Published online Dec 21, 2022. doi: 10.3748/wjg.v28.i47.6632
Peer-review started: September 3, 2022
First decision: October 20, 2022
Revised: October 23, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 21, 2022
An expanding range of advanced mucosal imaging technologies have been de
Core Tip: Advanced mucosal imaging enhances polyp detection and characterization. This detailed review summarises existing advanced mucosal imaging technologies to guide everyday colonoscopic practice for interventional and non-interventional endoscopists.
- Citation: Young EJ, Rajandran A, Philpott HL, Sathananthan D, Hoile SF, Singh R. Mucosal imaging in colon polyps: New advances and what the future may hold. World J Gastroenterol 2022; 28(47): 6632-6661
- URL: https://www.wjgnet.com/1007-9327/full/v28/i47/6632.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i47.6632
Colorectal cancer (CRC) accounts for 10% of cancer incidence and is the third leading cause of cancer-related death worldwide[1]. Whilst CRC incidence and mortality are increasing globally, there is now tangible evidence of the evolving efficacy of screening programs in developed countries including Australia, the United States, Iceland, New Zealand and Japan, where there have been improvements in both CRC incidence and mortality[2,3]. While these decreases are multifactorial and partly a result of lifestyle modification (reduction in smoking, weight loss, dietary changes), the implementation of population CRC screening programs has been integral to the prevention and early detection of CRC[4,5].
CRC develops through a well-documented adenoma-carcinoma cascade consisting of multiple differing pathways. Although underlying genetic mutations are diverse and heterogenous, most CRCs arise as either traditional tubular adenomas or serrated adenomas. Eventually these adenomas acquire additional carcinogenic mutations sufficient to develop invasive potential[6]. This sequence forms the basis of colonoscopic screening and surveillance programs. Not only can cancers be detected at an early stage where curative and non-invasive treatment is possible, but in many cases these pre-cancerous adenomas can be resected prior to their differentiation into carcinomas with invasive potential.
Unfortunately, interval CRCs still develop in patients who have undergone appropriate colonoscopic screening, accounting for 4.8%-7.9% of all CRCs[7-11]. Given that most adenomas take an estimated 5-15 years to develop into CRC, these interval cancers likely represent adenomas missed at the time of colonoscopy[12]. In fact, a 2019 meta-analysis found miss rates for adenomas to be as high as 26%[13]. Studies have consistently demonstrated that location in the proximal colon leads to an increased chance of missed adenomas, with interval cancers more than twice as likely to be proximally located[11]. Multiple factors contribute to this risk, as proximally located polyps are more likely to be flat, more likely to be sessile serrated polyps, more dysplastic whilst smaller and less likely to be hyperplastic polyps without malignant potential[14-16].
While certain polyp-related factors contribute to the likelihood of missed adenomas, overall adenoma detection rates (ADRs) are also highly operator-dependent. For example, a retrospective propensity-score matched study demonstrated an ADR of 44% for “high-ADR endoscopists” vs 26.9% for “low-ADR endoscopists” in the same Japanese screening population[17]. In this study, “high-ADR endoscopists” were more likely to detect proximal, non-protruding and high-risk adenomas. It is therefore not surprising that studies have demonstrated an inverse correlation between endoscopists” ADR and interval cancer development, with each 1% increase in ADR resulting in a 3% reduction in interval cancer risk[18,19]. Kaminski et al[19] also demonstrated an increase in interval cancer development in endoscopists with an ADR < 20%. Accordingly, societal guidelines recommend a minimum ADR of 25% (20% in women, 30% in men) as a means of ensuring quality control among colonoscopists[20]. More recently, the mean number of adenomas detected during colonoscopy has been raised as a possible alternative quality indicator, as the number of adenomas detected directly impacts surveillance intervals. Denis et al[21] found that even endoscopists with an ADR of more than 35% had considerable variation in mean adenoma detection over 42817 surveillance colonoscopies, from 0.36 to 0.98. The adenoma miss rate has also been demonstrated to vary considerably between high ADR endoscopists, instead correlating strongly with adenomas detected per colonoscopy[22].
Given the heterogeneity among proceduralists and the ongoing prevalence of interval CRCs, multiple add-on devices and techniques have been developed to increase mucosal visualisation and reduce adenoma miss rates. A 2020 network meta-analysis demonstrated that add-on devices such as “Endocuff vision” and techniques such as water-immersion colonoscopy do improve adenoma detection [relative risk (RR) 1.53 and 1.41 respectively] however they require additional equipment and cost while often increasing procedure times[23]. The addition of a transparent cap attached to the tip of the colonoscope has been demonstrated to improve adenoma detection while also reducing caecal intubation time[24-26]. However, a 2012 meta-analysis found the impact of these measures to be small, with a RR of 1.08 for adenoma detection and a mean 0.64 min reduction in caecal intubation time[27]. In the context of expansive population screening programs, small changes in equipment costs and procedure times have a considerable impact on a larger scale.
Advanced mucosal imaging techniques function by either improving image definition, application of dyes/altering the light source to enhance certain tissue features, digitally enhancing images in real time, or by providing “alerts” to the proceduralist for abnormal findings detected by artificial intelligence (AI). In doing so, these technologies aim to improve detection and characterisation of polyps without increasing equipment costs. This review aims to consider and summarise the numerous available advanced imaging technologies and examine their efficacy in both polyp detection and polyp characterisation. Whilst this is not a formal systematic review, it has been based largely on a structured interrogation of existing literature using Pubmed and Embase, with abstracts screened for relevance and reference lists searched for additional pertinent studies.
White light imaging (WLI) is the original unenhanced form of endoscopic imaging. Standard definition (SD-WLI) endoscopes produce a signal of up to 100000 to 400000 pixels, compared to high-definition (HD-WLI) endoscopes which produce from 850000 to more than 1 million pixels[28]. Despite this considerable improvement in image quality, studies comparing HD-WLI to SD-WLI have found an only marginal benefit in adenoma detection, with a 2020 meta-analysis of 6 randomised-controlled trials (RCTs) involving 4594 patients finding an ADR of 40% for HD-WLI vs 35% for SD-WLI (RR 1.13, P = 0.001)[29-31]. However, various studies have demonstrated a more significant increase in detection of flat adenomas (8.2%-9.5% vs 2.4%-3.8%), right sided adenomas (34% vs 19%) and sessile serrated polyps (RR 1.55, P = 0.03) with HD-WLI[29,31,32]. In the context of inflammatory bowel disease (IBD) where dysplasia detection is notoriously difficult, HD-WLI leads to increased likelihood of dysplasia on targeted biopsies, with an adjusted prevalence ratio of 2.99 (CI 1.16-7.79) in one 2013 study[33]. In fact, Krugliak et al[34] described 36 patients who underwent colectomy for dysplasia in IBD found using HD-WLI colonoscopy, in which no metachronous lesions were discovered that had not been detected endoscopically. While the overall benefit in adenoma detection may be marginal, the improved detection of high-risk, flat, right sided lesions, along with the fact that HD-WLI is now widely available, has led to almost universal uptake of HD-WLI in screening colonoscopy.
Chromoendoscopy involves topical application of dyes to enhance mucosal characterisation and improve detection of pathologic lesions. For adenoma detection during colonoscopy, the most commonly used dye is methylene blue, which is rapidly absorbed into healthy colonic mucosa and more slowly absorbed in dysplastic tissue[35]. More recently, chromoendoscopy using acetic acid has been described, acting as a mucolytic agent as well as increasing mucosal surface opacity[36].
Multiple studies have demonstrated the efficacy of chromoendoscopy for neoplasia detection (particularly proximal serrated lesions) during screening and surveillance colonoscopy, with a 2016 Cochrane review (7 studies, 2727 participants) finding an odds ratio (OR) of 1.53 for detection of at least one neoplastic lesion[37,38]. However, the incremental benefit in many of these studies has been marginal and not associated with any increase in detection of advanced adenomas or larger polyps[39,40]. The strongest evidence for the benefit of chromoendoscopy has been for detection of dysplasia in the IBD population. Compared to SD-WLI, multiple meta-analyses have demonstrated the superiority of chromoendoscopy, with a RR of up to 2.05 for dysplasia detection[41,42]. However, the utility of chromoendoscopy in IBD has become more controversial as more recent studies have not demonstrated a difference between chromoendoscopy and HD-WLI[41,43,44].
Chromoendoscopy has been shown to improve dysplasia detection in other high-risk populations, particularly in those with an increased risk of flat, right-sided lesions. A 2019 tandem study comparing HD-WLI and chromoendoscopy with indigo carmine in patients with serrated polyposis syndrome found a higher additional ADR (39% vs 22%, P < 0.001) in the chromoendoscopy group[45]. In hereditary non-polyposis colon cancer (HNPCC), a 2019 meta-analysis demonstrated improved adenoma detection with a relative risk (RR) of 1.53 (CI 1.07-2.17)[46]. However, again recent evidence has found the benefit of chromoendoscopy over HD-WLI to be marginal in this setting, with a 2021 meta-analysis of three RCTs not reaching statistical significance (OR 1.17, CI 1.81-1.70)[47-49].
Irrespective, widespread uptake of chromoendoscopy has been limited by the increase in procedure time required for dye application. A 2019 meta-analysis in IBD surveillance found the total procedure time to be a mean of 21.69 min (CI 9.01-34.38) longer for chromoendoscopy[50]. One method to counter this was described by Repici et al[51], using oral dye (methylene blue) ingested at the time of bowel preparation. Promisingly, this led to an 8.5% increase in ADR without increasing procedure times, although there was no difference in detection of larger or more advanced polyps.
Virtual, or electronic chromoendoscopy have been developed in attempt to digitally recreate the enhanced mucosal visualisation of chromoendoscopy without increasing procedure time. However, no form of virtual chromoendoscopy has been able to conclusively demonstrate a benefit with respect to polyp detection at colonoscopy.
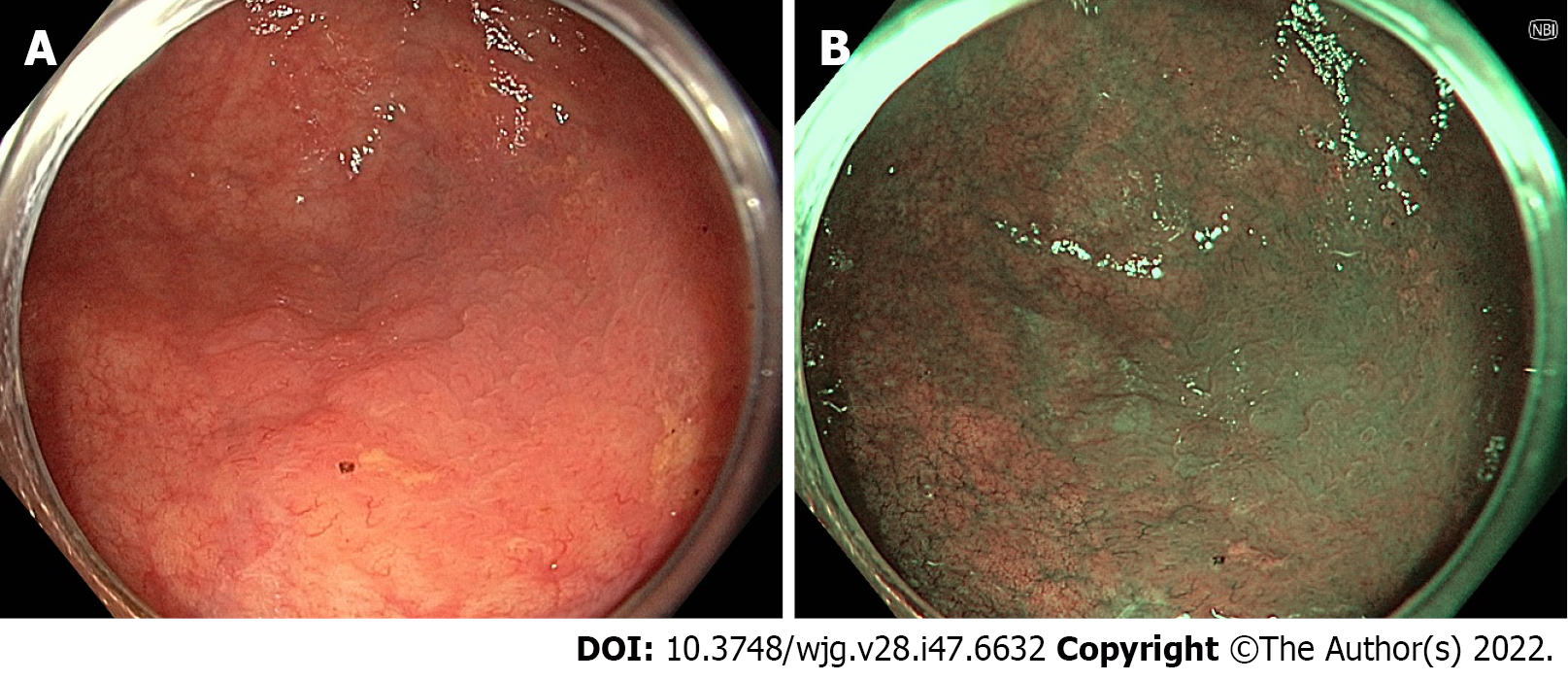
Narrow-band imaging (NBI) uses optical filters to produce two narrow bands of light centred at wavelengths of 415 nm and 540 nm, corresponding to the primary and secondary light absorption peaks of haemoglobin. Superficial capillaries appear brown, highlighted by the 415 nm wavelength, while deeper vessels in the mucosa and submucosa are cyan due to the deeper penetration of the 540 nm wavelength[52].
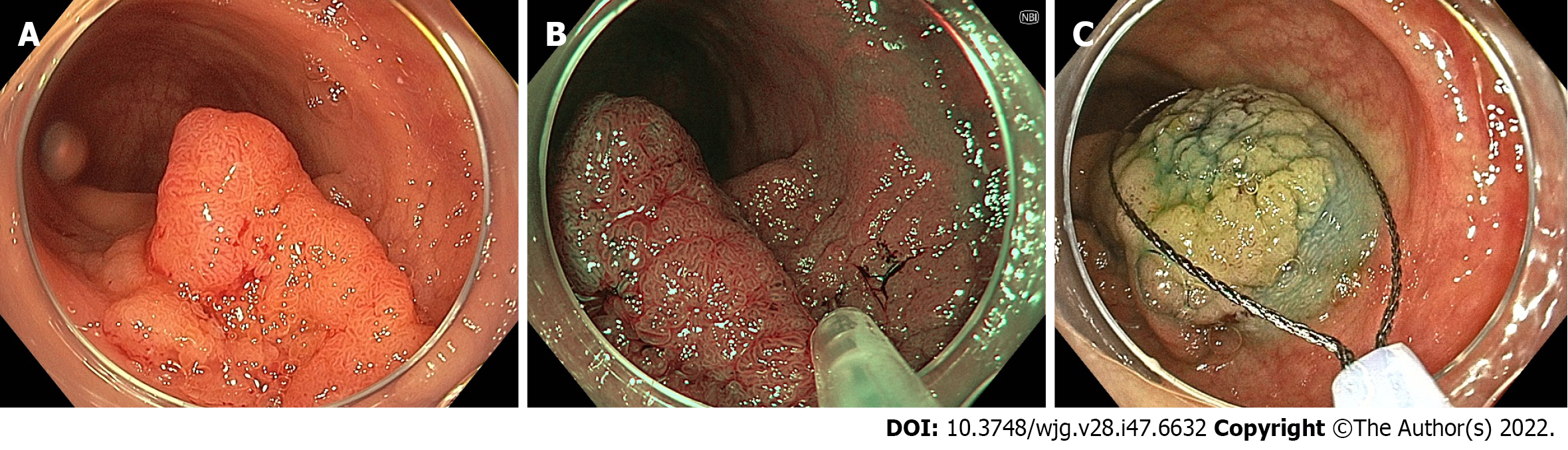
The role of NBI in adenoma detection during routine colonoscopy in the general population has been extensively studied. Studies that have found a benefit for NBI in this setting have demonstrated an improvement particularly in the detection of flat or depressed lesions (Figure 1), with a pooled RR of 1.96 in a 2012 meta-analysis[53-56]. However, the majority of studies, including a 2012 Cochrane review by Nagorni et al[57], have shown no difference in overall adenoma detection[32,57-61]. In fact, one 2017 RCT demonstrated a reduction in ADR with NBI when adjusted for increased withdrawal time[62].
