Abstract
Error-related negativity (ERN) has been used to investigate neural mechanisms underlying error processing and conflict monitoring. Recent evidence highlights that affective and motivational states modulate the ERN and that aversiveness of errors plays a vital role in error monitoring. Therefore, our primary objective was to systematically evaluate and describe the influence of affect state-related manipulations on the ERN. A total of 51 publications identified from PsyInfo, PubMed, and PsyArticles databases were included following the Prisma procedures for systematic reviews. Papers were analyzed using sample attributes, psychological paradigms, and states manipulations. The present study shows that the ERN component has recurrently appeared to be sensitive to manipulations of affective states in the reviewed literature. However, conclusive findings concerning the affect state-dependent properties of the ERN remain elusive. Results are discussed considering heterogeneity in paradigms, variables, and the state-trait interactions. Furthermore, recommendations for future high-quality studies are provided along with the necessity of upcoming high-power replication attempts and more studies with positive affect manipulations.
Similar content being viewed by others
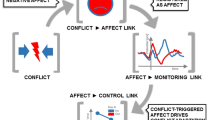
Cognitive control is the mental process that allows adaptive goal-directed behavior, and its principal function is to contain or inhibit prevalent responses to maintain focus on current goals (Inzlicht & Al-Khindi, 2012; Koechlin et al., 2003; Miller & Cohen, 2001). Convergent evidence from cognitive neuroscience points out that mental conflict generates control efforts (Inzlicht et al., 2015; Schiffer et al., 2015). Conflict monitoring theory suggests that the monitoring system is vital in analyzing the actual representations of action tendencies for potential conflicts. Thus, inhibitory mechanisms may be engaged to override the unwanted bias and promote active goal pursuit (Botvinick, 2007; Shenhav et al., 2016; Yeung, 2014).
Knowing that cognitive control begins with the appearance of conflict, it also is relevant to point out that conflict is not affectively neutral. Conflict represents an aversive event for the organism and includes negative affective states and emotional costs (Dreisbach & Fischer, 2012). Consistent with this view, Inzlicht et al. (2015) suggested that negative affect is an integral, instantiating aspect of cognitive control. Therefore, it states that cognitive control depends on emotion or its properties, such as arousal and valence.
Akin to conflict monitoring, error monitoring detects and signals errors to optimize behaviors across various tasks and situations. Error detection is necessary for adaptive behavioral adjustments (Moser et al., 2013). An organism can use it to inform behavioral strategies to achieve higher accuracy or preserve the executed task speed (Ridderinkhof et al., 2004; Zhou et al., 2019). For instance, error monitoring is correlated with the number of response alternatives in experimental tasks (Maier et al., 2010) and the difficulty of the chosen task. More complicated tasks provoke more errors (Riesel et al., 2013), and increasing the number of alternative responses in such tasks decreases the response monitoring mechanisms (Riesel et al., 2013). That is due to the cognitive overload on the different strategies, neural structures (Prefrontal Cortex, Motor Cortex, Basal Ganglia), and functions (hold and manipulate information) that are involved in error commissioning (Hoffmann & Beste, 2015).
As in conflict, the detection of committed errors is regularly accompanied by some negative emotional response (Hajcak et al., 2004; Spunt et al., 2012). Errors are unexpected and aversive endogenous events threatening the organism (Hajcak et al., 2012) and engage cognitive control to correct behavior and avoid more error commissioning. This threatening nature of errors suggests motivational salience and aversive properties (Jackson et al., 2015; Weinberg et al., 2012). Therefore, error commissioning could not be dissociated from negative affect states and the experience of aversiveness (Shackman et al., 2011). This perspective is consistent with findings linking errors to psychophysiological changes such as Heart Rate Variability (Mackersie & Calderon-Moultrie, 2016), Skin Conductance (Hajcak et al., 2003), and Electromyographic Activity (Dignath et al., 2019; Elkins-Brown et al., 2018; Hajcak & Foti, 2008).
Theoretical Frameworks of Error-Related Negativity
The Error-Related Negativity (ERN) is a component of event-related potentials (ERPs) that reaches maximum negative amplitude in frontocentral regions about 100 ms after an error has occurred in simple tasks of reaction time (Falkenstein et al., 1991; Gehring et al., 1993; Nieuwenhuis et al., 2001). The component marks the moment the brain indicates a motor error was committed, thus allowing the individual to adapt their responses and continue the task. Converging evidence from fMRI, EEG source modeling, and brain lesion research points to the dorsal portion of the anterior cingulate cortex as its site (Hajcak et al., 2012; Moser et al., 2013). The ERN has been identified as a valuable and reliable measure of partial or total detection of errors in healthy participants and individuals with various mental disorders (Maruo et al., 2016; Riesel et al., 2019; Weinberg et al., 2012).
Several cognitive and computational perspectives have attempted to explain the processing of errors and their neural signal, the ERN. According to mismatch theory, the error signal reflects a process that compares the neural representations of goal and actual responses (Bernstein et al., 1995; Coles et al., 2001; Falkenstein et al., 1991). From this perspective, the more significant the mismatch, the larger the ERN amplitude (Bernstein et al., 1995; Falkenstein et al., 1995). The reinforcement learning hypothesis (RFL) (Holroyd & Coles, 2002; Holroyd et al., 2005) suggests that errors are coded at the dopamine neurons from the basal ganglia (at the Ventral Tegmental Area) and alert the daMCC that outcomes of responses are worse than expected. Thus, the ERN is conceptualized as a reinforcement learning signal that trains the daMCC and the motor system. The role of the daMCC is to use the signal to adapt the response selection process for future better outcomes, acting as a control motor filter (Holroyd & Coles, 2002).
Another computational model, the conflict monitoring theory (Botvinick et al., 2001; Yeung et al., 2004), focuses on conflict detection rather than error detection, the ERN signals conflict. When a task requires selection among a set of responses, conflict emerges when overlapping preactivated (task representations) response sets exist. The daMCC reflects a signal of response conflict between correct and incorrect response processes. The response conflict signal (ERN) informs the Prefrontal Cortex of increasing cognitive control.
An additional, prominent model within the cognitive conceptualization of error processing is the predicted response- outcome model (Alexander & Brown, 2010). The PRO is a probabilistic model and focuses on the role of the Anterior Cingulate Cortex response–outcome based on models of reinforcement learning and their findings. The PRO model aims to explain various processes that include a more significant predicted activity for error, conflict, error likelihood, and unexpected outcomes in general (positive and negative results). Once an action is generated, the actual outcome is compared with the expected result, and any discrepancy leads to an update of the learned response–outcome predictions (Alexander & Brown, 2010).
Nonetheless, other exciting models suggest that the ERN reflects the motivational meaning or the motivational salience and aversiveness of errors (Hajcak, 2012; Hajcak & Foti, 2008; Jackson et al., 2015; Weinberg et al., 2012). The following section will account for its implications in recent literature regarding early error-detection processes.
ERN on affective states’ manipulations
When the monitoring processes evaluate error commissioning, those processes generate a signal, the ERN. Studies from the past decade have shown that this neural signal is influenced by affective and motivational factors (Hobson et al., 2014; Inzlicht & Al-Khindi, 2012; Jackson et al., 2015). In addition, errors are characterized as aversive events for the individual (Hajcak & Foti, 2008). Within this framework, the commission of errors is a distressing experience; it is perceived as threatening (Spunt et al., 2012) and engages a defensive motivational response (Hajcak, 2012; Weinberg et al., 2016). Thus, the ERN would indicate that the activation of the monitoring system is sensitive to the motivational significance and value of errors (Hajcak et al., 2005; Weinberg et al., 2016). This sensitiveness to affective and motivational context (de Bruijn et al., 2020; Riesel et al., 2012) may be a critical point in the path of understanding within and between subjects’ variations of the ERN (Levsen & Bartholow, 2018; Proudfit et al., 2013; Weinberg et al., 2012).
Two lines of research have emerged to unveil the relationships between affect and error-related negativity: manipulations with state affect and measurement of levels of trait affect. If the ERN is sensitive to state affect manipulations, its amplitudes will reflect responses to emotional context, as has been hypothesized (Inzlicht & Al-Khindi, 2012; Tullett et al., 2015; Wiswede et al., 2009; Wiswede et al., 2009). In contrast, measures of more stable levels of trait affect and individual differences would determine the ERN generation (Amodio et al., 2008; Hajcak, 2012; Riesel et al., 2019c; Weinberg & Hajcak, 2011).
Moreover, the literature extensively reported the ERN as stable and related to trait vulnerability. Trait affective measures of personality like perfectionism, neuroticism, and high in negative affect are significantly associated with increased error detection processes (Luu et al., 2000; Olvet & Hajcak, 2012; Weinberg et al., 2012; Weinberg & Hajcak, 2011). The considerable evidence of the state-independent characteristics of the ERN has even pointed it out to be considered as a reliable psychiatric endophenotype (Miller & Rockstroh, 2013; Riesel, 2019; Weinberg et al., 2012; Weinberg & Hajcak, 2011). Nonetheless, recent meta-analytic evidence (Saunders & Inzlicht, 2020) suggests that at least the relationship between trait anxiety and the ERN is smaller and more heterogenous than previously reported.
Furthermore, the connection between the ERN and affective state inductions has mixed and inconclusive data. There is growing evidence that stimuli with emotional significance can modulate the ERN (Boksem et al., 2011; Dreisbach & Fischer, 2012). Studies with experimental manipulations, such as evaluation of performance (Hajcak et al., 2005), disapproval in a social context (Boksem et al., 2011), the unpredictability of the stimuli (Jackson et al., 2015), and the administration of reward and punishment (Stürmer et al., 2011), have shown that experimental manipulations of affective states impact the ERN whether indicating the negative affect valence of errors (Aarts et al., 2013), an increasing threat value of errors (Meyer & Gawlowska, 2017; Weinberg et al., 2012), or changes in the reward prediction error (Bakic et al., 2014). In contrast, other authors have found an unaltered ERN by inducing diverse affective states (Elkins-Brown et al., 2018; Larson et al., 2013; Olvet & Hajcak, 2012).
