Abstract
Prior research has shown that the presence of another individual and type of attention cue (social gaze vs. nonsocial arrow) can modulate attention, with little done to integrate the two. We thus investigate the role of two social presence factors when completing a joint cueing task with either social (gaze) or nonsocial (arrow) cues. Familiarity was operationalized as participants engaged in a prompted conversation either before (n = 60 dyads) or after (n = 59 dyads) the task. To determine the effect of previous responder identity on attention, we contrasted trials where participants responded twice in a row (same responder) with switch trials (different responder), along with whether the previous target was in the same or a different location. Although familiarity only affected global speed and not magnitudes of cueing, we did find that attention to gaze and arrows was differentially affected by previous responder and previous target location. Specifically, for gaze cues muted cueing effects occurred for trials where the previous responder was different, while for arrow cues there was less muting of the cueing effect regardless of previous responder. Taken together, previous responder and previous target location both modulated attention, with the effect on attention dependent on the type of cue, gaze, or arrow.
Similar content being viewed by others
Introduction
Prior work suggests that attention varies with both the type of content we pay attention to as well as the environment we are in. By changing the type of content, such as comparing attention with gaze and arrow cues, some data suggest that social content is attentionally prioritized over other nonsocial content (e.g., Birmingham et al., 2008 ; Friesen et al., 2004; Hayward & Ristic, 2015; Marotta, Lupiàñez, Martella, et al., 2012b; but see, for example, Pereira et al., 2020, for no prioritization of social content). When looking at the environmental context, attention is also modulated based on various factors, including the presence of another individual (e.g., Cole et al., 2016; Risko & Kingstone, 2011; Skarratt et al., 2010) or different experimental contexts such as the lab or naturalistic situations (e.g., Foulsham et al., 2011; Gallup et al., 2012; Hayward et al., 2017). For instance, the presence of another individual has been shown to affect attention across a broad array of studies, from reducing the likelihood of looking at provocative items in a room (Risko & Kingstone, 2011), to yielding greater accuracy in search tasks when we share the task with a partner as compared with performing the task solo (e.g., Brennan & Enns, 2015; Niehorster et al., 2019). To the best of our knowledge, however, there has been little work investigating whether attention to social and nonsocial cues is differentially affected by the presence of another individual one has chatted with versus a complete stranger. Further, to obtain a more nuanced understanding of how others affect our attention, we looked at how performance changes from moment to moment while sharing a task by accounting for who responded on the previous trial and where the previous target was located (e.g., Dodd & Pratt, 2007; Hayward & Ristic, 2015; Jongen & Smulders, 2007; Welsh et al., 2005). Taken together, this study sought to investigate what social presence factors (familiarity, previous responder) influenced spatial attention to social and nonsocial cues.
Single-participant measures of attention in the lab
Attention is often measured in the laboratory using computerized tasks, such as the widely used Posner (1980) cueing task and variants thereof. In the classic cueing task, participants first see a central box and two peripheral boxes, one to the left and one to the right of fixation (Posner & Cohen, 1984). Next, one of the boxes will thicken or “brighten,” acting as a cue to orient attention and after a variable time (i.e., cue–target interval) a to-be-responded-to target will appear in one of the boxes. Importantly, the cue and target correspond or “match” for only 50% of trials, making the cue nonpredictive of the upcoming target location. Researchers compare how quickly and accurately participants respond to targets appearing in the cued location (cued trials) versus the uncued location (uncued trial) to discern how attention is shifted (e.g., spatial attention). Typically, for peripheral abrupt onsets, responses are faster for targets that appear soon after the cue (roughly 100 ms); however, when there is more time between the appearance of the cue and target (>300 ms), responses are slower for cued trials, known as inhibition of return (IOR; Posner & Cohen, 1984).
This late spatial inhibition effect is not usually seen when investigating attention to other types of more complex cues, including shapes (Ristic & Landry, 2015), numbers (Fischer et al., 2003), faces with averted eyes (Driver et al., 1999; Hayward & Ristic, 2017), and symbols like arrows (Hayward & Ristic, 2015; Ristic et al., 2002; Tipples, 2002). In the case of gaze or arrow cues, rather than a peripheral luminance change, a face with averted gaze or an arrow points in a direction to act as a cue to orient attention, again keeping the cue nonpredictive of the upcoming target location. In contrast to the notion that gaze is “special” (Downing et al., 2004; Driver et al., 1999; Friesen & Kingstone, 1998; Marotta et al., 2019), typical data indicate that both types of cues produce quick and sustained orienting of attention, in that responses are consistently faster for cued trials regardless of the cue–target interval, demonstrating facilitation without subsequent inhibition (Friesen et al., 2004; Hayward & Ristic, 2013b; Lassalle & Itier, 2015). Conversely, there is some research suggesting that the mechanisms underlying attention to gaze and arrows are not identical (e.g., Hayward & Ristic, 2015; Marotta, Lupiàñez, Martella, et al., 2012b; Vecera & Rizzo, 2006). For instance, while researchers have found that split-brain patients show cueing effects for arrows regardless of the hemifield in which the arrow is presented (Ristic et al., 2002), gaze cueing effects are only found when the face is presented in the hemifield specialized for processing upright faces (Kingstone et al., 2000; see also Marotta, Lupiàñez, & Casagrande, 2012a, for similar data in non-split-brain-patients). There has been other work showing differences in cueing effects for gaze and arrows, including work by Hayward and Ristic (2015), who found that after modifying the cueing task to reduce the influence of extraneous factors within the task (namely, tonic alertness and voluntary temporal preparation), gaze cueing emerged quickly but was fleeting, while arrow cueing was slower to emerge but longer lasting, along with other findings revealing smaller cueing effects for gaze than arrows (Bonato et al., 2008; Langdon & Smith, 2005, muted early gaze cueing effects in E1; Lockhofen et al., 2014). One potential reason for a dissociation between social and nonsocial attention is the idea that higher order processes such as the mental state or value of the social face can modulate basic social attention (Capozzi & Ristic, 2019; Kawai, 2011). In fact, previous work has found support for the notion that higher order mentalizing can affect spatial attention, as when participants were presented with a display containing a gaze cue and barriers that could block the face’s view of a target, participants only show intact gaze cueing when the central face could “view” the target (i.e., when there was no barrier blocking the face; Kawai, 2011), which was not replicated with arrow cues. Taken together, while attention to gaze and arrow cues seem to produce similar patterns of facilitation, there is some evidence supporting the notion that we attend to social and nonsocial content differently, especially when modifying the cueing task by showing lateralized cues, when removing extraneous cueing factors, or when accounting for higher order processes. This then opens the possibility that potential underlying differences between attention to gaze versus arrows may emerge when asking participants to complete a cueing task jointly with another person.