Multiple possible factors may contribute to the limitations of NBI in screening colonoscopy. Earlier-generation NBI resulted in a reduction in overall brightness due to the narrow bandwidths, which may limit overall visualisation in the wide colorectal lumen. The second-generation bright NBI has been developed to counter this, although recent studies have again demonstrated no difference in overall adenoma detection[58,63]. NBI also appears to be disproportionately affected by poor bowel preparation (which may also be in part due to reduced brightness), with a 2019 meta-analysis finding superior adenoma detection with second-generation NBI only in patients with maximal bowel preparation scores[64]. In addition, the colour spectrum of NBI is different to WLI and therefore may require experience and familiarity with the technology in order to be effective. This was demonstrated by Minamide et al[63] who retrospectively reviewed 1831 patients that underwent colonoscopy using second-generation bright NBI or WLI and found a higher polyp detection rate (PDR) with NBI (80.9% vs 71.4%, P = 0.02) in academic centres familiar with its use, while in community centres, there was actually a trend towards a higher PDR with WLI (51.1% vs 47.7%). Additionally, in the NBI group, the ADR for NBI-experienced proceduralists was 63.2% vs 39.2% for NBI-inexperienced proceduralists (P < 0.001).
i-SCAN is a software-based post-processing technology, which digitally enhances WLI output through surface and contrast enhancement (i-SCAN mode 1) as well as tone enhancement (i-SCAN modes 2 and 3)[52]. Evidence has again been inconsistent regarding its efficacy for adenoma detection. Multiple studies have found an improvement in polyp and adenoma detection, the largest of which demonstrated a non-statistically significant improvement in ADR from 27% to 33% (P = 0.33)[65-68]. As demonstrated by Kidambi et al[69] in 2019, this effect has mainly been due to improved detection of diminutive, flat, right-sided adenomas[68,69]. In terms of high-risk populations, Bisschops et al[70] found a reduction in adenoma miss rates from 62% to 12% using i-SCAN in 61 patients with HNPCC. On the contrary, a 2012 prospective back-to-back study comparing HD-WLI with i-SCAN modes 1 and 2 in 389 screening colonoscopies showed no difference in ADR or adenoma miss rates, while a 2014 meta-analysis also demonstrated no difference in ADR[71,72]. There is therefore insufficient evidence to recommend routine use of i-SCAN in screening colonoscopy at this stage.
Flexible spectral imaging colour enhancement (FICE) also involves digital enhancement of WLI images from the video processor, emphasising certain wavelengths which can be determined by the proceduralists according to 10 factory-determined pre-set modes[52]. FICE was developed with the goal of providing mucosal enhancement without compromising the familiarity of colour patterns from WLI. While one early back-to-back colonoscopy study in 2012 demonstrated reduced adenoma miss rate using FICE[73], multiple studies have demonstrated no significant impact, with the largest RCT in 2010 by Aminalai et al[74] finding no difference in ADR between FICE and HD-WLI over 1318 colonoscopies.
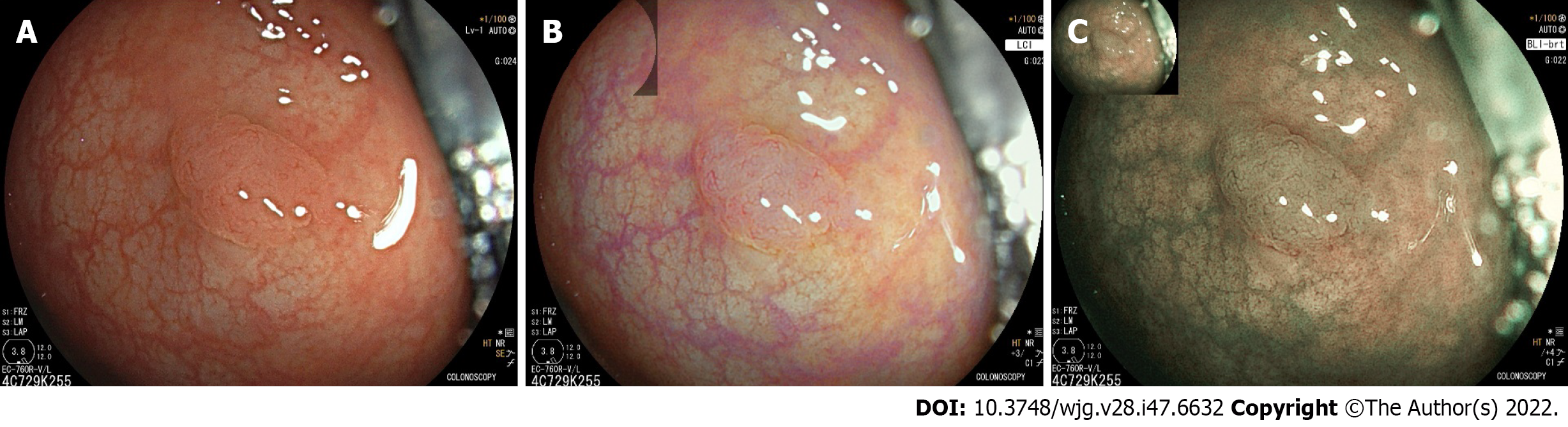
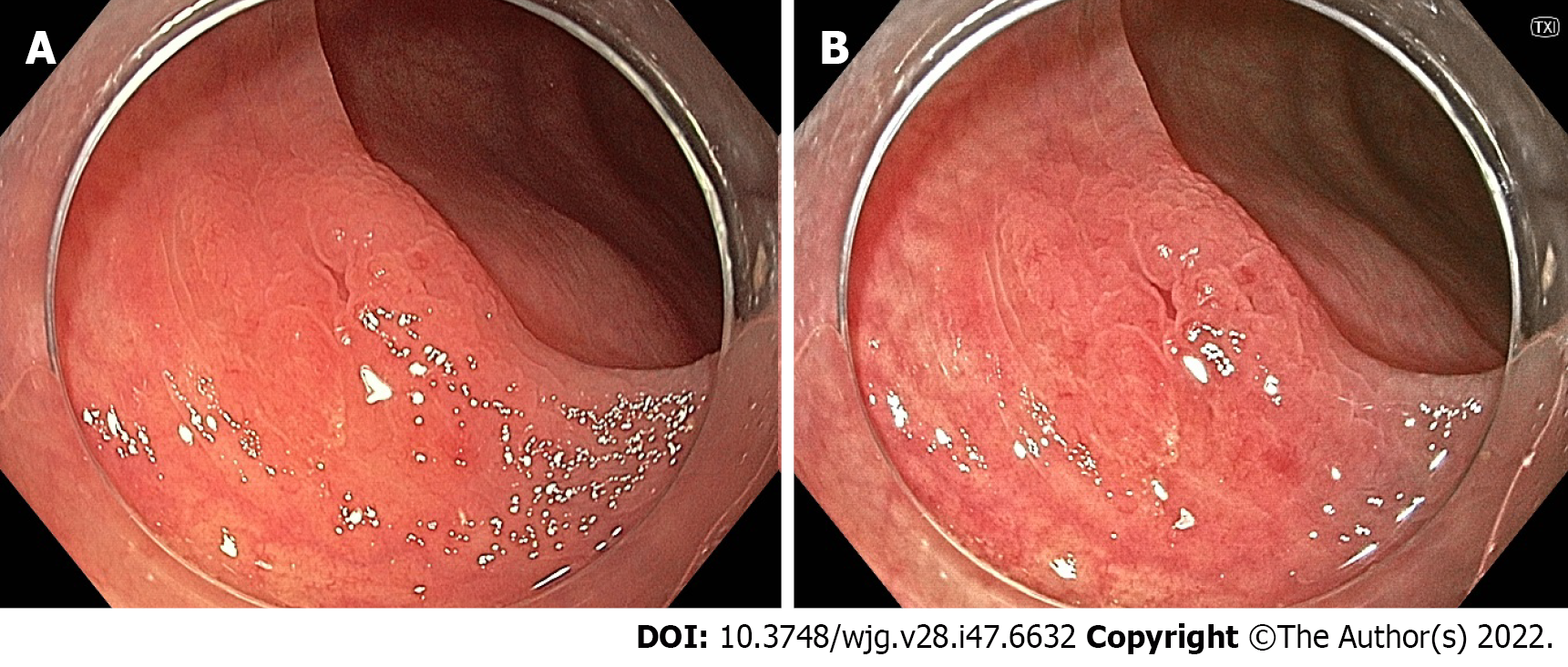
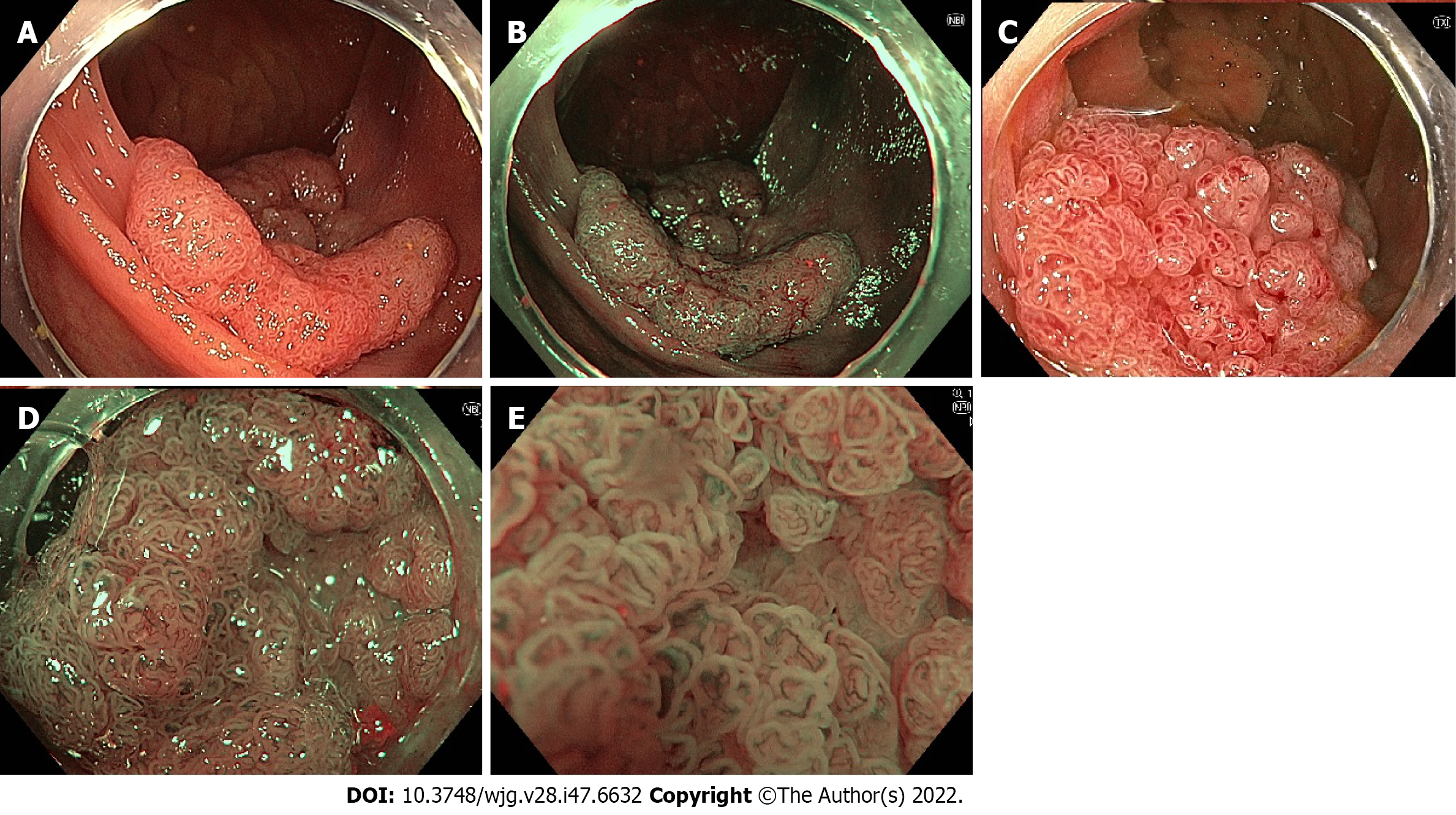
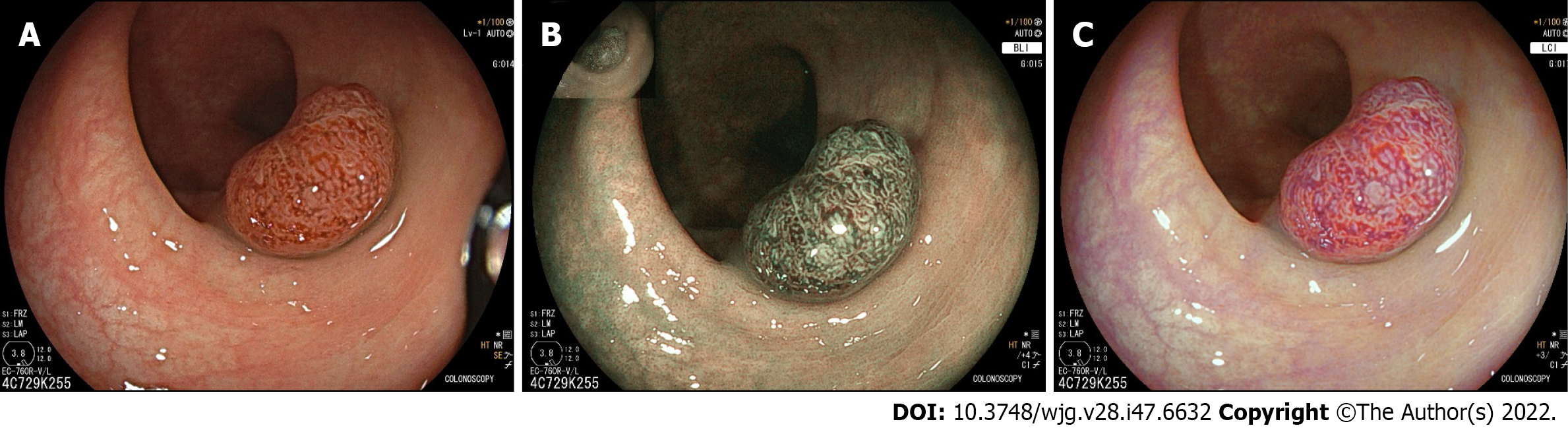
Linked colour imaging (LCI) uses both pre- and post-processing technology with narrow wavelength light to separate colours, increasing the vividity of the red and white colour spectrums and enhancing the contrast of mucosal surface patterns and superficial capillaries (Figure 2). It was developed with the aim of enhancing lesion visibility and surface characterisation without compromising brightness or familiarity of colour spectrums, offering perhaps the most promising early evidence for improved adenoma detection[75-77]. It has been demonstrated to improve lesion visibility in both video- and image-based studies when compared to HD-WLI, particularly for nongranular, flat lesions[75,78,79]. While evidence varies with regard to overall ADR, studies have found improvements in proximal adenoma detection and miss rates[80-84]. In addition, a 2020 meta-analysis of 7 studies including 3097 patients demonstrated improved adenoma detection (RR 1.26, P < 0.001), particularly in the right colon (RR 2.68, P < 0.001) and a mean of 0.27 additional adenomas detected per colonoscopy[85]. In a high-risk population of patients with HNPCC, LCI was found to improve ADR compared to HD-WLI (36.3% vs 25.6%, P = 0.04)[86]. Interestingly, while advanced imaging such as NBI appears to have a greater impact when used by experienced endoscopists, a 2021 study by Hasegawa et al[87] found a strong negative correlation between the baseline ADR with HD-WLI and the improvement ratio, indicating that perhaps the familiar colour pattern allows effective use by non-expert proceduralists.