State-trait interactions also have been regularly reported among studies. Sometimes, modulations of the ERN are not found exclusively by the experimental affective manipulations; however, when a trait is considered in the analysis, significant interactions emerge (Boksem et al., 2008; Olvet & Hajcak, 2012; Riesel et al., 2019c). Similarly, when experimental state affect inductions have significant modulations on the ERN, trait appear as a relevant moderator of the observed effect (Dikman & Allen, 2000; Maruo et al., 2016; Riesel et al., 2012). Nevertheless, additional factors, such as heterogeneity in task parameters, performance feedback, and task stimuli, seem to moderate the complex relationship between state-affect and trait-affect with the ERN (Gloe & Louis, 2021; Riesel et al., 2019a).
Insight into the role of state affect in cognitive conflict, and the ERN generation is critical to address theoretical questions and to define the nature of error processing. Therefore, this systematic review aims to synthesize the results in this area, investigating if the ERN is sensitive or can be influenced by state affect manipulations (i.e., state-dependent). We additionally explored the directions of the effects on the ERN and how the potential differences can be interpreted.
Method
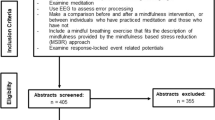
This systematic review was done following the Prisma guidelines (Page et al., 2021). We conducted an automated search up to October 2021 using the PubMed, PsycINFO, and PsyArticles databases. The search string “ERN” OR “Error related negativity” OR “Ne” OR “performance monitoring” OR “error monitoring” OR “conflict monitoring” AND “affect” OR “emotion” OR “mood” OR “reward” OR “punishment” OR “affective state” OR “aversiveness” was entered in the standard search field in all the databases. Also, for these searches of electronic databases, manual searches were performed using the reference sections of published texts to find eligible articles. Studies were included if they matched inclusion criteria: 1) Experimental manipulations of a broad framework of the so-called affective states (Russell, 2003; Russell & Barrett, 1999): core Affect (valence and arousal), emotion, mood, motivation (reward and punishment), and emotion regulation; 2) measurement of the ERN; 3) Nonclinical samples; 4) Original research published in peer-review journals written in English. Two reviewers (X.N.E.) and (L.Z.) independently assessed if studies met the initial criteria. In case of discordances, a third judge was consulted, and the three judges discussed again until all three agreed. After removing duplicates, exclusion criteria were applied through seeking titles and abstracts. Reasons for exclusion of studies included: (a) theoretical and review papers, (b) studies with clinical population and substance manipulations, (c) observational studies, (d) studies with children and adolescents, and (e) studies without EEG techniques. After removing studies that met exclusion criteria, the remaining papers were fully read to identify the studies’ manipulations and the results. Finally, studies that only reported analysis by trait affect were excluded. Figure 1 describes the four phases of this review process (identifying, screening, eligibility, and inclusion). Data extraction included: author, year, design, sample characteristics, paradigm used, type of affective state manipulation, main findings (behavioral outcomes and EEG effects), and conclusions. Data were only extracted from the included studies. An analysis table was constructed for the organization of the results. The two judges filled out the table. The experimental tasks were analyzed according to the type of manipulation.
Assessment of risk of bias
Two reviewers (X.N.E) and (R.M.) independently assessed the methodological quality of eligible studies using the JBI critical appraisal checklist for quasi-experimental studies (Tufanaru et al., 2020). In case of disagreements between the two judges, a third judge was consulted for the final decision. The JBI methodological checklist consists of 9 items concerning a study’s validity and overall assessment where items are rated as: “yes,” “no,” “unclear,” “no applicable.” The overall appraisal determines if an article is included or excluded for the analysis (Tufanaru et al., 2020). Only studies with High quality and Acceptable quality were included in this review. Additionally, we decided to rate the overall methodological quality of the studies as follows: “High quality (+ +)”: Majority of criteria met, Little or no risk of bias; OR Acceptable ( +) Most criteria met. Some flaws in the study with an associated risk of bias.” Special attention was given to reporting of internal consistency of the ERN, power sample calculations, and appropriate statistical procedures.
Results
After performing searches of the databases and removing duplicates, 299 articles were obtained. Figure 1 shows the electronic search steps that led to 51 articles being included in the systematic review. The exclusions based on the title and abstract (n = 155) were usually studies with the clinical population; articles that did not use ERN; studies with fMRI; studies that did not analyze ERN and affective states, and theoretical articles or systematic reviews. The main results are presented below, according to the sample’s characteristics, paradigms and designs, behavioral effects, variables, and EEG effects. A description of tasks and designs employed in the selected studies can be found in Table 1. A summary of the key findings is displayed in Table 2.
Study and sample characteristics
The 51 investigations contained 54 experimental studies. They employed 2284 subjects, resulting in an average sample size of 42.2 participants. Sample sizes varied widely, from 16 to 121 participants. Thirty-nine studies (72.2%) had a sample with less than 50 subjects; 13 (24.1%) had a sample size between 50 and 100 participants, and only 2 (3.7%) employed more than 100 participants. Ten (19.6%) articles reported sample size calculations for their studies. Forty-one studies (75.9%) had their samples composed of undergraduate students, of which 14 (25.9% of the total) were explicitly performed with undergraduate students of Psychology courses. The other 13 studies (24.1%) were conducted with a nonacademic population or did not specify the participants’ nature.
Concerning sample age, ten studies did not report the mean age in the final sample. Among the 44 studies reported (n = 1,966), the participants’ age ranged from 18.6 to 35.8 years, resulting in an average of 21.6 years.
Regarding other characteristics, such as gender and racial group, one study did not report the proportion between men and women in its final sample. Among the 47 studies that reported (n = 2,038), 62.7% (1,222) of the participants were female, and 37.3% (n = 816) were male. Three studies had a final sample composed of 100% women, and only a few studies included ethnicity data.
Paradigms and Designs
We observed more than 10 paradigms used in the 51 papers. Moreover, the paradigms are widely used in ERN and cognitive control studies. The distribution of the number of papers by paradigms used was as follows:
Flanker task (24): Letter version, arrow version, social version, and modified version; Go/No go Task (9), Stroop Task (3): six choices, spatial; Simon task (3); Probabilistic learning Task (3), Weapons Identification Task (2), Emotional Stop Signal Task, A combination of flanker and Simon task (1), and studies used other inhibitory control tasks (8): MSIT (multisource interference task), Two-choice Speeded Task, Punished Inhibitory Control Task, Modified Learning task, Emotional Stop signal Task, AX-CPT task, Faces identification task, and Picture naming task. Twenty-one experiments used between-subjects designs, 24 used within-subjects, and 9 were between and within-subjects analyses. We found 27 experiments that used feedback to encourage better Reaction Times and were contingent on trials or performance. Ten of the studies did not use feedback in their paradigms, and 17 did not report. The main description of the paradigms, designs and use of feedback can be found in Table 1.
On the other hand, we pooled publications according to the used affect manipulations to compare methodologies and results’ studies. A summary of extracted data is displayed in Table 2. The following sections will review the results of the included studies.
Manipulation’s check
Of the 54 studies (in the 51 papers), 39 studies described at least one state manipulation check, and 37 studies reported the effectiveness of the manipulation used.
Regarding the affective induction, a variety of stimuli and procedures were used. Nine studies, sensory stimuli (tones/noises, respiratory occlusion, electrocutaneous stimulation) were applied to elicit affective states. Eight more studies used an emotional regulation strategy (emotional attendance, suppression, or Reappraisal), and six manipulated financial punishment or reward. Six studies presented variables related to mood induction procedures (achieved using music, movies, guided imagery, or text passages), and three studies used a placebo. Some others reported unsolvable math tasks, encouraging and derogatory feedback, being or not being evaluated during the task, the Trier Social Stress Test (TSST), and Socially Evaluated Cold-Pressor Test (SECPT).
The most frequent measures used to assess the participant’s affect were the State-Trait Anxiety Inventory for state anxiety (STAI) (7 studies); Subjective valence and arousal ratings (7 studies); Profile of Mood State Scale (POMS) (4 studies); Self-reported mood state (4 studies); Self-Assessment Manikin (SAM) (4 studies), the Positive and Negative Affect Schedule (PANAS) (2 studies); and Self-reporting scales for discomfort, anxiety (2). All the following measures were reported by only one study: Self-reported emotional involvement; Religious Zeal Scale; Situational Motivational Scale (SIMS); Anxiety Sensitivity Index (ASI); Intrinsic Motivation Inventory (IMI); Helplessness questionnaire; Self-reported wakefulness state; Salivary cortisol, blood pressure, and Heart rate.
Risk of bias
Of the 51 articles included, 9 was rated as high quality (+ +), with the remaining 42 rated as acceptable ( +) (Table 2.)
Behavioral effects
RTs and accuracy by trial type and behavioral outcome.
Twenty studies reported a congruency effect with slower RTs and less accuracy on incongruent or high conflict trials (Boksem et al., 2008; Cano Rodilla et al., 2016; Clayson & Larson, 2019; Clayson et al., 2012; de Bruijn et al., 2020; Elkins-Brown et al., 2016; Hajcak et al., 2005; Inzlicht & Gutsell, 2007; Larson et al., 2006, 2013; Maruo et al., 2016; Nigbur & Ullsperger, 2020; Pfabigan et al., 2013; Potts, 2011; Rodeback et al., 2020; Saunders et al., 2015; Tan et al., 2019; van Wouwe et al., 2011; Wang et al., 2014; Wiswede et al., 2009; Wiswede et al., 2009).
