Abstract
Visual working memory (VWM) capacity is reduced in older adults. Research has shown age-related impairments to VWM encoding, but aging is likely to affect multiple stages of VWM. In the present study, we recorded the event-related potentials (ERPs) of younger and older adults during VWM maintenance and retrieval. We measured encoding-stage processing with the P1 component, maintenance-stage processing with the contralateral delay activity (CDA), and retrieval-stage processing by comparing the activity for old and new items (old–new effect). Older adults showed lower behavioral capacity estimates (K) than did younger adults, but surprisingly, their P1 components and CDAs were comparable to those of younger adults. This remarkable dissociation between neural activity and behavior in the older adults indicated that the P1 and CDA did not accurately assess their VWM capacity. However, the neural activity evoked during VWM retrieval yielded results that helped clarify the age-related differences. During retrieval, younger adults showed early old–new effects in frontal and occipital areas and a late central–parietal old–new effect, whereas older adults showed a late right-lateralized parietal old–new effect. The younger adults’ early old–new effects strongly resembled an index of perceptual fluency, suggesting that perceptual implicit memory was activated. The activation of implicit memory could have facilitated the younger adults’ behavior, and the lack of these early effects in older adults may suggest that they have much lower-resolution memory than do younger adults. From these data, we speculated that younger and older adults store the same number of items in VWM, but that younger adults store a higher-resolution representation than do older adults.
Similar content being viewed by others
Visual working memory (VWM) is the brief, limited capacity retention of visual information in the absence of visual stimuli. A wealth of research over the past decade has been dedicated to understanding factors that limit the capacity of VWM (Bays & Husain, 2008; Cowan, 2001; Vogel, McCollough, & Machizawa, 2005; Woodman, Vecera, & Luck, 2003; Zhang & Luck, 2008; for a review, see Fukuda, Awh, & Vogel, 2010). Consistently, the healthy aging process has been shown to significantly reduce VWM capacity (Brockmole, Parra, Della Sala, & Logie, 2008; Brown & Brockmole, 2010; Cowan, Naveh-Benjamin, Kilb, & Saults, 2006; Reuter-Lorenz & Sylvester, 2005; Vaughan & Hartman, 2009). How or what aspects of VWM capacity decreases over the course of normal aging remains unclear, but progress toward understanding this problem would be greatly benefited by examining how age affects multiple stages of VWM.
Why does advancing age reduce VWM capacity? This problem has been investigated in the context of two major theories of cognitive aging. One theory proposes that older adults are impaired in the ability to bind separate pieces of information together in a cohesive unit (Naveh-Benjamin, 2000), which is critical during VWM encoding (Wheeler & Treisman, 2002). Studies examining how older adults bind visual features during VWM encoding have reported mixed results, with some studies revealing a binding impairment (Brown & Brockmole, 2010; Cowan et al., 2006) and others revealing intact binding of information (Brockmole et al., 2008; Parra, Abrahams, Logie, & Della Sala, 2009). In contrast, recent event-related potential (ERP) studies have more consistently supported another theory, which proposes that older adults have a reduced ability to inhibit irrelevant information (Hasher & Zacks, 1988). For example, Gazzaley et al. (2008) found that older adults exhibited delayed inhibition of task-irrelevant information, relative to younger adults, during VWM encoding. In a more recent study using an ERP measure of VWM capacity called the contralateral delay activity (CDA), Jost, Bryck, Vogel, and Mayr (2011) found age-related differences in filtering out task-irrelevant information encoded into VWM. Both ERP studies suggest that inhibitory processes during VWM encoding are significantly delayed, but not abolished, during the course of healthy aging.
The studies described above suggest that age-related deficits in the cognitive processes deployed during VWM encoding (i.e., binding and inhibition) lead to reductions in VWM capacity. However, these studies did not directly examine whether older adults’ capacity is in fact reduced. In the present study, we examined VWM for simple colors to investigate whether age-related reductions in VWM capacity would still be observed when feature binding and inhibition during VWM encoding are not required. We also used a relatively long sample duration of 600 ms to further reduce the contribution of known VWM encoding deficits in older adults (Gazzaley et al., 2008; Jost et al., 2011; Vaughan & Hartman, 2009). Additionally, to ensure that participants were specifically using visual encoding processes, we included a verbal memory task and used a psychophysically balanced color set to discourage verbal encoding strategies. Although these methods reduced the need for encoding-related processes (i.e., binding and inhibition), we still monitored potential age-related differences in VWM encoding with the P1 component, which is closely related to sensory encoding (Warbrick, Arrubla, Boers, Neuner, & Shah, 2013). Although we did not predict group-related differences in encoding, the P1 would be sensitive to age-related deficits if they were present (Gazzaley et al., 2008).
Similar to other ERP studies of aging and VWM (Jost et al., 2011; Sander, Werkle-Bergner, & Lindenberger, 2011), we targeted the CDA in order to measure neural activity in older and younger adults during VWM maintenance. The CDA is a well-known online ERP measure of VWM capacity found by having participants view a brief, symmetrical sample display and instructing them to attend to and memorize only one side of the display (Vogel & Machizawa, 2004). Posterior activity time-locked to the sample is then used to index the contents of VWM while controlling for perceptually evoked activity. Specifically, the activity ipsilateral to the attended side of the display, presumably related to information that is perceived but not memorized, is subtracted from the activity contralateral to the attended side, presumably related to information that is perceived and memorized. The CDA is sustained after the display has offset, increases negatively and linearly with each item stored in VWM, and is highly predictive of individual VWM capacity (Vogel & Machizawa, 2004). Since the CDA is strongly correlated with individual capacity and since older adults typically show lower behavioral performance than younger adults do, we hypothesized that older adults would show a lower CDA and behavioral capacity estimates than would younger adults.
Although the CDA has been shown to correlate strongly with individual differences in behavioral estimates of VWM capacity, it is likely that age-related changes to the VWM process extend beyond the maintenance stage that the CDA measures. Relatively few studies have focused on neural activity during VWM retrievalFootnote 1 (Bledowski, Kaiser, Wibral, Yildiz-Erzberger, & Rahm, 2012; Danker et al., 2008; Hyun, Woodman, Vogel, Hollingworth, & Luck, 2009; Mathes, Schmiedt, Schmiedt-Fehr, Pantelis, & Basar-Eroglu, 2012), and to our knowledge there has not been any investigation into age-related differences in the neural mechanisms of VWM retrieval. However, several studies have compared older and younger adults in long-term memory retrieval. These studies were conducted under the framework of the dual-process model of long-term memory recognition, which proposes two distinct forms of recognition: familiarity, a vague sense that an item was previously encountered, and recollection, a vivid reexperiencing of encountering the item in a specific context (Yonelinas, 2002). Familiarity has been related to an early positive old–new effect in midfrontal areas (FN400), whereas recollection has been related to the late parietal old–new effect (or late positive complex, LPC; Ally & Budson, 2007; Rugg & Curran, 2007; Rugg et al., 1998; Woodruff, Hayama, & Rugg, 2006; Yonelinas, Aly, Wang, & Koen, 2010). Several recognition studies have shown that older adults exhibit intact familiarity but impaired recollection (Ally, Waring, et al., 2008; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Howard, Bessette-Symons, Zhang, & Hoyer, 2006; Yonelinas et al., 2007).
The approaches to understanding how aging affects long-term memory retrieval described above can be beneficially applied to investigating age-related capacity reductions in VWM, especially in light of emerging evidence that common neural mechanisms may serve retrieval from long-term memory and VWM. For example, Danker et al. (2008) elicited the FN400 and the LPC during retrieval from working memory. More recently, Bledowski et al. (2012) used magnetoencephalography (MEG) to examine VWM retrieval processes and found event-related fields (ERFs) analogous to the FN400. This recent work provides a compelling rationale to examine VWM retrieval with analyses borrowed from the long-term memory literature. We investigated VWM retrieval by targeting neural activity related to retrieval cues (probe) resembling items in memory (old) or not in memory (new) in anticipation of eliciting the FN400 and the LPC. In accordance with previous work showing intact familiarity processes in older adults and common neural mechanisms between long-term memory and working memory, we hypothesized that older and younger adults would both show the FN400, but older adults would show a reduced LPC relative to younger adults.