Blue light imaging (BLI) is form of digitally enhanced imaging which concentrates and enhances a specific wavelength of light between 410-450 nm, increasing the contrast of superficial micro-vessels and mucosal surface structures (Figure 2). BLI uses four independent light-emitting diodes rather than the xenon light used in NBI, which is postulated to improve brightness[88]. This new technology has not been as extensively studied, however a video-based 2015 study demonstrated improved visibility scores with BLI bright mode compared to WLI according to both expert and non-expert proceduralists[89]. On a smaller scale this translated into improved adenoma detection, with two studies (including 182 and 127 patients respectively), finding an improvement in ADR from 27.8% to 46.2% (P = 0.01) and a reduction in adenoma miss rate from 10% to 1.6% (P = 0.001) compared to HD-WLI[90,91]. In contrast, the largest prospective study to date, including 963 patients, did not find a difference in ADR, though did find a non-statistically significant increase in mean adenomas per patient (APP) (1.27 vs 1.01, P = 0.08)[92].
Texture and colour enhancement imaging (TXI) is a recently developed technology, where the HD-WLI image is split into two layers, each individually undergoing brightness enhancement, tone mapping and texture enhancement before the images are stacked (TXI mode 1) and undergo further colour enhancement (TXI mode 2)[93]. Similarly to LCI, this aims to enhance mucosal visualisation without compromising familiarity of colour patterns or brightness (Figure 3). As an only recently developed technology, clinical studies examining adenoma detection are not yet available, however preliminary studies have demonstrated improved visibility of adenomas and sessile serrated polyps using TXI compared to HD-WLI[94,95].
While virtual chromoendoscopy theoretically offers enhanced mucosa visualisation without the increase in procedure time required for dye-based chromoendoscopy, none of the currently available technologies have conclusively demonstrated a meaningful improvement in ADR compared to HD-WLI. These technologies may all have a role particularly in improving detection of flat, right sided adenomas and may be used as additional tools for examination during screening colonoscopy, but evidence is not yet sufficient for recommendation in societal guidelines. Data appear most promising for newer forms of post-processing technology where brightness and familiarity of color patterns are preserved, however additional research is required to confirm this efficacy.
Light of a specific wavelength induces cell autofluorescence produced by endogenous fluorophores, with varied characteristics between normal (green), inflamed (dark green) and neoplastic (magenta) tissue. Autofluorescence imaging (AFI) relies on the detection and delineation of this natural fluorescence after stimulating the mucosal cells with short wavelength light[96,97]. In doing so, AFI aims to detect neoplastic or dysplastic tissue even before it manifests as an anatomically distinguishable discrete lesion. McCallum et al[98] demonstrated that colonic adenomas have a significantly higher autofluorescence intensity than non-neoplastic polyps. It is therefore unsurprising that the greatest impact of AFI across multiple studies has been improved detection of flat, right sided polyps rather than elevated polypoid adenomas, with one RCT reporting an ADR for flat neoplasms of 42.5% vs 29.2% (P < 0.001)[99,100]. However, a 2015 meta-analysis found that while the adenoma and polyp miss rates were lower with AFI, there was no difference in overall ADR despite an average of 8 min longer procedural time for the AFI group[101].
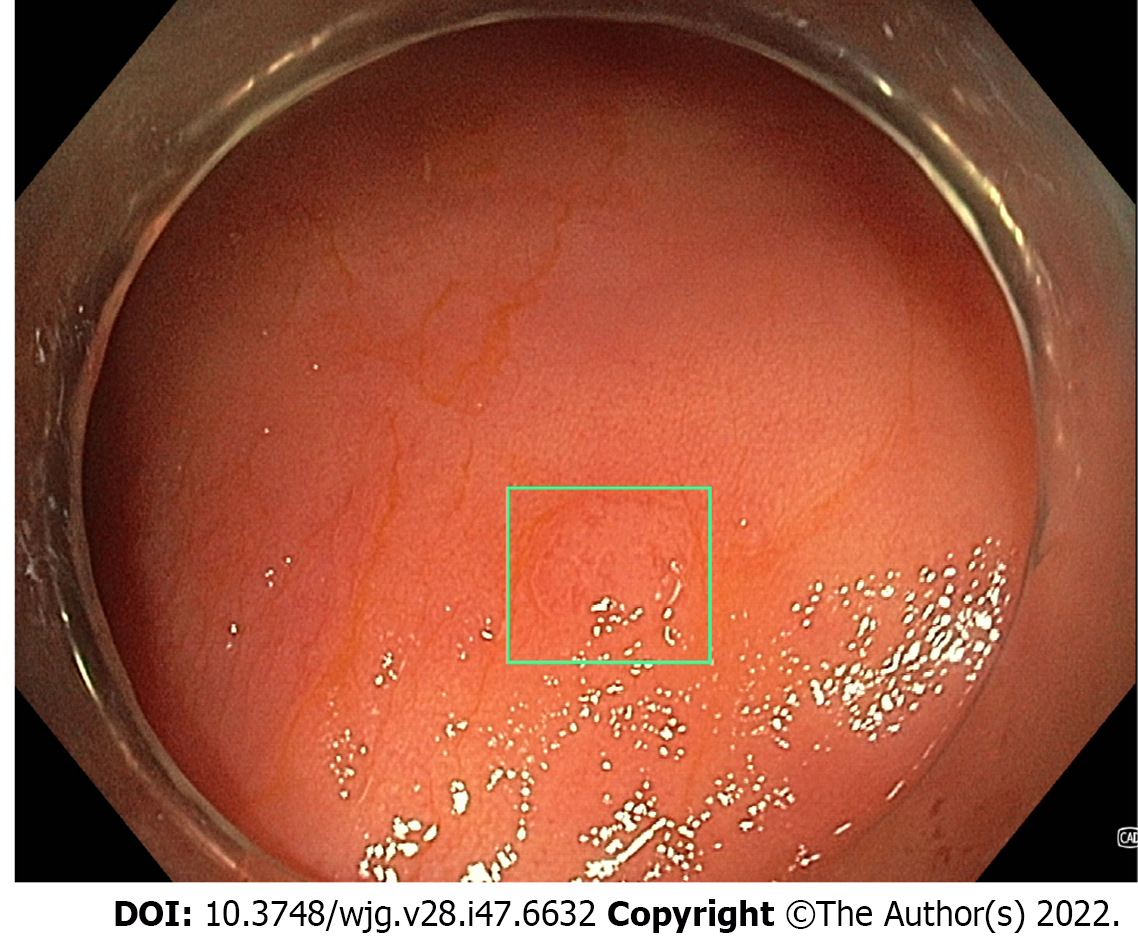
Multiple AI systems have been developed for polyp detection during surveillance colonoscopy, referred to as computer aided detection (CADe). These systems use convulational neural networks (CNNs) which are trained using still images and videos of polyps[102]. The most recent systems then output a real-time alert to the proceduralist to the presence of the polyp, most commonly with a square around the perimeters of the image output or around the polyp itself (Figure 4). CADe systems were initially analysed in still image- and video-based studies, demonstrating a sensitivity of 95%-99% and accuracy of 96%[103-107]. Subsequently, large studies by Repici et al[102] (2020) and Wang et al[108,109] (2019 and 2020) in real-time AI-assisted colonoscopy have demonstrated an increase in ADR (RR 1.61 vs 1.30), as well as a 1.46- to 1.72-fold increase in total adenomas detected. The adenoma miss rate in tandem colonoscopy studies has also been demonstrated to be lower with CADe-assisted colonoscopy (14%-20%) compared to WLI (31%-40%)[110,111]. Subsequently, multiple meta-analyses have consistently demonstrated improved ADR and adenomas detected per colonoscopy with CADe systems (Table 1)[112-115].
| Ref. | Studies, patients | ADR | Adenoma per patient | Withdrawal time | False positives | ||||
| AI | WLI | RR | AI | WLI | Mean difference | Mean difference CADe vs control | |||
| Aziz et al[112], 2020 | 3 studies, 2815 patients | 32.9% | 20.8% | 1.58 | 0.47 | 0.26 | 0.20 | 0.9 min (P = 0.03) | 4.87% (n = 137) |
| Hassan et al[113], 2021 | 5 studies, 4354 patients | 36.6% | 25.2% | 1.44 | 0.58 | 0.36 | 0.22 | 0.34 min (P = 0.13) | - |
| Spadaccini et al[114], 2021 | 6 studies, 5178 patients | 34.0% | 26.6% | 1.78 | - | - | - | No significant difference | - |
| Barua et al[115], 2021 | 5 studies, 4311 patients | 29.6% | 19.3% | 1.52 | 0.41 | 0.23 | 0.18 | 0.5 min | 11.2% |
An alternative role for AI-assistance in screening colonoscopy is based on quality assurance, employing AI to monitor withdrawal speed, endoscope slipping and blind spots to ensure consistency in colonoscopic practice. Gong et al[116] studied ENDOANGEL for this purpose and in a 2020 RCT involving 704 patients demonstrated an odds ratio of 2.3 for adenoma detection. Similar results were demonstrated by Su et al[117] using their Automatic Quality Control System (AQCS). Although not yet explored in studies, it may be that the combination of these AI systems using quality control and CADe may facilitate optimal adenoma detection. This is an area for further study as these systems become more widely available.
Fluorescence molecular endomicroscopy (FME) involves targeted fluorescent agents that bind to specific cellular components of dysplastic cells, allowing detection using a specialised near infra-red FME (NIR-FME) probe[118]. For example, Hartmans et al[119] developed a fluorescently-labelled antibody against vascular endothelial growth factor A (which is upregulated in colonic adenomas) and injected this intravenously 3 d prior to colonoscopy. In their pilot study, all 39 adenomas from 15 patients were detected using the NIR-FME probe, demonstrating the feasibility of this technique[119]. Alternatively, Joshi et al[120] identified a peptide sequence that binds specifically to sessile serrated adenomas/polyps (SSA/Ps) which was administered topically using a spray catheter to 38 subjects undergoing routine outpatient colonoscopy, distinguishing SSA/Ps from normal colonic mucosa with 89% sensitivity and 92% specificity[120].
As a result of the expanding range of advanced imaging technologies (Table 2) and improved adenoma detection, patients will increasingly meet societal guidelines for more frequent surveillance colonoscopy. To counter this, guidelines may eventually need to be adjusted to reduce the frequency of colonoscopy based on diminishing adenoma miss-rates. However, a 2014 study by Gómez et al[121] demonstrated no difference in adenoma detection at follow-up colonoscopy after prior procedures completed by higher ADR endoscopists using HD-WLI. Currently, the duration of use of these advanced technologies has been insufficient to analyse polyp detection at future surveillance, hence further research is required as experience grows.
| Modality | Strengths | Weaknesses | |
| HD-WLI | Widely available | Marginal incremental benefit over SD-WLI | |
| Increased detection of flat, right-sided adenomas and SSAs | |||
| Chromoendoscopy | Increased detection of small and flat adenomas | No significant increase in detection of advanced adenomas | |
| Increased dysplasia detection in IBD (compared to SD-WLI) | Increased procedural time | ||
| May increase polyp detection in high-risk syndromes (serrated polyposis syndrome, HNPCC) | |||
| Virtual chromoendoscopy | NBI | May improve flat lesion detection | Loss of brightness and familiarity of colour patterns |
| Effective in those with experience using NBI | No evidence of increased total adenoma detection | ||
| Less effective when used by proceduralists inexperienced in NBI | |||
| i-SCAN | May reduce miss-rates in high-risk populations | Not widely available | |
| No difference in adenoma detection in larger studies | |||
| Insufficient evidence to recommend use | |||
| FICE | Retains familiar colour patterns | Not widely available | |
| No difference in ADR | |||
| LCI | Retains familiar colour patterns | Not widely available | |
| Effective when used by non-LCI experienced proceduralists | Variable evidence regarding overall adenoma detection | ||
| Improve adenoma detection, particularly right sided and flat lesions | |||
| BLI | Improved adenoma detection and miss rate in smaller studies | Not widely available | |
| No difference in ADR in largest study to date | |||
| TXI | Retains familiar colour patterns | Not widely available | |
| New technology therefore insufficient evidence | |||
| AFI | Improved detection of flat/right sided polyps | Not widely available | |
| Increased procedure time | |||
| No difference in overall ADR | |||
| AI | Improves ADR | Expensive currently | |
| Improves consistency between proceduralists | Not widely available | ||
| Quality assurance | Some increase in procedure time | ||
| FME | In theory may improve detection of flat/poorly visible polyps | Insufficient evidence | |
| Requires injection/ingestion of tracer | |||
Polyp characterisation is critically important for both small and larger polyps. In the context of diminutive (< 5 mm) and small (< 10 mm) polyps, accurate characterisation has facilitated the “resect and discard” and “do not resect” strategies. For larger polyps, accurate endoscopic characterisation guides the selection of suitable polyps for endoscopic resection as well as the most appropriate resection technique.
Traditionally, all polyps identified during colonoscopy have been resected and examined histologically. However, as the accuracy of endoscopic identification of polyps has improved, the “resect and discard” or even “do not resect” strategies have been developed to minimise the resource consumption of routine histological analysis. These strategies were developed after large studies found that advanced histology (at least high-grade dysplasia) is present in as few as 1.7% of diminutive (≤ 5 mm) polyps, and only 6.6%-10.0% of small (< 10 mm) polyps[122,123]. In fact, a 2013 meta-analysis including 6280 polyps found only 56.7% of diminutive polyps are even neoplastic[124]. On this basis, the American Society of Gastrointestinal Endoscopy (ASGE) published the Preservation and Incorporation of Valuable endoscopic Innovations (PIVI) thresholds for adopting real-time endoscopic assessment of polyps for “resect and discard” and “do not resect”[125]. For diminutive polyps to be discarded without pathological assessment, endoscopic imaging should provide a ≥ 90% agreement in assignment of post-polypectomy surveillance. Polyps > 5 mm in size should be sent for histological assessment given the up to 10% frequency of more advanced histology which would alter surveillance intervals[123]. For diminutive rectosigmoid hyperplastic polyps, imaging should provide ≥ 90% negative predictive value for adenomatous histology. Even hyperplastic-appearing diminutive polyps proximal to the sigmoid colon should be resected as these polyps have a more than 10% chance of being SSA/Ps histologically[126]. These strategies would result in significant cost-savings to the healthcare sector. For example, Solon et al[127] examined the potential financial impact of this strategy for the National Health Service (NHS) in England in 2016, demonstrating potential annual cost savings of £141192057.
For larger polyps, endoscopic characterisation is critical to guiding suitability for endoscopic resection as well as appropriate resection techniques. Even for non-interventionalists who are not proceeding with immediate resection, accurate characterisation without the need for biopsy may be ideal to guide appropriate referral. Kuroha et al[128] highlighted this in a 2021 study examining predictors of success in 369 colorectal ESDs. Severe fibrosis was associated with increased mean procedure time, as well as lower en bloc and complete resection rates, with the greatest predictors of severe fibrosis on multivariate analysis being prior resection attempt (OR 175.4) and pre-treatment biopsy (OR 8.3)[128]. In addition, pre-resection biopsies can be inaccurate in large lesions, with false negative rates as high as 86% for adenocarcinoma, therefore characterisation with advanced imaging and upfront endoscopic resection may be more appropriate[129].