Paired-participant measures of attention in the lab
There is also evidence to suggest that eye movements or attention can be modified when performing tasks in pairs (Gobel & Giesbrecht, 2020; Hayes et al., 2009; Welsh et al., 2005). For example, Laidlaw and colleagues (2011) demonstrated the impact of how the potential for human interaction affects spatial attention by having participants wear an eye tracker while sitting in a waiting room. They found that those sitting in a room with a videotaped confederate were more likely to turn their head and fixate on that confederate, compared with when there was physically present confederate, indicating potential reluctance to engage in conversation with a stranger, and highlighting the dual functions of gaze as both a passive means of processing visual information as well as an active signal to indicate objects of interest to physically present individuals (e.g., Gobel et al., 2015; Risko et al., 2016). When looking specifically at two people mutually engaged in a task, Brennan and Enns (2015) asked pairs of participants complete a visual enumeration task separately and together. They found that team performance, measured using speed and accuracy, greatly exceeded individual performance which suggests that cooperative behaviour results in greater efficiency.
Turning specifically to studies employing variants of the cueing task, we find research has focused on how the identity of a previous responder and the spatial location of a previous target alters attention performance. Welsh and colleagues (2005) asked two participants to sit across from each other and take turns tapping an illuminated target (red dot) on a table, which could appear in one of two peripheral locations, to the left or right of a central fixation. Participants were instructed to respond to targets in a prearranged fashion where Participant A would respond twice followed by Participant B responding twice (AABBAABB . . .). This set-up yielded two types of “previous responder” trials: those where the same participant responded in the previous trial (AA, providing a measure of personal IOR), and those in which the participant responded after the other person (AB, providing a measure of social IOR, or sIOR). Highlighting the importance of target location, imagine Participant A has just responded to a left target; by contrasting how quickly Participant B then responds to a left versus a right target, we can obtain a measure of sIOR, indexing whether the spatial location of Participant A’s attention subsequently affects Participant B’s attention. The authors found IOR and sIOR effects of roughly the same magnitude, indicating that physically responding to a target in the same location twice and responding to a target location one’s partner had just responded to both lead to slowed response times (Welsh et al., 2005). Variants of this task have been used to determine that attention can be modulated when participants are told that a red dot acting as a cue represents a partner’s gaze but not when told the dot is randomly generated (Tufft et al., 2015), and when the gaze is believed to belong to an individual with higher (as compared with lower) perceived social rank (Gobel & Giesbrecht, 2020). One possible explanation for these previous-responder findings could be based on work looking at co-representation, which takes place when an individual shares another person’s mental representation of a task (Sebanz et al., 2005). Shared representations can contribute to joint action while completing cueing tasks, which may facilitate coordination between partners (Loehr et al., 2013) and may explain why personal characteristics of one’s partner could alter attention to the shared task. Taken together, there is evidence that spatial attention can be altered depending on who previously responded in a shared task, and that the personal qualities of one’s partner also influences our spatial attention.
The role of familiarity on attention
Finally, there is some evidence to suggest that level of familiarity can also influence attention (e.g., Chauhan et al., 2017; Dalmaso et al., 2020). For example, Dalmaso and colleagues (2016) set up a two-task study to explore whether joint attention in one task influenced attention for a subsequent gaze cueing task. In the joint attention task, some face identities would look in the same direction as the participant while others would look in the opposite direction; following this, participants completed a gaze cueing task with the different face identities. Results showed that strong gaze cueing effects emerged at short cue–target intervals only for those “joint-attention” faces, suggesting that familiarity, in the sense of a shared gaze location, can elicit larger magnitudes of attention (Dalmaso et al., 2016). Work has also been conducted looking at whether the magnitude of the gaze cueing effect is altered for personally familiar faces versus unfamiliar faces and found that, as compared with unfamiliar faces, participants were slower to look at and respond to targets that were cued by familiar faces, suggesting that familiarity may hold a person’s attention (Chauhan et al., 2017). Turning to manipulating familiarity of another person in dyadic tasks, there is some evidence that “familiarity,” broadly construed, can modulate attention. For example, Nafcha et al. (2020) operationalized “familiarity” in terms of group membership to a religion (in this case, Jewish or Muslim), where in-group pairings were composed of dyads with the same religious affiliation, and out-group pairings were comprised of dyads with different religious affiliations. Participants were seated across from each other with their own computer and keyboard and were asked to respond to the appearance of a target as part of a modified cueing task that also indicated where the other person was looking. The authors found that attention was only modulated by the other person (i.e., producing robust sIOR) when they were an in-group member, suggesting that familiarity in the sense of shared beliefs also affects attention. Furthermore, studies have shown that higher order factors such as mood and perceived relationship between two participants can also modify attention. Doneva et al. (2017) used a similar setup to Welsh et al. (2005) where a pair of participants sat across from each other and completed a cueing task. Critically, Doneva et al. (2017) employed a confederate who either acted positively (smiling and friendly) or negatively (indifferent and distant) toward their partner and found that sIOR was only produced when the confederate acted negatively, providing more evidence that a partner’s personality plays a role in one’s attention. It remains unknown, however, whether experimental task manipulations such as facilitating an unscripted conversation between two strangers before or after a joint task modulates attention.
