2010; Pongrácz et al., 2013; Scheider, Kaminski, Call, & Tomasello, 2013; Szetei et al., 2003). Crucially, just as children are able to override inaccurate physical cues (i.e., the marker placement in Jaswal et al., 2010), dogs can also learn to override inaccurate physical cues more quickly than they learn to override inaccurate communication (Petter et al., 2009). The difficulty dogs have overriding inaccurate communication may indicate that dogs – like human children – have a bias to defer to others’ direct communication.
However, it is also possible that dogs have trouble overriding inaccurate communication for entirely different reasons than human children. In particular, several researchers have argued that dogs interpret human communicative cues in a very different way from human infants, namely as imperatives or commands (e.g., Gergely & Csibra, 2013; Kaminski & Nitzschner, 2013; Petter et al., 2009; Topál, Gergely, Erdőhegyi, Csibra, & Miklósi, 2009). On this interpretation, dogs may have trouble overriding inaccurate information provided by a single informant, not because they have a child-like bias to defer to human communication, but because they see this communication as a command that must be followed.
To distinguish whether dogs have a tendency to follow human communicative cues because they see them as a command or because they have a child-like bias to defer to direct communication, it is necessary to use new methods that do not require dogs to disobey a single informant. One such method that has been used in developmental studies allows children to make a choice between two conflicting informants, rather than requiring them to override information provided by a single informant (e.g., Koenig & Harris, 2005; Johnston, Mills, & Landrum, 2015; Mills, 2013). When children are given a choice between an accurate and an inaccurate informant, they tend to go against the information provided by the inaccurate informant (e.g., Krogh-Jespersen & Echols, 2012; Vanderbilt, Heyman, & Liu, 2014).
Crucially, although children are more adept at overriding information provided by an inaccurate informant when they have the option of following an accurate one, they still show a bias to defer to direct communication, regardless of whether or not it is accurate (e.g., Couillard & Woodward, 1999; Palmquist et al., 2012; Palmquist & Jaswal, 2012). In one recent study (Palmquist et al., 2012), two experimenters provided children with conflicting information about the location of a sticker. One experimenter – the Knower – was knowledgeable about the location of the sticker, as she had witnessed the hiding process, and the other experimenter – the Guesser – was ignorant, as she had not witnessed the hiding process. When the two experimenters indicated their cup selections with non-communicative cues by placing a physical marker on the cups (i.e., Palmquist et al., 2012) or grasping the cups in a goal-directed way (Palmquist & Jaswal, 2012), children had no trouble choosing the Knower’s cup. However, when the experimenters indicated their cup selections by pointing, 4-year-olds were no longer able to selectively attend to the Knower’s information; instead they guessed randomly and chose each experimenter’s cup equally often (Palmquist et al., 2012; see also Palmquist & Jaswal, 2012). This suggests that children struggle to override inaccurate communication provided by a Guesser, even when they have the opportunity to follow accurate information provided by a Knower. Thus, children seem to override their understanding of how seeing leads to knowing and follow any available informant regardless of whether that informant is knowledgeable, as long as the informant provides information via pointing. More broadly, these findings suggest that children’s bias to defer to direct communication is so robust that they can lose sight of relevant information, such as whether the informant has the knowledge necessary to provide accurate information, even when they have a choice between two conflicting informants.
Although children receive fewer stickers in the experiment in which they fail to override the Guesser’s inaccurate pointing, when considered more broadly it is possible that this general tendency to defer to direct communication often aids children’s learning (e.g., Csibra & Gergely, 2011; Jaswal et al., 2010; Legare & Nielsen, 2015). Returning to the puzzle of complex human culture discussed earlier, children not only need to learn simple episodic information about their environment (e.g., the hiding location of a sticker), they also need to learn nuanced cultural information (e.g., how to use tools and perform intricate cultural rituals). Questioning everything they learn and critically evaluating every potential informant would greatly slow down children’s learning processes and possibly prevent them from absorbing the knowledge and tools of their culture. Considered in terms of the Guesser-Knower experiment just discussed, although children clearly have the ability to evaluate informant knowledge when informants use physical cues (e.g., Palmquist et al., 2012), they seem to stop paying attention to informant knowledge when the informants use communicative cues like pointing that indicate an intention to teach (e.g., Palmquist et al., 2012; Palmquist & Jaswal, 2012). Given that children typically learn from trustworthy caregivers who simply have a benevolent desire to teach accurately, a general bias to defer to others’ direct communication may enhance, rather than detract from, a child’s ability to quickly learn complex cultural information.
To examine whether children’s bias to defer to communicative cues has the potential to uniquely support our complex human culture, we examined whether this bias is unique to human learners or whether a similar bias was present in dogs. Specifically, we tested dogs in two experiments that were closely modeled off prior work with human children. In line with prior work with children (Palmquist et al., 2012; Palmquist & Jaswal, 2012), our experiments presented dogs with a cueing task in which they received conflicting information from a Guesser – who did not witness the hiding process – and a Knower – who did witness the hiding process. In each experiment, the Guesser and Knower indicated their cup selections either with a communicative cue (pointing) or a non-communicative cue, specifically a wooden marker in Experiment 1 (in line with Palmquist et al., 2012) or grasping the cups in a goal-directed way in Experiment 2 (in line with Palmquist & Jaswal, 2012).
Crucially, prior work with dogs has shown that dogs are able to preferentially follow information provided by a Knower over a Guesser when both individuals point to their respective cups (Catala, Mang, Wallis, & Huber, 2017; Cooper et al., 2003; Maginnity & Grace, 2014). Thus, in the current set of experiments, our primary goal was to examine whether dogs would be even more likely to follow information provided by the Knower when the informants signaled their cup selection with non-communicative cues, rather than communicative cues (used in prior work: Catala et al., 2017; Cooper et al., 2003; Maginnity & Grace, 2014). If dogs, like human children, are biased to defer to communicative cues (Palmquist et al., 2012; Palmquist & Jaswal, 2012), then they should be more likely to follow the Knower’s information when she and the Guesser provide information via non-communicative cues, rather than communicative cues. To clarify, if dogs are selectively biased to defer to communicative cues such as pointing, then they should only find conflicting communicative cues – not non-communicative cues – provided by the Guesser distracting. Thus, they should be more likely to follow the Knower over the Guesser when these conflicting communicative cues are eliminated and replaced with non-communicative cues, such as physical markers or goal-directed grasping. This pattern of results would suggest that our early human bias to defer to communicative cues is not unique and thus cannot explain our unique ability to sustain a complex culture. Instead, these findings would suggest that a bias to defer to communicative cues may arise from a more basic sensitivity to communicative cues. In contrast, if dogs are not distracted by the Guesser’s cues to a greater degree in the communicative condition – and thus follow the Knower’s information over the Guesser’s information equally often in the communicative and non-communicative conditions – this would suggest that dogs do not have a human-like bias to defer to communicative cues. This pattern of results would provide stronger evidence that this early bias to defer to communicative cues is unique to our species, and thus has the potential to help explain how humans are able to sustain a uniquely complex culture.
