Abstract
The present series of five flavor aversion experiments with rat subjects examined compound conditioning at varying CS–US intervals. Using a taste–taste design, Experiments 1A and 1B demonstrated overshadowing at a 0-min CS–US interval and potentiation at a 120-min CS–US interval, and these effects occurred with both tastes of the compound. Experiment 2 showed that the aversion to a single element is reduced when the CS–US interval is increased to 120 min, but the aversion for a compound taste is not. Experiments 3A and 3B explored odor + taste compound conditioning; the results demonstrated odor potentiation across the trace interval and a transition from taste overshadowing to taste potentiation. Collectively, the data show that the change from overshadowing to potentiation was not due to changes in the aversions produced by compound conditioning but, instead, was due to a more rapid loss of conditionability across a trace interval prior to the US in single-element conditioning. These experiments suggest that following compound conditioning, the aversion to each element represents generalization decrement from the configured compound, but the designation of overshadowing or potentiation actually depends on the status of conditioning in the single-element control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Typically, in classical conditioning, when two conditioned stimuli (CS A and CS X) are presented in compound prior to an unconditioned stimulus (US), learning to each cue is decreased, or overshadowed, as the cues compete for associative strength (e.g., Pavlov, 1927, pp. 269–270). The converse effect, potentiation, has been reported in the taste aversion learning literature when a taste cue and an odor cue are presented simultaneously before illness induction; the aversion to the odor is increased or potentiated relative to that shown by organisms that experienced only odor and illness (e.g., Rusiniak, Hankins, Garcia, & Brett, 1979). Considering that the same experimental design yields diametrically opposite results and that formal models of associative learning only anticipate overshadowing (e.g., Pearce & Hall, 1980; Rescorla & Wagner, 1972), there has been a need to identify the conditions that would produce competitive conditioning or synergistic conditioning and to determine whether both phenomena could be incorporated within a single theoretical framework.
Potentiation to a range of cues (e.g., odors, tastes, auditory cues, visual cues) within taste aversion conditioning has been reported since 1978 (e.g., Bouton, Dunlap, & Swartzentruber, 1987; Ellins, Cramer, & Whitmore, 1985; Galef & Osborne, 1978; Holder & Garcia, 1987; Lett, 1980), but evidence of potentiation in more traditional classical conditioning preparations has been scarce. Recently, however, Urcelay and Miller (2009) were the first to demonstrate potentiation in Pavlovian fear conditioning. Using cues that reliably produce overshadowing in their laboratory, their first experiment used a 2 × 3 design in which rats received either single-element fear conditioning (clicker) or compound fear conditioning (clicker + noise) with a 0-, 10-, or 20-s CS–US interval. During testing with the less salient clicker, they observed the expected overshadowing effect at the 0-s interval, but they also detected potentiation at the 20-s interval (i.e., stronger responding to the clicker after compound conditioning than after single-element conditioning). Notably, responding to the clicker in the single-element group decreased significantly as the CS–US interval increased (i.e., a trace interval effect), but responding to the clicker following compound conditioning appeared to increase across the CS–US interval. Their report provides an opportunity for further understanding of the mechanisms of overshadowing and potentiation, particularly if similar manipulations yield the same outcome in taste aversion learning.
Although an important next step would be to determine whether manipulations of the CS–US interval also produce an overshadowing-to-potentiation shift in a taste aversion preparation, there is some reason to question whether such a shift would occur in a taste aversion design. Specifically, in the fear conditioning design used by Urcelay and Miller (2009), the shift from overshadowing to potentiation was seen to the weaker cue of the compound (the clicker CS). Yet, in compound conditioning in the taste aversion learning design, overshadowing is often recorded to the stronger cue of the compound (e.g., Batsell & Best, 1992; Rusiniak, Palmerino, Rice, Forthman, & Garcia, 1982; Westbrook, Homewood, Horn, & Clarke, 1983). Moreover, there are few reports that have systematically investigated the effects of CS–US interval on potentiation. One study of this type, conducted by Palmerino, Rusiniak, and Garcia (1980, Experiment 2) demonstrated quite clearly that taste-potentiated odor aversion increases as the CS–US interval increases. Rats were given either an almond odor solution or an almond odor + saccharin compound, followed by illness induction at 0, 15, 30, 120, 240, 360, or 1,200 min. Significant differences in odor aversion were not observed at the 0-min interval, but significant potentiation effects were reported with CS–US intervals up to 120 min. Notably, the single-element odor aversion was weaker at later CS–US intervals: Evidence of a weak odor aversion was seen at the 15-min CS–US interval, but there was no suppression of drinking the odor solution once the CS–US interval was 30 min or longer. Because it may be difficult to maintain single-element odor conditioning beyond 15 min with the weak odor concentrations and conditioning parameters that have typically supported potentiation, this may limit the use of taste-mediated odor potentiation to replicate the work of Urcelay and Miller. To allow for exploration of the effects of increasing the CS–US interval on taste-mediated potentiation, a taste–taste compound conditioning design was adopted first in the present experiments because reliable taste aversions can be obtained with extended CS–US intervals (e.g., Garcia, Ervin, & Koelling, 1966) and three different laboratories have demonstrated taste-mediated taste potentiation in adult rats (e.g., Bouton et al., 1987; Davis, Best, & Grover, 1988; Kucharski & Spear, 1985).
The purpose of this research was to determine whether manipulations of the CS–US interval influenced the expression of overshadowing and potentiation in a taste aversion conditioning design in a manner similar to that in Urcelay and Miller (2009). Our initial experiments investigated taste-potentiated taste aversions, and the later experiments explored taste-potentiated odor aversions.
Prior to initiating these experiments, our laboratory was investigating the effects of intermixing single-element and compound conditioning trials in taste–taste conditioning with saccharin (SAC) and denatonium (DEN) flavors, and this entailed the use of a 0-min and a 120-min CS–US interval (Batsell & Wakefield, 2008). Although we recorded the expected overshadowing of DEN at a short CS–US interval, we were surprised to find significant potentiation of DEN by SAC at the 120-min interval—an effect consistent with the finding of Urcelay and Miller. Therefore, Experiments 1A and 1B were designed as a parametric investigation of the changes in aversion strength to each element following compound conditioning across a 120-min CS–US interval. Eight groups were tested in each experiment: Rats were exposed either to a single-element taste or to the taste–taste compound; illness was then induced 0, 30, 60, or 120 min later. Responding to DEN was tested in Experiment 1A, and responding to SAC was tested in Experiment 1B.
Experiments 1A and 1B
Experiment 1A method
Subjects
Subjects were 96 experimentally naïve, male, Holtzman rats (Rattus norvegicus, purchased from Harlan Sprague–Dawley, Indianapolis, IN) (weight range = 350–440 g). Rats were housed in groups of 3 in a double-sized cage until they exceeded 250 g, after which they were housed individually in suspended stainless steel cages (all cages from Unifab Corporation, Kalamazoo, MI). To ensure a constant environment, a 12:12-h light:dark cycle beginning at 0700 h was used. Rats were given LabDiet 5001 (TestDiet, Brentwood, MO) ad lib throughout the experiment, but access to water was progressively restricted over 4 days, beginning when the rats were moved to individual cages. During the study, rats were provided daily access to 40 ml of fluid for 20 min at 1000 h, except on conditioning and testing days, when the daily water maintenance was delayed 4 h after the last experimental manipulation. Kalamazoo College’s Institutional Animal Care and Use Committee approved all of the research in this report, and rats were treated in accordance with American Psychological Association guidelines.
Materials and procedure
All fluids were presented in 50-ml Nalgene centrifuge tubes fitted with rubber stoppers and ball bearing spouts. Consumption was measured as the difference between the tube weight before and after drinking. Amounts consumed are reported in milliliters, assuming 1 g = 1 ml.
Two taste cues were used: 0.01% DEN (denatonium saccharide solution [0.1 g/1 L room temperature tap water]) and a SAC + DEN compound that was comprised of 0.15% SAC and a 0.01% DEN (all chemicals from Sigma Chemical Co., St. Louis, MO). These solutions were used in concentrations previously used in our laboratory (e.g., Batsell & Best, 1992). Illness was induced via intraperitoneal (i.p.) injections of 0.075 M LiCl (12 ml/kg body weight); this concentration of LiCl was chosen to minimize floor effects in conditioning at the short CS–US interval.