Method
Participants
The participants were 39 members of the Nashville community, including 21 older adults (14 females, seven males) and 18 younger adults (14 females, four males). The older adults had a mean age of 66.6 years (SD = 4.89) and a mean education of 14.3 years (SD = 2.38), whereas the younger adults had a mean age of 22.9 years (SD = 1.89) and a mean education of 15.6 years (SD = 1.57). For reasons related to controlling for potential confounding eye movements (explained below), we excluded a number of participants from the final data set, which ultimately retained 11 older adults (mean age = 67.45 years, SD = 5.78; mean education = 14.45 years, SD = 1.97) and 13 younger adults (mean age = 22.93 years, SD = 2.08; mean education = 15.6 years, SD = 1.75). The neuropsychological performance for all older adults can be seen in Table 1. Participants were compensated $25/h for their participation. This study was approved by the Behavioral and Social Sciences Committee of the Vanderbilt University Institutional Review Board.
Stimuli and procedure
The primary stimuli were colored circles spanning 1.5 deg of visual angle (º) in diameter appearing on a gray background. The colored circles were randomly positioned on points of a 5 × 5 invisible grid spanning 4º × 4º, excluding positions in the central column of the grid. The position of each dot was then jittered to degrade the perception of a grid. The colors were equidistant points on a circular pattern centered around the white point in 1976 CIE L*a*b* space (using the following coordinates: Color 1, a* = 35.11, b* = 1.12; Color 2, a* = 30.86, b* = 24.14; Color 3, a* = 18.12, b* = 34.77; Color 4, a* = –2.20, b* = 40.29; Color 5, a* = –19.36, b* = 33.77; Color 6, a* = –33.62, b* = 18.31; Color 7, a* = –35.43, b* = –0.73; Color 8, a* = –26.43, b* = –19.39; Color 9, a* = –17.34, b* = –34.29; Color 10, a* = –3.11, b* = –40.31; Color 11, a* = 20.27, b* = –32.99; Color 12, a* = 33.90, b* = –18.70). The colors and gray background were set to a brightness of L* = 50. Six colors from a randomly selected half (every other color) of the color space could appear in a given trial, ensuring that the minimal perceptual difference between any color in the trial was not too difficult to discriminate. Appearing throughout a trial was a central bull’s eye stimulus spanning 1.5º in diameter. The stimuli were presented with E-Prime 2.0 on a Dell computer with an ASUS flat-screen computer monitor.
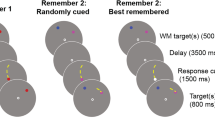
The task involved memorizing two numbers while performing a visual change detection task that required visual working memory. We instructed participants to strive for accuracy but not speed. Each trial began with the appearance of two white digits for 500 ms. These digits were vertically aligned above and below a central bull’s eye that remained visible throughout the trial. We instructed participants to memorize the digits for a memory test at the end of the trial. Following a 500-ms interstimulus interval (ISI), an arrow pointing left or right appeared inside the bull’s eye for 200 ms, followed by another 500-ms ISI. We instructed participants to attend to the side of the screen indicated by the arrow when the subsequent bilateral display appeared. The bilateral visual sample, containing two, three, or four colored circles in each lateral hemifield, appeared for 600 ms, followed by a 2,000-ms delay. We instructed participants to fixate on the central bull’s eye from the appearance of the arrow cue to the end of the delay. After the delay, a visual probe appeared in the form of one randomly selected circle from the cued hemifield. On half of the trials, the probe was the same color that had previously appeared in its location, whereas on the other half, the probe was a new color that had not previously appeared in the sample. The probe was visible until participants reported the color of the probe as being the “same” or “different” from its original appearance in the sample. A 500-ms ISI followed the offset of the probe, and then a single digit appeared inside the central fixation. This verbal probe was either one of the two digits from the verbal sample or a new, randomly selected digit. The verbal probe was visible until participants reported it to be the “same” or “different,” according to whether it was identical to one of the two digits in the verbal sample. Participants responded to the change detection and verbal memory tasks with a button box. Finally, the central bull’s eye offset for 1,000 ms before the next trial began. A schematic of the procedure is depicted in Fig. 1a. Participants performed 120 trials for each level of the Set Size factor (two, three, or four colors) across five blocks.
(a) Schematic of the trial procedure. (b) Topographies of the average event-related potential activity during the selected time of interest, to calculate the contralateral delay activity (CDA; 1,000–1,600 ms post-sample-onset) for both younger adults (left) and older adults (right). The topographies were used to guide the selection of our spatial regions of interest on the basis of peak negative activity in the posterior regions where the CDA is known to emerge. The topographies depict activity during Set Size 4 trials when the memory sample appeared in the left visual field. Red outlines illustrate the selected regions of interest. Topographies for all conditions can be found in the supplementary materials
EEG recording and analysis
We collected electroencephalographic (EEG) data from participants using an ActiveTwo biopotential measurement system (BioSemi, Amsterdam, Netherlands) consisting of 128 Ag–AgCl active electrodes connected to a head cap in a preconfigured montage, with electrodes being placed in equidistant concentric circles from 10–20 position Cz. We also placed flat-type electrodes on each mastoid process and, to record vertical and horizontal electrooculogram (EOG) activity, below the left eye and on the outer canthus of the left and right eyes. The EEG and EOG activity was amplified with a bandwidth of 0.03–35 Hz (–3-dB points) and digitized at a sampling rate of 256 Hz. Recordings were referenced to the vertex (Cz), but were later re-referenced to the common average montage in order to minimize the effects of reference-site activity and to accurately estimate the scalp topography of the measured electric fields (Ally & Budson, 2007; Curran, DeBuse, Woroch, & Hirshman, 2006; Dien, 1998).
The ERPs were constructed using EMSE Software Suite (Source Signal Imaging, San Diego, CA, USA). The ERPs were time-locked to the stimuli of interest (bilateral visual sample for the CDA analysis and the probe for the old–new analysis) and baseline corrected using the averaged activity from 200 ms prior to the stimulus onset. Electrode channels with excessively noisy activity were replaced with the weighted average of all other signals with heavier weight placed on proximal recording sites. We corrected contamination from eye-blink-related EOG activity using the EMSE Ocular Artifact Correction Tool. The tool builds a spatial filter from artifact-containing data and artifact-free data identified by the user and then uses the filter to remove artifacts from the data. Trials were discarded from the analyses if they contained baseline drift or eye movement greater than 90 μV. Although we used the ocular artifact correction procedure to remove noise related to eye blinks, it may not have removed contamination from saccades that threatened to artificially increase lateralized effects such as the CDA. To remove the contribution of saccade-related activity from the EEG, we excluded participants whose mean horizontal EOG activity exceeded 3 μV (Lins, Picton, Berg, & Scherg, 1993), resulting in the retention of 11 older adults and 13 younger adults. The mean trial counts for the remaining participants are reported in Tables 2 and 3. Greenhouse–Geisser corrections were used for all of the analyses.
The neural components related to encoding (P1) and maintenance (CDA) were obtained from the same ERP waveforms. These ERPs were time-locked to the bilateral visual sample and were constructed using correct trials only. We averaged the ERPs in four spatial regions of interest (ROIs) that divided scalp regions by left–right hemispheres and anterior–posterior areas. The posterior ROIs were established on the basis of where the CDA was the greatest. The ERP topographies revealed a different distribution of set-size-related posterior activity in each group, with the younger adults exhibiting a tighter cluster of posterior negativity than the older adults. On the basis of these topographies, we assembled slightly different posterior ROIs for each group, which can be viewed in Fig. 1b. A complementary analysis of the CDA using ROIs determined a priori was also conducted, and it showed results similar to those yielded using the topography-guided ROIs (see the supplementary materials).
Our analysis of ERPs related to VWM retrieval entailed time-locking the ERPs to the probe onset and examining differences related to previously viewed (“old”) items and novel (“new”) items. To correspond the change detection paradigm with long-term memory research on “old–new effects,” items to which participants reported “same” were considered “old,” and those reported as “different” were considered “new.” Old–new effects were qualified as “positive” or “negative,” depending on whether the old–new contrast produced a positive or negative value, respectively. The ERP data from correct trials only were averaged in ten spatial ROIs designed to detect old–new effects in components commonly found in studies of long-term memory recognition, including: left anterior inferior (LAI), central anterior inferior (CAI), right anterior inferior (RAI), left anterior superior (LAS), right anterior superior (RAS), left posterior superior (LPS), central posterior superior (CPS), right posterior superior (RPS), left posterior inferior (LPI), and right posterior inferior (RPI). Participants were allowed to move their eyes during the probe, so the data were collapsed across visual hemifields, since any lateralized effects would potentially be confounded by eye movement artifacts.