Experiment 1
Methods
Subjects
Forty dogs (18 males; MAge = 5.24; SDAge = 3.11) of varying ages, sexes, and breeds participated in Experiment 1 (see Online Supplementary Table 1 for specific information). Six additional dogs were excluded due to failure to pass the warm-up trials (4), making two “no choices” in a row (1), and the guardian’s request to stop the experiment (1). All dogs were pets whose guardians volunteered to participate by entering their dogs’ information into our online database. Prior to participation, all dogs visited the center at least once to make sure that they were comfortable in the center and had no aggressive tendencies.
Apparatus and testing setup
Dogs were tested in a large testing room (3.5 m × 3.15 m) in the presence of their guardian and three female experimenters. At the beginning of each trial, the dog subject sat in the corner of the room with their guardian, who acted as the dog’s handler. During testing, the guardian sat in a chair and held the dog’s leash, which was clipped to a built-in hook in the wall. Three experimenters – two informants and one baiter – sat on the floor 1.3 m away from the dog for the duration of the experiment (see Fig. 1).
Overhead view of the experimental setup for Experiments 1 and 2. The guardian held on the dog’s leash in the corner of the room at the beginning of each trial. The two informants (in red and blue shirts) sat behind the cups and the baiter sat in between the informants. The “V” shaped tape in front of the cups depicts the choice region for each cup
The experimental setup involved a removable occluder (92 cm × 60 cm) and two black plastic cups that rested upside down on top of white ceramic plates that were placed 50 cm apart. Both cups were false baited with a food reward, which was hidden in an inaccessible compartment to ensure that both cups smelled equally like food. We used 1-cm cubes of Natural Balance beef sausage as the food reward throughout the experiment. In cases where dogs were not motivated by these treats or were allergic to the ingredients in the treats, we used alternative treats of the same size provided by the dog’s guardian.
One informant sat behind each of the cups, and the baiter sat equidistant between the two informants. To ensure that dogs were able to distinguish between the two informants and remember which informant had witnessed the hiding process, the two informants wore different shirts: a plain red t-shirt and a plain blue t-shirt. We counterbalanced the informants’ side across dogs, such that the informant in the red shirt sat on the left side for half of dogs and on the right side for the other half of dogs. In the marker condition, the informants indicated their choices with blocks of wood (13 cm × 5 cm × 1.5 cm). The color of the wooden marker matched the informant’s shirt color (see Fig. 2f). Before beginning the experiment we passed images of our testing setup through an online image processing software (https://dog-vision.com/) to ensure that dogs would be able to distinguish between these two colors.
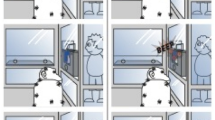
Procedure for Experiments 1 and 2. Figures (a) and (b) depict the hiding (a) and choice (b) phases for the warmup trials. Figures (c) and (d) depict the hiding phase for the visible (c) and hidden (d) test trials. In both Figures (c) and (d), the person in the red shirt is acting as the Knower and the person in the blue shirt is acting as the Guesser. Figures (e-g) depict the choice phase for the test trials for the pointing (e: Experiments 1 and 2), marker (f: Experiment 1), and grasping (g: Experiment 2) conditions
Procedure
All dogs participated in four warm-up trials followed by 16 test trials. During the warm-up trials, dogs were able to witness the hiding process and did not receive any information from the two informants. The goal of these warm-ups was to make sure that subjects did not have a bias toward cups on one side and that they were not distracted by the movement of the occluder. Once dogs passed these warm-up trials they proceeded to the test trials where they received conflicting information from the two informants about the location of a hidden treat. Half of the dogs were assigned to the pointing condition, where the informants each pointed to their selected cup, and the other half of dogs were assigned to the marker condition, where the informants placed a block of wood on their selected cup. We chose to conduct these conditions between-subjects, rather than within-subjects, because we did not want the communicative context of the pointing condition to spill over into the non-communicative context of the marker condition.
In each of these conditions, dogs received two trial types: visible trials, where they could see the hiding process and thus did not need the informants’ cues (see Fig. 2c), and hidden trials, where they could not see the hiding process and thus had to rely on information from the informants (see Fig. 2d). Thus, the test trials were presented in a 2 × 2 design, where condition (pointing vs. marker) was a between-subjects factor and trial type (visible vs. hidden) was a within-subjects factor.
The hidden trials allowed us to see whether dogs, like human children, have a bias to defer to communicative cues. If dogs, like human children, have a bias to defer to communicative cues, then they should be distracted by the Guesser’s cues to a greater degree in the pointing condition than in the marker condition. In this case, dogs should follow the Knower’s information less often in the pointing condition than in the marker condition because they are distracted by the Guesser’s conflicting communicative cues in the pointing condition. In contrast, if dogs are not biased to defer to communicative cues in the same way as human children, then they should not be distracted by the Guesser’s information, and thus they should be equally likely, or even more likely, to follow the Knower’s information in the pointing condition than in the marker condition. These results would suggest that dogs are not selectively distracted by conflicting communicative cues like pointing in the same way human children are. The visible trials allowed us to see how far this bias might extend. In particular, we tested whether dogs had a bias to defer to communicative cues even when those cues differed from their own knowledge about where the treat was hidden.
Warm-up trials. At the beginning of each warm-up trial, the baiter instructed the handler to hold the leash and close her eyes. Next, the baiter placed the occluder behind the two cups such that the occluder was between the cups and the baiter, rather than between the cups and the dog (see Figure 2a). This occluder placement allowed dogs to witness the hiding process, but ensured that the mere presence of the occluder would not distract dogs on the ensuing test trials. After placing the occluder, the baiter held a treat above the occluder and said, “[Subject’s Name], look!” while making eye contact with the subject (in line with prior work with children; Palmquist & Jaswal, 2012; Palmquist et al., 2012), and visibly placed the treat under one of the two cups. To place the treat under one of the two cups, the baiter reached around the side of the occluder with her hand and lifted the cup off of the plate, placed the treat down on the plate, and finally placed the cup back on top of the treat.
During the hiding process, the two informants faced forward and looked at a specific spot on the floor (indicated by a small piece of duct tape) 2 in. behind the two cups to avoid cueing the dog toward either cup (see Fig. 2a). This spot was an equal distance from both cups, but slightly behind the cups so that the informants could see the duct tape marking, even when the occluder was between them and the cups. To ensure that dogs did not develop a side bias, the baiter hid the treat under the left cup on two trials and under the right cup on the other two trials. The order of these trials was randomized across subjects. The side the two experimenters sat on was randomized between subjects, such that the experimenter in the red shirt sat on the left for half of dogs and the experimenter in blue sat on the left for the other half of dogs. Crucially, the exerimenters did not change sides across trials; all that changed across trials was whether the treat was hidden in the cup in front of the informant on the left or on the right.