The present study
Building upon existing literature concerning various social presence factors, the aims of our study were twofold. First, we aimed to determine whether attention to gaze and arrow cues is differentially modulated by a previous responder or based on the previous location of a target. Second, we investigated whether the degree of familiarity between two participants also modulated attention during cueing tasks. Familiarity was operationalized through manipulating whether participant pairs engaged in a short conversation before performing a partner cueing task or after the task. We anticipated that arrow cues would elicit facilitation, but that gaze cues would be more susceptible to our manipulations, leading to muted cueing effects. This prediction is based on some work showing smaller cueing effects for gaze than arrows (e.g., Bonato et al., 2008; Langdon & Smith, 2005; Lockhofen et al., 2014), that gaze cueing is only reliable for one hemifield rather than two (Kingstone et al., 2000; Marotta et al., 2012a, 2012b, and that higher order mentalizing processes may affect gaze cueing but not arrow cueing (e.g., Kawai, 2011). Regarding familiarity, we anticipate observing muted cueing effects for participants who are more familiar with the other participant. As those participants will have shared social information, we suspect that they may be more likely to pay attention to the other person’s responses, resulting in muted attention to the face on-screen.
Methods
Participants
Two hundred and fifty-two undergraduate students were recruited from a Psychology department Research Participation program. Participants were excluded if partners knew each other (i.e., if they rated their familiarity with their partner at least 5 out of 10 on the pretest partner scale, n = 14), resulting in 238 participants for data analysis (female–female pairs: n = 57 male–male pairs: n = 16, female–male pairs: n = 46; age range: 17–28 years, M = 19.5 years, SD = 2.19). Based on calculations by Gobel and Giesbrecht (2020), at least 45 participants were required per between-subjects factor in order to have 80% power to detect a medium-sized effect (d = 0.5), thus, for our design with two conditions in each of the cue type and familiarity between-subjects factors, at least 180 participants were required. Participants received 1 course credit, equivalent to 2% of their overall grade, for their time. The research procedure was reviewed and approved by the University Research Ethics Board, and the procedures used in the study adhere to the tenets of the Declaration of Helsinki. All participation occurred in the lab, and participants were assigned to each condition at random. Participants provided informed consent, signed up in pairs, and, in the event only one participant signed up, various lab volunteers filled in (n = 21; for three of these sessions, the same lab volunteer sat in).
Apparatus and stimuli
Eighteen-inch cathode ray tube (CRT) monitors were used to present the task, executed via MATLAB (The MathWorks, Natick, MA, USA) with Psychtoolbox-3 (Brainard, 1997) on Windows PCs.
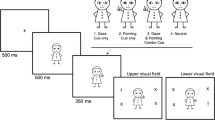
Figure 1 illustrates the social and nonsocial stimuli along with the task sequence. The social cue was a greyscale picture of one of two faces (one female, one male; with similar age (female = 23.7, male = 20.8) and attractiveness scores (female = 3.36, male = 3.65)) depicting a neutral expression, displayed on a white background. Faces were obtained from the Chicago Face Database (Version 2.0.3; Ma et al., 2015). Faces were displayed at 6.6° visual angle (VA; H) × 5.2°W VA, and pupils were 0.4° VA looking left or right, which acted as the directional attention cue. The nonsocial cue was an arrow pointing left or right (3.6°VA × 6.1°VA). The response targets were black capital letters T (1.3° VA × 1.1° VA) and L (1.3° VA × 0.9° VA). The target appeared 5° VA to the left or right of central fixation. Participants sat approximately 60 cm away from the screen.
Example task sequence for the social (a) and nonsocial (b) conditions. Trials began with a fixation screen that displayed either a plus sign or a horizontal line for 1,000 ms. Next, the face with averted gaze or arrow head and tail was displayed to cue a left or right location. After a variable cue–target interval, the target (a capital letter T or L) was displayed to the left or right of the cue, and participants made a discrimination response. Both the cue and target were displayed until response or 1,500 ms had passed. Cue direction was not predictive of upcoming target location, and the face identity in the social condition was pseudorandomly presented to appear equally in all conditions. Stimuli are not drawn to scale
Design
Participants first completed a cueing task with a partner, then solo (seeing either the gaze or arrow cue for both tasks), along with a 5-minute conversation. Since the purpose of this study was to investigate social presence and attention, we did not analyze the data from the individual task. During the conversation, participants were instructed to discuss their best and worst birthday experiences and then list a few aspects of each experience down on a sheet of paper. The conversation was meant to allow participants to chat and get to know one another, thus increasing familiarity. Roughly half of the participants engaged in a conversation with their partner first (n = 120) followed by the cueing task, while the other participants completed the partner cueing task first (n = 118), followed by the conversation. Before and after the conversation participants rated the extent to which they knew their partner (on a scale from 1 to 10), to ensure our familiarity manipulation worked. Roughly half of the participants saw the gaze cue (n = 120), while the rest saw the arrow cue (n = 118). Thus, this mixed design study included cue type (gaze, arrow) and familiarity (conversation first, conversation second) as between-subjects factors, and cue–target interval (100, 400, 700 ms) and cue validity (cued, uncued) as within-subjects factors. Participants completed three blocks of 144 trials, for a total of 432 trials.
Procedure
Participants shared a screen and keyboard. The cueing task ran with either a gaze or an arrow cue (see Fig. 1). Following a fixation screen, the cue appeared on the screen and participants were asked to fix their gaze on the center of the display. Next, one of two potential target letters appeared (a T or an L) to the left or right of the cue, and each participant was assigned one letter for which to respond. Participant side (sitting on left or right) and target response (T or L) were counterbalanced across sessions, with the participant on the left pressing the “z” key and the participant on the right pressing the “?” key. Participants were told that the two targets had an equal chance of appearing, and that they were equally likely to appear to the left or right of the cue. For example, a pair of participants sharing a computer and keyboard would be presented with an arrow cue. Subsequently, each participant would respond to the appearance of their designated letter (a T or an L). RTs were measured from target onset and based on keyboard press. Data are available through emailing the corresponding author.
Results
Data handling
As per typical conventions for discrimination cueing tasks, no response trials, response times faster than 150 ms or slower than 1,500 ms, along with incorrect responses (i.e., wrong key pressed) were removed from the data, resulting in less than 1% of trials removed. Inclusion criteria dictated that accuracy in the partner cueing task for each participant needed to be greater than chance (i.e., 50%). No participant was excluded for performance factors. Overall response accuracy for the partner cueing task was 95%. The data were coded based on prior trial, so that the previous responder (i.e., on trial n − 1) could either be the same or different as the responder on trial n, and the previous target location could either be the same or different as the target location on trial n.