All experimental procedures were conducted in the familiar home cages at 1000 h; the familiar home cage was employed to reduce any effects of context blocking. Rats were matched to one of eight groups that differed in terms of their CS–US interval (0, 30, 60, or 120 min) and conditioning fluid (DEN vs. SAC + DEN), and groups were labeled according to these factors (e.g., 30-SD). Mean water intake prior to experimental procedures was used for matching, and the intakes ranged from 17.2 to 18.2 ml. The experiment was completed in two replications. Because of limitations in animal numbers, the 19 rats in the second replication were distributed only across Groups 0-D, 0-SD, 120-D, and 120-SD; these groups now had 13 to 14 members.
On the conditioning day (day 1), Groups 0-D, 30-D, 60-D, and 120-D received 5 ml of DEN for 5 min, while Groups 0-SD, 30-SD, 60-SD, and 120-SD received 5-min access to 5 ml of the SAC + DEN compound. Rats were given a single i.p. injection of 0.075 M LiCl immediately after fluid presentation (Groups 0-D and 0-SD), 30 min after fluid presentation (Groups 30-D and 30-SD), 60 min after fluid presentation (Groups 60-D and 60-SD), or 120 min after fluid presentation (Groups 120-D and 120-SD). Taste testing began 5 days later on day 6. All rats were allowed 20-min access to 30 ml of DEN to test for a conditioned DEN aversion.
Experiment 1B method
Subjects, materials, and procedure
The subjects were 97 experimentally naïve, male, Holtzman rats purchased from the Harlan Sprague Dawley Co. (Indianapolis, IN). They were treated using the same procedures as in Experiment 1A. At the start of conditioning, the rats’ weights were in the range of 350–420 g.
Rats were matched to one of eight groups on the basis of their water intake during a 1-week period before conditioning (average water intake ranged from 17.3 to 18.1 ml). This study was also conducted in two replications, and the additional rats were included only in the 0- and 120-min groups. As in Experiment 1A, a 2 × 4 experimental design was used, with groups experiencing either single-element SAC (0.15% sodium saccharin solution) or the compound SAC + DEN solution during conditioning. Then groups of rats were exposed to SAC or SAC + DEN and were conditioned at each of the four CS–US intervals (0, 30, 60, or 120 min). For SAC testing on day 6, rats were given access to 30 ml of SAC for 20 min. All other conditioning, testing, and maintenance procedures were the same as in Experiment 1A.
Experiments 1A and 1B data analysis
For both Experiments 1A and 1B, a 2 × 4 factorial analysis of variance (ANOVA) with conditioning solution and CS–US interval as factors was used to analyze the test data. Because the detection of overshadowing or potentiation was the primary focus of these experiments, between-group t-tests were employed at each CS–US interval. The statistical criterion was set at .05 for all analyses.
Experiment 1A results
Conditioning
The rats were given 5-min access to 5 ml of their target solution during the conditioning period. Rats consumed less of the SAC + DEN solution (M = 2.2 ml) than of the DEN-alone solution (M = 3.7 ml), t(94) = 8.7, p < .001. This difference in conditioning intakes was seen in all of the subsequent experiments, and it has been observed in many previous compound flavor studies (cf. Bouton, Jones, McPhillips, & Swartzentruber, 1986; Bouton & Whiting, 1982; Rusiniak et al., 1979); the relation of conditioning intake to the observation of potentiation or overshadowing will be addressed in the Experiment 1 Discussion section.
DEN testing
The initial analysis was to determine whether there were any differences due to replication order. A 2 (replication 1 vs. replication 2) × 2 (fluid: DEN vs. SAC + DEN) × 2 (interval: 0 vs. 120 min) ANOVA was conducted on the test intakes. Neither the replication factor, F(1, 48) < 1, nor its various interactions (highest F score = 1.4) were statistically significant. Therefore, results were collapsed across replications for subsequent analyses.
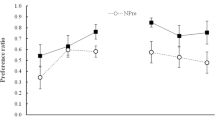
The upper panel of Fig. 1 displays the mean DEN test intakes for the eight groups. Overshadowing was observed at the shortest CS–US interval (0 min), since the single-element Group 0-D drank less than the compound Group 0-SD. However, as the CS–US interval increased, this pattern reversed to potentiation, since the compound groups (60-SD and 120-SD) drank less than their counterparts (60-D and 120-D). The statistical analyses confirm this interpretation.
A 2 × 4 factorial ANOVA with conditioning solution (DEN vs. SAC + DEN) and interval (0, 30, 60, or 120 min) as factors was conducted on these DEN intakes. The ANOVA yielded a significant interval effect, F(3, 88) = 15.6, p < .001, which was expected since the DEN aversion overall was stronger at the shorter CS–US intervals than at the longer CS–US intervals. Also, the solution × interval interaction was statistically significant, F(3, 88) = 5.4, p = .002; but the solution effect was not statistically significant, F(1, 88) = 3.5, p = .067.
To explore further the significant interaction effect, planned comparison t-tests were conducted at each CS–US interval to detect the presence of overshadowing, potentiation, or neither effect. A significant overshadowing effect was recorded at the 0-min CS–US interval, t(26) = 3.6, p = .001. A significant difference was not seen at the 30-min interval, t(18) < 1. Significant potentiation effects were seen at the 60-min interval, t(18) = 2.1, p = .04, and the 120-min interval, t(26) = 3.5, p = .002.
As a different means of investigating the significant interaction, post hoc one-way ANOVAs were conducted to analyze separately the single-element groups and the compound groups across the CS–US interval. For the DEN-only groups, the one-way ANOVA yielded a significant trace interval effect, F(3, 44) = 14.9, p < .001. Post hoc Student Newman–Keuls (SNK) tests confirmed that Group 0-D (M = 1.0 ml) drank significantly less than the other three groups, Groups 30-D (M = 6.2 ml) and 60-D (M = 5.6 ml) drank similar amounts, Group 120-D (M = 11.0 ml) drank significantly more than the other three groups, and Groups 30-D and 60-D did not differ significantly from each other. The one-way ANOVA on the test 1 DEN intakes of the four compound groups surpassed the statistical criterion, F(3, 44) = 3.2, p = .031, but no significant group differences were detected with the SNK tests (0-SD = 3.4 ml; 30-SD = 5.9 ml; 60-SD = 3.1 ml; 120-SD = 6.0 ml).
Experiment 1B results
Conditioning
As was expected, on the single conditioning trial, SAC consumption (3.9 ml) was significantly higher than DEN + SAC consumption (2.0 ml), t(95) = 10.9, p < .001.
SAC testing
As in the previous experiment, there were no significant effects related to replication, highest F(1, 50) = 0.6, p = .43, so groups were collapsed across replications.
The mean SAC intakes of the eight groups are portrayed in the lower panel of Fig. 1. Similar to Experiment 1A, the single-element groups drank less SAC than did the compound conditioning groups at shorter CS–US intervals (0 and 30 min), but the compound conditioning group drank less SAC than did the single-element group at the longest CS–US interval. The 2 × 4 ANOVA confirmed these interpretations, yielding a significant effect for interval, F(3, 89) = 16.9, p < .001, and a significant interaction of interval and solution, F(3, 89) = 9.7, p = .001. The solution main effect was not significant, F(1, 89) < 1. Independent group t-tests confirmed significant overshadowing at the 0-min interval, t(27) =3.9, p = .001, and a significant potentiation effect was recorded at the 120-min CS–US interval, t(27) = 3.6, p = .001. A statistically significant difference was not recorded at the 30-min interval, t(17) = 1.5, p = .145, or the 60-min CS–US interval, t(18) < 1.
As in Experiment 1A, the SAC intakes of the SAC-only groups and the SAC + DEN groups were analyzed separately. Concordant with the previous study, a significant trace interval effect was observed with the SAC-only groups, F(3, 43) = 24.4, p < .001. Post hoc tests showed that Group 120-S drank significantly more SAC than did the other three groups. Yet a significant trace interval effect was not observed across the SAC + DEN groups, F(3, 46) < 1.