After hiding the treat, the baiter removed the occluder and remained seated while placing the occluder against the wall to her right. The occluder was light enough that the baiter could simply reach around the back of the experimenter to her right in order to access the occluder on each trial. After removing the occluder, the baiter instructed the handler to open her eyes. While the handler was still holding the dog, the baiter counted to three, at which point the two experimenters fixed their gaze on the cup directly in front of them. Thus, the only cue the experimenters provided in the warm-up trials was gaze direction. The baiter then dropped her head down, signaling the handler to release the dog so that it was free to approach the cups (see Fig. 2b). If dogs approached the side of the baited cup first, the baiter lifted the cup and the dog was allowed to eat the treat from the plate. If the dog approached the incorrect cup first, the baiter lifted the incorrect cup and let the dog sniff the empty plate. Then, the handler was instructed to call the subject back to the starting position so the baiter could lift the correct cup and show the treat to the dog before placing it back in her treat pouch. If the dog did not make a choice within 30 s of being released, the baiter looked up and made eye contact with the subject while calling subject’s name. She also tapped the top of both cups simultaneously and looked down again. “No choice” was recorded if the subject still did not put its nose past either of the lines. In order to move on to the testing phase, dogs needed to choose the correct cup three out of four times. Four dogs were excluded for failing these warm-up trials.
Test trials. After passing the four warm-up trials, subjects completed 16 test trials. These test trials were administered in a partially repeated-measures design in which condition (pointing or marker) was a between-subjects variable, and the visibility of the hiding process (visible or hidden) was a within-subjects variable. Specifically, for half of dogs, the Guesser and Knower pointed across all 16 trials, and for the other half of dogs the Guesser and Knower indicated their selection with the wooden markers across all 16 trials. Within these 16 trials, each dog received eight trials in which the hiding process was visible to the dog because the occluder was placed behind the cups (see Fig. 2c) and eight trials in which the hiding process was hidden from the dog because the occluder was in front of the cups (see Fig. 2d). The location of the occluder was pseudo-randomized such that the occluder was not in the same location more than twice in a row.
At the beginning of each trial, the Guesser turned around such that her back was towards the subject, so she could not see the hiding process. The Knower remained facing forward and, as in the warm-up trials, always looked at the specific spot on the floor behind the two cups to avoid cueing the dog towards either of the cups during the hiding phase. The hiding process in the test trials was very similar to that of the warm-up trials. The baiter held the treat above the occluder and called the dog’s name. The baiter always hid the treat in the cup in front of the Knower. For visible trials, she baited the cup in the same exact way as in the warm-up trials. For hidden trials, she baited the cups behind the occluder so the dog could not witness the hiding process. After hiding the treat, the baiter removed the occluder and the Guesser turned back around (now facing the subject). After the Guesser turned around, the baiter told the handler she could open her eyes. The baiter then counted to three, at which point the Guesser and Knower simultaneously cued the cup that was directly in front of them. The Knower always cued the cup where the treat was hidden, and the Guesser always cued the empty cup. In the pointing condition, the Guesser and Knower simultaneously extended the hand that was closest to the baiter (i.e., the hand that was closest to the center of the setup) and touched the top of the cup with an extended index finger (see Fig. 2e). In the marker condition, the Guesser and Knower simultaneously placed a block of wood (that had previously been hidden in their laps) on the cup in front of them using the hand that was closest to the baiter (see Fig. 2f). In order to remain consistent with the warm-up trials, both conditions involved the Guesser and Knower looking at the cup as they extended their hand to point or place the wooden marker and then holding their gaze on the cup until the dog made its choice. After the Guesser and Knower pointed or placed the blocks of wood, the baiter dropped her head down, which signaled the handler to release the subject. Subjects had 30 s to choose a side, or their response was marked as a “no choice.” If a subject made two “no choice” responses in a row, the study session ended. One dog was excluded for this reason.
After the first eight test trials, dogs were given a short break where they could walk around outside before completing the last eight test trials. For half of each dog’s test trials, the informant on the left served as the Knower and for the other half of the test trials, the informant on the right served as the Knower. The experimenter playing the role of the Knower was pseudorandomized across trials such that the Knower was not the same experimenter for more than two trials in a row. Thus, dogs could not simply learn to follow one experimenter or the other, as each experimenter was the Knower on half of trials and the Guesser on the other half.
If dogs, like human children (Palmquist et al., 2012; Palmquist & Jaswal, 2012), are biased to defer to communicative cues, then they should be more biased to follow the Guesser’s information in the pointing condition than in the marker condition. In this case, they should be less likely to follow the Knower’s information in the pointing condition than in the marker condition. In contrast, if dogs do not have a human-like bias to defer to communicative cues, then they should be equally likely, or more likely, to follow the Knower’s information in the pointing condition than in the marker condition.
Coding and data analysis
The baiter live-coded each subject’s response at the end of every trial. To help with the reliability of this coding, we placed two strips of tape on the floor in the shape of a “V” to define the choice regions for the two cups (see Fig. 1). We defined a choice as the moment the dog’s nose first crossed the line in front of either the left or right cup during the choice phase. We defined accuracy as whether the subject first crossed the line in front of the baited (correct) or unbaited cup (incorrect), as well as whether the subject failed to make a choice in the first 30 s (no choice). An additional coder who was blind to the study’s hypothesis also coded for accuracy, as well as trial type, to make sure that the data was recorded accurately and that the trials were conducted correctly. Reliability was perfect for both accuracy (r = 100%) and trial type (r = 100%). Trials in which the subject failed to make a choice in the first 30 seconds (n = 2 trials) or the experimenter made an error (n = 1 trial) were excluded from analysis.
Statistical analyses were conducted using R statistical software (version 3.4.0, R Foundation for Statistical Computing, Vienna, Austria). Accuracy was analyzed with a generalized linear mixed model (GLMM) coded as a binary response term (correct cup = 1, incorrect cup = 0). Predictors of interest were trial number, condition (pointing or marker), and trial type (visible or hidden). Age (in years) was included as a covariate. The mixed models were conducted using R package ‘lme4’ (Bates, Maechler, & Bolker, 2012). First, we tested a null model that included only subject identity as a predictor for accuracy. We then compared the null model to full models with all predictor variables and their interactions, including age as a covariate. Based on the initial results of the GLMM, we then conducted follow-up t-tests looking at accuracy across the two different conditions (pointing vs. marker), broken down by trial type (visible vs. hidden).