First, we ran an omnibus mixed effects analysis or variance (ANOVA), with cue type (gaze, arrow) and familiarity (strangers, acquaintances) as between-subjects factors, and previous responder (same, different), previous location (same, different), cue validity (cued, uncued) and cue–target interval (100 ms, 400 ms, 700 ms) as within-subjects factors.Footnote 1 Following this, we calculated the cueing effect (uncued RTs − cued RTs) and ran two follow-up ANOVAs, split by previous responder, to further explore the interactions found in the omnibus ANOVA. Finally, we analyzed subjective measures of familiarity, comparing the pre- and postfamiliarity ratings across the two familiarity conditions.
Omnibus ANOVA: The effect of cue type and familiarity on the cueing effect
The repeated-measures ANOVA revealed main effects of familiarity, F(1, 234) = 6.3, p < .05, ηp2 = .03, previous responder, F(1, 234) = 11.3, p < .01, ηp2 = .046, previous location, F(1, 234) = 23.5, p < .001, ηp2 = .091, cue–target interval, F(2, 468) = 396.6, p < .001, ηp2 = .63, and cue validity, F(1, 234) = 32.6, p < .001, ηp2 = .12, with faster response times for those who did not previously have a conversation first, when the same person was responding for a second time, responding to the opposite location as the previous trial, for longer cue–target intervals, and for cued trials. The key question was which factors, if any, affected spatial attention; thus, next we report all interactions including the factor cue validity. We found two-way interactions between previous responder and cue validity, F(1, 234) = 6.4, p < .05, ηp2 = .027, and cue–target interval and cue validity, F(2, 468) = 5.7, p < .01, ηp2 = .024, with smaller magnitudes of cueing when the previous responder was a different participant, and larger magnitudes of cueing with increased cue–target times. In addition, we found a four-way interaction between previous location, cue–target interval, cue validity, and cue type, F(2, 468) = 4.6, p < .05, ηp2 = .019 (see Fig. 2, which we unpacked in follow-up analyses below).Footnote 2
Cueing effects (i.e., uncued RTs − cued RTs) are depicted, as a function of previous responder (same, different), previous location (same, different), familiarity (stranger, acquaintance), cue type (gaze, arrow), and cue–target interval (100, 400, 700 ms). Error bars denote standard error of the difference between the means
Analyses split by previous responder
To further explore the data, we conducted two follow-up ANOVAs, one for the same previous responder and one for the opposite previous responder, each with cue type, previous location, and cue–target interval as factors, collapsed across familiarity. To further reduce the number of factors, we calculated the magnitude of the cueing effect (subtraction of the cued RT from the uncued RT), which served as the new dependent variable.
When participants responded twice in a row (same previous responder), we found an interaction between previous location and cue type, F(1, 234) = 6.8, p < .05, ηp2 = .028, in that while the magnitude of cueing was larger for arrow cues when responding to the same location (13.5 ms) as compared with the opposite location (3.9 ms), the magnitude of cueing for gaze cues was comparable across the same (6.0 ms) and opposite (9.3 ms) locations (all other Fs < 2.5, ps > .08).
When the previous responder switched across trials (different previous responder), we found main effects of cue–target interval, F(2, 468) = 4.9, p < .01, ηp2 = .02, and cue type, F(1, 234) = 3.9, p = .05, ηp2 = .016, as the cueing effects increased as the cue–target time lengthened, and was larger for arrow (6.1 ms) as compared with gaze (0.7 ms) cues. Finally, we found an interaction between previous location, cue–target interval, and cue type, F(2, 468) = 5.4, p < .01, ηp2 = .023, revealing reversed cueing effects for gaze cues but not arrow cues, and an opposite pattern of cueing effect magnitudes across the two cue types and the cue–target intervals (see Fig. 3).
Across our analyses, the factor familiarity did not influence attention beyond varying overall speed, which could be an indication that our manipulation did not affect familiarity. To check this hypothesis, we conducted analyses to determine whether participants level of familiarity increased from the beginning to the end of the experimental session regardless of completing a conversation before or after the computer task. Specifically, we conducted two repeated-measures ANOVAs, one for gaze cues and one for arrow cues, as a function of partner rating (pre, post) and familiarity (strangers, acquaintances). Partner ratings were missing from two participants who were assigned to the gaze cue condition and four participants for the arrow cue condition, resulting in n = 118 for gaze cues and n = 114 for arrow cues.
For gaze cues, we found a main effect of partner rating, F(1, 116) = 353.0, p < .0001, ηp2 = .75, with higher familiarity scores after completing the task (3.6) as compared with beforehand (1.1); however, there was no main effect or interaction with familiarity (Fs < 1, ps > .5). For arrow cues, we again found a main effect of partner rating, F(1, 112) = 377.0, p < .0001, ηp2 = .77, again yielding higher ratings after completing the task (3.3) versus before (1.0). Again, there was no main effect, F(1, 112) = 3.4, p = .07, or interaction with familiarity, F(1, 112) = 3.4, p = .07, suggesting that the mere act of completing a computer task with another person leads to increased feelings of familiarity, regardless of participating in a conversation with them or not.
In sum, the results from the study demonstrated that gaze cues were more susceptible to our manipulations than arrow cues. Notably, when the responder changed from trial to trial (i.e., different responder), gaze cues had muted cueing effects compared with arrow cues.
Discussion
We sought to answer whether two social presence factors (previous responder and familiarity) affected attention to gaze and arrows when completing a two-person task. When looking at the effect of previous responder, we found two key findings. When participants responded twice in a row, arrow cues yielded larger cueing effects for targets appearing in the same location as compared with the opposite location, while gaze cues elicited similar cueing effects for both locations. In contrast, when participants responded after their partner a different pattern emerged, with arrow cues producing the largest cueing effects earlier when responding to the same location as compared with the opposite location, and gaze cues showing muted or reversed gaze cueing effects at early cue–target intervals and only producing intact gaze cueing at later cue–target intervals. When looking at the effect of familiarity, we found that although the acquaintance group was slower overall during the task, there was no effect of familiarity on cueing effect magnitudes, and in fact one’s subjective level of familiarity increased across both the stranger and acquaintance groups. We now discuss the main implications of this work.