Experiments 1A and 1B discussion
Experiments 1A and 1B provided a parametric investigation of the changes to each element of a two-taste compound across a range of CS–US intervals (0–120 min). In both experiments, at the shortest CS–US interval (0 min), the aversion to the tastes that had been conditioned alone was significantly stronger than the aversion to the tastes that had been conditioned in compound (i.e., overshadowing). In contrast, at the longest CS–US interval (120 min), the aversion to the tastes that had been conditioned alone was significantly weaker than the aversion to the tastes that had been conditioned in compound (i.e., potentiation). This pattern of results—a transition from overshadowing at the immediate CS–US interval to potentiation at the longest interval—replicates the results reported by Urcelay and Miller (2009) in Pavlovian fear conditioning.
It is first necessary to discuss the rather unexpected finding of significant overshadowing of SAC by DEN at the shortest CS–US intervals. Previous taste–taste compound conditioning studies using these stimuli have reported DEN potentiating the aversion to SAC with short CS–US intervals (e.g., Batsell & Best, 1992; Davis et al., 1988). At the present time, a specific factor that can account for why DEN overshadowed SAC at the short CS–US interval cannot be identified unequivocally.
In reviewing the factors that may have determined the transition from overshadowing to potentiation, it is first important to consider the role of conditioning intake. In all our experiments, during conditioning, rats given the flavor compound consumed significantly less than the rats given the single-element solution. This enhanced neophobia to the flavor compound, relative to controls, has been reported in many previous studies (e.g., Bouton et al., 1986; Bouton & Whiting, 1982; Rusiniak et al., 1979; Rusiniak et al., 1982; Slotnick, Westbrook, & Darling, 1997). Indeed, Bouton et al. concluded that conditioning intake was not the determining factor in the expression of overshadowing or potentiation because they saw decreased compound consumption associated with overshadowing in some experiments (Bouton & Whiting, 1982) and with potentiation in other experiments (Bouton et al., 1986). Similarly, the conditioning and test data from Experiments 1A and 1B support this claim. Therefore, it is apparent that some factor other than conditioning intake differences is responsible for overshadowing, potentiation, and the transition from overshadowing to potentiation observed in this report.
One explanation for the present results arises from the observation in both Experiments 1A and 1B that the aversion to the single-element taste decreased significantly as the CS–US interval increased, but a similar change was not observed if the target taste had been conditioned in compound with a second taste. Therefore, these data suggest a differential loss of conditionability across the CS–US interval to the single-element taste, as compared with the compound taste.Footnote 1 Although the idea that a more complex CS would be more resistant to trace interval manipulations than would a less complex CS is not novel, the application of this concept to compound aversion conditioning is unique. In the differential loss of conditonability hypothesis, exposure to the single-element CS or the compound CS establishes a memory or representation of that event, and the effectiveness of this representation will decline across the trace interval, reducing the extent to which presentation of the US can establish an aversion. However, this decline in effectiveness of conditionability occurs more rapidly across the trace interval for the single-element CS than for the compound CS. Indeed, in this approach, the changes responsible for the transition from overshadowing to potentiation are due to changes in the single-element cue, not the compound cue.
Experiment 2
In Experiments 1A and 1B, both DEN and SAC aversions were significantly weaker at the 120-min trace interval than at the 0-min interval; however, a similar significant difference at these trace intervals was not recorded to either of these tastes following compound conditioning. The differential loss of conditionability account was derived from these results, but direct evidence of the aversion to the compound itself across these same intervals is lacking. To confirm the differential loss of conditionability hypothesis, it is imperative to compare directly aversions to the compound and its elements at both short and long trace intervals; yet, to date, no such comparisons exist. Indeed, in the two closest studies of this type (cf. Kucharski & Spear, 1985, Experiment 1; Palmerino et al., 1980, Experiment 2), direct tests of the compound were omitted. Thus, the differential loss of conditionability account was tested by a comparison of aversion learning to the single-element flavors (SAC and DEN) and to the compound (SAC + DEN) at the immediate (0-min) and trace (120-min) intervals. The purpose of Experiment 2 was twofold: (1) to determine whether the rate of conditionability loss to the compound across the 120-min interval was different from the rate of loss to each single-element taste and (2) to determine whether aversion strength to the SAC + DEN compound decreases across the trace interval, using the intervals from Experiments 1A and 1B (0, 30, 60, and 120 min).
Method
Subjects, apparatus, and procedure
The subjects in Experiment 2 were 76 naïve male Holtzman rats (Harlan Sprague–Dawley, Indianapolis, IN). All housing and maintenance procedures were the same as previously described. Rats were matched to one of eight groups on the basis of the mean water intake for a 6-day period prior to conditioning. Groups were designated according to their CS–US interval (0, 30, 60, or 120 min) and the conditioning/ testing fluid (“S” for SAC, “D” for DEN, “SD” for the SAC + DEN compound). There were 10 rats each in Groups 0-S, 0-D, 0-SD, 120-S, 120-D, and 120-SD; there were 8 rats each in Groups 30-SD and 60-SD. Experimental procedures were conducted at 1000 h in the rat’s home cage.
Conditioning occurred on Day 1. During conditioning, rats were given 5-min access to 5 ml of their target solution. Groups 0-S and 120-S received SAC, Groups 0-D and 120-D received DEN, and Groups 0-SD, 30-SD, 60-SD, and 120-SD received the SAC + DEN compound solution. Following removal of the drinking tubes, LiCl injections (0.075 M LiCl at 1.2% of body weight) were administered on the basis of each rat’s group designation. Rats in Groups 0-S, 0-D, and 0-SD had a 0-min CS–US interval, Group 30-SD had a 30-min CS–US interval, Group 60-SD had a 60-min CS–US interval, and Groups 120-S, 120-D, and 120-SD had a 120-min CS–US interval.
Testing commenced on day 6 and lasted for 3 days. For testing, rats were given 20-min access to 30 ml of their target solution. Groups 0-S and 120-S received SAC, Groups 0-D and 120-D received DEN, and Groups 0-SD, 30-SD, 60-SD, and 120-SD received the SAC + DEN compound solution.
Results and discussion
Conditioning
The groups given the single-element solution (SAC or DEN) drank similar amounts during conditioning: The group means were 0-S = 3.7 ml, 120-S = 4.0 ml, 0-D = 3.7 ml, and 120-D = 3.8 ml. The groups given the SAC + DEN compound solution drank similar amounts, but less than the single-element groups: 0-SD = 1.8 ml, 30-SD = 2.1 ml, 60-SD = 1.9 ml, and 120-SD = 2.1 ml. A one-way ANOVA revealed a significant difference in conditioning intakes across fluids, F(2, 73) = 34, p < .001, since the compound groups drank significantly less SAC + DEN (M = 2.0 ml) than the single-element groups drank SAC (M = 3.9 ml) or DEN (M = 3.8 ml).
Testing
Figure 2 displays the mean fluid intake on test 1 of the 0-min groups and the 120-min groups. It is clear that a trace interval effect is seen across the SAC groups and the DEN groups, but there is no difference across the CS–US interval for the groups conditioned and tested with the compound solution. This interpretation is confirmed by a 2 × 3 ANOVA with interval (0 vs. 120 min) and solution (SAC, DEN, or SAC + DEN) as factors. Significant effects were obtained for interval, F(1, 54) = 24.7, p < .001, solution, F(2, 54) = 24.7, p < .001, and the interaction of these factors, F(2, 54) = 5.1, p = .009. In regard to the significant solution effect, post hoc Student Newman–Keuls tests confirmed that the SAC + DEN groups drank significantly less of their test solution than did the SAC alone or DEN alone groups, suggesting that the compound solution is more salient or complex than either single-element solution. The main experimental question in this study was to determine whether there was a differential loss in aversion strength as the trace interval increased (the trace interval effect), and this was explored with independent t-tests for each solution. As was noted above, there was a significant loss in aversion strength across the interval for the groups tested with SAC, t(18) = 3.4, p = .003, or DEN, t(18) = 3.9, p = .001, but not for the SAC + DEN groups, t(18) < 1.