Results and discussion
We examined whether the accuracy of dogs’ performance (the percentage of correct choices to the Knower’s cup) was affected by the cue used (pointing or marker cue), the visibility of the hiding process (visible or hidden), trial number, and subject age. Our model revealed that subjects’ tendency to favor the Knower over the Guesser was significantly predicted by both the cue used (pointing vs. marker; LRT: x2 = 13.89, p < .001) and trial type (visible vs. hidden: LRT: x2 = 87.89, p < .001). No other factors or interactions were significant predictors (LRT: ps > .077, see Fig. 3a).
Average number of times dogs selected the baited cup, indicated by the Knower, in Experiment 1 (a) and Experiment 2 (b). Dogs were able to witness the treat hiding process in the visible trials, but unable to witness the hiding process in hidden trials. The bar colors indicate how the informants conveyed their cup selection, either by placing a wooden marker on the cup, pointing to the cup, or grasping the cup. Error bars indicate standard error, and the horizontal dashed line indicates chance performance
Given that we found effects of both condition and trial type, we examined dogs’ performance in each of these contexts separately. First, we investigated whether dogs were biased to defer to communicative cues when they were only able to rely on informant knowledge (i.e., in hidden trials). In contrast to human children, who were more likely to be distracted by the Guesser in the pointing condition than in the marker condition (Palmquist et al., 2012), we found that dogs were less likely to be distracted by the Guesser, and thus were more likely to follow the Knower in the pointing condition (M = 4.60 out of eight total trials, SD = 1.54) than in the marker condition (M = 2.90 out of eight total trials; SD = 1.41), t(39) = 3.65, p < .001. In fact, although dogs were marginally more likely than chance to follow the Knower’s information in the pointing condition, t(19) = 1.75, p = .097, they were significantly less likely than chance to follow the Knower’s information in the marker condition, t(19) = 3.49, p = .002. This suggests that the pointing cues may have actually helped dogs override the Guesser’s inaccurate information and follow the Knower’s information instead. In particular, it seems that dogs may have generally been drawn to the Guesser’s information in the marker condition due to some extraneous cue, likely the fact that the Guesser had produced a significant amount of motion by turning around right before the choice phase. Although dogs were able to override this extraneous motion cue in the pointing condition, they were unable to do so in the marker condition.
In line with this possibility, dogs were also marginally more likely to follow the Knower’s information in the pointing condition (M = 6.90, SD = 1.17) than in the marker condition (M = 6.05, SD = 1.61) when the hiding process was visible, t(39) = 1.92, p = .064. Although dogs were able to choose the correct cup in the visible trials for both the pointing, t(19) = 11.13, p < .001, and marker conditions, t(19) = 5.71, p < .001, these findings generally suggest that pointing cues may have helped dogs override the Guesser’s inaccurate information, even in the visible trials.
Considered together, our results provide new evidence that dogs are not biased to defer to communicative cues like pointing in the same way as human children (Palmquist et al., 2012; Palmquist & Jaswal, 2012). Quite the contrary, communicative cues may help dogs correctly attend to information about informants, such as who is more knowledgeable. These findings provide initial evidence that humans may be unique in our bias to defer to communicative cues, regardless of whether they are provided by knowledgeable individuals or not.
Experiment 2
Experiment 1 suggested that dogs do not show a human-like bias to defer to communicative cues. Instead of biasing dogs to lose track of which informant was more knowledgeable, communicative cues (i.e., pointing) seemed to help dogs correctly attend to information about the informants. That said, even when the informants used communicative cues in the pointing condition, dogs still followed the Knower relatively infrequently on hidden trials (i.e., 58% of the time). Experiment 2 examined two reasons dogs may have followed the Knower relatively infrequently, even in the pointing condition. First, dogs may have been generally drawn toward the Guesser due to the extraneous motion cues she produced when turning back around before the choice phase. Second, dogs may have been biased to defer to communicative cues to some degree, even if they were more likely to follow the Knower when she provided communicative cues than non-communicative cues.
To examine these possibilities, Experiment 2 compared dogs’ tendency to follow the Knower’s information in the communicative pointing condition to a different non-communicative condition used with human children in prior work. Specifically, we compared a pointing condition to a non-communicative grasping condition where the two informants reached for their cup selection and grasped the cup. Although grasping is similar to pointing in many respects, it is distinct in one crucial way – in contrast to pointing, which is an intentional communicative signal for humans, grasping is a goal-directed action. Prior work has shown that children exhibit a selective bias to defer to intentional communicative cues – like pointing – but do not exhibit a similar bias to defer to non-communicative goal directed actions – like grasping (Palmquist & Jaswal, 2012). When considered in the context of human cultural learning, it makes sense that children’s bias to defer to social cues would be confined to intentionally conveyed communicative cues. Consider a child who watches an adult foraging for food. In one situation, the adult intentionally points to a particular foraging location, as if to teach the child that food is in that particular location. In another situation, the adult pays no attention to the child and simply reaches into the foraging area while searching for food independently of the child. In the first case – when the adult intentionally conveys information – the child has good reason to suspect that the indicated location has food because the adult has gone out of her way to provide this information to the child. However, in the second case, the child can make no such assumption, because the adult could simply be exploring the area in search of food, without having any intention to share that information with the child.
If dogs, like human children (e.g., Palmquist et al., 2012; Palmquist & Jaswal, 2012), are specifically biased to defer to communicative cues, then they should be less likely to override the Guesser’s information in the pointing condition than in the grasping condition. When considered in light of our results in Experiment 1, these findings would suggest that dogs may treat physical cues (i.e., the marker) differently than human children do, but that they may treat communicative cues (i.e., pointing) and non-communicative goal-directed actions (i.e., grasping) similarly. In contrast, if dogs are not biased to defer to communicative cues, like human children, then they should be no more likely to override the Guesser’s information in the grasping condition than in the pointing condition. In fact, if they treat the grasping cue like the marker cue in Experiment 1, they may even be more likely to override the Guesser’s information in the pointing condition than in the grasping condition. This pattern of results would suggest that communicative cues may actually help dogs correctly attend to which informant is more knowledgeable.
Methods
Subjects
Another 40 dogs (15 males; MAge = 6.75; SDAge = 3.59) participated in Experiment 2. Although dogs were recruited from the same online database as in Experiment 1, none of the dogs had previously participated in Experiment 1 (see Online Supplementary Table 1 for a full breakdown of breeds, ages, and sex). Ten additional dogs were excluded due to failure to pass the warm-up trials (9) and experimenter error (1).
Apparatus and testing setup
The apparatus and testing setup were identical to those of Experiment 1.