Regarding the behavioral outcome, 11 studies reported faster RTs on error trials (Hajcak et al., 2005; Hobson et al., 2014; Inzlicht & Al-Khindi, 2012; Meyer & Gawlowska, 2017; Olvet & Hajcak, 2012; Pfabigan et al., 2013; Riesel et al., 2012; Riesel et al., 2019b; Saunders et al., 2016; Wiswede et al., 2009; Wiswede et al., 2009), whereas 1 observed faster responses on correct trials (Compton et al., 2007). Finally, four studies in the Go/No go paradigm reported more commission than omission errors (Cano Rodilla et al., 2016; Elkins-Brown et al., 2018; Hobson et al., 2014; Inzlicht & Al-Khindi, 2012).
RTs and accuracy by conditions
Studies with groups and conditions differ along with findings by manipulations. Slower RTs and less accuracy were observed in five studies with punishment conditions (Leue et al., 2017; Meyer & Gawlowska, 2017; Potts, 2011; Riesel et al., 2012; Riesel et al., 2019b). Nevertheless, Saunders et al. (2015) observed higher accuracy in high conflict trials after punishment, whereas Maruo et al. (2016) reported higher accuracy for both reward and punishment than in control. Two studies found higher accuracy in the reward condition (Pailing & Segalowitz, 2004; Potts, 2011).
Regarding the negative affect manipulations, three studies found slower RTs in emotional faces condition (Sueyoshi et al., 2014), occlusion task (Tan et al., 2019), and with unpleasant pictures (Wiswede et al., 2009). However, two studies reported faster RTs to threatening visual stimuli (Senderecka, 2016, 2018). Three studies found better accuracy in the aversive conditions (Jackson et al., 2015; Senderecka, 2016, 2018).
After positive affect inductions, one study reported less accuracy in their smile faces condition (Wiswede et al., 2009), while the other found more accuracy in high conflict trials (van Wouwe et al., 2011). Studies that manipulated both negative and positive inductions reported better accuracy for neutral faces (Compton et al., 2007), slower RTs for emotionally arousing regardless of pleasant or unpleasant (Larson et al., 2006), and less accuracy following anxiety induction, especially on more difficult incongruent trials (Larson et al., 2013).
A considerable number of studies (n = 23) found no significant behavioral effects per condition or manipulation (Bakic et al., 2014; Boksem et al., 2008, 2011; Cano Rodilla et al., 2016; de Bruijn et al., 2020; Elkins-Brown et al., 2016, 2018; Ganushchak & Schiller, 2008; Glienke et al., 2015; Hajcak et al., 2005 (Experiments 1 and 2); Hobson et al., 2014; Inzlicht & Al-Khindi, 2012; Moser et al., 2005; Nigbur & Ullsperger, 2020; Olvet & Hajcak, 2012; Paul et al., 2017; Pfabigan et al., 2013; Rodeback et al., 2020; Tullett et al., 2015; Unger, et al., 2012; Valt et al., 2017; White et al., 2018).
Post-Error Slowing
Eighteen studies reported the presence of post-error slowing (PES) effect (Boksem et al., 2011; de Bruijn et al., 2020; Larson et al., 2013; Maruo et al., 2016; Meyer & Gawlowska, 2017; Nigbur & Ullsperger, 2020; Olvet & Hajcak, 2012; Paul et al., 2017; Pfabigan et al., 2013; Riesel et al., 2012; Riesel et al., 2019b; Rodeback et al., 2020; Stürmer et al., 2011; Sueyoshi et al., 2014; Wiswede et al., 2009; Wiswede et al., 2009; Wiswede et al., 2009). Specific PES effects were observed. Maruo et al. (2016) and Stürmer et al. (2011) reported that this effect was more pronounced in the reward condition or rewarded blocks. On the contrary, three studies found increased PES during punishment conditions (Meyer & Gawlowska, 2017; Riesel et al., 2012; Riesel et al., 2019b).
EEG Acquisition and ERPs Reduction
Methods and technologies to record EEG and calculate ERPs were diverse. Electrode numbers fluctuated between 6 to 129, with 128, 64, and 32 channel systems being the most frequent across studies (n = 23), distributed according to the 10–20 system. Most studies chose Biosemi Active Two system (Biosemi Inc.) and Electrical Geodesics System, including high-density arrays, to get ERPs components. For ocular movement artifacts correction, most of the studies (n = 21) used the procedure described by Gratton et al. (1983), Coles et al. (1988) and remotion with the independent component analysis (ICA) algorithm (18 studies). Components were quantified employing two measures: 39 studies selected mean amplitude (the most used brain activity measure); 9 used peak amplitude and latency; and 6 used peak-to-peak amplitude. Several authors across the studies cited the work of Luck (2005) and Clayson et al. (2013) as their primary criterion of choice for mean amplitude, as they identify this measure as a more reliable ERP measure than the peak amplitude. ERN amplitudes were mostly averaged across frontocentral electrode sites FCz, Fz, and Cz. Criteria to select EEG sites for analysis, electrodes, and latency ranges were due to the topographic properties of the dataset, previous works, and visual inspection of the grand-average waveform post response. A summary of the locations and time windows used in the reviewed studies is displayed in Table 2.
EGG effects
We reviewed 54 experimental studies of the 51 articles selected. Thirty-two studies reported significant effects of the state affect inductions on the ERN amplitudes, and 20 studies could not find significant effects or associations for the overall sample. However, 9 of the 20 studies that did not find significant effects for the overall sample by manipulation also assessed traits and reported moderation effects on the ERN. The following sections will review the results and the direction of the effects of the included studies, organized by experimental manipulation. Experimental manipulations with a negative valence of stimuli or aversive mood inductions were categorized as “negative affect.” Likewise, manipulations with a positive valence or mood inductions were labeled as “positive affect.” Some studies included both types of manipulations and were categorized as “negative and positive affect.”
An individualized category was created for manipulations with motivational factors, emotion regulation strategies, and state-traits interactions. Finally, some of the studies could appear in more than one category due to multiple variables manipulated (i.e., manipulation of the valence stimuli and motivational factors; studies containing two experiments with different manipulations). A summary of the extracted data is presented in Table 2.
Negative affect (18 studies)
Of the nine studies that reported significant effects, 8 studies identified enhanced ERN/ΔERN amplitudes manipulating negative affective states through mistakes that harmful others (de Bruijn et al., 2020), evaluation of performance (experiment 2) (Hajcak et al., 2005), unpredictable tones (Jackson et al., 2015), aversive respiratory occlusions (Jelinčić et al., 2020; Tan et al., 2019), feelings of helplessness (Pfabigan et al., 2013), randomness in readings (Tullett et al., 2015), and negative valence pictures (IAPS) (Wiswede et al., 2009). One study reported reduced amplitudes throughout a negative valence manipulation (angry faces) (Valt et al., 2017).
Conversely, nine studies did not find significant effects on the ERN for the overall sample (Glienke et al., 2015; Leue et al., 2017; Moser et al., 2005; Olvet & Hajcak, 2012; Rodeback et al., 2020; Senderecka, 2016, 2018; Sueyoshi et al., 2014; White et al., 2018). However, Sueyoshi et al. (2014) found positive correlations between the ERN and interoceptive sensitivity in disgusted face stimuli. Also, three of these studies reached significance when trait interactions were taken into account (Leue et al., 2017; Olvet & Hajcak, 2012; White et al., 2018), highlighting the relevance of looking for these changes in experimental manipulations of affect. (These results will be described in more detail in the state-trait interactions section.)
Positive Affect (5 studies)
Out of the five studies that manipulated positive affect, two studies reported significantly enhanced ERN through happy mood inductions (Bakic et al., 2014; Nigbur & Ullsperger, 2020), while two studies reported dampened ERN when manipulating movie clips (van Wouwe et al., 2011), and induced smile expressions (Wiswede et al., 2009). One study did not obtain significant main effects or interactions (Paul et al., 2017).
Negative and Positive Affect (6 studies)
Of the six studies with negative and positive affect manipulations, four observed enhanced ERN with different outcomes regarding the manipulation. Boksem et al. (2011) identified larger ERN for the disgusted faces condition, while Larson et al. (2006) for pleasant images. Clayson and Larson (2019) compared paradigms of previous studies: the overlaid flanker (Larson et al., 2006) and the interspersed flanker (Wiswede et al., 2009) and found enhanced amplitudes for high arousal images in an interspersed flanker task but dampened amplitudes in the overlaid task for more pleasant images (effect of valence). One study reported dampened ΔERN after less anxiolytic religious primes (Good et al., 2015), and two studies reported no significant effects on the ERN only due to mood (Larson et al., 2013) and stimulus emotionality manipulation (faces) (Compton et al., 2007). Nevertheless, Compton et al. (2007) observed an unexpected interaction effect between manipulation and state anxiety, with high anxious individuals showing smaller ERN for angry faces blocks and larger ERN after happy faces blocks.
Emotion regulation strategies (8 studies)
Six studies reported significant effects of emotion regulation manipulation with decreased overall ERN amplitudes (Hobson et al., 2014; Inzlicht & Al-Khindi, 2012; Inzlicht & Gutsell, 2007; Levsen & Bartholow, 2018; Saunders et al., 2016; Wang et al., 2014), suggesting that these regulatory strategies reduce affective reactivity and impact cognitive control through dampening the ERN. These studies used a down-regulation reappraisal condition (Hobson et al., 2014), suppression and reappraisal (Wang et al., 2014), meditation techniques (Saunders et al., 2016), and misattribution of the arousal paradigm (Inzlicht & Al-Khindi, 2012). The study by Levsen and Bartholow (2018) was composed of two experiments with informative moderators and interactions. In the first, a within-subjects analysis showed that only Reappraisal affected the ERN amplitudes. While in experiment 2, a between subjects’ analysis showed that both Reappraisal and Suppression diminished the ERN. The authors highlighted the importance of using between-subjects design when testing the effect of emotion regulation strategies to avoid cofounding effects. Another interesting finding from this study was the interaction effect observed between the used strategy and the black prime. In both experiments, larger ERNs were reported for Reappraisal and Suppression but only for black prime trials.