The SAC + DEN groups continued to show strong aversions on test 2 (0-SD = 3.0 ml [SEM = 0.9] and 120-SD = 4.7 ml [SEM = 1.2]), but extinction caused the trace interval effect to be lost across the SAC groups and the DEN groups (0-S = 16.7 ml [SEM = 0.9], 120-S = 15.0 ml [SEM = 1.8], 0-D = 14.8 ml [SEM = 2.3], and 120-D = 15.7 ml [SEM = 1.5]). The 2 × 3 factorial ANOVA with interval and conditioning solution revealed only a significant solution effect, F(2, 54) = 38.4, p < .001, since the SAC + DEN groups continued to drink significantly less solution than did the SAC groups and the DEN groups. Neither the interval effect nor the interval × solution interaction was statistically significant, both Fs < 1.
A final test was conducted on day 8. On this test, the single-element groups continued to drink substantial amounts of their target solution (0-S = 18.2 ml [SEM = 0.5], 120-S = 18.7 ml [SEM = 1.2], 0-D = 16. 9 ml [SEM = 1.4], and 120-D = 17.8 ml [SEM = 1.1]). The compound groups continued to drink much less of their test solution (0-SD = 9.3 ml [SEM = 2.4] and 120-SD = 13.9 ml [SEM = 2.0]). The 2 × 3 factorial ANOVA with interval and solution as factors revealed that only the solution factor was significant on this test, F(2, 54) = 10.9, p < .001, since the compound groups drank significantly less than the single-element groups, which did not differ.
The second purpose of Experiment 2 was to determine the time course of compound conditioning with the range of intervals used in Experiments 1A and 1B. In regard to this question, the mean SAC + DEN intakes across the CS–US interval were 0-SD = 1.0 ml (SEM = 0.4), 30-SD = 1.5 ml (SEM = 0.6), 60-SD = 1.2 ml (SEM = 0.5), and 120-SD = 1.2 ml (SEM = 0.4). A one-way ANOVA confirmed that these group intakes did not differ significantly, F(3, 32) < 1. The SAC + DEN groups continued to show strong aversions on test 2 (0-SD = 3.0 ml [SEM = 0.9], 30-SD = 3.7 ml [SEM = 1.0], 60-SD = 4.3 ml [SEM = 1.2], and 120-SD = 4.7 ml [SEM = 1.2]), and the one-way ANOVA conducted on these intakes confirmed there were still no group differences based on CS–US interval, F(3, 32) < 1. The compound groups continued to drink similar amounts on test 3 (0-SD = 9.3 ml [SEM = 2.4], 30-SD = 9.5 ml [SEM = 2.5], 60-SD = 12.8 ml [SEM = 3.0], and 120-SD = 13.9 ml [SEM = 2.0]). Analysis of the intakes of the compound solution groups across the 120-min CS–US with the one-way ANOVA continued to show no significant differences, F(3, 32) < 1.
In sum, the results from Experiment 2 are consistent with predictions from the differential loss of conditionability account. Specifically, a significantly weaker aversion was recorded to both single-element tastes (DEN and SAC) when the CS–US interval was increased to 120 min, but this pattern of differences was not seen to the DEN + SAC compound. Indeed, a direct comparison of the aversion to the compound at the 0-, 30-, 60-, and 120-min CS–US intervals showed no differences on any of the three tests. It is important to make clear that the results from Experiment 2 should not be interpreted to mean that a significant trace interval effect following aversion conditioning with a compound stimulus solution is not possible. Indeed, examination of the test 3 intakes of the four SAC + DEN groups demonstrates a pattern of increasing intake with increasing CS–US interval, yet these differences were not statistically significant; thus, a trace interval effect is likely with more testing or the use of a longer trace interval (e.g., 240 min). Instead, these data confirm that relative to the single-element control groups, the loss of conditionability to the compound stimulus across the trace interval occurs at a slower rate. As such, the results of Experiment 2 are supportive of the differential loss of conditionability hypothesis described above.
Although we have interpreted the differences in Experiment 2 as due to a differential loss in conditionability by the single-element tastes relative to the compound solution, it is possible that an alternative account based on differences in initial conditioning may also be applicable. In this alternative interpretation, the rate of decay across the trace interval is the same for both the single-element tastes and the compound solution, but the differences occur in initial salience or conditionability, with the compound having a higher conditionability. Because of this higher conditionability of the compound, the compound aversion is much stronger than the single-element aversions, and due to floor effects, no differences in compound aversion strength are recorded across the trace interval on the initial tests. Indeed, as was just noted, as compound testing continued in Experiment 2, aversion strength weakened across trials, and a nonsignificant trend for a trace interval effect began to emerge. At the present time, the data do not allow us to distinguish between these accounts; indeed, both may contribute to the present results.
Experiments 3A and 3B
Considering that manipulations of the trace interval revealed a transition from overshadowing to potentiation with two taste cues replicated the findings of Urcelay and Miller (2009), we next explored the effects of similar manipulations with an odor + taste compound. As was noted earlier, the majority of demonstrations of potentiation have involved an odor + taste compound, with the typical finding of potentiation of the odor aversion and, when taste data have been reported, overshadowing or no effect on the taste aversion (e.g., Bowman, Batsell, & Best, 1992; Westbrook et al., 1983). If the effects observed with a taste–taste compound also apply to an odor–taste compound, it was predicted that (1) odor potentiation would remain consistent across the trace intervals but (2) an overshadowed taste aversion at a short CS–US interval would transition to a odor-potentiated taste aversion at longer CS–US intervals. Therefore, Experiments 3A and 3B were performed to record responding to a taste–odor compound and its elements across a range of trace intervals (0, 30, 60, 120, and 240 min), and a few procedural decisions were made to promote testing of the predictions above. First, because a primary interest of this research was to assess odor, taste, and taste + odor compound conditioning across a range of trace intervals, we included groups that received conditioning and testing with the taste + odor compound in both experiments. Second, we chose a compound solution of 1% almond odor (AL Odor) and 0.01% denatonium taste solution that has been shown to result in odor potentiation, taste overshadowing, and little generalization between the cues (Bowman et al., 1992); also, Bowman et al. showed that nonreinforced controls would drink substantial amounts of these concentrations of AL Odor solution (means ranged from 17.3 to 19.9 ml) and DEN solution (means ranged from 15.7 to 18.6 ml) (see Experiments 2A and 2B). Third, to maximize the probability of detecting the novel transition to odor-mediated taste potentiation, we used the trace intervals from Experiment 1 in this report, along with a 240-min CS–US interval. Experiment 3A examined responding to the compound and the odor element, whereas Experiment 3B examined responding to the compound and the taste element.
Experiment 3A method
Subjects, materials, and procedure
The subjects were 116 experimentally naïve male Holtzman rats (weight range at conditioning = 300–398 g). The rats were maintained according to the husbandry protocols described in previous experiments. Rats were matched to 1 of 15 groups on the basis of their average water intake. The rats were designated by their CS–US interval (0, 30, 60, 120, or 240 min), their conditioning fluid (A or DA), and their test solution (A or DA). For example, Group 60-DA-A members received the DEN + AL compound solution (DA), followed 60 min later by the LiCl injection; subsequently, their aversion to the AL odor solution (A) was tested. The groups were 0-A-A (n = 10), 0-DA-A (n = 10), 0-DA-DA (n = 10), 30-A-A (n = 9), 30-DA-A (n = 9), 30-DA-DA (n = 9), 60-A-A (n = 10), 60-DA-A (n = 10), 60-DA-DA (n = 10), 120-A-A (n = 5), 120-DA-A (n = 5), 120-DA-DA (n = 5), 240-A-A (n = 5), 240-DA-A (n = 5), 240-DA-DA (n = 5). Due to the large number of animals required to conduct this research, Experiment 3A was completed in two replications. All experimental procedures were conducted at 1030 h, unless otherwise noted.
Conditioning occurred on day 1. During conditioning, all rats received 5-min access to 5 ml of their designated solution. The 5 A-A groups received the 1% AL Odor solution (10 ml of Adams Almond Extract mixed with 990 ml of room temperature tap water). The 5 DA-A groups and the 5 DA-DA groups were given the DEN + AL mixture (10 ml of Adams Almond Extract mixed with 990 ml of 0.01% room temperature denatonium solution). Following removal of the drinking tubes, rats were injected with LiCl (0.075 M at 1.2% of body weight) depending on their group designation; 0-min groups were injected immediately after tube removal, 30-min groups were injected 30 min later, 60-min groups were injected 60 min later, 120-min groups were injected 120 min later, and 240-min groups were injected 240 min later. Animals received their daily water maintenance 4 h later.