Procedure
The procedure for Experiment 2 was identical to Experiment 1, with one exception. Rather than comparing the pointing condition to a non-communicative marker condition, we compared the pointing condition to a non-communicative grasping condition in Experiment 2 (as in Palmquist & Jaswal, 2012). The grasping condition was identical to the pointing condition in Experiment 1, except that the Guesser and Knower reached for the cups and grasped them with the hand that was closer to the center of the experimental setup (see Fig. 2g). As in Experiment 1, we used a between-subjects design in which dogs were either assigned to the pointing condition or to the grasping condition. Moreover, as in Experiment 1, each dog received 16 total trials – eight visible and eight hidden trials. Visible and hidden trials were randomly intermixed, with the caveat that no trial type could be repeated more than twice in a row.
Coding and data analysis
As in Experiment 1, the baiter live-coded each subject’s response for accuracy at the end of every trial. An additional coder who was blind to the study’s hypothesis coded for accuracy and trial type, to make sure that the data was recorded accurately and that the trials were conducted correctly. Reliability was high for both accuracy (r = 98%) and trial type (r = 99%). For trials in which there was a discrepancy between the live coder and the blind coder (n = 7 trials), a third coder who was blind to hypothesis recoded the discrepant trials. In these rare cases of discrepancy, we used the third coder’s codes for these trials. Trials in which the subject failed to make a choice in the first 30 s (n = 1 trial) or the experimenter made an error (n = 3 trials) were excluded from analysis.
As in Experiment 1, accuracy was analyzed with a generalized linear mixed model (GLMM) coded as a binary response term (correct cup = 1, incorrect cup = 0). Predictors of interest were trial number, condition (pointing or grasping), and trial type (visible or hidden). As in Experiment 1, we also included age (in years) as a covariate. First, we tested a null model that included only subject identity as a predictor for accuracy. We then compared the null model to full models with all predictor variables and their interactions, including age as a covariate. Based on the initial results of the GLMM, we then conducted follow-up t-tests looking at accuracy across the two different trial types (visible vs. hidden).
Results and discussion
We first tested whether dogs’ accuracy was affected by condition (pointing or grasping), trial type (visible or hidden), trial number, and subject age. Our model revealed that subjects’ accuracy was significantly predicted by trial type (visible vs. hidden: LRT: x2 = 62.76, p < .001). No other factors or interactions were significant predictors (LRT: ps > .235, see Fig. 3b).
These findings demonstrate that dogs’ tendency to choose the cup indicated by the Knower was influenced by whether the location of the treat was visible during the hiding process or not, but not by the cue used (MPointing = 5.68 out of 8, SDPointing = 1.76, MGrasping = 5.35, SDGrasping = 1.59). Given that we found an effect of trial type, we examined subjects’ accuracy separately for the visible and hidden trials. As in Experiment 1, dogs chose the correct cup more often than chance when hiding process was visible to dogs (M = 6.65, SD = 1.05, t(39) = 15.95, p < .001). Moreover, as in the pointing condition of Experiment 1, dogs chose the correct cup indicated by the Knower marginally more often than chance when the hiding process was hidden (M = 4.38, SD = 1.39, t(39) = 1.71, p = .096).
Thus, although human children in prior work were only able to override the Guesser’s inaccurate information when the informants used non-communicative goal-directed actions (i.e., grasping; Palmquist & Jaswal, 2012), dogs were equally likely to override the Guesser’s information whether the informants used communicative pointing cues or non-communicative grasping cues. Taken together, these findings suggest that, unlike human children, dogs do not demonstrate a specific bias to defer to communicative cues.
General discussion
Across two experiments, we find clear evidence that dogs do not demonstrate a human-like bias to defer to communicative cues. In stark contrast to human children, who are less likely to critically evaluate informants in communicative contexts (Palmquist et al., 2012; Palmquist & Jaswal, 2012), dogs seem – if anything – to be more likely to critically evaluate informants in these communicative contexts. In particular, dogs in Experiment 1 were more likely to select the more knowledgeable informant when the informants used communicative cues (i.e., pointing) than non-communicative physical cues (i.e., a physical marker). In Experiment 2, dogs were no more likely to follow the knowledgeable informant when she used non-communicative goal-directed actions (i.e., grasping) than when she used communicative cues (i.e., pointing). These findings contrast with those of human children who show a general bias to defer to communicative cues, such that they often fail to engage their understanding of informant knowledge when both informants provide information communicatively (Palmquist et al., 2012; Palmquist & Jaswal, 2012). In other words, children struggle to override inaccurate information provided by an ignorant Guesser when she provides inaccurate information communicatively; in contrast, dogs are more likely to override inaccurate information provided by a Guesser in these communicative contexts. Together, our findings suggest that the bias for human learners to defer to communicative cues may be unique, given that dogs – a species that shows many basic aspects of human-like learning (e.g., Hare & Tomasello, 2005; Lakatos et al., 2009; Téglás et al., 2012; Topál et al., 2014) – do not demonstrate this bias.
More broadly, our findings suggest that dogs may show an advantage when learning from communicative cues and goal-directed actions, rather than a human-like bias to defer to communicative cues. Dogs in the pointing condition of Experiment 1 not only followed the Knower’s information more often than dogs in the marker condition, they also followed the Knower’s information marginally more often than chance. When considered in light of dogs’ performance in the marker condition of Experiment 1, it is particularly impressive that dogs were able to follow the Knower’s information in the pointing condition, even if it was to a marginal degree. In particular, dogs in the marker condition of Experiment 1 were significantly more likely to select the cup indicated by the Guesser than the Knower. Dogs’ preference for the Guesser in the marker condition suggests that dogs may have generally been drawn to the Guesser’s information at baseline, potentially due to extraneous motion cues the Guesser made when turning around before the choice phase. Given that dogs were drawn to the Guesser’s information in the marker condition, it is particularly impressive that dogs in Experiments 1 and 2 were able to override these extraneous cues and select the Knower’s cup marginally more often than chance in the pointing and grasping conditions. Although marginal, dogs’ ability to select the Knower’s cup more often in the pointing and grasping conditions suggests that dogs may be more likely to apply evidence regarding which informant is most likely to be accurate in contexts where the informants are performing a social behavior, whether that is a communicative cue or a goal-directed action.
Our findings are the first to suggest that dogs are more likely to follow knowledgeable informants in contexts where she is either explicitly communicating a hiding location to the dog (i.e., via pointing) or making a goal-directed motion towards the hiding location (i.e., grasping). Future work should investigate this possibility more closely. In particular, it would be informative to examine whether dogs continue to select the Knower more often in the pointing and grasping conditions than in the marker condition when they are tested in a method that does not bias them to follow the Guesser over the Knower at baseline. For example, Catala et al. (2017) recently developed a new method (i.e., the “glancing” method) that allows the Guesser and the Knower to turn their heads in the same direction (i.e., to the right or the left) on each trial. Because the baiter was always positioned in between the Guesser and Knower, only the Knower wound up looking at the baiter during the hiding process. If dogs continue to show an advantage for social and communicative cues – relative to physical cues – when the Guesser and Knower produce identical motions, this would provide stronger evidence that social and communicative cues enhance dogs’ ability to use informant knowledge.