Finally, one study identified an enhanced ERN for the emotion-focused group (Saunders et al., 2016), and two studies found no significant effects on the ERN (Cano Rodilla et al., 2016; Elkins-Brown et al., 2018). Cano Rodilla et al. (2016) and Elkins-Brown et al. (2018) were replications of the misattribution approach by Inzlicht and Al-Khindi (2012), and both failed to replicate the reduced amplitude of the ERN due to misattribution of arousal procedure, pointing out the original study as a possible false positive. However, it is relevant to note that these studies reported failing to manipulate state affect, so the data cannot provide evidence for or against the affective properties of the ERN.
Motivational Factors: Punishment and reward (16 studies)
Of the 16 studies that manipulated motivational factors, 10 found significant effects of condition on the ERN (Ganushchak & Schiller, 2008; Hajcak et al., 2005, experiment 2; Leue et al., 2017; Maruo et al., 2016; Potts, 2011; Riesel et al., 2012; Saunders et al., 2015; Stürmer et al., 2011; Unger et al., 2012; Wiswede et al., 2009). Eight studies reported larger ERN amplitudes under punishment or aversive feedback (Ganushchak & Schiller, 2008; Hajcak et al., 2005, experiment 2; Leue et al., 2017; Potts, 2011; Riesel et al., 2012; Saunders et al., 2015; Unger et al., 2012; Wiswede et al., 2009). One of these studies used a failure induction in two experiments investigating the effects of failure feedback and threatening self-worth (intelligence abilities) in deterministic and probabilistic conditions (Unger et al., 2012). The authors reported an enhancement of the ERN amplitudes resulting from the failure manipulation in the two learning conditions and decreasing ERN for individuals in the no-failure induction consequently (Unger et al., 2012).
Larger ERN amplitudes were also found in reward and punishment conditions (Maruo et al., 2016) and the reward blocks (Stürmer et al., 2011). In contrast, six studies reported no significant effects of salience and motivational manipulations (punishment or aversive feedback, and reward) on the ERN or ΔERN amplitudes (Clayson et al., 2012; Elkins-Brown et al., 2016; Meyer & Gawlowska, 2017; Pailing & Segalowitz, 2004; Riesel et al., 2019b). However, these studies reported trait interactions effects that are accounted for in the state-trait interactions section. The study conducted by Clayson et al. (2012) intended to replicate a previous study (Wiswede et al., 2009) that reported significant effects on the ERN amplitude but had a small sample composed only of 12 females. As mentioned, Clayson et al. (2012) failed to replicate those findings.
Sate-trait interactions (10 studies)
Nine studies reported a moderator effect of trait by enhancing the ERN amplitudes. These effects were reported depending on the experimental manipulation and the assessed trait. For instance, four studies observed an interaction effect in a punishment condition for high anxious individuals (Meyer & Gawlowska, 2017; Riesel et al., 2012; Riesel et al., 2019b), and high sensitivity to punishment (BIS) (Boksem et al., 2008). Specifically, Riesel et al. (2012) found significant differences by punishment for the overall sample, but further analysis corroborated that anxiety trait was moderating this effect with larger ERN amplitudes for punishment only in high anxious individuals.
Likewise, other state-trait interactions were reported. For instance, Olvet and Hajcak (2012) found no significant associations just by the mood induction, but high neuroticism moderated the relationship between mood changes (i.e., increase sadness) and Δ ERN. Regarding manipulations of the valence of the stimuli, Leue et al. (2017) also reported an emerged interactions between trait (lower BIS) and manipulation of valence. The authors found larger amplitudes for lower BIS individuals in the fearful faces condition (Leue et al., 2017). Other studies observed enhanced ERN associated with high reward drive responsiveness (Clayson et al., 2012), on high neuroticism and low conscientiousness on motivated trials with high monetary reward (Pailing & Segalowitz, 2004), and in high worry individuals that were exposed under uncertain evaluative feedback (White et al., 2018).
Conversely, one study that assessed trait affect under an emotion regulation manipulation did not find significant moderation effects on the ERN (Elkins-Brown et al., 2018).
Discussion
Overall findings
This study aimed to systematically review the literature searching for effects of affective state manipulations on the ERN event-related potential component. Of the 54 experiments reviewed, 34 (~ 63%) studies reported significant effects on the ERN solely by state affect manipulation. From the 20 studies that reported no significant effects by manipulation in the overall sample, 9 studies observed that trait affect significantly moderated the interactions with state affect. Notably, the most mixed results found in our review were seen in our label categorized as “negative affect.” Half of the studies reported no significant effects only by state manipulation. Interestingly, as previously stated, some of these studies assessed traits emphasizing emerging effects due to those state-trait interactions (Olvet & Hajcak, 2012; White et al., 2018).
Nevertheless, within the studies reporting modulations of the ERN component, amplitudes tended to be increased in negative affect manipulations and punishment and reduced with positive affect, and interventions that lessen negative affect. Although we observed heterogeneity across the studies that hinder comparisons between findings, the results from different manipulations suggested the sensitivity of the ERN to state affect contexts. It seems that this event-related component does not purely reflect the mismatch of goal and actual responses; instead, it can be informative about transient affective and motivational factors.
However, relevant issues emerged from studies that did not obtain significant effects. For instance, two studies (Cano Rodilla et al., 2016; Elkins-Brown et al., 2018) were replications of the Inzlicht and Al-Khindi (2012) paradigm, which had found a significant effect of misattribution of arousal on the ERN. These null findings highlighted the following important aspects: (a) the replication attempts used higher statistical power (more representative samples), with nonreplication findings confirmed by Bayesian Statistics; and (b) the possibility of a false positive of the original article (Inzlicht & Al-Khindi, 2012). Similarly, by including a larger sample, Clayson et al. (2012) failed to replicate the results from Wiswede et al.,(2009), and Clayson and Larson (2019) observed opposite patterns from the replicated studies (Larson et al., 2006; Wiswede et al., 2009). These results represent an opportunity to show the relevance of the high-powered replications of the ERN paradigms, methodologies, and theories.
Caution should be taken regarding significant effects reported in EEG low-powered studies, especially when treated as solid evidence of the nature of psychophysiological phenomena, such as the ERN (Button et al., 2013; Clayson, 2020; Gelman & Carlin, 2014). In our review, 72.2% of the studies had a sample with less than 50 subjects, 2 studies used more than 100 participants (Good et al., 2015; Larson et al., 2013), and 10 (19,6%) articles reported sample size calculations for their studies. Furthermore, 75.9% of the reviewed studies were conducted with samples composed of undergraduate students, and only a few reported ethnicity data. This fact leads us to the WEIRD (Western, educated, industrialized, rich, and democratic) sample problem (Henrich et al., 2016). Although empirical research publications usually report the characteristics of their samples as a limitation, few studies identified their target population adequately, and the risk of sampling bias remains despite the data collection issues (Muthukrishna et al., 2020). Nonetheless, the notable percentage of studies using undergraduate samples also can be seen as a strength in the homogeneity that allows future replications.
Importantly, given that widely different paradigm tasks were used to seek state affect effects on the ERN as a dependent variable, some methodological issues need to be acknowledged when considering the conflicting findings across studies. For instance, moderator factors, such as paradigm task, performance feedback, and task stimuli, have been shown to influence the effects and magnitude of the ERN in nonclinical and clinical samples (Gehring et al., 1993; Gloe & Louis, 2021; Riesel et al., 2019a). Our review identified 15 different paradigms to elicit the ERN; whereas the Flanker Task was the most frequent, at least 5 different arrays and modifications were used between and within subjects’ design. The heterogeneity of task parameters and methods limits the generalizability of results (Clayson et al., 2021) and leaves undetermined variability of the ERN unknown (Gloe & Louis, 2021).
Moreover, we explored the state manipulation check and its effectiveness. In our review, most of the studies presented a manipulation check with the STAI for state anxiety and Subjective valence/arousal ratings to be the most applied. Thirty-seven studies had a successful state affect induction, and in two, the manipulation did not influence affect (Cano Rodilla et al., 2016; Elkins-Brown et al., 2018); as a result, their data could not test the emotional properties of the ERN. However, usually, measurements were collected only after manipulation. Checking the effectiveness of manipulation is crucial, as it is the necessity of measurement before (when applicable) and after the experimental induction in order to establish the ERN state-dependent (or independent) characteristics.
What do the directions of the ERN say about state affect manipulations?
Knowing that errors are aversive endogenous events and that error commissioning is typically accompanied by negative emotions (Hajcak et al., 2004, 2012; Spunt et al., 2012); the aversive nature of errors and the emotional costs to the individual gave rise to the hypothesis that affective and motivational dimensions can influence performance monitoring (Maier & Steinhauser, 2016; Saunders et al., 2017). Following this line of thought, if the ERN reflects the valence (aversive) of performance monitoring processes, then negative affect and punishment manipulations would enhance the ERN amplitudes (Aarts et al., 2013; Wiswede et al., 2009). Likewise, manipulations, including positive affect, reward, and emotion regulation strategies, to lessen negative distress would prevent this aversive effect by dampening the ERN (van Steenbergen et al., 2015; Wiswede et al., 2009).
Supporting this view, 18 studies from our review found larger ERN amplitudes following negative affect and punishment, and 9 found smaller amplitudes after positive affect and emotion regulation manipulations. These results are in line with evidence of consistent integration of negative affect and cognitive control in the midcingulate cortex (MCC) (Braem et al., 2017; Shackman et al., 2011), the structure where the ERN is thought to be generated (Iannaccone et al., 2015; Ridderinkhof et al., 2004; Shenhav et al., 2016). In addition, positive correlations of the ERN amplitudes and aversive bodily sensations/interoception support the aversiveness of error commission reflected by the ERN (Jelinčić et al., 2020; Sueyoshi et al., 2014; Tan et al., 2019). However, the interpretation of the directions of these effects must be made considering the paradigms, designs, and variables compared.