Testing occurred on day 6. For testing, rats were given 20-min access to their test solution. Groups A-A and DA-A were tested with AL Odor solution, and Group DA-DA was tested with the DEN + AL solution. Rats received their daily water 4 h later.
Experiment 3B
Subjects, materials, and procedure
One-hundred fourteen experimentally naïve, white, male Holtzman rats were subjects in this experiment (weight range at conditioning = 291–399 g). Rats were matched to 1 of 15 groups on the basis of their mean water intake over a week-long period prior to conditioning (range = 18.1–19.7 ml). Rats were designated by their trace interval (0, 30, 60, 120, or 240 min), their conditioning solution (D or DA), and their test solution (D or DA). The 15 groups were 0-D-D (n = 10), 0-DA-D (n = 10), 0-DA-DA (n = 9), 30-D-D (n = 9), 30-DA-D (n = 9), 30-DA-DA (n = 8); 60-D-D (n = 10); 60-DA-D (n = 10), 60-DA-DA (n = 9), 120-D-D (n = 5), 120-DA-D (n = 5), 120-DA-DA (n = 5), 240-D-D (n = 5), 240-DA-D (n = 5), 240-DA-DA (n = 5). As with Experiment 3A, Experiment 3B was completed in two replications.
Conditioning occurred on Day 1, and testing occurred on Day 6; all experimental procedures were conducted in the familiar home cage. For conditioning, rats were given 5-min access to 5 ml of their designated conditioning solution (DEN or DEN + AL); these solutions were prepared as described for Experiment 3A. Following removal of the drinking tubes, the LiCl injection was delivered on the basis of each group’s designated CS–US interval (0, 30, 60, 120, or 240 min). For testing, rats were given 20-min access to their test solution (DEN or DEN + AL). Rats always received their daily water maintenance 4 h after any experimental manipulations.
Experiment 3A results
Conditioning
During conditioning, Group A-A drank AL, while Groups DA-A and DA-DA drank the DEN + AL compound. As was expected, AL consumption (N = 39; M = 3.3 ml) was significantly greater than DEN + AL consumption (N = 77; M = 2.7 ml), t(114) = 2.6, p = .011.
Testing
The initial statistical analysis was a 2 (replication: first vs. second) × 5 (interval: 0, 30, 60, 120, or 240 min) × 3 (group: A-A, DA-A, or DA-DA) between-groups factorial ANOVA on the test intakes to explore any effects due to replication. Neither the replication factor nor its interaction with the other factors surpassed the statistical criterion, all Fs < 1. As a result, the intakes across the replications were combined for subsequent data analyses.
For testing, all rats were given access to their target solution; Groups A-A and DA-A had access to the AL Odor solution, while Group DA-DA had the DEN + AL compound solution. The mean group fluid intakes are presented in the upper panel of Fig. 3. It can be seen at each trace interval that Group A-A drank the most solution and Group DA-DA drank the least solution. Group DA-A always drank an intermediate amount that varied along with the CS–US interval. A 5 × 3 factorial ANOVA with interval and group as factors confirmed these interpretations. The ANOVA yielded a significant interval effect, F(4, 101) = 51.7, p < .001, a significant group effect, F(2, 101) = 63.9, p < .001, and a significant interval × group interaction, F(8, 101) = 3.5, p = .001.
The upper panel displays the AL and DEN + AL mean intakes (+SEM) in Experiment 3A; the lower panel shows the DEN and DEN + AL mean intakes (+SEM) in Experiment 3B
To explore the significant interaction, one-way ANOVAs were conducted at each trace interval, and follow-up SNK tests were performed to detect taste-mediated odor potentiation. At the 0-min interval, the groups effect was significant, F(2, 27) = 8.2, p = .002. Potentiation was recorded, since Group A-A drank significantly more than Group DA-A and Groups DA-DA and DA-A drank similar amounts. Group differences were also recorded at the 30-min interval, F(2, 23) = 16.1, p < .001, and potentiation also occurred at this interval. Testing at the 60-min interval also yielded a significant group effect, F(2, 27) = 31.0, p < .001, and a significant potentiation effect. However, there was now a significant generalization decrement effect, since Group DA-A drank more solution than did Group DA-DA. At the 120-min interval, significant group, F(2, 12) = 8.1, p = .006, and potentiation effects were detected. Finally, at the 240-min interval, a significant group effect was obtained, F(2, 12) = 9.3, p = .004, since Group DA-DA drank less than the other two groups, but potentiation was not evident at this interval. Therefore, significant taste-mediated odor potentiation was recorded across a range of short to long trace intervals (0, 30, 60, and 120 min).
On the basis of the observations from the previous experiments, another aim of this study was to determine the rate of change in responding across the trace interval for each of the three groups. A one-way ANOVA performed on the AL intakes of Group A-A revealed a significant trace interval effect, F(4, 34) = 17.2, p < .001. Post hoc SNK tests revealed that a significant difference in AL Odor aversion strength occurred between the 0- and 30-min intervals (0-min A-A intake = 3.9 ml vs. 30-min A-A intake = 7.5 ml), which replicates the sharp decline in odor aversion conditioning reported by Palmerino et al. (1980). A significant trace interval effect was also seen with the test intakes of Group DA-A, F(4, 34) = 33.5, p < .001, but in this group, the significant loss in aversion strength did not appear until the 60-min trace interval (0-min DA-A intake = 1.8 ml vs. 60-min DA-A intake = 5.0 ml). Finally, the DA-DA groups also differed across the trace interval, F(4, 33) = 11.1, p < .001, but now the significant trace interval effect did not appear until the 120-min interval (0-min DA-DA intake = 1. 4 ml vs. 120-min DA-DA intake = 4.5 ml). Thus, these results are similar to those from the taste–taste experiments in that a significant trace interval effect was detected at earlier intervals following single-element conditioning, relative to later trace intervals following compound conditioning.
Experiment 3B results
Conditioning
During conditioning, groups consumed either DEN (D-D groups) or the AL + DEN compound (DA-D and DA-DA groups). DEN consumption (N = 39; M = 4.1 ml) was significantly greater than AL + DEN consumption (N = 75; M = 2.8 ml), t(112) = 6.5, p < .001.
Testing
As in the previous experiment, this experiment was conducted in two replications. A 2 × 5 × 3 between-groups ANOVA with order, interval, and group as factors, respectively, was conducted on the test 1 intakes. In this analysis, neither the order factor, F(1, 66) = 3.1, p = .08, nor any of its interactions with the other factors reached the statistical criterion [order × interval, F(2, 66) = 2.4, p = .10; order × group, F(2, 66) = 1.2, p = .3; order × interval × group, F(4, 66) < 1], so the intakes across replications were collapsed for the following analyses.
The lower panel of Fig. 3 displays the mean fluid intakes on test 1 of Groups D-D, DA-D, and DA-DA. It can be seen that Group D-D drank less DEN than did Group DA-D at the shortest interval (overshadowing) but Group D-D drank more DEN than did Group DA-D at later intervals (potentiation). A 5 × 3 ANOVA with interval and groups as factors yielded a significant interval effect, F(4, 99) = 28.6, p < .001, a significant group effect, F(2, 99) = 15.3, p < .001, and a significant interval × group interaction, F(8, 99) = 4.0, p < .001. To explore further the significant interaction, one-way ANOVAs were conducted on the intakes at each interval.
A significant group effect was obtained at the 0-min interval, F(2, 26) = 40.8, p < .001, and the post hoc SNK tests revealed a significant overshadowing effect (Group DA-D > Group D-D) and a significant generalization decrement effect (Group DA-D > Group DA-DA). At the 30-min interval, a significant group effect was obtained, F(2, 23) = 4.0, p = .032, since Group DA-DA drank significantly less than the other two groups. Notably, the pattern of consumption changed at the 60-min interval, F(2, 23) = 7.6, p = .003. Now, a significant potentiation effect was observed, since Group D-D drank significantly more than Group DA-D. This pattern of significant group differences, F(2, 12) = 7.2, p = .009, along with a significant potentiation effect, was replicated at the 120-min interval. Finally, at the 240-min interval, the group effect was not statistically significant, F(2, 12) = 2.4, p = .136.