Moreover, future work should more closely investigate whether dogs are evaluating the informants’ knowledge, per se, or whether they may instead be focusing on other extraneous cues. For instance, dogs could be learning which of the two informants is more likely to point to the hiding location of the treat based on the association between the location of the informant who turns around and the location of the treat. Although we did not find any evidence that dogs’ performance improved across trials, it is still possible that dogs were learning this contingency via associative learning, rather than reasoning about mental states. This would require dogs to learn a rule such as “the informant who faces forward points to the hiding location” or “the informant who turns around points to the incorrect location.” Dogs’ performance in the marker condition in Experiment 1 suggests they do no easily learn this association since they were more likely than chance to go to the incorrect cup. That said, it is still possible that dogs in the pointing condition were simply more likely to learn this association, rather than being more likely to use this information to evaluate informant knowledge. Future work should investigate this possibility more thoroughly.
Crucially, though, even if dogs were determining which informant to follow based purely on associative cues, our current findings clearly demonstrate that dogs do not have a human-like bias to defer to communicative cues. If dogs had a bias to defer to communicative cues, they should have shown a disadvantage for communicative cues in the current studies, rather than an advantage. That said, future work should investigate this possibility more closely. Although children in prior work demonstrated a bias to defer to communicative cues when pointing was the only cue used (i.e., in the absence of other communicative cues, such as eye contact or high-pitched speech; Palmquist et al., 2012; Palmquist & Jaswal, 2012), it could be that dogs would demonstrate a bias to defer to communicative cues if additional cues were used. Indeed, prior work has shown that dogs sometimes require additional communicative cues, such as eye contact (e.g., Kaminski et al., 2012; Téglás et al., 2012) and high-pitched speech (e.g., Téglás et al., 2012), to follow human pointing and gaze cues. Future work should therefore test how dogs evaluate these other communicative cues, but would need to use a design in which the Guesser and Knower inform the dog sequentially (as in prior work examining dogs’ understanding of reliability; e.g., Petter et al., 2009; Takaoka, Maeda, Hori, & Fujita, 2015), rather than simultaneously. If dogs begin to show a disadvantage for communicative cues when additional communicative cues are used (e.g., eye contact and high-pitched speech), this would suggest that dogs do demonstrate a bias to defer to communicative cues in some contexts. Although this bias would not be fully human-like – since it would require additional communicative input – it would further highlight the importance dogs place on communicative cues like eye contact and high-pitched speech.
Although additional work is necessary to fully establish the degree to which dogs show a greater tendency to follow the Knower when she uses communicative cues and goal-directed actions, the current findings clearly establish that dogs do not demonstrate a human-like bias to defer to communicative cues. These findings, along with several other recent findings (e.g., Johnston, Holden, & Santos, 2017; Topál et al., 2009), suggest that the human bias to defer to communicative cues may be unique. Not only do dogs fail to defer to communicative cues when they are able to discover more efficient solutions on their own (e.g., Johnston et al., 2017; Topál et al., 2009), but they may also be more likely to evaluate information provided by communicative cues than non-communicative cues (Experiments 1 and 2). Thus, it seems that although domestication has shaped human-like learning in dogs, it has shaped these learning capacities in fundamentally different ways.
Although this human bias to defer to pointing and other communicative cues may lead children to sub-optimal responding in the context of cleverly crafted experiments (e.g., Jaswal et al., 2010; Palmquist et al., 2012; Palmquist & Jaswal, 2012), it is possible that this bias generally helps children learn more efficiently about their environment. Children are tasked with learning about a vast array of complex cultural tools, rituals, and vocabulary. Having mechanisms to efficiently learn from others could help children skip the time-consuming task of needing to evaluate everything they are told and rediscover how to use these tools and perform these rituals on their own. Thus, it is possible that this bias to defer to communicative cues in childhood crucially supports our human ability to sustain a uniquely complex culture via highly efficient learning.
References
Bates, D., Maechler, M., & Bolker, B. (2012). lme4: Linear mixed-effects models using S4 classes (R Package Version 0.999999-0).
Behne, T., Carpenter, M., & Tomasello, M. (2005). One-year-olds comprehend the communicative intentions behind gestures in a hiding game. Developmental Science, 8(6), 492-499. https://doi.org/10.1111/j.1467-7687.2005.00440.x
Behne, T., Liszkowski, U., Carpenter, M., & Tomasello, M. (2012). Twelve-month-olds’ comprehension and production of pointing. British Journal of Developmental Psychology, 30(3), 359-375. https://doi.org/10.1111/j.2044-835X.2011.02043.x
Ben-Aderet, T., Gallego-Abenza, M., Reby, D., & Mathevon, N. (2017). Dog-directed speech: Why do we use it and do dogs pay attention to it? Proceedings of the Royal Society B, 284(1846), 20162429. https://doi.org/10.1098/rspb.2016.2429
Brand, R. J., Baldwin, D. A., & Ashburn, L. A. (2002). Evidence for ‘motionese’: Modifications in mothers’ infant-directed action. Developmental Science, 5(1), 72-83. https://doi.org/10.1111/1467-7687.00211
Call, J., Carpenter, M., & Tomasello, M. (2005). Copying results and copying actions in the process of social learning: Chimpanzees (Pan troglodytes) and human children (Homo sapiens). Animal Cognition, 8(3), 151-163. https://doi.org/10.1007/s10071-004-0237-8
Catala, A., Mang, B., Wallis, L., & Huber, L. (2017). Dogs demonstrate perspective taking based on geometrical gaze following in a Guesser–Knower task. Animal Cognition, 20(4), 1-9. https://doi.org/10.1007/s10071-017-1082-x
Cooper, J. J., Ashton, C., Bishop, S., West, R., Mills, D. S., & Young, R. J. (2003). Clever hounds: Social cognition in the domestic dog (Canis familiaris). Applied Animal Behaviour Science, 81(3), 229-244. https://doi.org/10.1016/S0168-1591(02)00284-8
Cooper R. P., Abraham J., Berman S., & Staska M. (1997). The development of infants’ preference for motherese. Infant Behavior & Development, 20, 477–488. https://doi.org/10.1016/S0163-6383(97)90037-0
Couillard, N. L., & Woodward, A. L. (1999). Children's comprehension of deceptive points. British Journal of Developmental Psychology, 17(4), 515-521. https://doi.org/10.1348/026151099165447
Csibra, G. (2010). Recognizing communicative intentions in infancy. Mind & Language, 25(2), 141-168. https://doi.org/10.1111/j.1468-0017.2009.01384.x
Csibra, G., & Gergely, G. (2011). Natural pedagogy as evolutionary adaptation. Philosophical Transactions of the Royal Society B, 366(1567), 1149-1157. https://doi.org/10.1098/rstb.2010.0319
Duranton, C., Range, F., & Virányi, Z. (2017). Do pet dogs (Canis familiaris) follow ostensive and non-ostensive human gaze to distant space and to objects? Royal Society Open Science, 4(7), 170349. https://doi.org/10.1098/rsos.170349
Farroni, T., Csibra, B., Simion, F., & Johnson, M. H. (2002). Eye contact detection in humans from birth. Proceedings of the National Academy of Science, 99(14), 9602-9605. https://doi.org/10.1073/pnas.152159999
Farroni, T, Mansfield, E., Lai, C., & Johnson, M. (2003). Infants perceiving and acting on the eyes: Tests of an evolutionary hypothesis. Journal of Experimental Child Psychology, 85(3), 199-212. https://doi.org/10.1016/s0022-0965(03)00022-5
Gácsi, M., Györi, B., Gyoöri, B., Virányi, Z., Kubinyi, E., Range, F., … Miklósi, A. (2009). Explaining dog wolf differences in utilizing human pointing gestures: Selection for synergistic shifts in the development of some social skills. PLoS One, 4, e6584. https://doi.org/10.1371/journal.pone.0006584
Gergely, G., & Csibra, G. (2013). Natural pedagogy. In M. R. Banaji & S. A. Gelman (Eds.), Navigating the social world: What infants, children, and other species can teach us (pp. 127–132). Oxford, England: Oxford University Press.