Accurate polyp characterisation using advanced mucosal imaging is impacted to some extent by proceduralist experience. A 2014 video-based study demonstrated that interventional endoscopists specialising in complex polypectomy were more accurate in identifying malignant polyps when compared to other endoscopists[130]. However, multiple studies support the efficacy of specialised training in advanced mucosal imaging for polyp characterisation, irrespective of endoscopist experience. Both Bae et al[131] and Patel et al[132] have studied the accuracy of endoscopists before and after a training module on identification diminutive rectal polyps, in whom the negative predictive value (NPV) for diminutive neoplastic polyps improved from 82.1% to 92.5%-94.7%, thus meeting the PIVI threshold. In addition, studies have demonstrated accurate characterisation after training even in medical residents with no endoscopy experience, while Basford et al[133] found no difference in the accuracy of interpretation of HD-WLI and i-SCAN images prior to specific training between consultant gastroenterologists, trainees and medical students[133-136]. Proceduralists should therefore engage in specific training in advanced mucosal imaging rather than relying on experience alone, in order to improve accuracy of polyp characterisation.
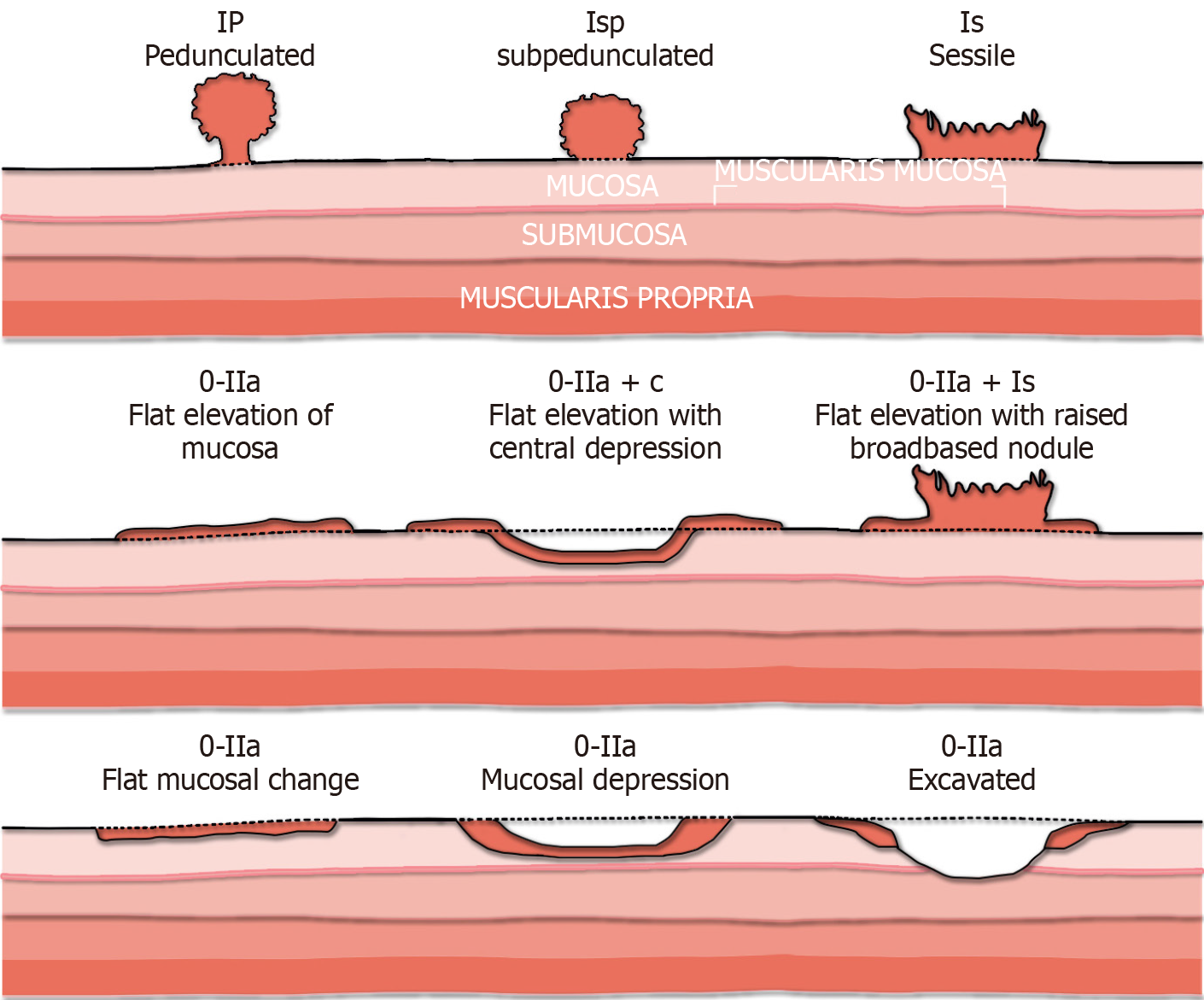
Multiple polyp classification systems have been developed to improve polyp characterisation (Table 3). While not reliant on advanced imaging, the Paris classification aids in risk stratification for larger polyps prior to consideration of endoscopic resection (Figure 5)[137]. A large multicentre 2017 study found that the presence of any 0-IIc (“depressed”) component predicted submucosal invasive cancer in almost 30% of patients. In laterally spreading tumours (LSTs), the presence of an elevated component (0-IIa + Is) predicted submucosal invasion in over 10% over patients vs 4.9% for those with flat lesions alone (0-IIa) (P < 0.001)[138]. However there is considerable inter-observer variability, particularly with regard to classification of lesions as flat vs sessile, with one study finding a kappa statistic of 0.42[139]. Van Doorn et al[139] proposed a simplified classification system of “pedunculated”, “elevated” (including flat and sessile) and “depressed” in order to address this, which resulted in improved interobserver agreement and 91.6% accuracy for prediction of invasive cancer[140].
| System | Imaging modality | Polyp features | Accuracy | Complexity | TA/TVAs included | SSAs included |
| Kudo | Any | Pits | AUC 0.94[143] | Complex | Yes | No |
| NICE | NBI | Vessels and pits | Sensitivity 98%, NPV 97.8%[145] | Moderate | Yes | No |
| JNET | NBI | Vessels and pits | AUC 0.97 for JNET 1, 0.84 for JNET 2A, 0.9 for JNET 3 but less accurate for JNET 2B (AUC 0.72)[152] | Moderate | Yes | No |
| BASIC | BLI | Vessels, pits and surface | Accurate surveillance prediction in 90%, NPV for rectosigmoid polyps 91%[160] | Moderate | Yes | No |
| WASP | Any | Pits, surface, shape | May improve SSA detection[162] | Simple | No | Yes |
| mSano | NBI | Vessels, pits and surface | AUC 0.92[169] | Simple | Yes | Yes |
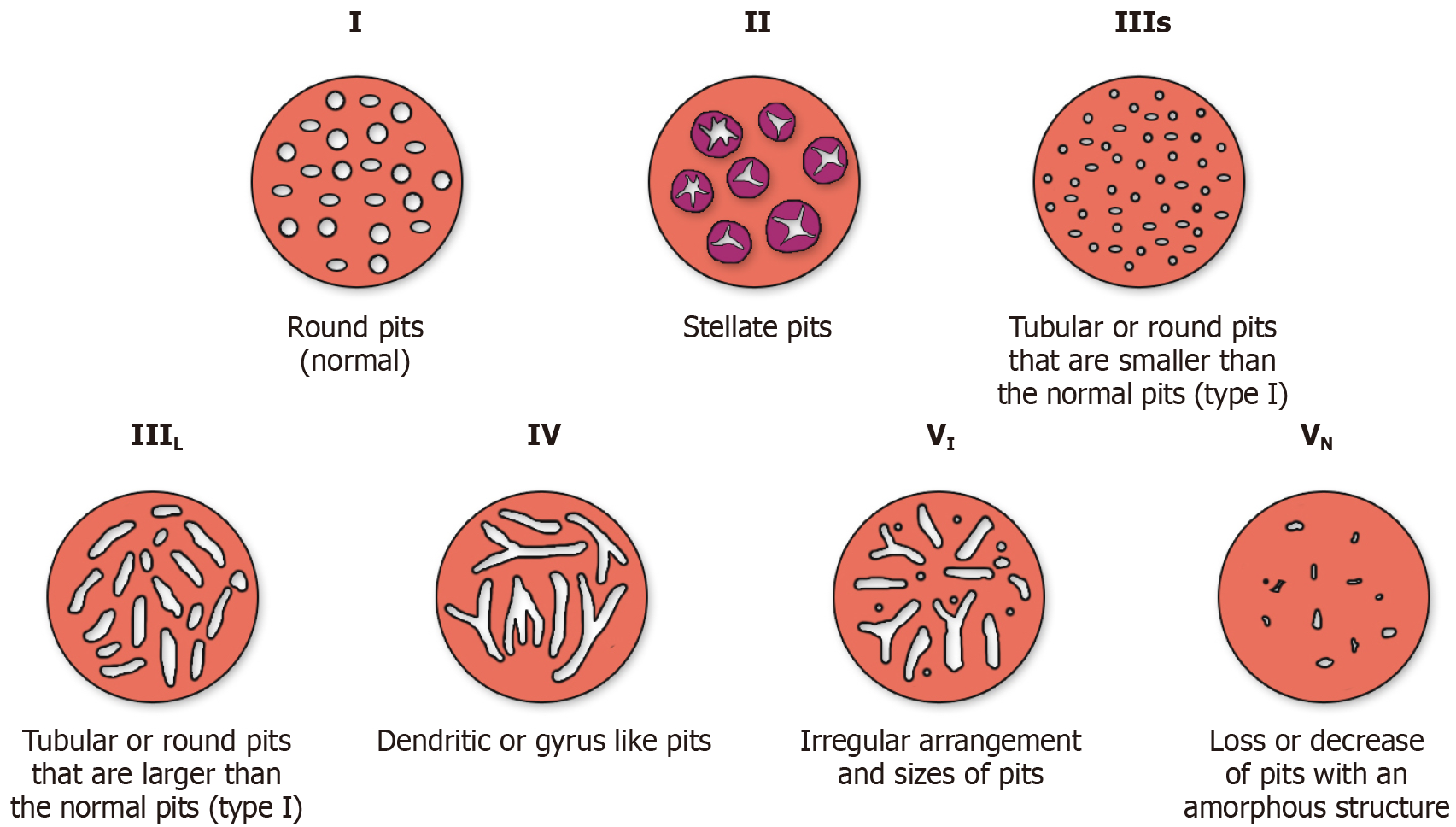
The Kudo classification (Figure 6) was developed in 1996 to classify polyps according to their “pit patterns” on magnifying endoscopy[141]. Type I pits appear round, while type II appear stellate or papillary, both representing benign changes (normal, hyperplastic or inflammatory). Type III-s pits are smaller, round, tubular pits while type III-L are larger tubular pits, representing tubular adenomas (TA). Type IV pits are branch-like or gyrus-like and represent tubulo-villous adenomas (TVA), while type V pits are non-structured representing HGD or cancer[142]. Multiple studies have assessed the accuracy of the Kudo classification, summarised by a 2014 meta-analysis of 20 studies, including 5,111 colorectal lesions[143]. Pit pattern classification differentiated neoplastic from non-neoplastic polyps with a pooled sensitivity of 89.00%, specificity of 85.78% and area under the receiver operating characteristic curve (AUC) of 0.94[144].
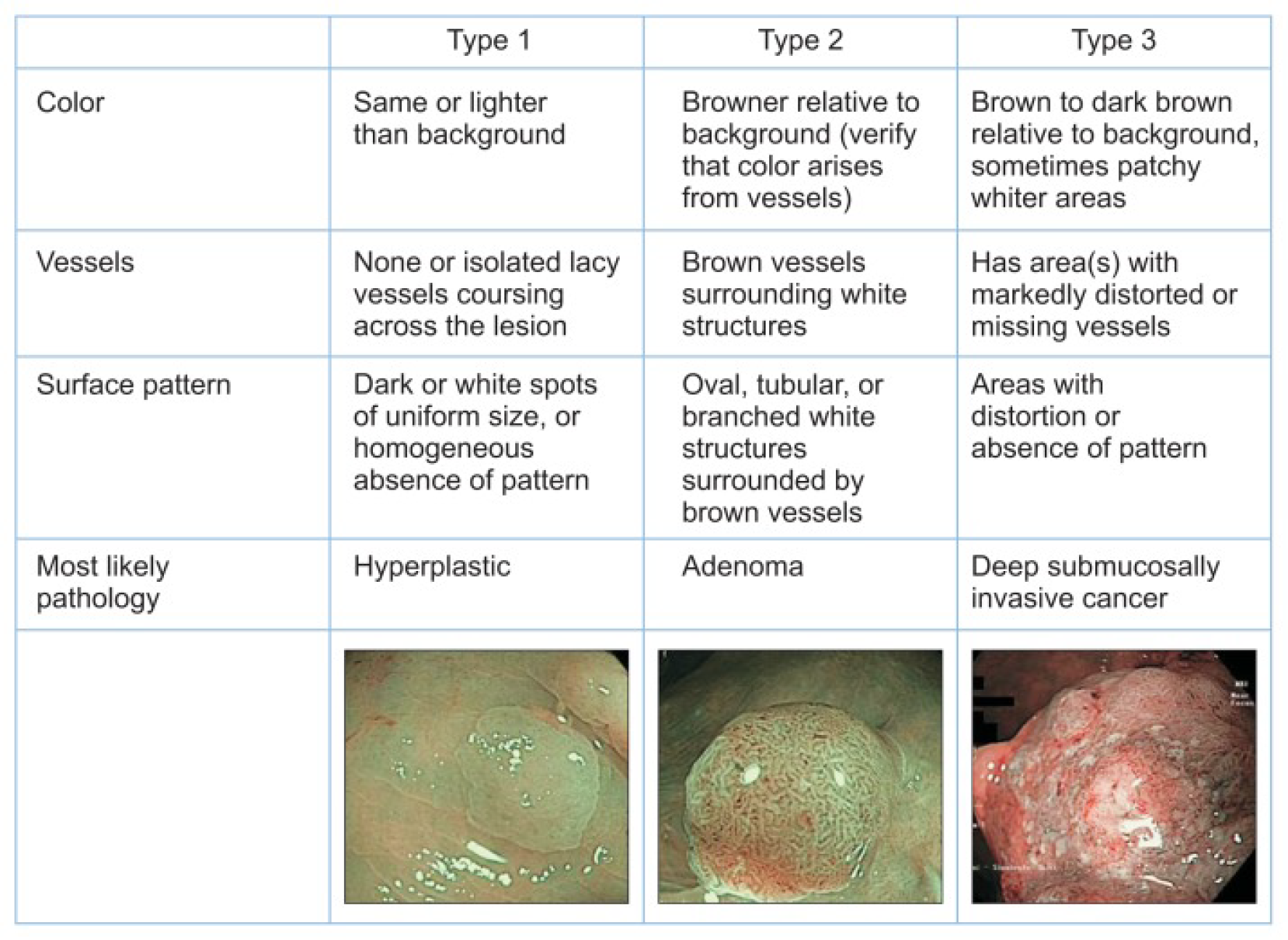
The NBI International Colorectal Endoscopic (NICE) classification was developed in 2012 with the goal of developing an international consensus for classification using NBI[145]. This classification takes into account the polyp colour, vessel pattern and surface pattern to characterise polyps into NICE type 1 (hyperplastic) type 2 (adenoma) and type 3 (invasive cancer) (Figure 7). Using this simplified classification results in highly accurate differentiation of neoplastic from non-neoplastic polyps, with sensitivity of 97%-99%, specificity of 85%-95% and accuracy of 89%-98% across 3 Large prospective studies[145-147]. In the 2012 validation study, 471 predominantly diminutive and small polyps were predicted with high-confidence with sensitivity of 98% and NPV of 97.7%, while 119 low-confidence predictions resulted in a sensitivity of 94.2% and NPV of 94.4%, both easily exceeding PIVI thresholds[145]. However, in a study of 2123 larger lesions, the NICE classification predicted deep invasive cancer with a sensitivity of just 58.4%. Nevertheless, due to low rates of deep invasion this was still associated with an NPV of 96.4% and specificity of 98.1%, therefore the authors suggested that even large NICE 1 and 2 Lesions should be considered for endoscopic resection[148]. The NICE classification has also been validated in a smaller cohort using i-SCAN rather than NBI, with similar results[149].