Same responder
When participants responded twice in a row, mimicking typical cueing task procedures, we found a surprising data pattern for arrow cues in that there was a reduced cueing effect when the target appeared in the opposite location as the previous trial. There are two main considerations based on this finding. One consideration pertains to the effect that selection history plays in shaping attention (e.g., Awh et al., 2012). Selection history encompasses one’s prior experiences, perhaps even at the level of the previous trial; in the current study, that selection history seemed to manifest as larger cueing effects when responding twice in a row to the same location, whereas responding to a new location reduced the cueing effect. One potential reason that specifically nonsocial attention was affected in this condition is a proposal put forth by Ristic and colleagues (Ristic & Kingstone, 2012; Ristic & Landry, 2015; Ristic et al., 2012), who state that the highly automatic-looking nature of arrow cueing could be due to overlearning the contingencies between the direction an arrow points and a target in the environment, leading to fast, automatic-like orienting that they term automated symbolic orienting. In contrast, we encounter averted gaze cues daily that are not linked to a specific target outcome, rendering their “utility” for navigating the environment less tightly linked (e.g., Gallup et al., 2012, found only 27% of passersby followed the upward gaze of a crowd). Perhaps cues that are more useful in everyday life generate larger selection history biases, which could explain our findings. Future work should systematically investigate the relationship between cue utility and strength of selection history on performance. The second consideration is with regards to the implications our findings place on the ways in which researchers analyze cueing paradigms. Often, cueing task analyses do not account for any potential effect of previous trial location on spatial attention (but see Dodd & Pratt, 2007; Jongen & Smulders, 2007, for examples of cueing tasks with previous-trial analyses). One study by Qian et al. (2012) looked at whether different previous trial factors affected attention to arrows and found that while previous target location did not matter, previous cue validity did; if the cue was invalid on trial n − 1, participants showed a smaller cueing effect on the current trial. It is thus possible that other prior work employing the cueing task with arrows may have also found similar data patterns of larger cueing effects when responding to the same location twice in a row as compared with different locations, however as typical analyses do not distinguish between those two cases, results were not reported. There is some evidence from the literature on reward that previous trial configurations alter attention capture (e.g., Hickey et al., 2010), thus, future work should keep this in mind when designing and analyzing their work.
Different responder
Rather than look at how overall performance is affected when completing a task solo versus with a partner (Brennan & Enns, 2015), we specifically focused on how attention is affected from one trial to the next across partners and found modulations of the cueing effect for both arrow and gaze cues, albeit more pronounced for gaze cues. Arrow cues yielded typical cueing effects that grew in magnitude with longer cue–target intervals, although the numerical values for the early cueing effects were close to zero. For gaze cues, however, cueing effects were abolished or reversed at early cue–target intervals, similar to prior findings investigating sIOR with simple luminance changes acting as spatial cues (Skarratt et al., 2010; Welsh et al., 2005). While prior work has demonstrated modulations in gaze cueing magnitudes based on factors including which hemifield contained the cue and target (Kingstone et al., 2000; Marotta, Lupiàñez, & Casagrande, 2012a), or whether there were distractors on-screen (Fan et al., 2018), our work shows that gaze cueing is also affected based on what happened in the previous trial, both regarding who responded as well as where the target appeared. To the best of our knowledge, there have not been other studies who have looked at the effect of previous responder on a joint gaze cueing task. We did find one conference proceeding that looked at previous trial effects in a solo gaze cueing task, where the authors found that the largest cueing effects occurred when the previous trial was a cued trial at a long cue–target interval (Qian et al., 2017). When looking at paired cueing tasks with abrupt onsets rather than gaze cues, some work suggests that attention varies due to “social” factors such as social rank. For instance, prior research shows that larger magnitudes of sIOR occurred when participants believed their partner’s gaze was controlling the location of a cue rather than a computer (Tufft et al., 2015), or when participants believed their partner was of higher social rank than them (Gobel & Giesbrecht, 2020; Experiment 2). With the rise of dyadic and group-based attention research (Schilbach et al., 2013; Wahn et al., 2018), researchers will need to be mindful of not only how individual variability affects solo attention (e.g., Bayliss & Tipper, 2005; Hayward & Ristic, 2017; Laidlaw et al., 2011), but also how the interaction of those individual personality traits may uniquely shape joint attention.
Familiarity and attention
In contrast to our original hypothesis, we did not observe modulations of spatial attention based on familiarity. Instead, we found that engaging in a conversation led to a global slowing of response times. While we cannot explicitly state why this may be the case, one potential reason for our findings could be that participants who got to know each other first perceived their partner as less of a threat and therefore felt less anxiety or pressure to perform well, leading to slower responses overall. The link between anxiety and attention has support in the literature (e.g., Bar-Haim et al., 2007), such as work by Süßenbach and Schönbrodt (2014), who asked participants to complete a modified gaze cueing task, where some faces were introduced as trustworthy and others not trustworthy, and measured participant levels of trait-anxiety. They found that the high-anxious group was unable to effectively prioritize attention towards trustworthy faces, suggesting anxiety can interfere with attention. Another potential reason for differences in response times across our familiarity manipulation could be based on the requirement that participants sit side by side; prior work has demonstrated that sitting close to a stranger may induce discomfort (e.g., Aiello, 1987), and the inability to move away from said stranger in our study could have resulted in participants shifting their attention away from the stranger (Szpak et al., 2016), or potentially attempting to complete the task as quickly as possible. Although we are unable to fully disentangle why increased familiarity resulted in slower global response times, future research may find value in further investigating how degree of familiarity between partners may modulate computer-task-based attention, whether the influence of perceived threat and anxiety plays a role, as well as how other social factors, such as physical proximity, may affect attention.