Grieser D. L., & Kuhl P. K. (1988). Maternal speech to infants in a tonal language: Support for universal prosodic features in motherese. Developmental Psychology, 24, 14–20. https://doi.org/10.1037/0012-1649.24.1.14
Hare, B., Brown, M., Williamson, C., & Tomasello, M. (2002). The domestication of social cognition in dogs. Science, 298(5598), 1634-1636. https://doi.org/10.1126/science.1072702
Hare, B., Plyusnina, I., Ignacio, N., Schepina, O., Stepika, A., Wrangham, R., & Trut, L. (2005). Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Current Biology, 15(3), 226-230. https://doi.org/10.1016/j.cub.2005.01.040
Hare, B., Rosati, A., Kaminski, J., Bräuer, J., Call, J., & Tomasello, M. (2010). The domestication hypothesis for dogs' skills with human communication: A response to Udell et al. (2008) and Wynne et al. (2008). Animal Behaviour, e1-e6. https://doi.org/10.1016/j.anbehav.2009.06.031
Hare, B., & Tomasello, M. (2005). Human-like social skills in dogs? Trends in Cognitive Sciences, 9(9), 439-444. https://doi.org/10.1016/j.tics.2005.07.003
Horner, V., & Whiten, A. (2005). Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Animal Cognition, 8(3), 164-181. https://doi.org/10.1007/s10071-004-0239-6
Itakura, S., Agnetta, B., Hare, B., & Tomasello, M. (1999). Chimpanzee use of human and conspecific social cues to locate hidden food. Developmental Science, 2(4), 448-456. https://doi.org/10.1111/1467-7687.00089
James, W. (1890). The principles of psychology. New York: Holt.
Jaswal, V. K., Croft, A. C., Setia, A. R., & Cole, C. A. (2010). Young children have a specific, highly robust bias to trust testimony. Psychological Science, 21(10), 1541-1547. https://doi.org/10.1177/0956797610383438
Johnston, A. M., Holden, P. C., & Santos, L. R. (2017). Exploring the evolutionary origins of overimitation: A comparison across domesticated and non-domesticated canids. Developmental Science, 20(4). https://doi.org/10.1111/desc.12460
Johnston, A. M., McAuliffe, K., & Santos, L. R. (2015). Another way to learn about teaching: What dogs can tell us about the evolution of pedagogy. Behavioral and Brain Sciences, 38, e44. https://doi.org/10.1017/S0140525X14000491
Johnston, A. M., Mills, C., & Landrum A. (2015). How do children weigh competence and benevolence when deciding whom to trust? Cognition, 144, 76-90. https://doi.org/10.1016/j.cognition.2015.07.015
Kaminski, J., & Nitzschner, M. (2013). Do dogs get the point? A review of dog-human communication ability. Learning and Motivation, 44(4), 294–302. https://doi.org/10.1016/j.lmot.2013.05.001
Kaminski, J., Riedel, J., Call, J., & Tomasello, M. (2005). Domestic goats, Capra hircus, follow gaze direction and use social cues in an object choice task. Animal Behaviour, 69(1), 11-18. https://doi.org/10.1016/j.anbehav.2004.05.008
Kaminski, J., Schulz, L., & Tomasello, M. (2012). How dogs know when communication is intended for them. Developmental Science, 15(2), 222-232. https://doi.org/10.1111/j.1467-7687.2011.01120.x
Koenig, M. A., & Harris, P. L. (2005). Preschoolers mistrust ignorant and inaccurate speakers. Child Development, 76(6), 1261-1277. https://doi.org/10.1111/j.1467-8624.2005.00849.x
Krogh-Jespersen, S., & Echols, C. H. (2012). The influence of speaker reliability on first versus second labeling. Child Development, 83(2), 581-590. https://doi.org/10.1111/j.1467-8624.2011.01713.x
Kuhl, P.K., Andruski, J.E., Chistovich, I.A., Chistovich, L.A., Kozhevnikova, E.V., Ryskina, V.L., Stolyarova, E.I., Sundberg, U., & Lacerda, F. (1997), Cross-language analysis of phonetic units in language addressed to infants. Science, 277, 684–686. https://doi.org/10.1126/science.277.5326.684
Kundey, S., De Los Reyes, A., Arbuthnot, J., Allen, R., Coshun, A., Molina, S., & Royer, E. (2010). Domesticated dogs’ (Canis familiaris) response to dishonest human points. International Journal of Comparative Psychology, 23, 201–215.