Results
Behavioral capacity estimates
We constructed behavioral capacity estimates (K) with the formula used by Cowan (2001): K = N × (hit rate + correct rejection rate – 1), where K is the capacity estimate and N is the number of target items in a trial. We constructed K estimates for each condition and averaged them across visual hemifields. Figure 2a depicts the means and standard errors and illustrates that the older adults had low capacity that did not increase with set size. In contrast, the younger adults showed a higher capacity that increased from set size 2 to 3, but not from set size 3 to 4. To confirm this pattern, we submitted the mean capacity estimates to a mixed-design analysis of variance (ANOVA) to examine the factors of group (older adults, younger adults) and set size (2, 3, 4), which revealed an effect of group [F(1, 22) = 28.15, p < .01, partial eta-squared (η P 2) = .56], no main effect of set size [F(2, 44) = 2.43, p = .10], and an interaction of group and set size [F(2, 44) = 3.89, p = .03, η P 2 = .15]. We examined the interaction of group and set size using paired t tests, which revealed that older adults failed to show increases in capacity from set size 2 to 3 [t(10) = 0.23, p = .82] or from set size 3 to 4 [t(10) = 1.05, p = .32]. In fact, we observed a decrease in capacity estimates from set sizes 2 to 4 that approached significance [t(10) = 2.06, p = .06]. In contrast, the younger adults showed a significant increase in capacity estimates from set size 2 to 3 [t(12) = 6.3, p < .01]. However, the younger adults showed no differences between set sizes 3 and 4 [t(12) = 1.14, p = .28] or set sizes 2 and 4 [t(12) = 1.64, p = .12]. A complementary ANOVA of the verbal memory task using the same design did not reveal effects of any factor (all F values < 1.07). Note that although the latter analysis examined changes in the verbal memory task across varying levels of the visual memory task set size, the number of items in the verbal memory task itself did not vary.
(a) Behavioral capacity estimates (top) and contralateral delay activity (CDA; bottom) for both older and younger adults. This illustrates the dissociation between behavioral and neural activity in the older adults, whereas younger adults demonstrate the common pattern of both capacity and CDA increasing with set size. (b) Posterior event-related potential (ERP) activity in both older adults (top) and younger adults (bottom). The magnitude of the difference between contralateral (gray) and ipsilateral (black) activity between 1,000 and 1,600 ms post-sample-onset (indicated by the open gray box) was used to construct the CDA. The gray areas near the beginning of the time courses indicate the time during which the sample was visible. (c) Waveforms for sample-locked ERP activity, averaged across all participants
P1 peaks
Encoding-stage processes were indexed by the peaks in the P1 component, which we isolated by taking the maximum positive amplitude of the waveform between 0 and 200 ms post-stimulus-onset from the posterior ROIs. The mean P1 peaks were averaged across hemispheres and submitted to a mixed-design ANOVA focused on the effects of group (older, younger adults), set size (2, 3, 4), and laterality (contralateral, ipsilateral). A main effect of set size [F(1.76, 38.65) = 4.16, p = .03, η P 2 = .16] was driven by less positive activity at set size 2 (1.80 μV, SEM = 0.25) than at set size 4 (2.24 μV, SEM = 0.28), and a main effect of laterality [F(1, 22) = 7.39, p = .013, η P 2 = .25] showed that contralateral activity was more positive (2.17 μV, SEM = 0.24) than ipsilateral activity (1.95 μV, SEM = 0.27). We found no other main effects or interactions (all F values < 1.0).
Contralateral delay activity (CDA)
Visual inspection of the contralateral and ipsilateral waveforms from both groups revealed two important features that informed the selection of our time of interest (Fig. 2b). First, the data revealed negative peaks appearing around 200 ms after the sample array offset that were most likely related to the visual offset of the sample display (Woodman, 2010). Second, the difference between the contralateral and ipsilateral activity at Set Size 4 appeared to diminish around 1,600 ms post-sample-onset for both groups, even though a substantial amount of time (1,000 ms) remained in the delay. We will describe our analysis of this attenuation in the next section. To avoid confounding VWM maintenance activity with the offset-related peaks while also capturing the maximum difference between contralateral and ipsilateral activity, we targeted the CDA from 1,000 to 1,600 ms post-sample-onset. Figure 2b depicts the posterior activity in both older and younger adults and shows similar magnitudes of separation between the contralateral and ipsilateral signals (see also Supplementary Fig. 1).
The data were submitted to a mixed-design ANOVA to examine the factors group (older, younger adults), region (anterior, posterior), set size (2, 3, 4), and laterality (contralateral, ipsilateral). The results revealed a main effect of group [F(1, 22) = 5.75, p = .025, η P 2 = .21], driven by less negative activity overall in the older adults (–0.13 μV, SEM = 0.10) than in the younger adults (–0.79 μV, SEM = 0.24). We found an interaction of region and group [F(1, 22) = 7.49, p = .012, η P 2 = .25], which was examined by comparing the overall activity between anterior and posterior regions in both groups. Older adults showed more positive activity in anterior regions (1.18 μV, SEM = 0.41) than in posterior regions (–1.43 μV, SEM = 0.31), t(10) = 3.74, p = .004. However, younger adults showed no difference in overall activity in the anterior (–0.74 μV, SEM = 0.38) and posterior regions (–0.83 μV, SEM = 0.39), t(12) = 0.14, p = .88. Remarkably, no other group-related interactions emerged (all other F values < 0.89), suggesting that although we observed some overall topographic differences between the groups, they did not affect the factors indexing the CDA (set size and laterality).
Importantly, we found a three-way interaction of region, set size, and laterality [F(1.91, 41.97) = 6.67, p = .003, η P 2 = .23], which we investigated by conducting separate ANOVAs examining the interaction of set size and laterality for the anterior and posterior regions. Activity in the anterior regions did not show an interaction of these factors [F(1.86, 40.96) = 0.62, p = .46, η P 2 = .03]. However, activity in posterior regions did show an interaction [F(1.99, 43.74) = 6.80, p = .003, η P 2 = 0.24], which we followed up with paired comparisons examining the differences in contralateral and ipsilateral activity for each level of set size. Contralateral activity was more negative than ipsilateral activity at set size 2, t(23) = 2.47, p = .02; set size 3, t(23) = 3.34, p = .003; and set size 4, t(23) = 4.89, p < .001. We then examined whether the contralateral and ipsilateral differences increased across levels of set size. We subtracted the ipsilateral from the contralateral activity and then compared these difference scores across levels of set size. The difference between contralateral and ipsilateral activity—that is, the CDA—increased between set sizes 2 and 3, t(23) = 2.19, p = .04, and between set sizes 2 and 4, t(23) = 3.63, p = .001.
CDA in later temporal epochs
As we mentioned in the previous section, visual inspection of the posterior waveforms in both groups suggested an attenuation of the CDA following the primary epoch of interest (1,000–1,600 ms). It was possible that the CDA diminished sooner in older than in younger adults after the primary epoch of interest, perhaps reflecting an earlier decay of VWM or an earlier depletion of the attentional resources necessary to rehearse the contents of VWM (Magen, Emmanouil, McMains, Kastner, & Treisman, 2009). Either of these accounts could help explain the dissociation between behavioral and ERP measures in the older adults. We split the remainder of the time course into 200-ms epochs and submitted it to a mixed-design ANOVA concerning the effects of group (older, younger adults), epoch (1,600–1,800, 1,800–2,000, 2,000–2,200, 2,200–2,400, and 2,400–2,600 ms), set size (2, 3, 4), and laterality (contralateral, ipsilateral). A main effect of group, driven by more negative activity in the older adults (–1.07 μV, SEM = 0.24) than in the younger adults (–0.37 μV, SEM = 0.28), approached significance [F(1, 22) = 3.41, p = .07, η P 2 = .13], but importantly, the group factor did not interact with any other factors (all other F values < 1.8). This suggested that the patterns of contralateral and ipsilateral activity were similar in both groups after our primary time of interest.
A main effect of laterality [F(1, 22) = 11.21, p = .003, η P 2 = .34] showed that contralateral activity (–0.80 μV, SEM = 0.21) was more negative than ipsilateral activity (–0.58 μV, SEM = 0.19). Also, an interaction of set size and laterality [F(1.6, 35.31) = 3.61, p < .05, η P 2 = .14] appeared to show differences between contralateral and ipsilateral activity that increased with set size, as was suggested by a linear trend in the interaction [F(1, 22) = 9.44, p = .006, η P 2 = .30]. We confirmed this pattern by submitting the difference between contralateral activity and ipsilateral activity at each level of set size to a one-way ANOVA focused on the set size factor, which was significant [F(1.6, 35.31) = 3.61, p < .05, η P 2 = .14] and was modeled well by a linear trend [F(1, 22) = 9.44, p = .006, η P 2 = .30]. These results mirror the same pattern of the CDA observed during our selected time of interest—that is, with contralateral activity being more negative than ipsilateral activity, and this difference increasing with set size.