Turning to our measure of subjective familiarity, we found evidence suggesting that participants’ subjective feelings of familiarity increased even when passively sitting beside another person while completing a shared task. While this finding suggests that our familiarity manipulation was not strong enough to result in higher familiarity ratings for the acquaintance group over the stranger group, it does suggest that familiarity increases even in passive situations such as sitting beside another person while completing a shared task; future work should be mindful of this potentially undesirable outcome when investigating performance during a shared task. Specifically, in cases where changes in familiarity are not desired, researchers should take steps to physically separate the two participants or minimize the duration of the task.
Another consideration regarding the familiarity manipulation is the measurements we collected. Specifically, although our experiment did not find an effect of familiarity on resultant cueing effects, it may be worthwhile to explore how familiarity was registered using different measures such as electroencephalography (EEG), as previous studies have found specific neural activity for social contexts similar to our familiarity manipulation (Rolison et al., 2020; Spapé et al., 2013). For example, there is some evidence that the mere presence of another individual modulates brain wave activity (Rolison et al., 2020), leaving the question open as to whether completing a joint task with individuals of differing familiarity affects underlying neural activity.
In sum, we found evidence of muted and reversed cueing effects as a function of previous responder, especially for gaze cues. While attention to arrows may be driven by their utility, affecting their usefulness in directing attention to the opposite location in the same responder condition, attention to gaze was modulated by previous responder identity. This dissociation between attention to gaze and arrow cues further suggests that attention is differentially modulated depending on various features including the cue itself (see also Hayward & Ristic, 2015, for another example of a dissociation between social and nonsocial cues). Regarding familiarity, our manipulation affected global speed rather than cueing effects; however, we did find evidence that the mere act of completing the task with another person in close proximity for an extended period of time led to increases in subjective familiarity. Taken together, our data suggest that both social presence factors affect some aspect of performance, with social attention being especially susceptible to the attention and actions of others.
Notes
Although there is little reason to believe that performance was significantly altered based on using an RA as a partner, we also ran this ANOVA removing all pairs who participated with an RA (n = 21 pairs). The ANOVA revealed virtually identical patterns of significant and nonsignificant results. The only numerical difference was the four-way interaction between previous location, cue–target interval, cue validity, and cue type, F(2, 388) = 2.54, p = .08.
For completion’s sake, here are the additional significant interactions that did not include the factor cue validity. Namely, we found two two-way interactions between previous responder and previous location, F(1, 234) = 158.4, p < .001, ηp2 = .40, and previous responder and cue-target interval, F(2, 468) = 3.4, p < .05, ηp2 = .014, where responses were faster when the same participant responded to the same location or when the opposite responder responded to the opposite location, and a changing foreperiod effect (i.e., slope of response times; see Hayward & Ristic, 2013a for a thorough explanation) for same versus different prior responder. We also found a four-way interaction between previous responder, previous location, cue–target interval, and cue type, F(2, 468) = 4.6, p < .05, ηp2 = .019; responses were slower for arrow cues than gaze cues, especially when the opposite previous responder was responding to the same target location.
References
Aiello, J. R. (1987). Human spatial behavior. Handbook of Environmental Psycholoy, 1, 389–504.
Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16(8), 437–443.
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van IJzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. https://doi.org/10.1037/0033-2909.133.1.1
Bayliss, A. P., & Tipper, S. P. (2005). Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. British Journal of Psychology, 96, 95–114.
Birmingham, E., Bischof, W. F., & Kingstone, A. (2008). Social attention and real-world scenes: The roles of action, competition and social content. The Quarterly Journal of Experimental Psychology, 61(7), 986–998.
Bonato, M., Priftis, K., Marenzi, R., & Zorzi, M. (2008). Modulations of hemispatial neglect by directional and numerical cues in the line bisection task. Neuropsychologia, 46, 426–433.
Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10(4), 433–436.
Brennan, A. A., & Enns, J. T. (2015). When two heads are better than one: Interactive versus independent benefits of collaborative cognition. Psychonomic Bulletin & Review, 22(4), 1076–1082. https://doi.org/10.3758/s13423-014-0765-4
Capozzi, F., & Ristic, J. (2019). How attention gates social interactions. Annals of the New York Academy of Sciences, 1426(1), 179–198. https://doi.org/10.1111/nyas.13854
Chauhan, V., di Oleggio, V., Castello, M., Soltani, A., & Gobbini, M. I. (2017). Social saliency of the cue slows attention shifts. Frontiers in Psychology, 8, 738. https://doi.org/10.3389/fpsyg.2017.00738
Cole, G. G., Skarratt, P. A., & Kuhn, G. (2016). Real person interaction in visual attention research. European Psychologist, 21(2), 141–149.
Dalmaso, M., Edwards, S. G., & Bayliss, A. P. (2016). Re-encountering individuals who previously engaged in joint gaze modulates subsequent gaze cueing. Journal of Experimental Psychology: Learning, Memory, and Cognition, 42(2), 271–284. https://doi.org/10.1037/xlm0000159
Dalmaso, M., Alessi, G., Castelli, L., & Galfano, G. (2020). Eye contact boosts the reflexive component of overt gaze following. Scientific Reports, 10, 4777. https://doi.org/10.1038/s41598-020-61619-6
Dodd, M. D., & Pratt, J. (2007). The effect of previous trial type on inhibition of return. Psychological Research, 71, 411–417. https://doi.org/10.1007/s00426-005-0028-0
Doneva, S. P., Atkinson, M. A., Skarratt, P. A., & Cole, G. G. (2017). Action or attention in social inhibition of return? Psychological Research, 81, 43–54. https://doi.org/10.1007/s00426-015-0738-x
Downing, P. E., Dodds, C. M., & Bray, D. (2004). Why does the gaze of others direct visual attention? Visual Cognition, 11(1), 71–79. https://doi.org/10.1080/13506280344000220
Driver, J., Davis, G., Ricciardelli, P., Kidd, P., Maxwell, E., & Baron-Cohen, S. (1999). Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6(5), 509–540.
Fan, L., Yu, H., Zhang, X., Feng, Q., Sun, M., & Xu, M. (2018). Conflict tasks of different types divergently affect the attentional processing of gaze and arrow. i-Perception, 9(3), 1–19. https://doi.org/10.1177/2041669518771713
Fischer, M. H., Castel, A. D., Dodd, M. D., & Pratt, J. (2003). Perceiving numbers causes spatial shifts of attention. Nature Neuroscience, 6, 555–556.