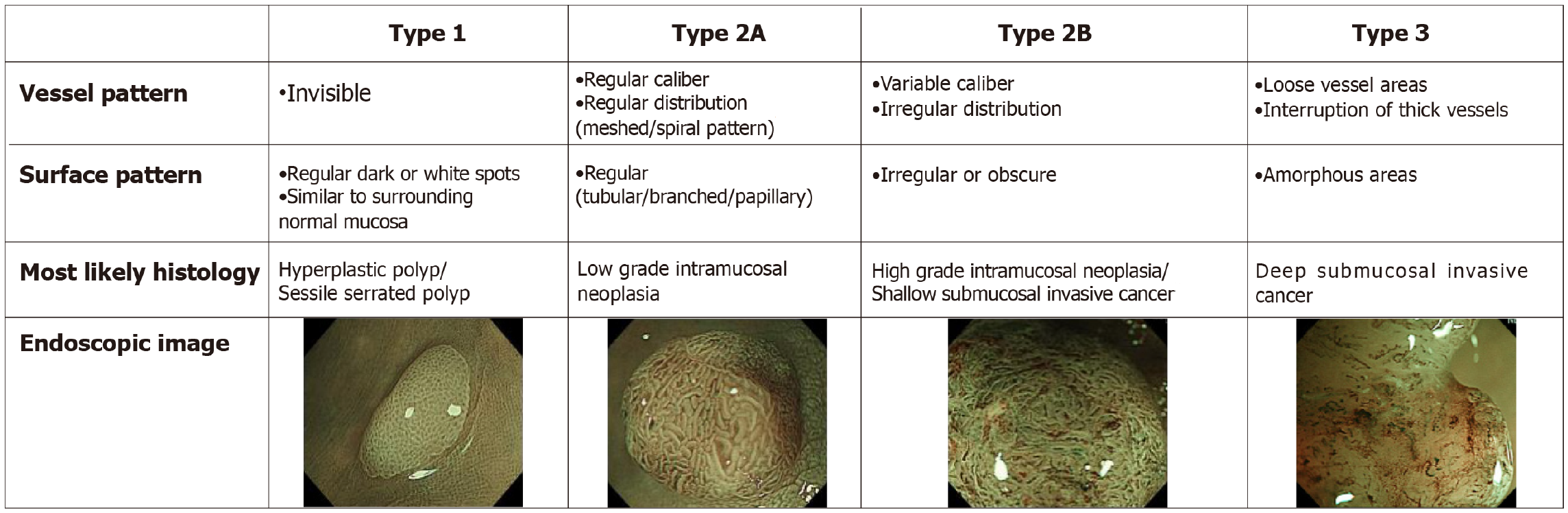
More recently, the Japan NBI Expert Team developed the Japan NBI expert team (JNET) classification specifically for the classification of colorectal polyps based on their appearance on magnification NBI using a combination of vessel and surface pattern analysis (Figure 8)[150,151]. The JNET classification is highly accurate for differentiating neoplastic vs non-neoplastic polyps, with an AUC of 0.97 for JNET 1 (hyperplastic/SSA/Ps) and 0.84 for JNET 2A (adenoma with LGD) in a 2020 meta-analysis[152]. In a retrospective 2020 study, this resulted in an increase in the number of adenomas resected per colonoscopy (1.7 vs 1.2, P < 0.01) and a reduction in resection of non-neoplastic lesions (8.9% vs 17.0%, P < 0.01)[153]. It is also highly specific in predicting deep invasive cancer in JNET 3 Lesions, with specificity of 100% and an AUC of 0.9[152]. In addition, unlike other systems, the JNET classification has been validated for characterisation of dysplasia within SSA/Ps, with Murakami et al[154] finding that the presence of JNET 2A/B/3 foci within a JNET 1 Lesion is 83.9% sensitive, 95.5% specific and 94.5% accuracy for detection of dysplasia within sessile serrated lesions. However, the main limitation of the JNET classification is in the interpretation of JNET 2B lesions, with studies demonstrating a wide range of advanced pathology, from HGD to superficial invasive cancer and even deep invasive cancer in JNET 2B polyps, with an AUC of 0.72[152,155,156]. This was highlighted in a recent study that retrospectively reviewed 297 colorectal adenocarcinomas, in which the probability of deep invasion was only 1.8% for JNET 2A, 30.1% for JNET 2B and 96.6% for JNET 3[157,158]. In this study, JNET 2B lesions were then further analysed using chromoendoscopy and Kudo”s classification of pit patterns. In Kudo non-V lesions, the risk of deep invasion was only 4.3%. Overall, JNET differentiates accurately for JNET 1, 2A, and 3 lesions, however proceduralists should consider further examination with magnified chromoendoscopy for JNET 2B lesions to improve accuracy of histology prediction.
The BLI adenomas serrated international classification (BASIC) classification was developed in 2018 for classification of polyps using BLI, based on assessment of surface, pit patterns and vessels, classifying polyps as either hyperplastic, traditional adenomatous, sessile serrated or cancer[159]. In the largest prospective validation study of 748 diminutive polyps this classification reached PIVI thresholds with accurate surveillance prediction in 90% and an NPV for rectosigmoid polyps of 91%[160].
The dutch workgroup serrAted polypS & polyposis (WASP) classification was developed in 2016 to facilitate accurate differentiation of SSA/Ps from hyperplastic and traditional adenomatous polyps as many existing classification systems did not allow for inclusion of SSA/Ps[161]. It’s accuracy has been validated by Lee et al[162] who demonstrated that the implementation of a specific training program in the WASP classification led to a statistically significant increase in SSA/P resection over the 6-mo training period, from 4.5% to 8% (P = 0.003).
The Sano classification (Figure 9) characterises polyps according to their capillary pattern, with barely visible honeycomb pattern capillaries in type I (normal or hyperplastic), larger elongated capillaries in type II [adenoma with low-grade dysplasia (LGD)] and irregular branching vessels in type III [high-grade dysplasia (HGD)] or adenocarcinoma)[163,164]. In a validation study, 97% of Sano II lesions were diagnosed as LGD while 87% of Sano III lesions were HGD or invasive cancer[165]. In 2013, this system was modified by Singh et al[166] (mSano classification) to include type IIo lesions in order to distinguish hyperplastic from sessile serrated polyps (Figure 7). Across multiple studies, the overall accuracy of the mSano classification has been between 90%-97%, with near-perfect interobserver agreement (k 0.89)[166,167]. The NPV for diminutive rectosigmoid polyps is as high as 100% and the accuracy for post-polypectomy surveillance 97%, exceeding the PIVI thresholds described above[167]. mSano as a standalone classification system was compared to the combination of the WASP and JNET classification in 2020, with superior high-confidence predictions (85% vs 69%, P < 0.05) and equivalent interobserver reliability[168]. It was also compared to the NICE classification in a 2018 RCT including 348 colonoscopies, with an AUC of 0.92 for prediction of neoplasia by mSano vs 0.78 for NICE (P = 0.02) and an AUC of 0.92 for prediction of suitability for endoscopic resection vs 0.83 for NICE (P = 0.04)[169]. The mSano is therefore a highly accurate standalone criteria for characterisation of colonic polyps including differentiation of neoplasia (including SSA/Ps) as well as invasive cancer.
There appears to be some incremental benefit from examination with HD-WLI alone vs SD-WLI for polyp characterisation, although this may be smaller than expected. In the largest direct comparison from Rastogi et al[31] in 2011, HD-WLI improved sensitivity for characterisation of small adenomas from 51.7% to 66.8% (P < 0.001) however the overall accuracy did not change. Minimal evidence exists comparing the accuracy of HD-WLI to SD-WLI for characterisation and prediction of invasion in larger polyps, however with the vast expansion of advanced imaging technologies, evidence increasingly supports the use of ancillary technology over HD-WLI in this context[170].
Chromoendoscopy has been demonstrated to be highly effective in differentiating neoplastic from non-neoplastic small colonic polyps, with overall diagnostic accuracy of greater than 99%[171-173]. However, with increasingly accurate forms of virtual chromoendoscopy for assessment of these diminutive and small polyps, the procedure time required for chromoendoscopy is likely to limit its ongoing use. Instead, the main ongoing role for chromoendoscopy may be in the prediction of invasion depth in larger lesions to guide resection techniques[174]. For example, the European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend the use of chromoendoscopy for pit pattern analysis in JNET 2B lesions (where NBI lacks accuracy), in order to further qualify the risk of deep invasion according to Kudo’s classification as described above[175]. This recommendation has been supported by Hosotani et al[157] in their 2021 study which demonstrated a PPV of 76% for invasive cancer in the presence of a “VH” pit pattern and a NPV of 96% for non-V pit patterns. Even in this context however, a recent prospective study including 400 patients found that there was no overall incremental benefit for the use of chromoendoscopy in addition to HD-WLI and NBI for the characterisation of large nonpedunculated polyps[176]. Novel indications for chromoendoscopy include the use of acetic acid chromoendoscopy or submucosal methylene blue injection (Figure 10) to clearly delineate polyp margins prior to resection[177-179].
While virtual chromoendoscopy has not been conclusively demonstrated to improve polyp detection, an expanding body of evidence supports its use for polyp characterisation to guide endoscopic resection strategies, as well as the “resect and discard” and “do not resect” strategies in diminutive polyps. For classification of highly prevalent small and diminutive polyps where dye-based chromoendoscopy may no longer be efficient on a population level, virtual chromoendoscopy has been demonstrated to have equivalent accuracy with a reduction in median procedural and interpretation time[180].
For diminutive colorectal polyps, multiple studies have shown that characterisation using NBI is able to easily exceed PIVI thresholds, with correct surveillance interval prediction in 92%-99% of cases and an NPV for diminutive rectosigmoid polyps of 91%-92%[62,124,181,182]. While many of these studies have been performed by expert endoscopists with experience in NBI, it has also been demonstrated that non-interventional endoscopists are able to achieve significant improvement following specific training, with Higashi et al[133] reporting an overall accuracy of 90% for non-interventionalists following a single training module[133,183].
Additionally, NBI has been used for the characterisation and prediction of invasion depth within larger colonic polyps (Figure 11). As early as 2008, Katagiri et al[165] demonstrated that an irregular capillary pattern (designated CP III) on NBI predicted a 65.6% (21/31) rate of invasive adenocarcinoma. Subsequently, Ikematsu et al[184] differentiated CP III into IIIA (characterised by high microvessel density with a lack of uniformity, blind ending, branching and curtailed irregularly) and IIIB (characterised by the presence of a nearly avascular or loose microvascular area). They found that IIIA lesions defined adenomas, intramucosal cancers and superficial submucosal invasive cancer, while IIIB lesions defined deep submucosal invasive cancers, with a sensitivity of 84.8%, specificity of 88.7% and overall accuracy of 87.7%. NBI has also been examined for detection of dysplasia and cancer within SSA/Ps. Tate et al[185] found that the presence of an adenomatous (NICE II) pattern within an SSA had 95% accuracy and a 98.1% NPV for detection of dysplasia within SSA/Ps, while Chino et al[186] demonstrated 100% sensitivity and 99% specificity with NBI for detection of cancers within SSA/Ps.
FICE has also been demonstrated to be highly accurate for the characterisation of colorectal polyps, with sensitivity of 89.4%-94.7%, specificity of 81.0%-89.2% and accuracy of 87.0%-89.4%[172,187-190]. However, Yoshida et al[187] did demonstrate its accuracy to be inferior to that of chromoendoscopy (89.4% vs 94.7%, P < 0.05). While minimal direct comparative data exists between modalities of virtual chromoendoscopy, Akarsu et al[191] found the NPV of FICE (80%) to be inferior to that of NBI (96.3%, P < 0.001), although there was no difference in overall accuracy.
i-SCAN has achieved similar results with respect to diminutive and small colorectal polyp categorisation, with sensitivity and specificity consistently above 90% across multiple studies[149,192-194]. It also appears to be an accessible form of advanced imaging for non-experts, with junior residents achieving similar accuracy to experts in one study after a 30-min training session[149]. There have been two RCTs directly comparing the accuracy of NBI and i-SCAN for polyp characterisation, both of which have found no difference in accuracy between these modalities but did demonstrate superiority for both NBI and i-SCAN when compared to HD-WLI[195,196].
LCI was developed in conjunction with BLI, aiming to improve polyp detection while BLI aimed to improve characterisation (Figure 12). Accordingly, minimal evidence exists regarding the accuracy of LCI for polyp characterisation. However, in 2017 Wu et al[197] employed the NICE classification using LCI, and reported a sensitivity of 96.5%, specificity of 83.8% and NPV of 93.9% for neoplastic lesion prediction.
The accuracy of BLI for polyp characterisation has been more extensively studied. Both retrospective and prospective studies have demonstrated the superiority of BLI over WLI for the characterisation of < 10 mm colonic polyps, with the largest 2019 prospective randomised study by Rondonotti et al[198] finding the overall accuracy for BLI to be 92% vs 84% for WLI (P = 0.01)[198-200]. BLI has also been compared to NBI using the JNET classification in a retrospective study where there was no significant difference in accuracy (92.1% for BLI vs 91.7% for NBI)[201].
While studies on AFI have been promising regarding polyp detection, its role in polyp characterisation appears limited. A 2011 RCT comparing HD-WLI, AFI and NBI did find that the overall accuracy of AFI is equivocal to that of NBI for distinguishing adenoma from hyperplastic polyps (84.9% vs 88.4%)[202]. However, the interobserver agreement for NBI with magnification is superior to that of AFI, while a 2017 meta-analysis demonstrated inferior specificity using AFI (44%) compared to NBI (69%, P = 0.031)[203,204].
As the most recently developed form of advanced mucosal imaging, TXI has yet to be studied in the context of polyp characterisation. Given the familiarity of color patterns, it may have some role for differentiation of neoplastic from non-neoplastic diminutive polyps which may increase its uptake during population screening. In addition, this familiarity may benefit proceduralists during resection by more clearly delineating polyp margins without compromising visualisation.