Although, in general, manipulations of negative affect and negative valence stimuli were related to larger ERN amplitudes, some studies reported reduced ERN (Compton et al., 2007; Valt et al., 2017) and enhanced ERN for positive valence images (Larson et al., 2006) and in positive mood manipulations (Bakic et al., 2014; Nigbur & Ullsperger, 2020). Reconciling these findings is a difficult task. However, these differences in outcomes may be interpreted considering the impact of valence and arousal in attentional processes (Clayson & Larson, 2019; Saunders et al., 2017; Weinberg et al., 2012), the differences in cognitive and physiological costs of emotion regulation (Saunders et al., 2016; Wang et al., 2014) the affective context of manipulations (if related or unrelated to the task: prior or superimposed) (Larson et al., 2013; Wiswede et al., 2009), the different nature of the task and its specific effects (Riesel et al., 2013; Suzuki et al., 2020), and the response mode (Gloe & Louis, 2021; Senderecka, 2018).
The directions of the ERN when manipulating the stimuli valence or affect induction may vary in function of the context manipulation and motivational significance of errors (Bakic et al., 2014; Clayson & Larson, 2019). Therefore, different directions should not be interpreted as isolated events on a unidimensional line from positive–negative affective states (Nigbur & Ullsperger, 2020). Studies in our review found larger ERN amplitudes for the rewarded blocks, for both motivational conditions (reward and punishment) (Maruo et al., 2016; Stürmer et al., 2011), and in emotional regulation strategies when errors underly racial bias (i.e., highly salient errors are more difficult to regulate) (Levsen & Bartholow, 2018). These results are consistent with the notion that affective/motivational influences are not linear and that transient affect can dynamically alter error monitoring (Larson et al., 2013).
Furthermore, the divergent findings may also be understood within the Reinforcement Learning Theory (Holroyd & Coles, 2002). In their theoretical framework, the authors state that the ERN is a reward prediction error signal; in other words, a signal that carries information about expectancy violation. Enhanced or reduced amplitudes would emerge depending on the affective context and the existing discrepancy between predicted and actual rewards. For instance, positive mood may increase reward prediction error when the probability of getting a reward is high (Bakic et al., 2014) or with task-irrelevant pleasant backgrounds during error commission (Larson et al., 2006).
In general, the reviewed studies that found affective and motivational influences on the ERN support the hypothesis that the ERN modulations account for the expectancy violation (Holroyd & Coles, 2002) and the specific value or motivational saliency of errors (Hajcak & Foti, 2008; Hajcak et al., 2005). However, findings with no influences of affective manipulations and non-replication attempts suggest that state affect accounts only for some variability in the ERN. Likewise, informative results from emerging interaction trait effects in studies that found no significant effects solely by state affect manipulation need to be addressed.
State-trait interactions
Nine studies found moderator effects of trait on the ERN throughout the different state manipulations. Interestingly, all studies reported enhanced ERN amplitudes for the assessed traits. High anxiety, worry, neuroticism, and BIS moderated the effects in negative affect and punishment manipulations, except in one study where the interaction effect was with lower BIS trait (Leue et al., 2017). These findings are part of a growing body of evidence that explains the ERN (generation and modulation) by stable levels of trait personality and individual differences (Hajcak, 2012; Riesel, 2019; Weinberg & Hajcak, 2011). Analysis by trait allowed recognition of subtle state affect differences that could not be detected in the overall sample (Pailing & Segalowitz, 2004) and explained the significant differences in state manipulation within the sample (Riesel et al., 2012). Thus, individual differences are relevant when examining the effects of state manipulation, as trait affect is a moderator variable in state or motivational changes (Dikman & Allen, 2000; Luu et al., 2000; Masaki et al., 2017).
In this sense, state manipulation effects would be conditional to the moderator effect of trait personality; therefore, studies would be dealing with unaccounted ERN variability if there is no trait measurement. Future studies should include trait measures in their analysis as variability in the ERN might depend on affective/motivational states and dispositional traits (Hajcak & Foti, 2008; Leue et al., 2017; Olvet & Hajcak, 2012; Park & Kitayama, 2014). Especially in light of recent meta-analytic evidence suggesting that trait findings may be more heterogenous and influenced by publication bias (Saunders & Inzlicht, 2020) and that other moderators may be affecting the sizes effects of the ERN (Clayson et al., 2021; Gloe & Louis, 2021).
Limitations of the present study
While the review findings suggest that the ERN may be sensitive to state-specific affect manipulations, heterogeneity of the paradigms and variables studied (i.e., tasks employed, performance feedback, designs) and a considerable number of studies with nonmodulations of the ERN made it challenging to draw more decisive conclusions. Likewise, most of the reviewed studies had a sample composed of less than 50 participants with no precise sample size calculations. We identified that some higher power studies could not replicate previous findings that reported significant effects with lower power samples. In addition, caution should be taken concerning our conclusions since they are based on qualitative analysis, and the effect sizes were not examined. Therefore, future studies should include similar variables and consider the possibility of conducting a meta-analytic review of the affect states’ manipulations and their effects on the ERN to unravel the nature of their associations.
Recommendations and future directions
This systematic review identified the need for further research in state affect-related changes and the error-related negativity. Our results showed that future studies should increase their statistical power due to nonreplication findings with higher sizes samples (Cano Rodilla et al., 2016; Elkins-Brown et al., 2018; Larson et al., 2013). To facilitate future high-power replication attempts in this area, it is relevant to explicitly describe the protocol: variables, scales, and methodologies, such as detail or explicit justification of the sample sizes, procedures, and statistical tools. Furthermore, studies should include evidence of complete preregistration (description of the content of the hypothesis, primary analysis, and exclusion criteria determined before data collection). We observed that it is not common to report statistical power estimations for sample size and effect sizes, the final sample’s complete characteristics (i.e., mean age, standard deviation, gender, ethnicity), and the internal consistency of ERN scores. Although inferring the reliability of the ERN from previous studies is a conventional practice, ERN scores’ internal consistency is dependent on studies’ contextual factors and should be reported based on each study (Clayson, 2020). As reproducible research is needed, adequate reporting behavior across studies is vital in human electrophysiological studies (Clayson et al., 2019; Larson & Carbine, 2017).
In addition, we identified that most studies tended to report marginally statistical effects, especially when reporting trends. This flexibility is problematic, because it could mislead the reader to an interpretation of effects that have low evidential value (false positives) (Pritschet et al., 2016), and not less important, they are associated with lower reproducibility (Olsson-Collentine et al., 2019). In consequence, researchers should avoid this practice. Likewise, state induction validation is essential in determining the evidence for/against the state affect-independent properties of the ERN (Cano Rodilla et al., 2016; Elkins-Brown et al., 2018). Future studies manipulating state affect should always register the effectiveness of the affect’s inductions throughout a manipulation check (Rodeback et al., 2020).
Regarding affective manipulations, we found conflicting results in the modulations of the ERN with positive valence stimuli and positive mood manipulations. In general, more studies with positive affect are needed since its specific relationship with the ERN remains unclear. Finally, future studies should include an assessment of trait in their samples. In line with previous and recent research, trait has been identified as a crucial moderator factor regarding the state affect relations with the ERN. Thus, this aspect should not be ruled out of the analysis if the objective is to account for the ERN variability in affective state manipulations.
Conclusions
We extracted 51 articles exploring the effects of state affective manipulations on the ERN. Findings with significant effects indicated a tendency towards the sensitivity of the ERN to state-related affect manipulations, and more specifically, results support the motivational value and aversiveness of errors. Likewise, manipulations of increasing negative affect or punishment usually led to an enhancement of the ERN, while manipulations that lessen negative affect (i.e., emotion regulation) or with positive affect tended to reduce the ERN. However, conflicting results with different effects, null findings in recent replications, and trait as crucial moderator factor informed the complex relationship between electrophysiological indices of error processing, individual differences, and state affective manipulations. Future research is needed with more statistical power and replicability on state-related manipulations.