Foulsham, T., Walker, E., & Kingstone, A. (2011). The where, what and when of gaze allocation in the lab and the natural environment. Vision Research, 51, 1920–1931.
Friesen, C. K., & Kingstone, A. (1998). The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review, 5(3), 490–495. https://doi.org/10.3758/BF03208827
Friesen, C. K., Ristic, J., & Kingstone, A. (2004). Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance, 30(2), 319–329.
Gallup, A. C., Hale, J. J., Sumpter, D. J. T., Garnier, S., Kacelnik, A., Krebs, J. R., & Couzin, I. D. (2012). Visual attention and the acquisition of information in human crowds. Proceedings of the National Academy of Sciences of the United States of America, 109(19), 7245–7250.
Gobel, M. S., & Giesbrecht, B. (2020). Social information rapidly prioritizes overt but not covert attention in a joint spatial cueing task. Acta Psychologica, 211, 103188. https://doi.org/10.1016/j.actpsy.2020.103188
Gobel, M. S., Kim, H. S., & Richardson, D. C. (2015). The dual function of social gaze. Cognition: International Journal of. Cognitive Science, 136, 359–364. https://doi.org/10.1016/j.cognition.2014.11.040
Hayes, S. J., Hansen, S., & Elliott, D. (2009). Between-person effects on attention and action: Joe and Fred revisited. Psychological Research, 2010(74). https://doi.org/10.1007/s00426-009-0250-2
Hayward, D. A., & Ristic, J. (2013a). Measuring attention using the Posner cuing paradigm: The role of across and within trial target probabilities. Frontiers in Human Neuroscience, 7, 205. https://doi.org/10.3389/fnhum.2013.00205
Hayward, D. A., & Ristic, J. (2013b). The uniqueness of social attention revisited: working memory load interferes with endogenous but not social orienting. Experimental Brain Research, 231(4), 405–414.
Hayward, D. A., & Ristic, J. (2015). Exposing the cuing task: the case of gaze and arrow cues. Attention, Perception, & Psychophysics, 77(4), 1088–1104.
Hayward, D. A., & Ristic, J. (2017). Feature and motion-based gaze cuing is linked with reduced social competence. Scientific Reports, 7, 44221. https://doi.org/10.1038/srep44221
Hayward, D. A., Voorhies, W., Morris, J. L., Capozzi, F., & Ristic, J. (2017). Staring reality in the face: A comparison of social attention across laboratory and real world measures suggests little common ground. Canadian Journal of Experimental Psychology, 71(3), 212–225. https://doi.org/10.1037/cep0000117
Hickey, C., Chelazzi, L., & Theeuwes, J. (2010). Reward changes salience in human vision via the anterior cingulate. The Journal of Neuroscience, 30(33), 11096–11103.
Jongen, E. M. M., & Smulders, F. T. Y. (2007). Sequence effects in a spatial cueing task: Endogenous orienting is sensitive to orienting in the preceding trial. Psychological Research, 71, 516–523. https://doi.org/10.1007/s00426-006-0065-3
Kawai, N. (2011). Attentional shift by eye gaze requires joint attention: Eye gaze cues are unique to shift attention. Japanese Psychological Research, 53(3), 292–301.
Kingstone, A., Friesen, C. K., & Gazzaniga, M. S. (2000). Reflexive joint attention depends on lateralized cortical connections. Psychological Science, 11(2), 159–166.
Laidlaw, K. E. W., Foulsham, T., Kuhn, G., & Kingstone, A. (2011). Potential social interactions are important to social attention. Proceedings of the National Academy of Sciences of the United States of America, 108(14), 5548–5553.
Langdon, R., & Smith, P. (2005). Spatial cueing by social versus nonsocial directional signals. Visual Cognition, 12(8), 1497–1527.
Lassalle, A., & Itier, R. J. (2015). Autistic traits influence gaze-oriented attention to happy but not fearful faces. Social Neuroscience, 10(1), 70–88.
Lockhofen, D. E. L., Gruppe, H., Ruprecht, C., Gallhofer, B., & Sammer, G. (2014). Hemodynamic response pattern of spatial cueing is different for social and symbolic cues. Frontiers in Human Neuroscience, 8, 912. https://doi.org/10.3389/fnhum.2014.00912
Loehr, J. D., Sebanz, N., & Knoblich, G. (2013). Joint action: From perception-action links to shared representations Action science: Foundations of an emerging discipline (p. 333). MIT Press.
Ma, D. S., Correll, J., & Wittenbrink, B. (2015). The Chicago Face Database: A free stimulus set of faces and norming data. Behavior Research Methods, 47(4), 1122–1135. https://doi.org/10.3758/s13428-014-0532-5
Marotta, A., Lupiàñez, J., & Casagrande, M. (2012a). Investigating hemispheric lateralization of reflexive attention to gaze and arrow cues. Brain and Cognition, 80, 361–366.
Marotta, A., Lupiàñez, J., Martella, D., & Casagrande, M. (2012b). Eye gaze versus arrows as spatial cues: Two qualitatively different modes of attentional selection. Journal of Experimental Psychology: Human Perception and Performance, 38(2), 326–335.
Marotta, A., Lupiàñez, J., Román-Caballero, R., Narganes-Pineda, C., & Martín-Arévalo, E. (2019). Are eyes special? Electrophysiological and behavioural evidence for a dissociation between eye-gaze and arrows attentional mechanisms. Neuropsychologia, 129, 146–152. https://doi.org/10.1016/j.neuropsychologia.2019.03.017
Nafcha, O., Morshed-Sakran, A., Shamay-Tsoory, S., & Gabay, S. (2020). The effect of co-actor group membership on the social inhibition of return effect. Acta Psychologica, 208, 1–8. https://doi.org/10.1016/j.actpsy.2020.103119
Niehorster, D. C., Cornelissen, T., Holmqvist, K., & Hooge, I. (2019). Searching with and against each other: Spatiotemporal coordination of visual search behavior in collaborative and competitive settings. Attention, Perception, & Psychophysics, 81(3), 666–683. https://doi.org/10.3758/s13414-018-01640-0
Pereira, E. J., Birmingham, E., & Ristic, J. (2020). The eyes do not have it after all? Attention is not automatically biased towards faces and eyes. Psychological Research, 84, 1407–1423. https://doi.org/10.1007/s00426-018-1130-4
Posner, M. I. (1980). Orienting of attention. The Quarterly Journal of Experimental Psychology, 32(1), 3–25. https://doi.org/10.1080/00335558008248231
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. Erlbaum.