Importantly, we observed an interaction of epoch and laterality [F(1.65, 36.26) = 8.74, p = .001, η P 2 = .28]. This trend appeared to be driven by decreasing differences between contralateral and ipsilateral activity across levels of epoch, as is suggested by the means (see Fig. 3) and by a significant linear trend in the interaction [F(1, 22) = 12.06, p = .002, η P 2 = .35]. We confirmed this pattern by submitting the difference between contralateral and ipsilateral activity to a one-way ANOVA examining the effect of epoch (1,600–1,800, 1,800–2,000, 2,000–2,200, 2,200–2,400, and 2,400–2,600 ms), which was significant [F(1.65, 36.26) = 8.74, p = .001, η P 2 = .28]. Confirming the decrease in the contralateral-to-ipsilateral difference, a linear trend in the epoch factor emerged [F(4, 88) = 12.06, p = .002, η P 2 = .35]. Higher-order models were not significant (all F values < 2.7).
Probe-locked ERPs
Probe-locked ERPs were analyzed by dividing the data into 200-ms epochs beginning from 300 ms post-probe-onset, and then submitting the data from each epoch into a mixed-effects ANOVA using factors group (older, younger), ROI (LAI, CAI, RAI, LAS, RAS, LPS, CPS, RPS, LPI, and RPI), set size (2, 3, 4), and item (old, new). The results revealed two important effects that are described below in separate sections. For brevity, we will only report important interactions and the appropriate follow-up tests.
Old–new effects
A three-way interaction of group, ROI, and item found in most epochs showed topographically distinct old–new effects for younger and older adults. We examined this interaction by collapsing the data across levels of set size and analyzing the two-way interaction of ROI and item separately for the older and younger adults with F tests. The interactions were further examined with paired t tests at the Bonferroni-corrected alpha level (see Fig. 4).
Waveforms depicting the old–new effect appear on the left for older adults and on the right for younger adults. Gray areas indicate epochs during which old–new effects were significant. This activity was collapsed across all levels of set size. At the bottom, scalp topographies depicting the old–new contrast appear for younger (top row) and older (bottom row) adults.
The three-way interactions of group, ROI, and item was first evident during the 300- to 500-ms epoch, during which early old–new effects such as the midfrontal FN400 are typically found [F(2.62, 57.60) = 4.49, p < .01, η P 2 = .17]. Follow-up F tests revealed an interaction of ROI and item in younger adults [F(3.47, 41.63) = 9.85, p < .01, η P 2 = 0.45], but not in older adults (F < 1). Paired t tests on the younger-adult data showed that old items elicited more positive activity than did new items in region RAS [t(12) = 5.28, p < .01], whereas old items elicited more negative activity than did new items in occipital areas LPI [t(12) = 5.95, p < .01] and RPI [t(12) = 3.86, p < .01].
The three-way interaction of group, ROI, and item was also observed reliably in later epochs during which the LPC and later effects are often observed, including 700–900 ms [F(2.65, 58.36) = 3.63, p = .02, η P 2 = .14], 1,100–1,300 ms [F(2.43, 53.42) = 3.27, p < .05, η P 2 = .13], and 1,300–1,500 ms [F(2.89, 77.42) = 3.10, p = .03, η P 2 = .12]. Follow-up F tests showed an interaction of ROI and item for the older adults only during the 700- to 900-ms epoch [F(2.05, 20.54) = 5.82, p = .01, η P 2 = .37]. Older adults showed negative old–new effects at frontal regions LAI [t(10) = 4.04, p < .01] and LAS [t(10) = 4.39, p < .01], and positive old–new effects at the parietal region RPS [t(10) = 4.50, p < .01]. In contrast, younger adults showed the interaction of ROI and item at 1,100–1,300 ms [F(2.88, 34.59) = 4.34, p = .01, η P 2 = .27], which was driven by a positive old–new effect in region CPS [t(12) = 4.54, p < .01]. Younger adults also showed an ROI-by-item interaction at 1,300–1,500 ms [F(3.23, 38.73) = 4.38, p < .01, η P 2 = .27], driven by a positive old–new effect in midfrontal region RAS [t(12) = 3.76, p < .01] and a negative old–new effect in occipital region LPI [t(12) = 4.34, p < .01].
Although a three-way interaction of group, ROI, and item was not observed during the 900- to 1,100-ms epoch, we did find a significant interaction of ROI and item [F(2.72, 59.95) = 6.22, p < .01, η P 2 = .22], which indicated topographically similar old–new effects between older and younger adults during this epoch. After we collapsed the data across both groups, paired t tests showed a negative old–new effect in frontal region LAI [t(23) = 4.26, p < .01] and a positive old–new effect in parietal region RPS [t(23) = 4.14, p < .01].
In summary, these results showed early (300–500 ms) and late (700–1,500 ms) old–new effects for younger adults, but only late (700–1,100 ms) old–new effects for the older adults. The old–new effects were largely distinct between the groups, with the exception of topographically similar old–new effects during the 700- to 900-ms epoch.
Set-size effects
An interaction of ROI and set size observed in most epochs revealed an important set-size effect that was common to both groups. We examined this interaction by collapsing the data across the levels of group and item, which revealed set-size effects in frontal (LAI, CAI, RAI) and parietal (LPS, CPS, RPS) scalp areas with paired t tests at the Bonferroni-corrected alpha level. These analyses revealed that, as set size increased, frontal areas showed decreasing negative activity, whereas parietal areas showed decreasing positive activity (see Fig. 5).
Waveforms depicting the set-size effect in each region of interest (ROI). This activity is collapsed across groups and old–new status. The gray areas indicate when set-size effects were significant, with symbols indicating the specific contrasts that were significant. The map at right indicates the locations of the ROIs
The interaction of ROI and set size was reliably observed at 700–900 ms [F(5.46, 120.03) = 7.11, p < .01, η P 2 = .24], 900–1,100 ms [F(6.09, 133.96) = 9.28, p < .01, η P 2 = .30], and 1,100–1,300 ms [F(18, 396) = 3.14, p = .01, η P 2 = .13]. During the 700- to 900-ms epoch, activity became less negative from set size 2 to set size 4 at frontal regions CAI [t(23) = 5.72, p < .01] and RAI [t(23) = 5.26, p < .01]. In contrast, activity in region CPS became less positive from set sizes 2 to 3 [t(23) = 4.22, p < .01] and from set sizes 2 to 4 [t(23) = 5.13, p < .01]. A similar pattern was observed in region RPS (set sizes 2–3, p < .01; set sizes 2–4, p < .01). During the 900- to 1,100-ms epoch, frontal activity again showed decreasing positivity in regions LAI (set sizes 2–3, p < .01), CAI (set sizes 2–3, p < .01; set sizes 2–4, p < .01), and RAI (set sizes 2–4, p < .01). Parietal region LPS showed decreasing positive activity from set sizes 3 to 4 (p < .01) and set sizes 2 to 4 (p < .01). Significant differences were found between all levels of set size at region CPS (all p values < .01) and between set sizes 2 and 3 (p < .01) and set sizes 2 and 4 (p < .01) for region RPS. This pattern continued in the 1,100- to 1,300-ms epoch, in which decreasingly negative activity was observed in frontal regions CAI (set sizes 2–3, p = .03; set sizes 2–4, p = .003) and RAI (set sizes 2–3, p = .04; set sizes 2–4, p = .004). In contrast, parietal areas showed decreasing positive activity as set size increased at regions CPS (set sizes 2–4, p < .01) and RPS (set sizes 2–4, p < .01).
In summary, both groups showed a common neural set-size effect, consisting of decreasingly negative activity in frontal regions, concurrent with decreasingly positive activity in parietal regions, as the task set size increased.