References
Aarts, K., De Houwer, J., & Pourtois, G. (2013). Erroneous and correct actions have a different affective valence: Evidence from ERPs. Emotion, 13(5), 960–973. https://doi.org/10.1037/a0032808
Alexander, W. H., & Brown, J. W. (2010). Computational models of performance monitoring and cognitive control. Topics in Cognitive Science, 2(4), 658–677. https://doi.org/10.1111/j.1756-8765.2010.01085.x
Amodio, D. M., Master, S. L., Yee, C. M., & Taylor, S. E. (2008). Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology, 45(1), 11–19. https://doi.org/10.1111/j.1469-8986.2007.00609.x
Bakic, J., Jepma, M., De Raedt, R., & Pourtois, G. (2014). Effects of positive mood on probabilistic learning: Behavioral and electrophysiological correlates. Biological Psychology, 103, 223–232. https://doi.org/10.1016/j.biopsycho.2014.09.012
Bernstein, P. S., Scheffers, M. K., & Coles, M. G. H. (1995). “Where did I go wrong?” A psychophysiological analysis of error detection. Journal of Experimental Psychology Human Perception and Performance, 21(6), 1312–1322. https://doi.org/10.1037//0096-1523.21.6.1312
Boksem, M. A. S., Ruys, K. I., & Aarts, H. (2011). Facing disapproval: Performance monitoring in a social context. Social Neuroscience, 6(4), 360–368. https://doi.org/10.1080/17470919.2011.556813
Boksem, M. A. S., Tops, M., Kostermans, E., & De Cremer, D. (2008). Sensitivity to punishment and reward omission: Evidence from error-related ERP components. Biological Psychology, 79(2), 185–192. https://doi.org/10.1016/j.biopsycho.2008.04.010
Botvinick, M. M. (2007). Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cognitive, Affective & Behavioral Neuroscience, 7(4), 356–366. https://doi.org/10.3758/cabn.7.4.356
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. https://doi.org/10.1037/0033-295X.108.3.624
Braem, S., King, J. A., Korb, F. M., Krebs, R. M., Notebaert, W., & Egner, T. (2017). The Role of Anterior Cingulate Cortex in the Affective Evaluation of Conflict. Journal of Cognitive Neuroscience, 29(1), 137–149. https://doi.org/10.1162/jocn_a_01023
Button, K. S., Ioannidis, J. P. A., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S. J., & Munafò, M. R. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience, 14(5), 365–376. https://doi.org/10.1038/nrn3475
Cano Rodilla, C., Beauducel, A., & Leue, A. (2016). Error-Related Negativity and the Misattribution of State-Anxiety Following Errors: On the Reproducibility of Inzlicht and Al-Khindi (2012). Frontiers in Human Neuroscience, 10https://doi.org/10.3389/fnhum.2016.00475
Clayson, P. E. (2020). Moderators of the internal consistency of error-related negativity scores: A meta-analysis of internal consistency estimates. Psychophysiology, 57(8), e13583. https://doi.org/10.1111/psyp.13583
Clayson, P. E., Baldwin, S. A., & Larson, M. J. (2013). How does noise affect amplitude and latency measurement of event-related potentials (ERPs)? A methodological critique and simulation study. Psychophysiology, 50(2), 174–186. https://doi.org/10.1111/psyp.12001
Clayson, P. E., Carbine, K. A., Baldwin, S. A., & Larson, M. J. (2019). Methodological reporting behavior, sample sizes, and statistical power in studies of event-related potentials: Barriers to reproducibility and replicability. Psychophysiology, 56(11), e13437. https://doi.org/10.1111/psyp.13437
Clayson, P. E., Clawson, A., & Larson, M. J. (2012). The effects of induced state negative affect on performance monitoring processes. Social Cognitive and Affective Neuroscience, 7(6), 677–688. https://doi.org/10.1093/scan/nsr040
Clayson, P. E., Kappenman, E. S., Gehring, W. J., Miller, G. A., & Larson, M. J. (2021). A commentary on establishing norms for error-related brain activity during the arrow flanker task among young adults. NeuroImage, 234, 117932. https://doi.org/10.1016/j.neuroimage.2021.117932
Clayson, P. E., & Larson, M. J. (2019). The impact of recent and concurrent affective context on cognitive control: An ERP study of performance monitoring. International Journal of Psychophysiology, 143, 44–56. https://doi.org/10.1016/j.ijpsycho.2019.06.007
Coles, M. G. H., Gratton, G., & Donchin, E. (1988). Detecting early communication: Using measures of movement-related potentials to illuminate human information processing. Biological Psychology, 26(1–3), 69–89. https://doi.org/10.1016/0301-0511(88)90014-2.
Coles, M. G., Scheffers, M. K., & Holroyd, C. B. (2001). Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology, 56(3), 173–189. https://doi.org/10.1016/s0301-0511(01)00076-x
Compton, R. J., Carp, J., Chaddock, L., Fineman, S. L., Quandt, L. C., & Ratliff, J. B. (2007). Anxiety and error monitoring: Increased error sensitivity or altered expectations? Brain and Cognition, 64(3), 247–256. https://doi.org/10.1016/j.bandc.2007.03.006
de Bruijn, E. R. A., Jansen, M., & Overgaauw, S. (2020).Enhanced error-related brain activations for mistakes that harm others: ERP evidence from a novel social performance-monitoring paradigm. NeuroImage, 204https://doi.org/10.1016/j.neuroimage.2019.116238
Dignath, D., Berger, A., Spruit, I. M., & van Steenbergen, H. (2019). Temporal dynamics of error-related corrugator supercilii and zygomaticus major activity: Evidence for implicit emotion regulation following errors. International Journal of Psychophysiology, 146, 208–216. https://doi.org/10.1016/j.ijpsycho.2019.10.003
Dikman, Z. V., & Allen, J. J. (2000). Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology, 37(1), 43–54.
Dreisbach, G., & Fischer, R. (2012). Conflicts as aversive signals. Brain and Cognition, 78(2), 94–98. https://doi.org/10.1016/j.bandc.2011.12.003
Elkins-Brown, N., Saunders, B., & Inzlicht, M. (2016). Error-related electromyographic activity over the corrugator supercilii is associated with neural performance monitoring. Psychophysiology, 53(2), 159–170. https://doi.org/10.1111/psyp.12556
Elkins-Brown, N., Saunders, B., & Inzlicht, M. (2018). The misattribution of emotions and the error-related negativity: A registered report. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 109, 124–140. https://doi.org/10.1016/j.cortex.2018.08.017
Falkenstein, M., Hohnsbein, J., & Hoormann, J. (1995). Event-related potential correlates of errors in reaction tasks. Electroencephalography and Clinical Neurophysiology. Supplement, 44, 287–296.
Falkenstein, M., Hohnsbein, J., Hoormann, J., & Blanke, L. (1991). Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography & Clinical Neurophysiology, 78(6), 447–455. https://doi.org/10.1016/0013-4694(91)90062-9
Ganushchak, L. Y., & Schiller, N. O. (2008). Motivation and semantic context affect brain error-monitoring activity: An event-related brain potentials study. NeuroImage, 39(1), 395–405. https://doi.org/10.1016/j.neuroimage.2007.09.001
Gehring, W. J., Goss, B., Coles, M. G., Meyer, D. E., & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–390. https://doi.org/10.1111/j.1467-9280.1993.tb00586.x
Gelman, A., & Carlin, J. (2014). Beyond Power Calculations: Assessing Type S (Sign) and Type M (Magnitude) Errors. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 9(6), 641–651. https://doi.org/10.1177/1745691614551642
Glienke, K., Wolf, O. T., & Bellebaum, C. (2015). The impact of stress on feedback and error processing during behavioral adaptation. Neuropsychologia, 71, 181–190. https://doi.org/10.1016/j.neuropsychologia.2015.04.004
Gloe, L. M., & Louis, C. C. (2021). The Error-Related Negativity (ERN) in Anxiety and Obsessive-Compulsive Disorder (OCD): A Call for Further Investigation of Task Parameters in the Flanker Task. Frontiers in Human Neuroscience, 15, 779083. https://doi.org/10.3389/fnhum.2021.779083
Gratton, G., Coles, M. G., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology, 55(4), 468–484. https://doi.org/10.1016/0013-4694(83)90135-9.
Good, M., Inzlicht, M., & Larson, M. J. (2015). God will forgive: Reflecting on God’s love decreases neurophysiological responses to errors. Social Cognitive and Affective Neuroscience, 10(3), 357–363. https://doi.org/10.1093/scan/nsu096
Hajcak, G. (2012). What we’ve learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science, 21(2), 101–106. https://doi.org/10.1177/0963721412436809
Hajcak, G., & Foti, D. (2008). Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science, 19(2), 103–108. https://doi.org/10.1111/j.1467-9280.2008.02053.x
Hajcak, G., McDonald, N., & Simons, R. F. (2003). To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40(6), 895–903. https://doi.org/10.1111/1469-8986.00107
Hajcak, G., McDonald, N., & Simons, R. F. (2004). Error-related psychophysiology and negative affect. Brain and Cognition, 56(2), 189–197. https://doi.org/10.1016/j.bandc.2003.11.001
Hajcak, G., Moser, J. S., Yeung, N., & Simons, R. F. (2005). On the ERN and the significance of errors. Psychophysiology, 42(2), 151–160. https://doi.org/10.1111/j.1469-8986.2005.00270.x
Hajcak, G., Weinberg, A., MacNamara, A., & Foti, D. (2012). ERPs and the study of emotion. In The Oxford handbook of event-related potential components (pp. 441–472). Oxford University Press.