As in the previous experiments, we also separately explored changes in aversion strength across the trace interval for each of the groups. A significant group difference was obtained for Group D-D, F(4, 34) = 11.5, p < .001, and a significant trace interval effect was evident by the 60-min interval (0-min D-D intake = 1.9 ml vs. 60-min D-D intake = 9.8 ml). Similarly, for Group DA-D, a significant group effect was recorded, F(4, 34) = 6.0, p = .001, but the significant trace interval effect was not evident until the 240-min interval (0-min DA-D intake = 3.6 ml vs. 240-min DA-D intake = 10.2 ml). A significant group effect was also obtained for Group DA-DA, F(4, 31) = 41.2, p < .001. For Group DA-DA, the significant trace interval effect became evident at the 120-min interval (0-min DA-DA = 0.6 ml vs. 120-min DA-DA intake = 4.7 ml), which replicates the trace interval for the complimentary Group DA-DA in Experiment 3A. Overall, the pattern of differences across the trace interval for the single-element and compound conditioning groups was consistent with the previous experiments.
Experiments 3A and 3B discussion
The results of Experiment 3A show the effects of odor potentiation, taste + odor conditioning, and single-element odor aversion conditioning across a range of CS–US intervals. The odor potentiation results replicated the work of Palmerino et al. (1980) in showing that taste-mediated odor potentiation could be obtained across a range of trace intervals. In regard to single-element odor aversion conditioning, the decay rate of conditionability across the CS–US interval of single-element odor conditioning was steeper than that of taste + odor compound conditioning. In contrast to the results of Palmerino et al., the results of Experiment 3A suggest that single-element odor aversions can be produced with a 30-min or longer trace interval: Rats at the 30-min and 60-min trace intervals had odor aversions that were significantly stronger than the odor aversions of the 240-min group. These results are in agreement with work from Slotnick et al. (1997) and Desgranges, Sevelinges, Bonnefond, Levy, Ravel and Ferreira (2009) that single-element aversions can be produced with CS–US intervals beyond 30 min. One notable procedural difference between these experiments is that Palmerino et al. presented the odor cue distally from the taste cue, whereas the odor was mixed in solution with the taste in the other studies, a procedure that produces stronger odor conditioning (Bouton et al., 1986).
There are two notable findings in the results of Experiment 3B. First, the results reveal the predicted transition from taste overshadowing at the shortest trace interval to taste potentiation at the 60- and 120-min intervals. The latter result replicates the outcome obtained in Experiments 1A and 1B of this report and extends the finding of the transition from overshadowing to potentiation to taste + odor compounds. Second, the demonstration of odor-mediated taste potentiation in Experiment 3B and the fact that it co-occurred with taste-mediated odor potentiation (i.e., reciprocal potentiation) have implications for the theoretical analysis of potentiation. To date, odor-mediated taste potentiation with adult rats have been reported twice (Peterson, Valliere, Misanin, & Hinderliter, 1985; Slotnick et al., 1997), but with conditions that were very different from those in our experiments. The first documentation of this effect was by Peterson et al., who examined taste aversions following odor + taste preexposure and subsequent compound conditioning with a 0-min CS–US interval in rats of differing ages (weanling, young adult, and old age). They reported odor-mediated taste potentiation in the young-adult group, but no significant taste potentiation effects in the weanling or old-age groups. However, it is not explicitly clear why they obtained this outcome with these conditioning parameters when similar studies observed taste overshadowing (e.g., Rusiniak et al., 1982; Westbrook et al., 1983). The second demonstration came from Slotnick et al., who demonstrated odor-mediated taste potentiation after they manipulated the concentrations of the flavor cues (saccharin and amyl acetate odor). Specifically, in their Experiment 4, they observed that the detection of overshadowing or potentiation was dependent on the relative concentrations of the cues. During odor testing, a strong saccharin solution (0.1%) overshadowed their strongest amyl acetate solution (0.00025%), had no effect on moderate concentrations of amyl acetate solution (0.00005% and 0.00001%), and potentiated the weakest concentration of amyl acetate (0.000005%). Similarly, the strong amyl acetate odor solution (0.1%) overshadowed the strongest saccharin concentration (0.05%) but potentiated weaker concentrations of saccharin (0.025%, 0.0125%, and 0.006%). Therefore, the concentrations that were necessary to produce potentiation of one element were not capable of producing potentiation to the other element. These results provided some of the strongest evidence to date that potentiation depends on the relative salience of the different flavor cues.
On the basis of their results and similar findings from other laboratories (e.g., Bouton et al., 1986), Slotnick et al. (1997) argued that the conditions necessary to produce potentiation do not depend on the identity of the cues, as had been proposed by Garcia, Lasiter, Bermudez-Rattoni, and Deems (1985), a conclusion that is bolstered by our Experiment 3B results. Furthermore, Slotnick et al. suggested that potentiation was dependent on the relative salience of the cues in that it was necessary to have a compound of a cue that was strongly associated with illness paired with a cue that was weakly associated with illness. If this claim is valid, one would predict that a concentration of odor that is capable of being potentiated by taste would not also be able to potentiate that same taste (i.e., reciprocal potentiation). Yet reciprocal potentiation was obtained at both the 60- and 120-min intervals in Experiments 3A and 3B, and it was also observed at the 120-min interval in Experiments 1A and 1B. These demonstrations of reciprocal potentiation with both taste–taste and odor–taste compounds invalidates the salience rule that potentiation is obtained only when a strong cue potentiates a weak cue. Instead, it appears that the strong-cue–weak-cue dynamic simply favors the detection of potentiation, but it is not necessary to produce potentiation.
Collectively, the results from these experiments have further implications for theoretical accounts of potentiation. The results from Experiments 3A and 3B are also difficult to explain with regard to the within-compound association model of potentiation provided by Durlach and Rescorla (1980). In this elemental approach to potentiation, the organism forms three associations during compound conditioning (AX–US). One association forms between A and the US, a second association forms between X and the US, and the third association is the within-compound association that forms between A and X. Potentiation is produced when testing A activates the direct association of A–US and the indirect A → X → US chain. The response to A in this case is potentiated because of the summation of the direct and indirect pathways, which results in a significantly stronger response than that of a single-element control group that only has the direct A–US association. The within-compound association approach to potentiation, however, has always been difficult to reconcile with the many demonstrations that flavor-compound conditioning was often an “asymmetrical phenomenon” with increased conditioning (potentiation) to the odor and decreased conditioning (overshadowing) to the taste (e.g., Rusiniak et al., 1982; Westbrook et al., 1983; see also the 0-min conditions of the present Experiments 3A and 3B). If odor potentiation occurred because the odor could recruit additional strength via its within-compound association to the taste, why was the taste prohibited from using this same pathway to also increase responding? A possible resolution to this quandary was that the within-compound association that produced odor potentiation was unidirectional and could benefit only the weak cue (i.e., odor); however, such a claim leads to the prediction that reciprocal odor–taste potentiation is not possible. Clearly, the results from Experiment 3 refute such a claim. Moreover, if the argument was advanced that separate within-compound associations are formed (i.e., a taste-to-odor association and an odor-to-taste association), it is difficult to justify why the within-compound association that favors odor potentiation appears to form immediately, whereas the within-compound association that favors taste potentiation requires at least more than 30 min (see Fig. 3). Therefore, like many other recent explorations of the mechanism of potentiation (e.g., Batson, Watkins, Doyle, & Batsell, 2008), the present results are most consistent with a configural interpretation of potentiation, and the application of this approach to potentiation and the overshadowing-to-potentiation transition will be discussed in the next section.
General discussion
This series of experiments explored the effects of manipulating CS–US interval on flavor-compound aversion conditioning, particularly to explore the generality of the overshadowing-to-potentiation transition reported by Urcelay and Miller (2009) in a fear conditioning design. Experiments 1A and 1B demonstrated that overshadowing of each element of the taste–taste compound was recorded at the immediate CS–US interval, but at the 120-min interval, potentiation was observed, since the aversion to either element following compound conditioning was significantly stronger than the aversion to each element conditioned alone. Moreover, in these experiments, it was shown that the aversion to each taste following single-element conditioning decreased as the CS–US interval increased (i.e., the trace interval effect), but no significant change in aversion strength across the CS–US interval was recorded to either taste following compound conditioning. The absence of a trace interval effect following compound conditioning was explored in Experiment 2. The results confirmed the earlier finding that both a SAC aversion and a DEN aversion decrease significantly across a 120-min CS–US interval, but importantly, the aversion to the SAC + DEN compound does not decrease across the 120-min trace interval. Experiments 3A and 3B examined odor + taste compound conditioning to determine whether similar changes occurred across an extended trace interval. Indeed, confirmatory evidence was recorded, since odor potentiation was detected across a range of trace intervals and taste overshadowing at a short CS–US interval transitioned to taste potentiation at longer CS–US intervals. We will first consider different explanations for the overshadowing-to-potentiation transition and then will review the implications of the present results for understanding mechanisms of overshadowing and potentiation in flavor aversion learning.