Extensive research has been undertaken in recent times into the development of AI systems for characterisation of colonic polyps, designated computer aided diagnosis (CADx) (Table 4). These systems have proven to be highly accurate in assessment of diminutive polyps, with a 2020 meta-analysis demonstrating a pooled AUC of 0.96 (CI 0.95-0.98) and a pooled NPV of 95.1%[104]. Interestingly, across multiple studies, CADx systems have not proven to be superior to expert endoscopists regarding histology prediction, although they have consistently led to improved histology prediction in non-expert endoscopists, nearing that of experts[205-208]. In these studies, the NPV for diminutive polyps has been 90%-97%, with an accurate surveillance interval in 93-94%, well surpassing PIVI thresholds[205,206,208-220].
| Ref. | Study type | Imaging modality | Number of patients/polyps | Sensitivity | Specificity | NPV | Accurate surveillance interval |
| Kominami et al[209], 2016 | Retrospective | NBI | 41 patients, 118 polyps | 93% | 95% | 93% | 92.7% |
| Chen et al[210], 2018 | Retrospective | NBI | 284 polyps | 96% | 78% | 90% | - |
| Mori et al[211], 2018 | Prospective | NBI | 325 patients, 466 polyps | 93% | 90% | 95% | - |
| Renner et al[212], 2018 | Retrospective | WLI, NBI | 100 polyps | 92% | 63% | 90% | - |
| Byrne et al[213], 2019 | Retrospective | NBI | 125 polyps | 98% | 83% | 97% | - |
| Min et al[214], 2019 | Prospective | LCI | 91 patients, 217 polyps | 83% | 70% | 71% | - |
| Sánchez-Montes et al[206], 2019 | Retrospective | WLI | 225 polyps | 92% | 89% | 87% | - |
| Horiuchi et al[215], 2019 | Prospective | AFI | 95 patients, 258 polyps | 80% | 95% | 93% | - |
| Ozawa et al[216], 2020 | Retrospective | WLI, NBI | 309 polyps | 97% for NBI, 90% for WLI | - | 91% for NBI, 85% for WLI | - |
| Jin et al[205], 2020 | Retrospective | NBI | 300 polyps | 83% | 90% | 94% | - |
| Zacharia et al[208], 2020 | Retrospective | WLI, NBI | 524 polyps | 96% | 90% | 93% | 94% |
| Rodriguez-Diaz et al[217], 2021 | Retrospective | NBI | 119 patients, 280 polyps | 96% | 84% | 91% | 94% |
| Van der Zander et al[218], 2021 | Retrospective | WLI, BLI | 54 patients, 60 polyps | 96% | 93% | 88% | - |
| Yoshida et al[220], 2021 | Retrospective | BLI | 25 patients, 100 polyps | 91% | 85% | 92% | - |
| Sakamoto et al[219], 2022 | Retrospective | WLI, BLI | 604 polyps | 96% for WLI, 96% for BLI | 84% for WLI, 89% for BLI | - | - |
There are fewer studies examining the efficacy of AI for delineation of submucosal invasive adenocarcinoma to guide resection strategies. Lu et al[221] found the accuracy of their AI model “Endo-CRC” to be 93.78% for polyps with and 91.71% for polyps without advanced CRC. Lui et al[222] developed an AI model to classify polyps more than 2 cm in size as being endoscopically resectable (less than 1 mm submucosal invasion, no lymphovascular invasion and no more than well-differentiated adenocarcinoma) or non-resectable. The overall accuracy was 85.5% for prediction of endoscopically resectable lesions, but improved to 94.3% when the AI system was interpreting NBI images. However, while AI models have been more effective than non-expert interventionalists for detection of invasive carcinoma, in each of these studies AI was not superior to expert endoscopists, suggesting the main role of AI for larger polyps may be in improving inter-endoscopist consistency as well as perhaps aiding in selection of suitable referrals to interventionalists by non-expert endoscopists[221,222].
Emerging technologies have been developed with the goal of achieving in vivo histological diagnosis, termed “optical biopsy”. Accurate optical biopsies would allow endoscopists to not only surpass PIVI thresholds for small and diminutive polyps but would also allow accurate endoscopic diagnosis for larger polyps and LSTs where existing mucosal imaging technology may have deficiencies.
Endocytoscopy is a novel technology that allows in vivo visualisation of tissue at the cellular level in real-time[223]. The device can either be incorporated into the endoscope or comes as a probe-based system, utilising a high-power fixed-focus objective lens to achieve ultra-high magnification in excess of 450 ×, generally following methylene blue staining[224]. Studies have demonstrated superior accuracy compared to advanced mucosal imaging and chromoendoscopy, with accuracy as high as 93.3%-96.8% for distinction of neoplastic vs non-neoplastic diminutive polyps[225]. Endocytoscopy has been shown to be similarly highly accurate for larger polyps in detection of submucosal invasion, with an overall accuracy of 85.8%-97.0%[226-229]. The main limiting factors for this technology are the requirement for specific equipment, as well as the time and training required to facilitate accurate interpretation of the images. However, its uptake may evolve with the development of AI technologies which could allow effective use by inexperienced proceduralists. Misawa et al[230] developed and published a new AI system for interpretation of endocytoscopy images (using NBI rather than methylene blue staining) named “EndoBRAIN” in 2016. Their study demonstrated overall sensitivity, specificity, and accuracy for high-confidence predictions of 97.6%, 95.8%, and 96.9% respectively. In 2020, Kudo et al[231] compared “EndoBRAIN” to trainee and expert endoscopists using both dye-based and virtual chromoendoscopy and found the AI system to be superior to both groups, with sensitivity of 96.9%, specificity of 100% and overall accuracy of 98%.
Multiphoton microscopy is based on the detection of signals at specific emission wavelengths after laser excitation, offering real-time high-resolution visualisation. The use of longer photons allows deeper tissue penetration and visualisation up to a depth of several hundred microns[232]. Recently, Terradillos et al[233] developed an AI system for interpretation of multiphoton microscopy images of colorectal polyps, with a specificity of 91% and sensitivity of 82% for malignant colorectal lesions. Further study is clearly required into the application of this technology, however the greater depth of visualisation may allow in vivo assessment of invasion depth for submucosal invasive adenocarcinoma.
New and existing advanced mucosal imaging technologies facilitate improved adenoma detection and characterisation in both expert and non-expert endoscopists (Table 5). The use of virtual chromoendoscopy for polyp detection has been limited by reduced brightness and loss of familiarity of color patterns, however new technologies such as LCI and TXI enhance visualisation without significantly altering color patterns and may lead to more consistent improvement in polyp detection. Additionally, the availability of AI systems is increasing and may improve consistency between expert and non-expert endoscopists. Advanced mucosal imaging also allows accurate in vivo assessment of polyps to guide resection techniques, while clearly exceeding PIVI thresholds for the “resect and discard” and “do not resect” strategies. NBI has been at the forefront of polyp characterisation, improving delineation of neoplastic from non-neoplastic diminutive and small polyps, while improving prediction of invasion depth in larger polyps. AI technologies are yet to surpass expert endoscopists for histology prediction but facilitate accurate prediction by non-experts to rival that of expert endoscopists. Effective use of these advanced mucosal imaging technologies is not out of reach of any endoscopist following brief but dedicated training programs, thereby maximising the efficacy of everyday colonoscopy and improving patient outcomes.
| Modality | Detection | Characterisation | Comment | |
| HD-WLI | Advantages: Marginal benefit in overall adenoma detection; and improved detection of right-sided, flat polyps, and SSAs1 | Advantage: Marginal benefit for small adenomas; disadvantage: Insufficient evidence for large polyps | Advantage: Widely available | |
| Chromo-endoscopy | Advantage: Increases polyp detection; disadvantage: Increases withdrawal time | Advantages: Highly effective for small polyps (although inefficient); and useful in prediction of invasion depth for large polyps1 | Disadvantage: Increases procedural time | |
| Virtual chromo-endoscopy | NBI | Disadvantage: No significant difference in ADR | Advantages: Accurate for distinguishing neoplastic from non-neoplastic small and diminutive polyps; and accurate for prediction of invasion depth1 | Disadvantage: Loss of brightness; neutral: Greater efficacy when used by expert proceduralists |
| i-SCAN | Neutral: Variable results, increased detection of flat and right-sided polyps | Advantage: Effective for diminutive and small polyps | ||
| FICE | Disadvantage: No significant difference in ADR | Disadvantage: Inferior to NBI | Advantage: Familiar colour spectrum | |
| LCI | Advantages: Improves adenoma detection; and effective for non-expert proceduralists1 | Disadvantage: Insufficient evidence | Advantage: Familiar colour spectrum | |
| BLI | Disadvantage: No significant difference in ADR | Advantage: Similar to NBI in terms of colour spectrum and accuracy1 | Advantage: Similar colour spectrum to NBI | |
| TXI | Advantage: Increases polyp visibility in image-based studies | Disadvantage: Insufficient evidence | Disadvantage: Insufficient evidence; advantage: Familiar colour spectrum | |
| AFI | Disadvantage: Insufficient evidence; advantage: Improves detection of flat, right-sided polyps and reduces miss rates | Disadvantage: Inferior to NBI | Disadvantage: Not widely available | |
| AI | Advantage: Increases adenoma detection; no significant difference in withdrawal time1 | Advantages: Highly accurate; superior to non-expert endoscopists for histology prediction; not superior to experts using NBI1 | Disadvantage: Not yet widely available | |
| FME | Disadvantages: Expensive; insufficient evidence | Disadvantage: Not widely available | ||
| Endo-cystoscopy | Neutral: Accurate but requires expertise for interpretation; advantages: Uptake may increase with incorporation of AI | Disadvantages: Requires additional equipment; and not widely available | ||
| Multiphoton microscopy | Disadvantage: Insufficient evidence | Disadvantage: Requires additional equipment | ||
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report”s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Osera S, Japan; Romo JA, Colombia; Tadros M, United States S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366-378. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-180. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158:291-302. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Arain MA, Sawhney M, Sheikh S, Anway R, Thyagarajan B, Bond JH, Shaukat A. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189-1195. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588-2596. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Gorski TF, Rosen L, Riether R, Stasik J, Khubchandani I. Colorectal cancer after surveillance colonoscopy: false-negative examination or fast growth? Dis Colon Rectum. 1999;42:877-880. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044-3052. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Samadder NJ, Curtin K, Tuohy TM, Pappas L, Boucher K, Provenzale D, Rowe KG, Mineau GP, Smith K, Pimentel R, Kirchhoff AC, Burt RW. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950-960. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551-2557. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Zhao S, Wang S, Pan P, Xia T, Chang X, Yang X, Guo L, Meng Q, Yang F, Qian W, Xu Z, Wang Y, Wang Z, Gu L, Wang R, Jia F, Yao J, Li Z, Bai Y. Magnitude, Risk Factors, and Factors Associated With Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:1661-1674.e11. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Puri N, Walia S, Olafsson S, Jackson C. Right-sided Colon Polyps Have Worse Histology and are More Often Sessile Than Left-sided Polyps. This Argues for Colonoscopy Being Used for Screening Rather Than Sigmoidoscopy and Fecal Occult Blood Testing. A Retrospective Single Center VA Hospital Study. Am J Gastroenterol. 2010;105:S557-S558. [DOI] [Cited in This Article: ] |
| 15. | Qumseya BJ, Coe S, Wallace MB. The effect of polyp location and patient gender on the presence of dysplasia in colonic polyps. Clin Transl Gastroenterol. 2012;3:e20. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Gupta S, Balasubramanian BA, Fu T, Genta RM, Rockey DC, Lash R. Polyps with advanced neoplasia are smaller in the right than in the left colon: implications for colorectal cancer screening. Clin Gastroenterol Hepatol. 2012;10:1395-1401.e2. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Toyoshima O, Nishizawa T, Yoshida S, Sekiba K, Kataoka Y, Hata K, Watanabe H, Tsuji Y, Koike K. Expert endoscopists with high adenoma detection rates frequently detect diminutive adenomas in proximal colon. Endosc Int Open. 2020;8:E775-E782. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72-90. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Denis B, Sauleau EA, Gendre I, Exbrayat C, Piette C, Dancourt V, Foll Y, Ait Hadad H, Bailly L, Perrin P. The mean number of adenomas per procedure should become the gold standard to measure the neoplasia yield of colonoscopy: a population-based cohort study. Dig Liver Dis. 2014;46:176-181. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Aniwan S, Orkoonsawat P, Viriyautsahakul V, Angsuwatcharakon P, Pittayanon R, Wisedopas N, Sumdin S, Ponuthai Y, Wiangngoen S, Kullavanijaya P, Rerknimitr R. The Secondary Quality Indicator to Improve Prediction of Adenoma Miss Rate Apart from Adenoma Detection Rate. Am J Gastroenterol. 2016;111:723-729. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Aziz M, Fatima R, Lee-Smith W, Khuder S, Nawras A. Comparing endoscopic interventions to improve serrated adenoma detection rates during colonoscopy: a systematic review and network meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol. 2020;32:1284-1292. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, Gaddam S, Singh V, Sharma P. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut. 2012;61:402-408. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | de Wijkerslooth TR, Stoop EM, Bossuyt PM, Mathus-Vliegen EM, Dees J, Tytgat KM, van Leerdam ME, Fockens P, Kuipers EJ, Dekker E. Adenoma detection with cap-assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426-1434. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Rzouq F, Gupta N, Wani S, Sharma P, Bansal A, Rastogi A. Cap assisted colonoscopy for the detection of serrated polyps: a post-hoc analysis. BMC Gastroenterol. 2015;15:11. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | ASGE Technology Committee. High-definition and high-magnification endoscopes. Gastrointest Endosc. 2014;80:919-927. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Tziatzios G, Gkolfakis P, Lazaridis LD, Facciorusso A, Antonelli G, Hassan C, Repici A, Sharma P, Rex DK, Triantafyllou K. High-definition colonoscopy for improving adenoma detection: a systematic review and meta-analysis of randomized controlled studies. Gastrointest Endosc. 2020;91:1027-1036.e9. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499-505. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, Jonnalagadda SS, Hovis CE, Gaddam S, Wani SB, Edmundowicz SA, Sharma P. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593-602. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Roelandt P, Demedts I, Willekens H, Bessissow T, Braeye L, Coremans G, Cuyle PJ, Ferrante M, Gevers AM, Hiele M, Osselaer M, Tack J, Tejpar S, Ulenaers M, Van Assche G, Van Cutsem E, Van Gool S, Vannoote J, Vermeire S, Bisschops R. Impact of endoscopy system, high definition, and virtual chromoendoscopy in daily routine colonoscopy: a randomized trial. Endoscopy. 2019;51:237-243. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Subramanian V, Ramappa V, Telakis E, Mannath J, Jawhari AU, Hawkey CJ, Ragunath K. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350-355. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Krugliak Cleveland N, Colman RJ, Rodriquez D, Hirsch A, Cohen RD, Hanauer SB, Hart J, Rubin DT. Surveillance of IBD Using High Definition Colonoscopes Does Not Miss Adenocarcinoma in Patients with Low-grade Dysplasia. Inflamm Bowel Dis. 2016;22:631-637. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | ASGE Technology Committee; Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JM, Disario JA, Mishkin DS, Shah RJ, Somogyi L, Tierney WM, Petersen BT. Chromoendoscopy. Gastrointest Endosc. 2007;66:639-649. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Tribonias G, Theodoropoulou A, Stylianou K, Giotis I, Mpitouli A, Moschovis D, Komeda Y, Manola ME, Paspatis G, Tzouvala M. Irrigating Acetic Acid Solution During Colonoscopy for the Detection of Sessile Serrated Neoplasia: A Randomized Controlled Trial. Dig Dis Sci. 2022;67:282-292. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Brown SR, Baraza W, Din S, Riley S. Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database Syst Rev. 2016;4:CD006439. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Hurt C, Ramaraj R, Farr A, Morgan M, Williams N, Philips CJ, Williams GT, Gardner G, Porter C, Sampson J, Hillier S, Heard H, Dolwani S; CONSCOP Clinical Research Consortium. Feasibility and economic assessment of chromocolonoscopy for detection of proximal serrated neoplasia within a population-based colorectal cancer screening programme (CONSCOP): an open-label, randomised controlled non-inferiority trial. Lancet Gastroenterol Hepatol. 2019;4:364-375. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Kahi CJ, Anderson JC, Waxman I, Kessler WR, Imperiale TF, Li X, Rex DK. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol. 2010;105:1301-1307. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Kim SY, Park HJ, Kim HS, Park DI, Cha JM, Park SJ, Choi H, Shin JE, Eun CS, Kim JO, Kim HG, Kim SE, Park CH, Kim TI, Hong SN. Cap-Assisted Chromoendoscopy Using a Mounted Cap Versus Standard Colonoscopy for Adenoma Detection. Am J Gastroenterol. 2020;115:465-472. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Wan J, Wang X, Yang ZP, Wu KC. Systematic review with meta-analysis: Chromoendoscopy versus white light endoscopy in detection of dysplasia in patients with inflammatory bowel disease. J Dig Dis. 2019;20:206-214. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Subramanian V, Mannath J, Ragunath K, Hawkey CJ. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:304-312. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Coelho-Prabhu N, Bruining DH, Faubion WA, Kane SV, Kisiel JB, Papadakis KA, Pardi DS, Raffals LE, Schroeder KW, Tremaine WJ, Fruth K, Harmsen WS, Loftus EV. A 1-Year Cross-sectional Inflammatory Bowel Disease Surveillance Colonoscopy Cohort Comparing High-definition White Light Endoscopy and Chromoendoscopy. Inflamm Bowel Dis. 2021;27:594-602. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Iacucci M, Kaplan GG, Panaccione R, Akinola O, Lethebe BC, Lowerison M, Leung Y, Novak KL, Seow CH, Urbanski S, Minoo P, Gui X, Ghosh S. A Randomized Trial Comparing High Definition Colonoscopy Alone With High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy. Am J Gastroenterol. 2018;113:225-234. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | López-Vicente J, Rodríguez-Alcalde D, Hernández L, Riu Pons F, Vega P, Herrero Rivas JM, Santiago García J, Salces Franco I, Bustamante Balén M, López-Cerón M, Pellisé M; Endoscopy for High Risk Cancer Conditions group of the Spanish Gastroenterological Association and Spanish Digestive Endoscopy Society. Panchromoendoscopy Increases Detection of Polyps in Patients With Serrated Polyposis Syndrome. Clin Gastroenterol Hepatol. 2019;17:2016-2023.e6. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Har-Noy O, Yung DE, Koulaouzidis A, Eliakim R, Kopylov U, Avidan B, Katz LH. Chromoendoscopy or white light endoscopy for neoplasia detection in Lynch syndrome, a meta-analysis. Dig Liver Dis. 2019;51:1515-1521. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Haanstra JF, Dekker E, Cats A, Nagengast FM, Hardwick JC, Vanhoutvin SA, de Vos Tot Nederveen Cappel WH, Vasen HF, Kleibeuker JH, Koornstra JJ. Effect of chromoendoscopy in the proximal colon on colorectal neoplasia detection in Lynch syndrome: a multicenter randomized controlled trial. Gastrointest Endosc. 2019;90:624-632. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Houwen BBSL, Mostafavi N, Vleugels JLA, Hüneburg R, Lamberti C, Rivero-Sánchez L, Pellisé M, Stoffel EM, Syngal S, Haanstra JF, Koornstra JJ, Dekker E, Hazewinkel Y. Dye-Based Chromoendoscopy in Patients With Lynch Syndrome: An Individual Patient Data Meta-Analysis of Randomized Trials. Am J Gastroenterol. 2021;116:825-828. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Montale A, Buttitta F, Pierantoni C, Ferrari C, Cameletti M, Colussi D, Miccoli S, Bazzoli F, Turchetti D, Ricciardiello L. Chromoendoscopy Is Not Superior to White Light Endoscopy in Improving Adenoma Detection in Lynch Syndrome Cohort Undergoing Surveillance with High-Resolution Colonoscopy: A Real-World Evidence Study. Dig Dis. 2022;40:517-525. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Feuerstein JD, Rakowsky S, Sattler L, Yadav A, Foromera J, Grossberg L, Cheifetz AS. Meta-analysis of dye-based chromoendoscopy compared with standard- and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc. 2019;90:186-195.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Repici A, Wallace MB, East JE, Sharma P, Ramirez FC, Bruining DH, Young M, Gatof D, Irene Mimi Canto M, Marcon N, Cannizzaro R, Kiesslich R, Rutter M, Dekker E, Siersema PD, Spaander M, Kupcinskas L, Jonaitis L, Bisschops R, Radaelli F, Bhandari P, Wilson A, Early D, Gupta N, Vieth M, Lauwers GY, Rossini M, Hassan C. Efficacy of Per-oral Methylene Blue Formulation for Screening Colonoscopy. Gastroenterology. 2019;156:2198-2207.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | ASGE Technology Committee; Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JT, Murad FM, Siddiqui UD, Wallace MB, Banerjee S. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249-261. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Jin XF, Chai TH, Shi JW, Yang XC, Sun QY. Meta-analysis for evaluating the accuracy of endoscopy with narrow band imaging in detecting colorectal adenomas. J Gastroenterol Hepatol. 2012;27:882-887. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Rex DK, Clodfelter R, Rahmani F, Fatima H, James-Stevenson TN, Tang JC, Kim HN, McHenry L, Kahi CJ, Rogers NA, Helper DJ, Sagi SV, Kessler WR, Wo JM, Fischer M, Kwo PY. Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 2016;83:166-171. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Ikematsu H, Saito Y, Tanaka S, Uraoka T, Sano Y, Horimatsu T, Matsuda T, Oka S, Higashi R, Ishikawa H, Kaneko K. The impact of narrow band imaging for colon polyp detection: a multicenter randomized controlled trial by tandem colonoscopy. J Gastroenterol. 2012;47:1099-1107. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Paggi S, Radaelli F, Amato A, Meucci G, Mandelli G, Imperiali G, Spinzi G, Terreni N, Lenoci N, Terruzzi V. The impact of narrow band imaging in screening colonoscopy: a randomized controlled trial. Clin Gastroenterol Hepatol. 2009;7:1049-1054. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Kim H, Goong HJ, Ko BM, Myung YS, Ho Jung Y, Jeon SR, Kim HG, Lee MS. Randomized, back-to-back trial of a new generation NBI with a high-definition white light (HQ290) for detecting colorectal polyps. Scand J Gastroenterol. 2019;54:1058-1063. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363-70; quiz 371. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, Schröder A, Scheel M, Wiedenmann B, Rösch T. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology. 2009;136:410-6.e1; quiz 715. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406-1412. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Singh R, Cheong KL, Zorron Cheng Tao Pu L, Mangira D, Koay DSC, Kee C, Ng SC, Rerknimitr R, Aniwan S, Ang TL, Goh KL, Ho SH, Lau JY. Multicenter randomised controlled trial comparing the high definition white light endoscopy and the bright narrow band imaging for colon polyps. World J Gastrointest Endosc. 2017;9:273-281. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Minamide T, Sashiyama H, Muramatsu Y, Yada T, Matsumura T, Takeda S, Suzuki T, Kakimoto T, Yano T, Yoshii K, Arai M, Uemura N, Yamaguchi T, Ikematsu H. Second-generation narrow-band imaging to detect colorectal adenomas: A prospective study including community hospitals. J Gastroenterol Hepatol. 2021;36:3084-3091. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Atkinson NSS, Ket S, Bassett P, Aponte D, De Aguiar S, Gupta N, Horimatsu T, Ikematsu H, Inoue T, Kaltenbach T, Leung WK, Matsuda T, Paggi S, Radaelli F, Rastogi A, Rex DK, Sabbagh LC, Saito Y, Sano Y, Saracco GM, Saunders BP, Senore C, Soetikno R, Vemulapalli KC, Jairath V, East JE. Narrow-Band Imaging for Detection of Neoplasia at Colonoscopy: A Meta-analysis of Data From Individual Patients in Randomized Controlled Trials. Gastroenterology. 2019;157:462-471. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Bowman EA, Pfau PR, Mitra A, Reichelderfer M, Gopal DV, Hall BS, Benson ME. High Definition Colonoscopy Combined with i-SCAN Imaging Technology Is Superior in the Detection of Adenomas and Advanced Lesions Compared to High Definition Colonoscopy Alone. Diagn Ther Endosc. 2015;2015:167406. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827-833. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Testoni PA, Notaristefano C, Vailati C, Di Leo M, Viale E. High-definition colonoscopy with i-Scan: better diagnosis for small polyps and flat adenomas. World J Gastroenterol. 2012;18:5231-5239. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Kim WJ, Park SY, Park I, Lee WJ, Park J, Chon N, Oh TG, Kim KH. Increased Detection of Colorectal Polyps in Screening Colonoscopy Using High Definition i-SCAN Compared with Standard White Light. Clin Endosc. 2016;49:69-75. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Kidambi TD, Terdiman JP, El-Nachef N, Singh A, Kattah MG, Lee JK. Effect of I-scan Electronic Chromoendoscopy on Detection of Adenomas During Colonoscopy. Clin Gastroenterol Hepatol. 2019;17:701-708.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Bisschops R, Tejpar S, Willekens H, De Hertogh G, Van Cutsem E. Virtual chromoendoscopy (I-SCAN) detects more polyps in patients with Lynch syndrome: a randomized controlled crossover trial. Endoscopy. 2017;49:342-350. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Omata F, Ohde S, Deshpande GA, Kobayashi D, Masuda K, Fukui T. Image-enhanced, chromo, and cap-assisted colonoscopy for improving adenoma/neoplasia detection rate: a systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:222-237. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Hong SN, Choe WH, Lee JH, Kim SI, Kim JH, Lee TY, Lee SY, Cheon YK, Sung IK, Park HS, Shim CS. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc. 2012;75:1011-1021.e2. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Kiriyama S, Matsuda T, Nakajima T, Sakamoto T, Saito Y, Kuwano H. Detectability of colon polyp using computed virtual chromoendoscopy with flexible spectral imaging color enhancement. Diagn Ther Endosc. 2012;2012:596303. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Aminalai A, Rösch T, Aschenbeck J, Mayr M, Drossel R, Schröder A, Scheel M, Treytnar D, Gauger U, Stange G, Simon F, Adler A. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J Gastroenterol. 2010;105:2383-2388. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Yoshida N, Naito Y, Murakami T, Hirose R, Ogiso K, Inada Y, Dohi O, Kamada K, Uchiyama K, Handa O, Konishi H, Siah KTH, Yagi N, Fujita Y, Kishimoto M, Yanagisawa A, Itoh Y. Linked color imaging improves the visibility of colorectal polyps: a video study. Endosc Int Open. 2017;5:E518-E525. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Kanzaki H, Takenaka R, Kawahara Y, Kawai D, Obayashi Y, Baba Y, Sakae H, Gotoda T, Kono Y, Miura K, Iwamuro M, Kawano S, Tanaka T, Okada H. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int Open. 2017;5:E1005-E1013. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Shinozaki S, Osawa H, Hayashi Y, Lefor AK, Yamamoto H. Linked color imaging for the detection of early gastrointestinal neoplasms. Therap Adv Gastroenterol. 2019;12:1756284819885246. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Yoshida N, Hisabe T, Ikematsu H, Ishihara H, Terasawa M, Inaba A, Sato D, Cho H, Ego M, Tanaka Y, Yasuda R, Inoue K, Murakami T, Inada Y, Itoh Y, Saito Y. Comparison Between Linked Color Imaging and Blue Laser Imaging for Improving the Visibility of Flat Colorectal Polyps: A Multicenter Pilot Study. Dig Dis Sci. 2020;65:2054-2062. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Suzuki T, Hara T, Kitagawa Y, Takashiro H, Nankinzan R, Sugita O, Yamaguchi T. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc. 2017;86:692-697. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Paggi S, Mogavero G, Amato A, Rondonotti E, Andrealli A, Imperiali G, Lenoci N, Mandelli G, Terreni N, Conforti FS, Conte D, Spinzi G, Radaelli F. Linked color imaging reduces the miss rate of neoplastic lesions in the right colon: a randomized tandem colonoscopy study. Endoscopy. 2018;50:396-402. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Paggi S, Radaelli F, Senore C, Maselli R, Amato A, Andrisani G, Di Matteo F, Cecinato P, Grillo S, Sereni G, Sassatelli R, Manfredi G, Alicante S, Buscarini E, Canova D, Milan L, Pallini P, Iwatate M, Rondonotti E, Repici A, Hassan C. Linked-color imaging versus white-light colonoscopy in an organized colorectal cancer screening program. Gastrointest Endosc. 2020;92:723-730. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Oliveira Dos Santos CE, Malaman D, Pereira-Lima JC, de Quadros Onófrio F, Ribas Filho JM. Impact of linked-color imaging on colorectal adenoma detection. Gastrointest Endosc. 2019;90:826-834. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Miyaguchi K, Takabayashi K, Saito D, Tsuzuki Y, Hirooka N, Hosoe N, Ohgo H, Ashitani K, Soma H, Miyanaga R, Kimura K, Tokunaga S, Mitsui T, Miura M, Ozaki R, Nakamoto H, Kanai T, Hisamatsu T, Ogata H, Imaeda H. Linked color imaging versus white light imaging colonoscopy for colorectal adenoma detection: A randomized controlled trial. J Gastroenterol Hepatol. 2021;36:2778-2784. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Kudo T, Horiuchi A, Kyodo R, Horiuchi I, Arai N, Kajiyama M, Tanaka N. Linked colour imaging versus white-light colonoscopy for the detection of flat colorectal lesions: A randomized controlled trial. Colorectal Dis. 2021;23:1414-1420. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Shinozaki S, Kobayashi Y, Hayashi Y, Sakamoto H, Sunada K, Lefor AK, Yamamoto H. Colon polyp detection using linked color imaging compared to white light imaging: Systematic review and meta-analysis. Dig Endosc. 2020;32:874-881. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Houwen BBSL, Hazewinkel Y, Pellisé M, Rivero-Sánchez L, Balaguer F, Bisschops R, Tejpar S, Repici A, Ramsoekh D, Jacobs MAJM, Schreuder RM, Kaminski MF, Rupinska M, Bhandari P, van Oijen MGH, Koens L, Bastiaansen BAJ, Tytgat KM, Fockens P, Vleugels JLA, Dekker E. Linked Colour imaging for the detection of polyps in patients with Lynch syndrome: a multicentre, parallel randomised controlled trial. Gut. 2022;71:553-560. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Hasegawa I, Yamamura T, Suzuki H, Maeda K, Sawada T, Mizutani Y, Ishikawa E, Ishikawa T, Kakushima N, Furukawa K, Ohno E, Kawashima H, Nakamura M, Fujishiro M. Detection of Colorectal Neoplasms Using Linked Color Imaging: A Prospective, Randomized, Tandem Colonoscopy Trial. Clin Gastroenterol Hepatol. 2021;19:1708-1716.e4. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Desai M, Kennedy K, Aihara H, Van Dam J, Gross S, Haber G, Pohl H, Rex D, Saltzman J, Sethi A, Waxman I, Wang K, Wallace M, Repici A, Sharma P. External validation of blue light imaging (BLI) criteria for the optical characterization of colorectal polyps by endoscopy experts. J Gastroenterol Hepatol. 2021;36:2728-2734. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Yoshida N, Hisabe T, Hirose R, Ogiso K, Inada Y, Konishi H, Yagi N, Naito Y, Aomi Y, Ninomiya K, Ikezono G, Terasawa M, Yao K, Matsui T, Yanagisawa A, Itoh Y. Improvement in the visibility of colorectal polyps by using blue laser imaging (with video). Gastrointest Endosc. 2015;82:542-549. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Ang TL, Li JW, Wong YJ, Tan YJ, Fock KM, Tan MTK, Kwek ABE, Teo EK, Ang DS, Wang LM. A prospective randomized study of colonoscopy using blue laser imaging and white light imaging in detection and differentiation of colonic polyps. Endosc Int Open. 2019;7:E1207-E1213. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Shimoda R, Sakata Y, Fujise T, Yamanouchi K, Tsuruoka N, Hara M, Nakayama A, Yamaguchi D, Akutagawa T, Fujimoto K, Iwakiri R. The adenoma miss rate of blue-laser imaging vs. white-light imaging during colonoscopy: a randomized tandem trial. Endoscopy. 2017;49:186-190. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Ikematsu H, Sakamoto T, Togashi K, Yoshida N, Hisabe T, Kiriyama S, Matsuda K, Hayashi Y, Matsuda T, Osera S, Kaneko K, Utano K, Naito Y, Ishihara H, Kato M, Yoshimura K, Ishikawa H, Yamamoto H, Saito Y. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc. 2017;86:386-394. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Sato T. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J Healthc Eng. 2021;2021:5518948. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Nishizawa T, Toyoshima O, Yoshida S, Uekura C, Kurokawa K, Munkhjargal M, Obata M, Yamada T, Fujishiro M, Ebinuma H, Suzuki H. TXI (Texture and Color Enhancement Imaging) for Serrated Colorectal Lesions. J Clin Med. 2021;11. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Yoshida N, Inoue K, Dohi O, Kobayashi R, Tomita Y, Hashimoto H, Sugino S, Hirose R, Murakami T, Inada Y, Morinaga Y, Itoh Y. Analysis of Texture and Color Enhancement Imaging for Improving the Visibility of Non-polypoid Colorectal Lesions. Dig Dis Sci. 2022;. [PubMed] [DOI] [Cited in This Article: ] |