Discussion
In the present study, we set out to investigate age-related differences in behavioral and neural activity during VWM encoding, maintenance, and retrieval using ERPs. Although we attempted to minimize the effect of known age-related impairments on VWM encoding processes, we monitored these processes using the P1 component. Older and younger adults did not show amplitude differences in the P1 component. During maintenance, we hypothesized that both behavioral and neural estimates of VWM capacity would be significantly lower in older than in younger adults. However, our results were not completely in line with this prediction. Whereas the older adults showed lower behavioral capacity estimates than did the younger adults, they exhibited a CDA that was comparable to that of younger adults. The dissociations between behavioral capacity estimates and neural measures of VWM encoding and maintenance in the older adults indicated that the P1 and CDA measures did not capture all of the factors affecting older adults’ VWM. Therefore, in addition to encoding and maintenance, we also examined neural activity related to retrieval. As with previous studies of retrieval in both long-term memory and working memory, our results showed early and late stages of neural processing related to VWM retrieval. On the basis of research from long-term memory and the idea that common retrieval mechanisms may mediate both long-term memory and VWM, we predicted that both older and younger adults would show an early FN400 old–new effect, but that older adults would fail to show a late parietal old–new effect (LPC) relative to younger adults. Although our results showed the younger adults to exhibit activity resembling the early FN400 and late parietal LPC old–new effects, the older adults failed to exhibit activity resembling either the FN400 or the LPC. These results will be discussed in terms of two-stage models of recognition.
Recent research has shown that the P1 component is more closely related to sensory processing than other early components like the N1 (Warbrick et al., 2013). Consistent with this notion, our analysis of the P1 peaks showed that its amplitude increased with set size—that is, as more visual information was viewed. Additionally, the P1 is enhanced for relevant information and reduced for irrelevant information (Gazzaley et al., 2008). Our results were consistent with this aspect as well, since P1 amplitudes were more positive for contralateral (memory-related) items than ipsilateral (to-be-ignored) items. Importantly, we found no group differences in these modulations of the P1, suggesting that the older adults exhibited no impairment in sensory processing or filtering during VWM encoding. However, the lack of group differences in this early component is inconsistent with the significant decrease in behavioral capacity estimates in the older relative to the younger adults. Together, these results suggest that age-related capacity limitations arise at a stage later than VWM encoding.
We also found a clear dissociation between the behavioral capacity estimates and the CDA, which is a well-known measure of VWM capacity, in the older adults. To our knowledge, this is the first study to show a clear dissociation between behavior and electrophysiological correlates of working memory capacity in older adults. Although the CDA is often highly correlated with behavioral performance of change detection (Jost et al., 2011; Vogel & Machizawa, 2004), some previous studies have reported its dissociation from behavior in younger adults. For example, Ikkai, McCollough, and Vogel (2010) measured the CDA while participants performed a change detection task using high- or low-contrast colors, and showed that behavioral capacity estimates for high-contrast colors were superior to those of low-contrast colors. However, the CDA magnitude measured during the low-contrast condition was just as large as that of the high-contrast condition. These results indicated that although the same numbers of items were stored in VWM for both conditions, the lower-quality memory of the low-contrast colors impaired discrimination between the sample and the probe (i.e., the comparison process; see also Exp. 4 of Luria, Sessa, Gotler, Jolicœur, & Dell’Acqua, 2009). This account is consistent with a growing number of studies showing that the CDA indexes the quantity, but not the quality, of items in VWM (Gao, Yin, Xu, Shui, & Shen, 2011; Luria & Vogel, 2011). This is further supported by neuroimaging studies indicating that separate neuroanatomical regions index the quality and quantity of VWM contents (Xu & Chun, 2006, 2009).
In light of this previous research, it is possible that our maintenance results reflect older adults storing the same quantity or number of items in VWM as younger adults, but having lower quality or resolution of VWM representations than do younger adults. To illustrate, a trial could have had three circles in the sample, with one of them being colored red. Both younger and older adults might have stored three items, but older adults were less able than younger adults to remember the specific shade of colors. When the probe appeared in the same location as the red item, but in a slightly different shade of red, younger adults were more likely than older adults to discriminate the subtle differences between the shades of red in the sample and the probe. In other words, the low-resolution representations maintained by the older adults impaired their ability to effectively compare these representations to the probe. This low-resolution storage account is supported by one recent aging study that used mixture modeling to directly show that older adults store lower-resolution VWM representations than do younger adults (Noack, Lövdén, & Lindenberger, 2012).
The set-size effect observed in the probe-locked ERP activity also suggests that older and younger adults maintain the same numbers of items in VWM. Both groups exhibited a parietal-based P3 component that decreased in magnitude with increasing set size. One theory proposes that P3 amplitudes increase as the contents of working memory are updated (Donchin & Coles, 1988). While viewing the single-item probe in our task, participants may have automatically updated the information in VWM related to the probe. Updating this single item would require less of an update to the total contents of VWM at large than at small set sizes, resulting in P3 amplitudes being inversely related to set size. Importantly, the lack of group differences may suggest that older and younger adults shared a similar quantity of total VWM contents to be updated at the time of the probe.
An alternative account of the dissociation between the CDA and behavior in older adults is that the CDA reflects attention-based rehearsal of items in VWM. Neuroimaging has long shown that visual attention could be used to rehearse and facilitate the representation of objects in VWM (Awh & Jonides, 2001; Nobre et al., 2004). In support of this attentional rehearsal account, research has shown that the CDA increases with the number of objects attended during multiple-object tracking (Drew, Horowitz, & Vogel, 2013). In our study, it is possible that the CDA reflected attentional rehearsal that increased with the number of items in VWM, and that both older and younger adults applied the same amount of attention to rehearsal. However, despite using the same amount of attention as younger adults, the older adults’ performance could have remained low due to low-resolution representations. This account does not differ much from the low-resolution storage account, except that it proposes that the CDA reflects the rehearsal rather than the memory of items.
It should be noted that finding a similar CDA between older and younger adults conflicts with previous studies that have shown age-related changes in the CDA (Jost et al., 2011; Sander et al., 2011), but these discrepant findings may be due to top-down control factors. Sander et al. reported that age-related differences in the CDA could be attributed to greater top-down control that younger adults have over older adults (and children). In our study, it is possible that concurrent performance of the verbal memory task removed the advantage in top-down control on VWM that the younger adults typically have over older adults, resulting in a comparable CDA across groups. Importantly, previous work has shown that engagement of a concurrent task does not impair older adults more than younger adults on VWM (Brown & Brockmole, 2010). Also, Jost et al. used the CDA to find age-related impairments to an early filtering process involved in VWM. However, later in the time course (after 550 ms post-sample-onset), they reported that “the CDA effects for old and young adults are virtually indistinguishable” (p. 1153). It is possible that impaired top-down control in the older adults led to differences in filtering that were observable in an early segment of the CDA. However, once the filtering process completed, older and younger adults were equated on the degrees of top-down control that they exerted on VWM, and the CDAs became comparable across groups.
The dissociation of the CDA and behavioral capacity estimates in older adults also potentially indicates that the CDA does not capture all of the factors that determine their ultimate behavioral performance. To further investigate other factors that could affect behavior, we analyzed the ERP activity time-locked to the test probe in order to examine processes related to VWM retrieval. At a glance, the younger adults showed an early negative old–new effect over occipital regions and an early positive old–new effect over midfrontal bilateral regions (300–500 ms postprobe). In a later time interval (900–1,500 ms), the younger adults showed a sustained positive old–new effect over right and central parietal areas. In contrast, older adults only showed a sustained old–new effect with a right parietal and occipital distribution, accompanied by an inverse dipole in left frontal areas (700–1,500 ms). In summary of the ERP effects, there appeared to be both early and late stages of neural processing related to VWM retrieval, but group differences showed that whereas younger adults exhibited both stages, older adults only exhibited a late stage of processing.