Henrich, J., Heine, S. J., & Norenzayan, A. (2016). Most people are not WEIRD (p. 114). American Psychological Association. https://doi.org/10.1037/14805-007
Hobson, N. M., Saunders, B., Al-Khindi, T., & Inzlicht, M. (2014). Emotion down-regulation diminishes cognitive control: A neurophysiological investigation. Emotion, 14(6), 1014–1026. https://doi.org/10.1037/a0038028
Hoffmann, S., & Beste, C. (2015). A perspective on neural and cognitive mechanisms of error commission. Frontiers in Behavioral Neuroscience, 9, 50. https://doi.org/10.3389/fnbeh.2015.00050
Holroyd, C. B., & Coles, M. G. H. (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. https://doi.org/10.1037/0033-295X.109.4.679
Holroyd, C. B., Yeung, N., Coles, M. G. H., & Cohen, J. D. (2005). A Mechanism for Error Detection in Speeded Response Time Tasks. Journal of Experimental Psychology: General, 134(2), 163–191. https://doi.org/10.1037/0096-3445.134.2.163
Iannaccone, R., Hauser, T. U., Staempfli, P., Walitza, S., Brandeis, D., & Brem, S. (2015). Conflict monitoring and error processing: New insights from simultaneous EEG–fMRI. NeuroImage, 105, 395–407. https://doi.org/10.1016/j.neuroimage.2014.10.028
Inzlicht, M., & Al-Khindi, T. (2012). ERN and the placebo: A misattribution approach to studying the arousal properties of the error-related negativity. Journal of Experimental Psychology: General, 141(4), 799–807. https://doi.org/10.1037/a0027586
Inzlicht, M., Bartholow, B. D., & Hirsh, J. B. (2015). Emotional foundations of cognitive control. Trends in Cognitive Sciences, 19(3), 126–132. https://doi.org/10.1016/j.tics.2015.01.004
Inzlicht, M., & Gutsell, J. N. (2007). Running on empty: Neural signals for self-control failure. Psychological Science, 18(11), 933–937. https://doi.org/10.1111/j.1467-9280.2007.02004.x
Jackson, F., Nelson, B. D., & Proudfit, G. H. (2015). In an uncertain world, errors are more aversive: Evidence from the error-related negativity. Emotion (washington, D.c.), 15(1), 12–16. https://doi.org/10.1037/emo0000020
Jelinčić, V., Torta, D. M., Van Diest, I., & von Leupoldt, A. (2020). Error-related negativity relates to the neural processing of brief aversive bodily sensations. Biological Psychology, 152, 107872. https://doi.org/10.1016/j.biopsycho.2020.107872
Koechlin, E., Ody, C., & Kounelher, F. (2003). The Architecture of Cognitive Control in the Human Prefrontal Cortex. Science, 302(5648), 1181–1185. https://doi.org/10.1126/science.1088545
Larson, M. J., & Carbine, K. A. (2017). Sample size calculations in human electrophysiology (EEG and ERP) studies: A systematic review and recommendations for increased rigor. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 111, 33–41. https://doi.org/10.1016/j.ijpsycho.2016.06.015
Larson, M. J., Gray, A. C., Clayson, P. E., Jones, R., & Kirwan, C. B. (2013). What are the influences of orthogonally-manipulated valence and arousal on performance monitoring processes? The effects of affective state. International Journal of Psychophysiology, 87(3), 327–339. https://doi.org/10.1016/j.ijpsycho.2013.01.005
Larson, M. J., Perlstein, W. M., Stigge-Kaufman, D., Kelly, K. G., & Dotson, V. M. (2006). Affective context-induced modulation of the error-related negativity. NeuroReport, 17(3), 329–333. https://doi.org/10.1097/01.wnr.0000199461.01542.db
Leue, A., Rodilla, C. C., & Beauducel, A. (2017). Worry-inducing stimuli in an aversive Go/NoGo task enhance reactive control in individuals with lower trait-anxiety. Biological Psychology, 125, 1–11. https://doi.org/10.1016/j.biopsycho.2017.02.003
Levsen, M. P., & Bartholow, B. D. (2018). Neural and behavioral effects of regulating emotional responses to errors during an implicit racial bias task. Cognitive, Affective & Behavioral Neuroscience, 18(6), 1283–1297. https://doi.org/10.3758/s13415-018-0639-8
Luck, S. J. (2005). An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press.
Luu, P., Collins, P., & Tucker, D. M. (2000). Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General, 129(1), 43–60. https://doi.org/10.1037/0096-3445.129.1.43
Mackersie, C. L., & Calderon-Moultrie, N. (2016). Autonomic Nervous System Reactivity During Speech Repetition Tasks: Heart Rate Variability and Skin Conductance. Ear and Hearing, 37(Suppl 1), 118S-S125. https://doi.org/10.1097/AUD.0000000000000305
Maier, M. E., & Steinhauser, M. (2016). Error significance but not error expectancy predicts error-related negativities for different error types. Behavioural Brain Research, 297, 259–267. https://doi.org/10.1016/j.bbr.2015.10.031
Maier, M. E., Steinhauser, M., & Hübner, R. (2010). Effects of response-set size on error-related brain activity. Experimental Brain Research, 202(3), 571–581. https://doi.org/10.1007/s00221-010-2160-3
Maruo, Y., Schacht, A., Sommer, W., & Masaki, H. (2016). Impacts of motivational valence on the error-related negativity elicited by full and partial errors. Biological Psychology, 114, 108–116. https://doi.org/10.1016/j.biopsycho.2015.12.004
Masaki, H., Maruo, Y., Meyer, A., & Hajcak, G. (2017). Neural Correlates of Choking Under Pressure: Athletes High in Sports Anxiety Monitor Errors More When Performance Is Being Evaluated. Developmental Neuropsychology, 42(2), 104–112. https://doi.org/10.1080/87565641.2016.1274314
Meyer, A., & Gawlowska, M. (2017). Evidence for specificity of the impact of punishment on error-related brain activity in high versus low trait anxious individuals. International Journal of Psychophysiology, 120, 157–163. https://doi.org/10.1016/j.ijpsycho.2017.08.001
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
Miller, G. A., & Rockstroh, B. (2013). Endophenotypes in psychopathology research: Where do we stand? Annual Review of Clinical Psychology, 9, 177–213. https://doi.org/10.1146/annurev-clinpsy-050212-185540
Moser, J. S., Hajcak, G., & Simons, R. F. (2005). The effects of fear on performance monitoring and attentional allocation. Psychophysiology, 42(3), 261–268. https://doi.org/10.1111/j.1469-8986.2005.00290.x
Moser, J. S., Moran, T. P., Schroder, H. S., Donnellan, M. B., & Yeung, N. (2013). On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7https://doi.org/10.3389/fnhum.2013.00466
Muthukrishna, M., Bell, A. V., Henrich, J., Curtin, C. M., Gedranovich, A., McInerney, J., & Thue, B. (2020). Beyond Western, Educated, Industrial, Rich, and Democratic (WEIRD) Psychology: Measuring and Mapping Scales of Cultural and Psychological Distance. Psychological Science, 31(6), 678–701. https://doi.org/10.1177/0956797620916782
Nieuwenhuis, S., Ridderinkhof, K. R., Blom, J., Band, G. P., & Kok, A. (2001). Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology, 38(5), 752–760.
Nigbur, R., & Ullsperger, M. (2020). Funny kittens: Positive mood induced via short video-clips affects error processing but not conflict control. International Journal of Psychophysiology, 147, 147–155. https://doi.org/10.1016/j.ijpsycho.2019.11.007
Olsson-Collentine, A., van Assen, M. A. L. M., & Hartgerink, C. H. J. (2019). The Prevalence of Marginally Significant Results in Psychology Over Time. Psychological Science, 30(4), 576–586. https://doi.org/10.1177/0956797619830326
Olvet, D. M., & Hajcak, G. (2012). The error-related negativity relates to sadness following mood induction among individuals with high neuroticism. Social Cognitive and Affective Neuroscience, 7(3), 289–295. https://doi.org/10.1093/scan/nsr007
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71https://doi.org/10.1136/bmj.n71
Pailing, P. E., & Segalowitz, S. J. (2004). The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology, 41(1), 84–95. https://doi.org/10.1111/1469-8986.00124
Park, J., & Kitayama, S. (2014). Interdependent selves show face-induced facilitation of error processing: Cultural neuroscience of self-threat. Social Cognitive and Affective Neuroscience, 9(2), 201–208. https://doi.org/10.1093/scan/nss125
Paul, K., Walentowska, W., Bakic, J., Dondaine, T., & Pourtois, G. (2017). Modulatory effects of happy mood on performance monitoring: Insights from error-related brain potentials. Cognitive, Affective & Behavioral Neuroscience, 17(1), 106–123. https://doi.org/10.3758/s13415-016-0466-8
Pfabigan, D. M., Pintzinger, N. M., Siedek, D. R., Lamm, C., Derntl, B., & Sailer, U. (2013). Feelings of helplessness increase ERN amplitudes in healthyindividuals. Neuropsychologia, 51(4), 613–621. https://doi.org/10.1016/j.neuropsychologia.2012.12.008
Potts, G. F. (2011). Impact of reward and punishment motivation on behavior monitoring as indexed by the error-related negativity. International Journal of Psychophysiology, 81(3), 324–331. https://doi.org/10.1016/j.ijpsycho.2011.07.020
Pritschet, L., Powell, D., & Horne, Z. (2016). Marginally Significant Effects as Evidence for Hypotheses: Changing Attitudes Over Four Decades. Psychological Science, 27(7), 1036–1042. https://doi.org/10.1177/0956797616645672
Proudfit, G. H., Inzlicht, M., & Mennin, D. (2013). Anxiety and error monitoring: The importance of motivation and emotion. Frontiers in Human Neuroscience, 7https://doi.org/10.3389/fnhum.2013.00636
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., & Nieuwenhuis, S. (2004). The Role of the Medial Frontal Cortex in Cognitive Control. Science, 306(5695), 443–447. https://doi.org/10.1126/science.1100301
Riesel, A. (2019). The erring brain: Error-related negativity as an endophenotype for OCD—A review and meta-analysis. Psychophysiology, 56(4), e13348. https://doi.org/10.1111/psyp.13348
Riesel, A., Kathmann, N., & Klawohn, J. (2019a). Flexibility of error-monitoring in obsessive-compulsive disorder under speed and accuracy instructions. Journal of Abnormal Psychology, 128(7), 671–677. https://doi.org/10.1037/abn0000463
Riesel, A., Kathmann, N., Wüllhorst, V., Banica, I., & Weinberg, A. (2019b). Punishment has a persistent effect on error-related brain activity in highly anxious individuals twenty-four hours after conditioning. International Journal of Psychophysiology, 146, 63–72. https://doi.org/10.1016/j.ijpsycho.2019.09.014