Urcelay and Miller (2009) observed a similar transition from overshadowing to potentiation in their fear conditioning experiments, and they proposed changes in stimulus processing as the source of this effect. Specifically, the stimulus processing account favored by Urcelay and Miller was the flexible encoding hypothesis—based on earlier human work by Williams, Sagness, and McPhee (1994) and Melchers, Shanks, and Lachnit (2008)—in which rats utilize an elemental encoding strategy at the short interval (which yields overshadowing) and a configural encoding strategy at the long interval (which produces potentiation). Unfortunately, as was noted by Urcelay and Miller, many of the manipulations that can distinguish between elemental and configural processing are not viable with a taste aversion preparation because of the speed of conditioning and amount of generalizability in taste aversion studies. Therefore, it appears that there is no apparent empirical means to distinguish between elemental and configural processing in the present experiments, but there may be a logical means for assessing the flexible encoding approach. Obviously, at a specific point in time, the rat can process the compound stimulus only in one mode, either elementally or configurally. This interpretation of the flexible encoding hypothesis is consistent with the Experiment 1 results at the 0-min interval when elemental encoding yields overshadowing of both tastes and the results at the 120-min interval when configural encoding produces potentiation of both tastes (see Fig. 1), but it is difficult to identify the mode of processing at the 30- and 60-min intervals. Specifically, significant overshadowing of SAC was recorded at the 30-min interval, implicating elemental processing, but overshadowing of DEN is not present at this interval. Similarly, at the 60-min interval, significant potentiation of DEN was obtained, suggesting that configural encoding occurred, but potentiation of SAC was not present. This concern is more evident regarding the results of Experiments 3A and 3B. As can be seen in Fig. 3, taste overshadowing and odor potentiation were both recorded at the 0-min interval in these studies, a combination of outcomes that has been seen in other reports (Bowman et al., 1992; Rusiniak et al., 1982; Westbrook et al., 1983). Considering that the evidence supports simultaneous overshadowing and simultaneous potentiation, it does not seem possible that the taste is processed elementally while the odor is processed configurally.Footnote 2
Because changes in stimulus processing cannot account for the transition from overshadowing to potentiation, the best explanation appears to be differential loss of conditionability across time. The present results appear to suggest the following interpretation: When a rat experiences a flavor compound, these cues may be configured into a highly salient, unitary stimulus, which retains its conditionability across extended CS–US intervals with minimal loss in aversion strength (cf. Experiments 2, 3A, and 3B). Following compound conditioning, if the aversion to one of these cues is assessed, the aversion will be weaker than the aversion to the compound itself, representing generalization decrement from the configural stimulus (cf. Experiments 3A and 3B). However, because the aversion to the configured compound remains relatively consistent across the CS–US interval, the generalization decrement to either cue also remains consistent across this interval. Indeed, although overshadowing is defined as a “decreased” conditioned response and potentiation is defined as an “increased” conditioned response, the transition from overshadowing to potentiation was not accompanied by a recovery of responding to the overshadowed cue (cf. Batsell & Best, 1992). In Experiments 1A, 1B, and 3B, there was no statistically significant difference between the aversion that was labeled overshadowing at the 0-min interval and the aversion labeled potentiation at a later interval. Although the conditionability of the compound changes little across the trace interval, the conditionability of single-element cues change more rapidly across time. As was observed across all experiments, the single-element groups always showed a significant trace interval effect at an earlier time point than did the groups that received compound conditioning, regardless of whether the latter groups were tested with the compound or one of its elements. Therefore, the transition from overshadowing to potentiation in the present experiments appears to be due to changes in conditionability to the single-element cue relative to the changes to the compound cue across the trace interval.
Finally, this interpretation suggests a possible means for incorporating overshadowing and potentiation within the same theoretical framework, which is noteworthy because, as was described in the introduction, researchers have often proposed unique mechanisms to accommodate the presence of potentiation in situations that favor overshadowing (see Batsell & Paschall, 2009; Batson et al., 2008; Durlach & Rescorla, 1980; Garcia et al., 1985). A tentative proposal is offered: When two flavor cues (e.g., taste + odor; taste + taste) are presented simultaneously, these cues may be configurally processed depending on a range of factors, including previous experience with either cue alone, the relative salience of the cues, the perceptual mixing of the stimulus modality (i.e., two cues of the same modality may be more easily configured than cues of different modalities), and, possibly, number of exposures to the compound. If these conditions favoring configuration are present, conditioning to this salient, configured stimulus will be stronger than that to each element by itself. Then, responding to each element of the compound will reflect the generalization decrement from the compound to that element. As such, the determination of overshadowing or potentiation depends on two factors: (1) the extent of generalization decrement from the compound to a given element and (2) conditioning to the single-element cue. As was demonstrated in the present experiments, lengthening the CS–US interval acts differentially to weaken conditioning in the single-element control group, thus escalating its difference from the compound group and favoring the statistically significant detection of potentiation. On this interpretation, potentiation and overshadowing do not require unique explanations, because they both arise from the same mechanism; they both arise following generalization decrement after configural processing. If the conditions that favor configuration are not present, however, the two flavor stimuli will be processed elementally, and factors that influence the expression of overshadowing, such as stimulus intensity, salience, familiarity, or predictive value, will determine the extent of overshadowing. Finally, a configural analysis of flavor preference learning similar to the one above has been recently offered by Dwyer, Haselgrove, and Jones (2011), and their configural approach also accommodates both overshadowing and potentiation effects. Therefore, it appears that recent work in flavor preference learning and flavor aversion learning converges to suggest that a configural analysis may be best suited to explain compound flavor interactions.
In conclusion, the present set of experiments demonstrated that extending the CS–US interval produces a shift from overshadowing to potentiation of the aversion in a two-flavor compound. It appears that this shift is due to differential loss of conditionability to the single-element taste, relative to any changes in conditionability to the compound stimulus across the trace interval. These results suggest a framework for potentiation and overshadowing in flavor aversion conditioning in which both phenomena arise following configural processing of the compound stimulus, but the detection of potentiation or overshadowing is primarily determined by the aversion strength of the single-element control. Although this analysis appears applicable to a range of potentiation experiments that used flavor cues (e.g., Bouton et al., 1986; Bouton & Whiting, 1982; Palmerino et al., 1980; Slotnick et al., 1997; Trost & Batsell, 2004), it remains to be determined whether this analysis is appropriate for potentiation with nonflavor cues (e.g., Ellins et al., 1985), augmentation effects in the A+/AX + design (e.g., Batsell & Batson, 1999; Batsell, Paschall, Gleason, & Batson, 2001), or potentiation effects in spatial learning (e.g., Pearce, Graham, Good, Jones, & McGregor, 2006).
Notes
During this work, we have considered various terms to describe the differential changes to the single-element flavors and the compound solution across the trace interval. Because the focus of this research was to evaluate mechanisms of potentiation and overshadowing—and we did not conduct experiments to isolate the nature of the changes of the various solutions—we have chosen to use the term conditionability. We recognize the limitations of using a neutral term such as conditionability that is simply reflective of subsequent aversion strength, but the goal in selecting this term is to acknowledge that the present experiments do not address whether the changes across the trace interval involve alterations in the perceptual structure, the associability (see Jones & Haselgrove, 2011), salience, or some other component of the cues.