Lakatos, G., Soproni, K., Dóka, A., & Miklósi, Á. (2009). A comparative approach to dogs’ (Canis familiaris) and human infants’ comprehension of various forms of pointing gestures. Animal Cognition, 12(4), 621-631. https://doi.org/10.1007/s10071-009-0221-4
Legare, C. H., & Nielsen, M. (2015). Imitation and innovation: The dual engines of cultural learning. Trends in Cognitive Sciences, 19(11), 688-699. https://doi.org/10.1016/j.tics.2015.08.005
Maginnity, M. E., & Grace, R. C. (2014). Visual perspective taking by dogs (Canis familiaris) in a Guesser–Knower task: Evidence for a canine theory of mind? Animal Cognition, 17(6), 1375-1392. https://doi.org/10.1007/s10071-014-0773-9
Miklósi, Á., Polgárdi, R., Topál, J., & Csányi, V. (1998). Use of experimenter-given cues in dogs. Animal Cognition, 1(2), 113-121. https://doi.org/10.1007/s100710050016
Mills, C. M. (2013). Knowing when to doubt: Developing a critical stance when learning information from others. Developmental Psychology, 49(3), 404-418. https://doi.org/10.1037/a0029500
Palmquist, C. M., Burns, H. E., & Jaswal, V. K. (2012). Pointing disrupts preschoolers’ ability to discriminate between knowledgeable and ignorant informants. Cognitive Development, 27(1), 54-63. https://doi.org/10.1016/j.cogdev.2011.07.002
Palmquist, C. M., & Jaswal, V. K. (2012). Preschoolers expect pointers (even ignorant ones) to be knowledgeable. Psychological Science, 23(3), 230-231. https://doi.org/10.1177/0956797611427043
Petter, M., Musolino, E., Roberts, W. A., & Cole, M. (2009). Can dogs (Canis familiaris) detect human deception? Behavioural Processes, 82(2), 109-118. https://doi.org/10.1016/j.beproc.2009.07.002
Pongrácz, P., Hegedüs, D., Sanjurjo, B., Kővári, A., & Miklósi, Á. (2013). “We will work for you”–Social influence may suppress individual food preferences in a communicative situation in dogs. Learning and Motivation, 44(4), 270–281. https://doi.org/10.1016/j.lmot.2013.04.004
Povinelli, D. J., Reaux, J. E., Bierschwale, D. T., Allain, A. D., & Simon, B. B. (1997). Exploitation of pointing as a referential gesture in young children, but not adolescent chimpanzees. Cognitive Development, 12(4), 423-461. https://doi.org/10.1016/S0885-2014(97)90017-4
Proops, L., Walton, M., & McComb, K. (2010). The use of human-given cues by domestic horses, Equus caballus, during an object choice task. Animal Behaviour, 79(6), 1205-1209. https://doi.org/10.1016/j.anbehav.2010.02.015
Riedel, J., Schumann, K., Kaminski, J., Call, J., & Tomasello, M. (2008). The early ontogeny of human-dog communication. Animal Behaviour, 74(3), 1003-1014. https://doi.org/10.1016/j.anbehav.2007.08.010
Rossano, F., Nitzschner, M., & Tomasello, M. (2014). Domestic dogs and puppies can use human voice direction referentially. Proceedings of the Royal Society of London B: Biological Sciences, 281(1785), 20133201. https://doi.org/10.1098/rspb.2013.3201
Scheider, L., Grassmann, S., Kaminski, J., & Tomasello, M. (2011). Domestic dogs use contextual information and tone of voice when following a human pointing gesture. PLoS One, 6(7), e21676. https://doi.org/10.1371/journal.pone.0021676
Scheider, L., Kaminski, J., Call, J., & Tomasello, M. (2013). Do domestic dogs interpret pointing as a command? Animal Cognition, 16(3), 361–372. https://doi.org/10.1007/s10071-012-0577-8
Soproni, K., Miklósi, Á., Topál, J, & Csányi, V. (2001). Comprehension of human communicative signs in pet dogs (Canis familiaris). Journal of Comparative Psychology, 115(2), 122-126. https://doi.org/10.1037//0735-7036.115.2.122
Szetei, V., Miklósi, Á., Topál, J., & Csányi, V. (2003). When dogs seem to lose their nose: An investigation on the use of visual and olfactory cues in communicative context between dog and owner. Applied Animal Behaviour Science, 83(2), 141–152. https://doi.org/10.1016/S0168-1591(03)00114-X
Takaoka, A., Maeda, T., Hori, Y., & Fujita, K. (2015). Do dogs follow behavioral cues from an unreliable human? Animal Cognition, 18(2), 475-483. https://doi.org/10.1007/s10071-014-0816-2
Téglás, E, Gergely, A., Kupán, K., Miklósi, Á., & Topál, J. (2012). Dogs’ gaze following is tuned to human communicative signals. Current Biology, 22(3), 209-212. https://doi.org/10.1016/j.cub.2011.12.018
Tomasello, M. (2008). Origins of human communication. Cambridge, MA: MIT Press.
Tomasello, M., Call, J., & Gluckman, A. (1997). Comprehension of novel communicative signs by apes and human children. Child Development, 68(6), 1067-1080. https://doi.org/10.1111/j.1467-8624.1997.tb01985.x
Tomasello, M., Carpenter, M., Call, J., Behne, T., & Moll, H. (2005). Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences, 28(05), 675-691. https://doi.org/10.1017/S0140525X05000129
Topál, J., Gergely, G., Erdőhegyi, A., Csibra, G., & Miklósi, Á. (2009). Differential sensitivity to human communication in dogs, wolves, and human infants. Science, 325(5945), 1269-1272. https://doi.org/10.1126/science.1176960
Topál, J., Kis, A., & Oláh, K. (2014). Dogs’ sensitivity to human ostensive cues: A unique adaptation. The social dog: Behavior and cognition. Elsevier, San Diego, pp. 319-346.
Vanderbilt, K. E, Heyman, G. D., & Liu, D. (2014). In the absence of conflicting testimony children trust inaccurate informants. Developmental Science, 17(3), 443-451. https://doi.org/10.1111/desc.12134
Virányi, Z., Gácsi, M., Kubinyi, E., Topál, J., Belényi, B., Ujfalussy, D., & Miklósi, Á. (2008). Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris). Animal Cognition, 11, 373–387. https://doi.org/10.1007/s10071-007-0127-y
Acknowledgements
We would like to thank the members of the Canine Cognition Center at Yale for their helpful assistance and feedback, particularly Alondra Arguello, Mikey Bogese, Arianna Neal, Astrid Hengartner, Jack Schleifer, Kacie Saxer-Taulbee, Lena Nasrallah, Molly Byrne, Nele Löecher, Nikita Cotzias, and Emily Reagan. AMJ was supported by a National Science Foundation Graduate Research Fellowship under Grant No. DGE-1122492, YH was supported by a University of Rochester Discover Grant for Undergraduate Research, and the Canine Cognition Center at Yale was supported by an NSF REU grant (SMS-1659085). All procedures performed were in accordance with the ethical standards of and the approved by the Institutional Animal Care and Use Committee of Yale University (#2014-11616).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
(DOCX 32.8 kb)
Rights and permissions
About this article
Cite this article
Johnston, A.M., Huang, Y. & Santos, L.R. Dogs do not demonstrate a human-like bias to defer to communicative cues. Learn Behav 46, 449–461 (2018). https://doi.org/10.3758/s13420-018-0341-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-018-0341-2