Under the classic dual-process model of recognition memory, these results could suggest that younger adults potentially relied on familiarity and recollection to support VWM decisions, whereas older adults had deficits in these processes. The early occipital effect exhibited by younger adults strongly resembled the N300, recently related to perceptual fluency (Leynes & Zish, 2012; Ryals, Yadon, Nomi, & Cleary, 2011; Voss & Paller, 2009) that can be used to support recognition (Ko, Duda, Hussey, & Ally, 2013). In the same epoch, younger adults also exhibited the well-known FN400 effect typically associated with familiarity-based recognition (Ally & Budson, 2007; Rugg & Curran, 2007; Rugg et al., 1998; Woodruff et al., 2006; Yonelinas et al., 2010). In the later epoch, the younger adults’ positive old–new effect strongly resembled the late parietal effect, or LPC, which has long been related to memorial recollection (Rugg & Curran, 2007). Although this effect had a later onset and lasted longer than is typically observed for the LPC, these differences might have been due to task-related differences between change detection and long-term recognition tests. In contrast, older adults failed to show activity resembling the FN400 and LPC, so it is possible that familiarity and recollection processes were not recruited. Instead, the older adults exhibited a late right-lateralized effect that topographically resembled activity related to postretrieval monitoring and verification (Ally & Budson, 2007). This interpretation complements the absence of the LPC in older adults, because postretrieval monitoring and verification processes tend to be stronger when recollection processes are weak or absent (Ally & Budson, 2007; Ally, McKeever, Waring, & Budson, 2009; Budson et al., 2005). The possible absence of recollection in older adults, as suggested by the lack of LPC-like activity, is also consistent with our earlier supposition that older adults may maintain the same quantity of information in VWM but suffer from a lower quality of information than is present in younger adults. Recent research has also suggested that recollection entails—primarily due to the role of parietal areas—a subjective “reexperiencing” of the memory encoding (Ally, Simons, McKeever, Peers, & Budson, 2008; Ciaramelli, Rosenbaum, Solcz, Levine, & Moscovich, 2010; Simons, Peers, Mazuz, Berryhill, & Olson, 2010). This subjective reexperience might be crucial for the change detection task, because the retrieved contents of VWM must be explicitly compared to the probe, and lack of this reexperience in older adults would lower their ability to make the comparison.
Although the dual-process model has helped to frame early- and late-stage ERP activity in several studies of memory recognition, the use of both familiarity and recollection processes during retrieval in the change detection paradigm seems unlikely and led us to consider an alternative interpretation. Recently, researchers have reinterpreted early- and late-stage ERPs observed during memory recognition within the fluency heuristic framework (Jacoby & Dallas, 1981; Voss, Lucas, & Paller, 2012). Participants experience fluency when an item is relatively easy to process and report—for example, when a word is read more quickly under clear versus difficult viewing conditions (for a review, see Alter & Oppenheimer, 2009). Fluency often accompanies behavioral priming, indicating that it can be evoked by activated implicit memory representations (Alter & Oppenheimer, 2009). The fluency heuristic model proposes that participants often experience fluency when a retrieval cue is similar to a previously viewed item, and that they attribute this experience of fluency to memory (Jacoby & Dallas, 1981; Rajaram & Geraci, 2000). Often, the fluency heuristic increases memory accuracy when the cue matches a studied item, but it can lead to false memory decisions (Jacoby & Whitehouse, 1989). Recent research has related early old–new ERP effects to the emergence of fluency that later informs a recognition process (familiarity or recollection) indicated by the late LPC (Voss, Lucas, & Paller, 2012). For example, the FN400 has been reinterpreted to reflect conceptual fluency (Voss, Lucas, & Paller, 2010). Several studies have related perceptual fluency to a negative N300 effect observed over occipital recording sites (Ko et al., 2013; Leynes & Zish, 2012; Ryals et al., 2011; Voss & Paller, 2009; although positive posterior effects have also been observed by Kurilla & Gonsalves, 2012; Nessler, Mecklinger, & Penney, 2005; Woollams, Taylor, Karayanidis, & Henson, 2008).
In our results, younger adults showed concurrent activation of the FN400 and N300 during VWM retrieval. In the context of the fluency heuristic model, it is possible that younger adults used fluency to inform their change detection response. The FN400 could have reflected conceptual fluency related to the verbal memory load. The N300 was likely to have reflected perceptual fluency related to the visual sample. In other words, younger adults may have retrieved a perceptual implicit memory of the visual sample stored concurrently with the visual working memory representation (e.g., Jiang, Haxby, Martin, Ungerleider, & Parasuraman, 2000). This early signal of perceptual fluency would then be used to inform a later familiarity or recollection process as indicated by the LPC. The idea that fluency affected the younger adults’ memory decisions is further supported by the observation that the younger adults’ responses (collapsed across levels of set size, median response time = 819.31 ms, SEM = 43.25) were made prior to their LPC (900–1,500 ms), suggesting a form of behavioral priming. Unlike the younger adults, older adults did not exhibit either the FN400 or the N300, suggesting that they did not experience fluency that could have informed their behavioral responses. Although older adults exhibit intact priming (Fleischman, 2007), they may rely on long-term semantic representations (e.g., everyday objects or easily labeled colors) for visual priming (Soldan, Hilton, Cooper, & Stern, 2009), which would not have been activated in the present study. As with the dual-process model, the fluency heuristic model would interpret the late-stage neural activity in older adults as a failure to activate a familiarity or recollection process, but as successful activation of postretrieval monitoring and verification processes.
How do the results obtained from VWM encoding, maintenance, and retrieval fit together? Of the possibilities that we have discussed, the low-resolution storage account of the encoding- and maintenance-related activity and the fluency heuristic model of the retrieval-related activity appear to complement each other. During encoding, older and younger adults engage in similar sensory processing. During maintenance, younger and older adults represent the same number of items in VWM, but older adults represent these items at a lower quality. At retrieval, the probe activates a perceptual implicit memory in the younger adults, providing them with a subjective sense of fluency that they use to facilitate their memory decision. This difference in retrieval between the groups could provide younger adults with a high-resolution memory of recently viewed items that facilitates their change detection accuracy. In contrast, older adults would have a low-resolution visual memory that impairs their change detection accuracy below that of younger adults. Additionally, older adults do not strongly engage a familiarity or recollection process, and must compensate with enhanced post-retrieval monitoring and verification.
We appreciate the fact that the number of participants in each group was relatively low, as compared to other aging studies. However, this resulted from an exclusion criterion that was necessary in order to avoid confounding our CDA effects with eye movement artifacts. Despite this low number, we note that the critical aspects of our data are the finding of significant set-size changes in the CDA, in conjunction with a lack of set-size effects in the behavior of older adults, as well as important group differences between younger and older adults in the retrieval-stage ERPs. In other words, our primary conclusions are not based on null effects in the results.
In conclusion, a more comprehensive examination of multiple VWM stages has provided important insight to understanding why healthy aging involves a reduction in VWM capacity. Older and younger adults use similar sensory processes during encoding. During maintenance, both groups store the same number of items, resulting in comparable neural measures of VWM maintenance, but older adults store them at a lower resolution than do younger adults, resulting in impaired behavior. The consequence of these differences in resolution is apparent during retrieval, when younger adults are able to use robust perceptual implicit memory to facilitate the comparison of VWM contents to the probe. These data could suggest that younger and older adults have the same number of “slots” in VWM (Zhang & Luck, 2008) but store information in these slots at very different resolutions (Bays & Husain, 2008). Further investigation and a prospective look on how the stages of VWM interact might help illuminate how memory changes in healthy aging. As the results of this study indicate, understanding how VWM capacity is reduced with age cannot be accomplished by focusing on a single stage of the VWM process. Instead, the multiple stages of encoding, maintenance, and retrieval must be taken in account.
Notes
We use the term “retrieval” to refer to the access and use of a VWM representation to complete a task. We do not specify whether an effortful process, like a memory search, is necessary to access the VWM representation. Some theories conceive of working memory as an activated portion of long-term memory, a subset of which resides in the focus of attention (Cowan, 2001; Öztekin, Davachi, & McElree, 2010). A process to access information in the focus of attention is not necessary, because it is already “online,” but the other information in the activated portion may undergo an access process (Öztekin, McElree, Staresina, & Davachi, 2009). In visuospatial change detection tasks, it is sometimes believed that all items in a sample are within the focus of attention under subthreshold conditions (e.g., number of items <4; Cowan, 2001), so we might assume that an access process is unnecessary in the current task. In this introduction, it is important for us to be agnostic as to the nature of the access in this task, because, as our results will show, retrieval-stage processes are different between younger and older adults. In accord with formal models (Johnson, Spencer, Luck, & Schöner, 2009) and empirical studies (Awh, Barton, & Vogel, 2007; Bledowski et al., 2012), we have assumed that the use of the representation entails comparing it to the perceived test probe.
References
Adjutant General’s Office. (1944). Army individual test battery: Manual of directions and scoring. Washington, DC: War Department.