Riesel, A., Klawohn, J., Grützmann, R., Kaufmann, C., Heinzel, S., Bey, K., Lennertz, L., Wagner, M., & Kathmann, N. (2019c). Error-related brain activity as a transdiagnostic endophenotype for obsessive-compulsive disorder, anxiety and substance use disorder. Psychological Medicine, 49(7), 1207–1217. https://doi.org/10.1017/S0033291719000199
Riesel, A., Weinberg, A., Endrass, T., Kathmann, N., & Hajcak, G. (2012). Punishment has a lasting impact on error-related brain activity. Psychophysiology, 49(2), 239–247. https://doi.org/10.1111/j.1469-8986.2011.01298.x
Riesel, A., Weinberg, A., Endrass, T., Meyer, A., & Hajcak, G. (2013). The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biological Psychology, 93(3), 377–385. https://doi.org/10.1016/j.biopsycho.2013.04.007
Rodeback, R. E., Hedges-Muncy, A., Hunt, I. J., Carbine, K. A., Steffen, P. R., & Larson, M. J. (2020). The Association Between Experimentally Induced Stress, Performance Monitoring, and Response Inhibition: An Event-Related Potential (ERP) Analysis. Frontiers in Human Neuroscience, 14, 189. https://doi.org/10.3389/fnhum.2020.00189
Russell, J. A. (2003). Core affect and the psychological construction of emotion. Psychological Review, 110(1), 145–172. https://doi.org/10.1037/0033-295x.110.1.145
Russell, J. A., & Barrett, L. F. (1999). Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. Journal of Personality and Social Psychology, 76(5), 805–819. https://doi.org/10.1037//0022-3514.76.5.805
Saunders, B., & Inzlicht, M. (2020). Assessing and adjusting for publication bias in the relationship between anxiety and the error-related negativity. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 155, 87–98. https://doi.org/10.1016/j.ijpsycho.2020.05.008
Saunders, B., Lin, H., Milyavskaya, M., & Inzlicht, M. (2017). The emotive nature of conflict monitoring in the medial prefrontal cortex. International Journal of Psychophysiology, 119, 31–40. https://doi.org/10.1016/j.ijpsycho.2017.01.004
Saunders, B., Milyavskaya, M., & Inzlicht, M. (2015). What does cognitive control feel like? Effective and ineffective cognitive control is associated with divergent phenomenology. Psychophysiology, 52(9), 1205–1217. https://doi.org/10.1111/psyp.12454
Saunders, B., Rodrigo, A. H., & Inzlicht, M. (2016). Mindful awareness of feelings increases neural performance monitoring. Cognitive, Affective & Behavioral Neuroscience, 16(1), 93–105. https://doi.org/10.3758/s13415-015-0375-2
Schiffer, A.-M., Waszak, F., & Yeung, N. (2015). The role of prediction and outcomes in adaptive cognitive control. Journal of Physiology, Paris, 109(1–3), 38–52. https://doi.org/10.1016/j.jphysparis.2015.02.001
Senderecka, M. (2016). Threatening visual stimuli influence response inhibition and error monitoring: An event-related potential study. Biological Psychology, 113, 24–36. https://doi.org/10.1016/j.biopsycho.2015.11.003
Senderecka, M. (2018). Emotional enhancement of error detection—The role of perceptual processing and inhibition monitoring in failed auditory stop trials. Cognitive, Affective & Behavioral Neuroscience, 18(1), 1–20. https://doi.org/10.3758/s13415-017-0546-4
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–167. https://doi.org/10.1038/nrn2994
Shenhav, A., Cohen, J. D., & Botvinick, M. M. (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience, 19(10), 1286–1291. https://doi.org/10.1038/nn.4384
Spunt, R. P., Lieberman, M. D., Cohen, J. R., & Eisenberger, N. I. (2012). The Phenomenology of Error Processing: The Dorsal ACC Response to Stop-signal Errors Tracks Reports of Negative Affect. Journal of Cognitive Neuroscience, 24(8), 1753–1765. https://doi.org/10.1162/jocn_a_00242
Stürmer, B., Nigbur, R., Schacht, A., & Sommer, W. (2011).Reward and Punishment Effects on Error Processing and Conflict Control. Frontiers in Psychology, 2https://doi.org/10.3389/fpsyg.2011.00335
Sueyoshi, T., Sugimoto, F., Katayama, J., & Fukushima, H. (2014). Neural correlates of error processing reflect individual differences in interoceptive sensitivity. International Journal of Psychophysiology, 94(3), 278–286. https://doi.org/10.1016/j.ijpsycho.2014.10.001
Suzuki, T., Ait Oumeziane, B., Novak, K., Samuel, D. B., & Foti, D. (2020). Error-monitoring across social and affective processing contexts. International Journal of Psychophysiology, 150, 37–49. https://doi.org/10.1016/j.ijpsycho.2020.01.009
Tan, Y., Vandeput, J., Qiu, J., Van den Bergh, O., & von Leupoldt, A. (2019). The error-related negativity for error processing in interoception. NeuroImage, 184, 386–395. https://doi.org/10.1016/j.neuroimage.2018.09.037
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. (2020). Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. Available from. https://synthesismanual.jbi.global
Tullett, A. M., Kay, A. C., & Inzlicht, M. (2015). Randomness increases self-reported anxiety and neurophysiological correlates of performance monitoring. Social Cognitive and Affective Neuroscience, 10(5), 628–635. https://doi.org/10.1093/scan/nsu097
Unger, K., Kray, J., & Mecklinger, A. (2012). Worse than feared? Failure induction modulates the electrophysiological signature of error monitoring during subsequent learning. Cognitive, Affective & Behavioral Neuroscience, 12(1), 34–51. https://doi.org/10.3758/s13415-011-0061-y
Valt, C., Palazova, M., & Stürmer, B. (2017). Processing of Internal and External Signals for Performance Monitoring in the Context of Emotional Faces. Advances in Cognitive Psychology, 13(3), 190–200. https://doi.org/10.5709/acp-0219-5
van Steenbergen, H., Band, G. P. H., Hommel, B., Rombouts, S. A. R. B., & Nieuwenhuis, S. (2015). Hedonic Hotspots Regulate Cingulate-driven Adaptation to Cognitive Demands. Cerebral Cortex (new York, N.y.: 1991), 25(7), 1746–1756. https://doi.org/10.1093/cercor/bht416
van Wouwe, N. C., Band, G. P. H., & Ridderinkhof, K. R. (2011). Positive affect modulates flexibility and evaluative control. Journal of Cognitive Neuroscience, 23(3), 524–539. https://doi.org/10.1162/jocn.2009.21380
Wang, Y., Yang, L., & Wang, Y. (2014). Suppression (but not reappraisal) impairs subsequent error detection: An ERP study of emotion regulation’s resource-depleting effect. PLoS ONE, 9(4), e96339. https://doi.org/10.1371/journal.pone.0096339
Weinberg, A., & Hajcak, G. (2011). Longer term test-retest reliability of error-related brain activity. Psychophysiology, 48(10), 1420–1425. https://doi.org/10.1111/j.1469-8986.2011.01206.x
Weinberg, A., Hilgard, J., Bartholow, B. D., & Hajcak, G. (2012a). Emotional targets: Evaluative categorization as a function of context and content. International Journal of Psychophysiology, 84(2), 149–154. https://doi.org/10.1016/j.ijpsycho.2012.01.023
Weinberg, A., Klein, D. N., & Hajcak, G. (2012b). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology, 121(4), 885–896. https://doi.org/10.1037/a0028270
Weinberg, A., Meyer, A., Hale-Rude, E., Perlman, G., Kotov, R., Klein, D. N., & Hajcak, G. (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–385. https://doi.org/10.1111/psyp.12538
Weinberg, A., Riesel, A., & Hajcak, G. (2012c). Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion, 36(1), 84–100. https://doi.org/10.1007/s11031-011-9269-y
White, E. J., Grant, D. M., Taylor, D. L., Frosio, K. E., Mills, A. C., & Judah, M. R. (2018). Examination of evaluative threat in worry: Insights from the error-related negativity (ERN). Psychiatry Research: Neuroimaging, 282, 40–46. https://doi.org/10.1016/j.pscychresns.2018.10.006
Wiswede, D., Münte, T. F., Goschke, T., & Rüsseler, J. (2009a). Modulation of the error-related negativity by induction of short-term negative affect. Neuropsychologia, 47(1), 83–90. https://doi.org/10.1016/j.neuropsychologia.2008.08.016
Wiswede, D., Münte, T. F., Krämer, U. M., & Rüsseler, J. (2009b). Embodied emotion modulates neural signature of performance monitoring. PLoS One, 4(6), e5754. https://doi.org/10.1371/journal.pone.0005754
Wiswede, D., Münte, T. F., & Rüsseler, J. (2009c). Negative affect induced by derogatory verbal feedback modulates the neural signature of error detection. Social Cognitive and Affective Neuroscience, 4(3), 227–237. https://doi.org/10.1093/scan/nsp015
Yeung, N. (2014). Conflict monitoring and cognitive control. In The Oxford handbook of cognitive neuroscience, Vol. 2: The cutting edges (pp. 275–299). Oxford University Press.
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychological Review, 111(4), 931–959. https://doi.org/10.1037/0033-295X.111.4.931
Zhou, S., Xiong, S., Cheng, W., & Wang, Y. (2019). Flanker paradigm contains conflict and distraction factors with distinct neural mechanisms: An ERP analysis in a 2–1 mapping task. Cognitive Neurodynamics, 13(4), 341–356. https://doi.org/10.1007/s11571-019-09529-w
Acknowledgements
The authors thank Dr. Roberto Nohoray for feedback on the analysis of EEG extraction methodologies, and Dr. (C) Sergio Lopez for feedback on the interpretation of studies’ statistical analysis and critical appraisal. This manuscript is part of the first author’s master’s thesis. Funding that made this research possible was granted by CNPq (National Council for Scientific and Technological Development) and CAPES Foundation (Coordination for the Improvement of Higher Education Personnel).
All persons who meet authorship criteria are listed as authors. RMMA, GG, and XNE conceived the study and methodological strategy. XNE and LZB conducted the literature searches. XNE and LZ independently conducted the first two screening phases of the studies. XNE and RMMA conducted the quality assessment. XNE wrote the manuscript, with all authors contributing significantly to manuscript revision. All authors certify that they have participated sufficiently in work to take public responsibility for the content, including participation in the manuscript’s concept, design, analysis, writing, or revision. All authors have approved the final article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Nuñez-Estupiñan, X., Berticelli, L.Z., de Almeida, R.M.M. et al. Aversiveness of errors and the error-related negativity (ERN): A systematic review on the affective states’ manipulations findings. Cogn Affect Behav Neurosci 22, 754–776 (2022). https://doi.org/10.3758/s13415-022-01002-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-022-01002-2