Nonetheless, it is important to note that although the flexible encoding account offered by Urcelay and Miller (2009) is not applicable to the present data, these two reports may be reconciled. Their description of the shift from elemental processing to configural processing occurred across a matter of seconds (0–20 s). Conceivably, this shift may have happened due to the rapid decay of auditory cues in echoic memory, or it may occur because the change in encoding strategy is quite rapid. Even though a very brief conditioning regime was attempted in the present experiments, the present design far exceeded the parameters from the Urcelay and Miller studies. Conservatively speaking, in the present experiments, the rat had a 5-min exposure to the target solution; allowing for removal of the drinking tube, the earliest a rat received the LiCl injection may have been 1 min. Moreover, since the toxic experience of LiCl is somewhat delayed, the actual interval between CS termination and illness experience may have actually been 5 min or more in the present experiments, and a configural encoding strategy may already be active.
References
Batsell, W. R., Jr., & Batson, J. D. (1999). Augmentation of taste conditioning by a preconditioned odor. Journal of Experimental Psychology. Animal Behavior Processes, 25, 374–388.
Batsell, W. R., Jr., & Best, M. R. (1992). Variations in the retention of taste aversions: Evidence for retrieval competition. Animal Learning & Behavior, 20, 146–159.
Batsell, W. R., Jr., & Paschall, G. Y. (2009). Mechanisms of compound conditioning in flavor- aversion conditioning. In S. Reilly & T. R. Schachtman (Eds.), Conditioned taste aversion: Behavioral and neural processes (pp. 159–178). Oxford: Oxford University Press.
Batsell, W. R., Jr., Paschall, G. Y., Gleason, D. I., & Batson, J. D. (2001). Taste preconditioning augments odor-aversion learning. Journal of Experimental Psychology. Animal Behavior Processes, 27, 30–47.
Batsell, W. R., Jr., & Wakefield, E. (2008, March). Inflation of the elements of a taste–taste compound. Poster presented at the 15th International Conference on Comparative Cognition. Melbourne, FL.
Batson, J. D., Watkins, J. H., Doyle, K., & Batsell, W. R., Jr. (2008). Differences in taste- potentiated odor aversions with O+/OT + versus OT+/O + conditioning: Implications for configural associations. Learning & Behavior, 36, 267–278.
Bouton, M. E., Dunlap, C. M., & Swartzentruber, D. (1987). Potentiation of taste by another taste during compound aversion learning. Animal Learning & Behavior, 15, 433–438.
Bouton, M. E., Jones, D. L., McPhillips, S. A., & Swartzentruber, D. (1986). Potentiation and overshadowing in odor-aversion learning: Role of method of odor presentation, the distal-proximal cue distinction, and the conditionability of odor. Learning and Motivation, 17, 115–138.
Bouton, M. E., & Whiting, M. R. (1982). Simultaneous odor–taste and taste–taste compounds in poison-avoidance learning. Learning and Motivation, 13, 472–494.
Bowman, M. T., Batsell, W. R., Jr., & Best, M. R. (1992). Evidence that stimulus generalization does not determine taste-mediated odor potentiation. Bulletin of the Psychonomic Society, 30, 241–243.
Davis, S. F., Best, M. R., & Grover, C. A. (1988). Toxicosis-mediated potentiation in a taste/taste compound: Evidence for within-compound associations. Learning and Motivation, 19, 183–205.
Desgranges, B., Sevelinges, Y., Bonnefond, M., Levy, F., Ravel, N., & Ferreira, G. (2009). Critical role of insular cortex in taste but not odour aversion memory. European Journal of Neuroscience, 29, 1654–1662.
Durlach, P. J., & Rescorla, R. A. (1980). Potentiation rather than overshadowing in flavor-aversion learning: An analysis in terms of within-compound associations. Journal of Experimental Psychology. Animal Behavior Processes, 6, 175–187.
Dwyer, D. M., Haselgrove, M., & Jones, P. M. (2011). Cue interactions in flavor preference learning: A configural analysis. Journal of Experimental Psychology. Animal Behavior Processes, 37, 41–57.
Ellins, S. R., Cramer, R. E., & Whitmore, C. (1985). Taste potentiation of auditory aversions in rats: A case for spatial contiguity. Journal of Comparative Psychology, 99, 108–111.
Galef, B. G., & Osborne, B. (1978). Novel taste facilitation of the association of visual cues with toxicosis in rats. Journal of Comparative and Physiological Psychology, 92, 907–916.
Garcia, J., Ervin, F. R., & Koelling, R. A. (1966). Learning with prolonged delay of reinforcement. Psychonomic Science, 5, 121–122.
Garcia, J., Lasiter, P. S., Bermudez-Rattoni, F., & Deems, D. A. (1985). A general theory of aversion learning. In N. S. Braveman & P. Bronstein (Eds.), Experimental assessments and clinical applications of conditioned food aversions (Annals of the New York Academy of Sciences (Vol. 443, pp. 8–21). New York: New York Academy of Sciences.
Holder, M. D., & Garcia, J. (1987). Role of temporal order and odor intensity in taste- potentiated odor aversions. Behavioral Neuroscience, 101, 158–163.
Jones, P. M., & Haselgrove, M. (2011). Overshadowing and associability change. Journal of Experimental Psychology. Animal Behavior Processes, 37, 287–299.
Kucharski, D., & Spear, N. E. (1985). Potentiation and overshadowing in preweanling and adult rats. Journal of Experimental Psychology. Animal Behavior Processes, 11, 15–34.
Lett, B. T. (1980). Taste potentiates color-sickness associations in pigeons and quail. Animal Learning & Behavior, 8, 193–198.
Melchers, K. G., Shanks, D. R., & Lachnit, H. (2008). Stimulus coding in human associative learning: Flexible representations of parts and wholes. Behavioural Processes, 77, 413–427.
Palmerino, C. C., Rusiniak, K. W., & Garcia, J. (1980). Flavor-illness aversions: The peculiar roles of odor and taste in memory for poison. Science, 208, 753–755.
Pavlov, I. J. (1927). Conditioned reflexes. London: Oxford University Press.
Pearce, J. M., Graham, M., Good, M. A., Jones, P. M., & McGregor, A. (2006). Potentiation, overshadowing, and blocking of spatial learning based on the shape of the environment. Journal of Experimental Psychology. Animal Behavior Processes, 32, 201–214.
Pearce, J. M., & Hall, G. (1980). A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review, 87, 532–552.
Peterson, C. S., Valliere, W. A., Misanin, J. R., & Hinderliter, C. F. (1985). Age differences in the potentiation of taste aversion by odor cues. Physiological Psychology, 13, 103–106.
Rescorla, R. A., & Wagner, A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning II: Current research and theories (pp. 64–99). New York: Appleton-Century-Crofts.
Rusiniak, K. W., Hankins, W. G., Garcia, J., & Brett, L. P. (1979). Flavor-illness aversions: Potentiation of odor by taste in rats. Behavioral and Neural Biology, 25, 1–17.
Rusiniak, K. W., Palmerino, C. C., Rice, A. G., Forthman, D. L., & Garcia, J. (1982). Flavor– illness aversions: Potentiation of odor by taste with toxin but not shock in rats. Journal of Comparative and Physiological Psychology, 96, 527–539.
Slotnick, B. M., Westbrook, F., & Darling, F. M. C. (1997). What the rat’s nose tells the rat’s mouth: Long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Animal Learning & Behavior, 25, 357–369.
Trost, C. A., & Batsell, W. R., Jr. (2004). Taste + odor interactions in compound aversion conditioning. Learning & Behavior, 32, 440–453.
Urcelay, G. P., & Miller, R. R. (2009). Potentiation and overshadowing in Pavlovian fear conditioning. Journal of Experimental Psychology. Animal Behavior Processes, 35, 340–356.
Westbrook, R. F., Homewood, J., Horn, K., & Clarke, J. C. (1983). Flavour–odour compound conditioning: Odour-potentiation and flavour-attenuation. Quarterly Journal of Experimental Psychology, 35B, 13–33.
Williams, D. A., Sagness, K. E., & McPhee, J. E. (1994). Configural and elemental strategies in predictive learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20, 694–709.
Author Note
The authors thank Dana Allswede for her help with this research, and they thank Gonzalo Urcelay, Bob Boakes, and John Batson for their helpful conversations and feedback on earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batsell, W.R., Wakefield, E., Ulrey, L.A. et al. CS–US interval determines the transition from overshadowing to potentiation with flavor compounds. Learn Behav 40, 180–194 (2012). https://doi.org/10.3758/s13420-011-0054-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-011-0054-2