Ally, B. A., & Budson, A. E. (2007). The worth of pictures: Using high density event-related potentials to understand the memorial power of pictures and the dynamics of recognition memory. NeuroImage, 35, 378–395. doi:10.1016/j.neuroimage.2006.11.023
Ally, B. A., McKeever, J. D., Waring, J. D., & Budson, A. E. (2009). Preserved frontal memorial processing for pictures in patients with mild cognitive impairment. Neuropsychologia, 47, 2044–2055. doi:10.1016/j.neuropsychologia.2009.03.015
Ally, B. A., Simons, J. S., McKeever, J. D., Peers, P. V., & Budson, A. E. (2008a). Parietal contributions to recollection: Electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia, 46, 1800–1812. doi:10.1016/j.neuropsychologia.2008.02.026
Ally, B. A., Waring, J. D., Beth, E. H., McKeever, J. D., Milberg, W. P., & Budson, A. E. (2008b). Aging memory for pictures: Using high-density event-related potentials to understand the effect of aging on the picture superiority effect. Neuropsychologia, 46, 679–689. doi:10.1016/j.neuropsychologia.2007.09.011
Alter, A. L., & Oppenheimer, D. M. (2009). Uniting the tribes of fluency to form a metacognitive nation. Personality and Social Psychology Review, 13, 219–235. doi:10.1177/1088868309341564
Awh, E., Barton, B., & Vogel, E. K. (2007). Visual working memory represents a fixed number of items regardless of complexity. Psychological Science, 18, 622–628. doi:10.1111/j.1467-9280.2007.01949.x
Awh, E., & Jonides, J. (2001). Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences, 5, 119–126. doi:10.1016/S1364-6613(00)01593-X
Bays, P. M., & Husain, M. (2008). Dynamic shifts of limited working memory resources in human vision. Science, 321, 851–854. doi:10.1126/science.1158023
Bledowski, C., Kaiser, J., Wibral, M., Yildiz-Erzberger, K., & Rahm, B. (2012). Separable neural bases for subprocesses of recognition in working memory. Cerebral Cortex, 22, 1950–1958. doi:10.1093/cercor/bhr276
Brockmole, J. R., Parra, M. A., Della Sala, S., & Logie, R. H. (2008). Do binding deficits account for age-related decline in visual working memory? Psychonomic Bulletin & Review, 15, 543–547. doi:10.3758/PBR.15.3.543
Brown, L. A., & Brockmole, J. R. (2010). The role of attention in binding visual features in working memory: Evidence from cognitive ageing. Quarterly Journal of Experimental Psychology, 63, 2067–2079. doi:10.1080/17470211003721675
Budson, A. E., Droller, D. B., Dodson, C. S., Schacter, D. L., Rugg, M. D., Holcomb, P. J., & Daffner, K. R. (2005). Electrophysiological dissociation of picture versus word encoding: The distinctiveness heuristic as a retrieval orientation. Journal of Cognitive Neuroscience, 17, 1181–1193. doi:10.1162/0898929055002517
Ciaramelli, E., Rosenbaum, R. S., Solcz, S., Levine, B., & Moscovitch, M. (2010). Mental space travel: Damage to posterior parietal cortex prevents egocentric navigation and reexperiencing of remote spatial memories. Journal of Experimental Psychology: Learning, Memory, and Cognition, 36, 619–634. doi:10.1037/a0019181
Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–114. doi:10.1017/S0140525X01003922. disc. 114–185.
Cowan, N., Naveh-Benjamin, M., Kilb, A., & Saults, J. S. (2006). Life-span development of visual working memory: When is feature binding difficult? Developmental Psychology, 42, 1089–1102. doi:10.1037/0012-1649.42.6.1089
Curran, T., DeBuse, C., Woroch, B., & Hirshman, E. (2006). Combined pharmacological and electrophysiological dissociation of familiarity and recollection. Journal of Neuroscience, 26, 1979–1985. doi:10.1523/JNEUROSCI.5370-05.2006
Danker, J. F., Hwang, G. M., Gauthier, L., Geller, A., Kahana, M. J., & Sekuler, R. (2008). Characterizing the ERP old–new effect in a short-term memory task. Psychophysiology, 45, 784–793. doi:10.1111/j.1469-8986.2008.00672.x
Daselaar, S. M., Fleck, M. S., Dobbins, I. G., Madden, D. J., & Cabeza, R. (2006). Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex, 16, 1771–1782. doi:10.1093/cercor/bhj112
Dien, J. (1998). Addressing misallocation of variance in principal components analysis of event-related potentials. Brain Topography, 11, 43–55.
Donchin, E., & Coles, M. G. H. (1988). Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences, 11, 357–374.
Drew, T., Horowitz, T. S., & Vogel, E. K. (2013). Swapping or dropping? Electrophysiological measures of difficulty during multiple object tracking. Cognition, 126, 213–223. doi:10.1016/j.cognition.2012.10.003
Fleischman, D. A. (2007). Repetition priming in aging and Alzheimer’s disease: An integrative review and future directions. Cortex, 43, 889–897.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6
Fukuda, K., Awh, E., & Vogel, E. K. (2010). Discrete capacity limits in visual working memory. Current Opinion in Neurobiology, 20, 177–182. doi:10.1016/j.conb.2010.03.005
Gao, Z., Yin, J., Xu, H., Shui, R., & Shen, M. (2011). Tracking object number or information load in visual working memory: Revisiting the cognitive implication of contralateral delay activity. Biological Psychology, 87, 296–302. doi:10.1016/j.biopsycho.2011.03.013
Gazzaley, A., Clapp, W., Kelley, J., McEvoy, K., Knight, R. T., & D’Esposito, M. (2008). Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Sciences, 105, 13122–13126. doi:10.1073/pnas.0806074105
Hasher, L., & Zacks, R. T. (1988). Working memory, comprehension, and aging: A review and a new view. In G. H. Bower (Ed.), The psychology of learning and motivation: Advances in research and theory (Vol. 22, pp. 193–225). San Diego, CA: Academic Press. doi:10.1016/S0079-7421(08)60041-9
Howard, M. W., Bessette-Symons, B., Zhang, Y., & Hoyer, W. J. (2006). Aging selectively impairs recollection in recognition memory for pictures: Evidence from modeling and receiver operating characteristic curves. Psychology and Aging, 21, 96–106. doi:10.1037/0882-7974.21.1.96
Hyun, J.-S., Woodman, G. F., Vogel, E. K., Hollingworth, A., & Luck, S. J. (2009). The comparison of visual working memory representations with perceptual inputs. Journal of Experimental Psychology: Human Perception and Performance, 35, 1140–1160. doi:10.1037/a0015019
Ikkai, A., McCollough, A. W., & Vogel, E. K. (2010). Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of Neurophysiology, 103, 1963–1968. doi:10.1152/jn.00978.2009
Jacoby, L. L., & Dallas, M. (1981). On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General, 110, 306–340. doi:10.1037/0096-3445.110.3.306
Jacoby, L. L., & Whitehouse, K. (1989). An illusion of memory: False recognition influenced by unconscious perception. Journal of Experimental Psychology: General, 118, 126–135. doi:10.1037/0096-3445.118.2.126
Jiang, Y., Haxby, J. V., Martin, A., Ungerleider, L. G., & Parasuraman, R. (2000). Complementary neural mechanisms for tracking items in human working memory. Science, 287, 643–646. doi:10.1126/science.287.5453.643
Johnson, J., Spencer, J., Luck, S., & Schöner, G. (2009). A dynamic neural field model of visual working memory and change detection. Psychological Science, 20, 568–577.
Jost, K., Bryck, R. L., Vogel, E. K., & Mayr, U. (2011). Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cerebral Cortex, 21, 1147–1154. doi:10.1093/cercor/bhq185
Ko, P. C., Duda, B., Hussey, E., & Ally, B. A. (2013). Electrophysiological distinctions between recognition memory with and without awareness. Neuropsychologia, 51, 642–655.
Kurilla, B. P., & Gonsalves, B. D. (2012). An ERP investigation into the strategic regulation of the fluency heuristic during recognition memory. Brain Research, 1442, 36–46. doi:10.1016/j.brainres.2011.12.060
Leynes, P. A., & Zish, K. (2012). Event-related potential (ERP) evidence for fluency-based recognition memory. Neuropsychologia, 50, 3240–3249. doi:10.1016/j.neuropsychologia.2012.10.004
Lins, O. G., Picton, T. W., Berg, P., & Scherg, M. (1993). Ocular artifacts in EEG and event-related potentials I: Scalp topography. Brain Topography, 6, 51–63.
Luria, R., Sessa, P., Gotler, A., Jolicœur, P., & Dell’Acqua, R. (2009). Visual short-term memory capacity for simple and complex objects. Journal of Cognitive Neuroscience, 22, 496–512. doi:10.1162/jocn.2009.21214
Luria, R., & Vogel, E. K. (2011). Shape and color conjunction stimuli are represented as bound objects in visual working memory. Neuropsychologia, 49, 1632–1639. doi:10.1016/j.neuropsychologia.2010.11.031
Mack, W. J., Freed, D. M., Williams, B. W., & Henderson, V. W. (1992). Boston Naming Test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology, 47, 154–P158.