Qian, Q., Shinomori, K., & Song, M. (2012). Sequence effects by non-predictive arrow cues. Psychological Research, 76, 253–262. https://doi.org/10.1007/s00426-011-0339-2
Qian, Q., Wang, X., Song, M., & Wang, F. (2017). Gazes induce similar sequential effects as arrows in a target discrimination task. Paper presented at the IFIP Advances in Information and Communication Technology, Shanghai, China.
Risko, E. F., & Kingstone, A. (2011). Eyes wide shut: implied social presence, eye tracking and attention. Attention, Perception, & Psychophysics, 73, 291–296.
Risko, E. F., Richardson, D. C., & Kingstone, A. (2016). Breaking the fourth wall of cognitive science: Real-world social attention and the dual function of gaze. Current Directions in Psychological Science, 25(1), 70–74. https://doi.org/10.1177/0963721415617806
Ristic, J., & Kingstone, A. (2012). A new form of human spatial attention: Automated symbolic orienting. Visual Cognition, 20(3), 244–264.
Ristic, J., & Landry, M. (2015). Combining attention: a novel way of conceptualizing the links between attention, sensory processing, and behavior. Attention, Perception, & Psychophysics, 77(1), 36–49.
Ristic, J., Friesen, C. K., & Kingstone, A. (2002). Are eyes special? It depends on how you look at it. Psychonomic Bulletin & Review, 9(3), 507–513.
Ristic, J., Landry, M., & Kingstone, A. (2012). Automated symbolic orienting: The missing link. Frontiers in Psychology, 3, 560. https://doi.org/10.3389/fpsyg.2012.00560
Rolison, M. J., Naples, A. J., Rutherford, H. J. V., & McPartland, J. C. (2020). The presence of another person influences oscillatory cortical dynamics during dual brain EEG recording. Frontiers in Psychiatry, 11, 246. https://doi.org/10.3389/fpsyt.2020.00246
Schilbach, L., Timmermans, B., Reddy, V., Costall, A., Bente, G., Schlicht, T., & Vogeley, K. (2013). Toward a second-person neuroscience. Behavioral and Brain Sciences, 36, 393–462. https://doi.org/10.1017/S0140525X12000660
Sebanz, N., Knoblich, G., & Prinz, W. (2005). How to share a task: Corepresenting stimulus–response mappings. Journal of Experimental Psychology: Human Perception and Performance, 31(6), 1234–1246. https://doi.org/10.1037/0096-1523.31.6.1234
Skarratt, P. A., Cole, G. G., & Kingstone, A. (2010). Social inhibition of return. Acta Psychologica, 134, 48–54. https://doi.org/10.1016/j.actpsy.2009.12003
Spapé, M. M., Kivikangas, J. M., Järvelä, S., Kosunen, I., Jacucci, G., & Ravaja, N. (2013). Keep your opponents close: Social context affects EEG and fEMG linkage in a turn-based computer game. PLOS ONE, 8(11), e78795. https://doi.org/10.1371/journal.pone.0078795
Süßenbach, F., & Schönbrodt, F. (2014). Not afraid to trust you: Trustworthiness moderates gaze cueing but not in highly anxious participants. Journal of Cognitive Psychology, 26(6), 670–678.
Szpak, A., Nicholls, M. E. R., Thomas, N. A., Laham, S. M., & Loetscher, T. (2016). “No man is an island”: Effects of interpersonal proximity on spatial attention. Cognitive Neuroscience, 7(1/4), 45–54. https://doi.org/10.1080/17588928.2015.1048677
Tipples, J. (2002). Eye gaze is not unique: Automatic orienting in response to uninformative arrows. Psychonomic Bulletin & Review, 9(2), 314–318.
Tufft, M. R. A., Gobel, M. S., & Richardson, D. C. (2015). Social eye cue: How knowledge of another person’s attention changes your own. In M. G. P. Bello, M. McShane, & B. Scassellati (Eds.), Proceedings of the cognitive science society. Cogntive Science Society.
Vecera, S. P., & Rizzo, M. (2006). Eye gaze does not produce reflexive shifts of attention: Evidence from frontal-lobe damage. Neuropsychologia, 44(1), 150–159.
Wahn, B., Czeszumski, A., & König, P. (2018). Performance similarities predict collective benefits in dyadic and triadic joint visual search. PLOS ONE, 13(1), 15–19. https://doi.org/10.1371/journal.pone.0191179
Welsh, T. N., Elliott, D., Anson, J. G., Dhillon, V., Weeks, D. J., Lyons, J. L., & Chua, R. (2005). Does Joe influence Fred’s action? Inhibition of return across different nervous systems. Neuroscience Letters, 385, 99–104. https://doi.org/10.1016/j.neulet.2005.05.013
Acknowledgements
We would like to thank Raman Dhaliwal, Jamie Cundal, and members of the VASP lab who acted as RA partners.
Funding
This research was partially funded through internal start-up funds (D.A.H.) and an NSERC Discovery Grant (D.A.H.).
Author information
Authors and Affiliations
Contributions
D.A.H. developed the initial study concept and study design. J.H. implemented the study, completed data collection, and both authors processed the data. D.A.H. conducted the data analyses. J.H wrote the first draft of the manuscript with feedback from D.A.H. Both authors wrote the manuscript and approve the final version.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Approval was obtained from the ethics committee of University C. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ha, J., Hayward, D.A. Are you paying attention to me? The effect of social presence on spatial attention to gaze and arrows. Atten Percept Psychophys 85, 41–51 (2023). https://doi.org/10.3758/s13414-022-02618-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-022-02618-9