Magen, H., Emmanouil, T.-A., McMains, S. A., Kastner, S., & Treisman, A. (2009). Attentional demands predict short-term memory load response in posterior parietal cortex. Neuropsychologia, 47, 1790–1798. doi:10.1016/j.neuropsychologia.2009.02.015
Mathes, B., Schmiedt, J., Schmiedt-Fehr, C., Pantelis, C., & Basar-Eroglu, C. (2012). New rather than old? For working memory tasks with abstract patterns the P3 and the single-trial delta response are larger for modified than identical probe stimuli. Psychophysiology, 49, 920–932.
Monsch, A. U., Bondi, M. W., Butters, N., Salmon, D. P., Katzman, R., & Thal, L. J. (1992). Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology, 49, 1253–1258.
Morris, J. C., Heyman, A., Mohs, R. C., Hughes, J. P., van Belle, G., Fillenbaum, G., & Clark, C. (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39, 1159–1165.
Naveh-Benjamin, M. (2000). Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 1170–1187. doi:10.1037/0278-7393.26.5.1170
Nessler, D., Mecklinger, A., & Penney, T. B. (2005). Perceptual fluency, semantic familiarity and recognition-related familiarity: An electrophysiological exploration. Cognitive Brain Research, 22, 265–288. doi:10.1016/j.cogbrainres.2004.03.023
Noack, H., Lövdén, M., & Lindenberger, U. (2012). Normal aging increases discriminal dispersion in visuospatial short-term memory. Psychology and Aging, 27, 627–637. doi:10.1037/a0027251
Nobre, A. C., Coull, J. T., Maquet, P., Frith, C. D., Vandenberghe, R., & Mesulam, M. M. (2004). Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience, 16, 363–373. doi:10.1162/089892904322926700
Öztekin, I., Davachi, L., & McElree, B. (2010). Are representations in working memory distinct from representations in long-term memory? Neural evidence in support of a single store. Psychological Science, 21, 1123–1133. doi:10.1177/0956797610376651
Öztekin, I., McElree, B., Staresina, B. P., & Davachi, L. (2009). Working memory retrieval: Contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. Journal of Cognitive Neuroscience, 21, 581–593. doi:10.1162/jocn.2008.21016
Parra, M. A., Abrahams, S., Logie, R. H., & Della Sala, S. (2009). Age and binding within-dimension features in visual short-term memory. Neuroscience Letters, 449, 1–5. doi:10.1016/j.neulet.2008.10.069
Rajaram, S., & Geraci, L. (2000). Conceptual fluency selectively influences knowing. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 1070–1074. doi:10.1037/0278-7393.26.4.1070
Reuter-Lorenz, P. A., & Sylvester, C.-Y. C. (2005). The cognitive neuroscience of working memory and aging. In R. Cabeza, L. Nyberg, & D. Park (Eds.), Cognitive neuroscience of aging: Linking cognitive and cerebral aging (pp. 186–217). New York, NY: Oxford University Press.
Rugg, M. D., & Curran, T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11, 251–257. doi:10.1016/j.tics.2007.04.004
Rugg, M. D., Mark, R. E., Walla, P., Schloerscheidt, A. M., Birch, C. S., & Allan, K. (1998). Dissociation of the neural correlates of implicit and explicit memory. Nature, 392, 595–598. doi:10.1038/33396
Ryals, A. J., Yadon, C. A., Nomi, J. S., & Cleary, A. M. (2011). When word identification fails: ERP correlates of recognition without identification and of word identification failure. Neuropsychologia, 49, 3224–3237. doi:10.1016/j.neuropsychologia.2011.07.027
Sander, M. C., Werkle-Bergner, M., & Lindenberger, U. (2011). Contralateral delay activity reveals life-span age differences in top-down modulation of working memory contents. Cerebral Cortex, 21, 2809–2819. doi:10.1093/cercor/bhr076
Simons, J. S., Peers, P. V., Mazuz, Y. S., Berryhill, M. E., & Olson, I. R. (2010). Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex, 20, 479–485. doi:10.1093/cercor/bhp116
Soldan, A., Hilton, H. J., Cooper, L. A., & Stern, Y. (2009). Priming of familiar and unfamiliar visual objects over delays in young and older adults. Psychology and Aging, 24, 93–104. doi:10.1037/a0014136
Vaughan, L., & Hartman, M. (2009). Aging and visual short-term memory: Effects of object type and information load. Aging, Neuropsychology, and Cognition, 17, 35–54. doi:10.1080/13825580903009063
Vogel, E. K., & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. doi:10.1038/nature02447
Vogel, E. K., McCollough, A. W., & Machizawa, M. G. (2005). Neural measures reveal individual differences in controlling access to working memory. Nature, 438, 500–503. doi:10.1038/nature04171
Voss, J. L., Lucas, H. D., & Paller, K. A. (2010). Conceptual priming and familiarity: Different expressions of memory during recognition testing with distinct neurophysiological correlates. Journal of Cognitive Neuroscience, 22, 2638–2651. doi:10.1162/jocn.2009.21341
Voss, J. L., Lucas, H. D., & Paller, K. A. (2012). More than a feeling: Pervasive influences of memory without awareness of retrieval. Cognitive Neuroscience, 3, 193–207. doi:10.1080/17588928.2012.674935
Voss, J. L., & Paller, K. A. (2009). An electrophysiological signature of unconscious recognition memory. Nature Neuroscience, 12, 349–355. doi:10.1038/nn.2260
Warbrick, T., Arrubla, J., Boers, F., Neuner, I., & Shah, N. J. (2013). Attention to detail: Why considering task demands is essential for single-trial analysis of BOLD correlates of the visual P1 and N1. Journal of Cognitive Neuroscience. doi:10.1162/jocn_a_00490. Advance online publication.
Wheeler, M. E., & Treisman, A. M. (2002). Binding in short-term visual memory. Journal of Experimental Psychology: General, 131, 48–64. doi:10.1037/0096-3445.131.1.48
Woodman, G. F. (2010). A brief introduction to the use of event-related potentials in studies of perception and attention. Attention, Perception, & Psychophysics, 72, 2031–2046. doi:10.3758/BF03196680
Woodman, G. F., Vecera, S. P., & Luck, S. J. (2003). Perceptual organization influences visual working memory. Psychonomic Bulletin & Review, 10, 80–87. doi:10.3758/BF03196470
Woodruff, C. C., Hayama, H. R., & Rugg, M. D. (2006). Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research, 1100, 125–135. doi:10.1016/j.brainres.2006.05.019
Woollams, A. M., Taylor, J. R., Karayanidis, F., & Henson, R. N. (2008). Event-related potentials associated with masked priming of test cues reveal multiple potential contributions to recognition memory. Journal of Cognitive Neuroscience, 20, 1114–1129. doi:10.1162/jocn.2008.20076
Xu, Y., & Chun, M. M. (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature, 440, 91–95. doi:10.1038/nature04262
Xu, Y., & Chun, M. M. (2009). Selecting and perceiving multiple visual objects. Trends in Cognitive Sciences, 13, 167–174. doi:10.1016/j.tics.2009.01.008
Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46, 441–517. doi:10.1006/jmla.2002.2864
Yonelinas, A. P., Aly, M., Wang, W.-C., & Koen, J. D. (2010). Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus, 20, 1178–1194. doi:10.1002/hipo.20864
Yonelinas, A. P., Widaman, K., Mungas, D., Reed, B., Weiner, M. W., & Chui, H. C. (2007). Memory in the aging brain: Doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus, 17, 1134–1140. doi:10.1002/hipo.20341
Zhang, W., & Luck, S. (2008). Discrete fixed-resolution representations in visual working memory. Nature, 453, 233–235. doi:10.1038/nature06860
Author Note
This research was supported by National Institute on Aging Grant Nos. K23AG031925 and R01AG0347 to B.A.A., and F32AG044076 to P.C.K.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(JPEG 104 kb)
Supplementary Figure 2
(JPEG 100 kb)
ESM 1
(DOCX 88 kb)
Rights and permissions
About this article
Cite this article
Ko, P.C., Duda, B., Hussey, E. et al. Understanding age-related reductions in visual working memory capacity: Examining the stages of change detection. Atten Percept Psychophys 76, 2015–2030 (2014). https://doi.org/10.3758/s13414-013-0585-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-013-0585-z