| 96. | Filip M, Iordache S, Săftoiu A, Ciurea T. Autofluorescence imaging and magnification endoscopy. World J Gastroenterol. 2011;17:9-14. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Takehana S, Kaneko M, Mizuno H. Endoscopic diagnostic system using autofluorescence. Diagn Ther Endosc. 1999;5:59-63. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | McCallum AL, Jenkins JT, Gillen D, Molloy RG. Evaluation of autofluorescence colonoscopy for the detection and diagnosis of colonic polyps. Gastrointest Endosc. 2008;68:283-290. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Takeuchi Y, Sawaya M, Oka S, Tamai N, Kawamura T, Uraoka T, Ikematsu H, Moriyama T, Arao M, Ishikawa H, Ito Y, Matsuda T. Efficacy of autofluorescence imaging for flat neoplasm detection: a multicenter randomized controlled trial (A-FLAT trial). Gastrointest Endosc. 2019;89:460-469. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Moriichi K, Fujiya M, Sato R, Watari J, Nomura Y, Nata T, Ueno N, Maeda S, Kashima S, Itabashi K, Ishikawa C, Inaba Y, Ito T, Okamoto K, Tanabe H, Mizukami Y, Saitoh Y, Kohgo Y. Back-to-back comparison of auto-fluorescence imaging (AFI) versus high resolution white light colonoscopy for adenoma detection. BMC Gastroenterol. 2012;12:75. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Zhao ZY, Guan YG, Li BR, Shan YQ, Yan FH, Gao YJ, Wang H, Lou Z, Fu CG, Yu ED. Detection and miss rates of autofluorescence imaging of adenomatous and polypoid lesions during colonoscopy: a systematic review and meta-analysis. Endosc Int Open. 2015;3:E226-E235. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Repici A, Badalamenti M, Maselli R, Correale L, Radaelli F, Rondonotti E, Ferrara E, Spadaccini M, Alkandari A, Fugazza A, Anderloni A, Galtieri PA, Pellegatta G, Carrara S, Di Leo M, Craviotto V, Lamonaca L, Lorenzetti R, Andrealli A, Antonelli G, Wallace M, Sharma P, Rosch T, Hassan C. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology. 2020;159:512-520.e7. [PubMed] [DOI] [Cited in This Article: ] |
| 103. | Urban G, Tripathi P, Alkayali T, Mittal M, Jalali F, Karnes W, Baldi P. Deep Learning Localizes and Identifies Polyps in Real Time With 96% Accuracy in Screening Colonoscopy. Gastroenterology. 2018;155:1069-1078.e8. [PubMed] [DOI] [Cited in This Article: ] |
| 104. | Lui TKL, Guo CG, Leung WK. Accuracy of artificial intelligence on histology prediction and detection of colorectal polyps: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:11-22.e6. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Misawa M, Kudo SE, Mori Y, Hotta K, Ohtsuka K, Matsuda T, Saito S, Kudo T, Baba T, Ishida F, Itoh H, Oda M, Mori K. Development of a computer-aided detection system for colonoscopy and a publicly accessible large colonoscopy video database (with video). Gastrointest Endosc. 2021;93:960-967.e3. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Zhao SB, Yang W, Wang SL, Pan P, Wang RD, Chang X, Sun ZQ, Fu XH, Shang H, Wu JR, Chen LZ, Chang J, Song P, Miao YL, He SX, Miao L, Jiang HQ, Wang W, Yang X, Dong YH, Lin H, Chen Y, Gao J, Meng QQ, Jin ZD, Li ZS, Bai Y. Establishment and validation of a computer-assisted colonic polyp localization system based on deep learning. World J Gastroenterol. 2021;27:5232-5246. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Becq A, Chandnani M, Bharadwaj S, Baran B, Ernest-Suarez K, Gabr M, Glissen-Brown J, Sawhney M, Pleskow DK, Berzin TM. Effectiveness of a Deep-learning Polyp Detection System in Prospectively Collected Colonoscopy Videos With Variable Bowel Preparation Quality. J Clin Gastroenterol. 2020;54:554-557. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, Liu P, Li L, Song Y, Zhang D, Li Y, Xu G, Tu M, Liu X. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813-1819. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | Wang P, Liu X, Berzin TM, Glissen Brown JR, Liu P, Zhou C, Lei L, Li L, Guo Z, Lei S, Xiong F, Wang H, Song Y, Pan Y, Zhou G. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol. 2020;5:343-351. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | Glissen Brown JR, Mansour NM, Wang P, Chuchuca MA, Minchenberg SB, Chandnani M, Liu L, Gross SA, Sengupta N, Berzin TM. Deep Learning Computer-aided Polyp Detection Reduces Adenoma Miss Rate: A United States Multi-center Randomized Tandem Colonoscopy Study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 2022;20:1499-1507.e4. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Wang P, Liu P, Glissen Brown JR, Berzin TM, Zhou G, Lei S, Liu X, Li L, Xiao X. Lower Adenoma Miss Rate of Computer-Aided Detection-Assisted Colonoscopy vs Routine White-Light Colonoscopy in a Prospective Tandem Study. Gastroenterology. 2020;159:1252-1261.e5. [PubMed] [DOI] [Cited in This Article: ] |
| 112. | Aziz M, Fatima R, Dong C, Lee-Smith W, Nawras A. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: A systematic review with meta-analysis. J Gastroenterol Hepatol. 2020;35:1676-1683. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, Antonelli G, Yu H, Areia M, Dinis-Ribeiro M, Bhandari P, Sharma P, Rex DK, Rösch T, Wallace M, Repici A. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:77-85.e6. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Spadaccini M, Iannone A, Maselli R, Badalamenti M, Desai M, Chandrasekar VT, Patel HK, Fugazza A, Pellegatta G, Galtieri PA, Lollo G, Carrara S, Anderloni A, Rex DK, Savevski V, Wallace MB, Bhandari P, Roesch T, Gralnek IM, Sharma P, Hassan C, Repici A. Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:793-802. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Barua I, Vinsard DG, Jodal HC, Løberg M, Kalager M, Holme Ø, Misawa M, Bretthauer M, Mori Y. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53:277-284. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Gong D, Wu L, Zhang J, Mu G, Shen L, Liu J, Wang Z, Zhou W, An P, Huang X, Jiang X, Li Y, Wan X, Hu S, Chen Y, Hu X, Xu Y, Zhu X, Li S, Yao L, He X, Chen D, Huang L, Wei X, Wang X, Yu H. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5:352-361. [PubMed] [DOI] [Cited in This Article: ] |
| 117. | Su JR, Li Z, Shao XJ, Ji CR, Ji R, Zhou RC, Li GC, Liu GQ, He YS, Zuo XL, Li YQ. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest Endosc. 2020;91:415-424.e4. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Nagengast WB, Hartmans E, Garcia-Allende PB, Peters FTM, Linssen MD, Koch M, Koller M, Tjalma JJJ, Karrenbeld A, Jorritsma-Smit A, Kleibeuker JH, van Dam GM, Ntziachristos V. Near-infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut. 2019;68:7-10. [PubMed] [DOI] [Cited in This Article: ] |
| 119. | Hartmans E, Tjalma JJJ, Linssen MD, Allende PBG, Koller M, Jorritsma-Smit A, Nery MESO, Elias SG, Karrenbeld A, de Vries EGE, Kleibeuker JH, van Dam GM, Robinson DJ, Ntziachristos V, Nagengast WB. Potential Red-Flag Identification of Colorectal Adenomas with Wide-Field Fluorescence Molecular Endoscopy. Theranostics. 2018;8:1458-1467. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Joshi BP, Dai Z, Gao Z, Lee JH, Ghimire N, Chen J, Prabhu A, Wamsteker EJ, Kwon RS, Elta GH, Stoffel EM, Pant A, Kaltenbach T, Soetikno RM, Appelman HD, Kuick R, Turgeon DK, Wang TD. Detection of Sessile Serrated Adenomas in the Proximal Colon Using Wide-Field Fluorescence Endoscopy. Gastroenterology. 2017;152:1002-1013.e9. [PubMed] [DOI] [Cited in This Article: ] |
| 121. | Gómez V, Racho RG, Heckman MG, Diehl NN, Wallace MB. High-definition white-light (HDWL) colonoscopy and higher adenoma detection rate and the potential for paradoxical over surveillance. Dig Dis Sci. 2014;59:2749-2756. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100-1105. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343-348. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | McGill SK, Evangelou E, Ioannidis JP, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013;62:1704-1713. [PubMed] [DOI] [Cited in This Article: ] |
| 125. | Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, Lieberman DA. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [PubMed] [DOI] [Cited in This Article: ] |
| 126. | Sano W, Sano Y, Iwatate M, Hasuike N, Hattori S, Kosaka H, Ikumoto T, Kotaka M, Fujimori T. Prospective evaluation of the proportion of sessile serrated adenoma/polyps in endoscopically diagnosed colorectal polyps with hyperplastic features. Endosc Int Open. 2015;3:E354-E358. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Solon C, Klausnitzer R, Blissett D, Ihara Z. Economic value of narrow band imaging versus white light endoscopy for the characterization of diminutive polyps in the colon: systematic literature review and cost-consequence model. J Med Econ. 2016;19:1040-1048. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Kuroha M, Shiga H, Kanazawa Y, Nagai H, Handa T, Ichikawa R, Onodera M, Naito T, Moroi R, Kimura T, Endo K, Kakuta Y, Kinouchi Y, Shimosegawa T, Masamune A. Factors Associated with Fibrosis during Colorectal Endoscopic Submucosal Dissection: Does Pretreatment Biopsy Potentially Elicit Submucosal Fibrosis and Affect Endoscopic Submucosal Dissection Outcomes? Digestion. 2021;102:590-598. [PubMed] [DOI] [Cited in This Article: ] |
| 129. | Chen CH, Wu KL, Hu ML, Chiu YC, Tai WC, Chiou SS, Chuah SK. Is a biopsy necessary for colon polyps suitable for polypectomy when performing a colonoscopy? Chang Gung Med J. 2011;34:506-511. [PubMed] [Cited in This Article: ] |
| 130. | Aziz Aadam A, Wani S, Kahi C, Kaltenbach T, Oh Y, Edmundowicz S, Peng J, Rademaker A, Patel S, Kushnir V, Venu M, Soetikno R, Keswani RN. Physician assessment and management of complex colon polyps: a multicenter video-based survey study. Am J Gastroenterol. 2014;109:1312-1324. [PubMed] [DOI] [Cited in This Article: ] |
| 131. | Bae JH, Lee C, Kang HY, Kwak MS, Doo EY, Seo JY, Song JH, Yang SY, Yang JI, Lim SH, Yim JY, Lim JH, Chung GE, Chung SJ, Jin EH, Park B, Kim JS. Improved Real-Time Optical Diagnosis of Colorectal Polyps Following a Comprehensive Training Program. Clin Gastroenterol Hepatol. 2019;17:2479-2488.e4. [PubMed] [DOI] [Cited in This Article: ] |
| 132. | Patel SG, Schoenfeld P, Kim HM, Ward EK, Bansal A, Kim Y, Hosford L, Myers A, Foster S, Craft J, Shopinski S, Wilson RH, Ahnen DJ, Rastogi A, Wani S. Real-Time Characterization of Diminutive Colorectal Polyp Histology Using Narrow-Band Imaging: Implications for the Resect and Discard Strategy. Gastroenterology. 2016;150:406-418. [PubMed] [DOI] [Cited in This Article: ] |
| 133. | Basford P, Brown J, Cooper S, Bhandari P. Endoscopic characterization of small colonic polyps: baseline performance of experienced endoscopists is no different to that of medical students. Endosc Int Open. 2019;7:E403-E411. [PubMed] [DOI] [Cited in This Article: ] |
| 134. | Koehn C, Rex DK, Allen J, Bhatti U, Bhavsar-Burke I, Thoguluva Chandrasekar V, Challa A, Duvvuri A, Dakhoul L, Ha J, Hamade N, Hicks SB, Jansson-Knodell C, Krajicek E, Das Kundumadam S, Nutalapati V, Phatharacharukul PP, Razmdjou S, Saito A, Sarkis F, Sutton R, Wehbeh A, Sharma P, Desai M. Optical diagnosis of colorectal polyps using novel blue light imaging classification among trainee endoscopists. Dig Endosc. 2022;34:191-197. [PubMed] [DOI] [Cited in This Article: ] |
| 135. | Higashi R, Uraoka T, Kato J, Kuwaki K, Ishikawa S, Saito Y, Matsuda T, Ikematsu H, Sano Y, Suzuki S, Murakami Y, Yamamoto K. Diagnostic accuracy of narrow-band imaging and pit pattern analysis significantly improved for less-experienced endoscopists after an expanded training program. Gastrointest Endosc. 2010;72:127-135. [PubMed] [DOI] [Cited in This Article: ] |
| 136. | Basford P, Longcroft-Wheaton G, Higashi R, Uraoka T, Bhandari P. Colonic lesion characterisation skills among UK endoscopists and the impact of a brief training intervention. Frontline Gastroenterol. 2017;8:2-7. [PubMed] [DOI] [Cited in This Article: ] |
| 137. | . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] [DOI] [Cited in This Article: ] |
| 138. | Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, Mahajan H, McLeod D, Bourke MJ. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732-742.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 139. | van Doorn SC, Hazewinkel Y, East JE, van Leerdam ME, Rastogi A, Pellisé M, Sanduleanu-Dascalescu S, Bastiaansen BA, Fockens P, Dekker E. Polyp morphology: an interobserver evaluation for the Paris classification among international experts. Am J Gastroenterol. 2015;110:180-187. [PubMed] [DOI] [Cited in This Article: ] |
| 140. | Cocomazzi F, Gentile M, Perri F, Merla A, Bossa F, Piazzolla M, Ippolito A, Terracciano F, Giuliani AP, Cubisino R, Marra A, Carparelli S, Mileti A, Paolillo R, Fontana A, Copetti M, Di Leo A, Andriulli A. Interobserver agreement of the Paris and simplified classifications of superficial colonic lesions: a Western study. Endosc Int Open. 2021;9:E388-E394. [PubMed] [DOI] [Cited in This Article: ] |
| 141. | Mathews AA, Draganov PV, Yang D. Endoscopic management of colorectal polyps: From benign to malignant polyps. World J Gastrointest Endosc. 2021;13:356-370. [PubMed] [DOI] [Cited in This Article: ] |
| 142. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [PubMed] [DOI] [Cited in This Article: ] |
| 143. | Li M, Ali SM, Umm-a-OmarahGilani S, Liu J, Li YQ, Zuo XL. Kudo's pit pattern classification for colorectal neoplasms: a meta-analysis. World J Gastroenterol. 2014;20:12649-12656. [PubMed] [DOI] [Cited in This Article: ] |
| 144. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [PubMed] [DOI] [Cited in This Article: ] |
| 145. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 146. | Hamada Y, Tanaka K, Katsurahara M, Horiki N, Yamada R, Yamada T, Takei Y. Utility of the narrow-band imaging international colorectal endoscopic classification for optical diagnosis of colorectal polyp histology in clinical practice: a retrospective study. BMC Gastroenterol. 2021;21:336. [PubMed] [DOI] [Cited in This Article: ] |
| 147. | Hattori S, Iwatate M, Sano W, Hasuike N, Kosaka H, Ikumoto T, Kotaka M, Ichiyanagi A, Ebisutani C, Hisano Y, Fujimori T, Sano Y. Narrow-band imaging observation of colorectal lesions using NICE classification to avoid discarding significant lesions. World J Gastrointest Endosc. 2014;6:600-605. [PubMed] [DOI] [Cited in This Article: ] |
| 148. | Puig I, López-Cerón M, Arnau A, Rosiñol Ò, Cuatrecasas M, Herreros-de-Tejada A, Ferrández Á, Serra-Burriel M, Nogales Ó, Vida F, de Castro L, López-Vicente J, Vega P, Álvarez-González MA, González-Santiago J, Hernández-Conde M, Díez-Redondo P, Rivero-Sánchez L, Gimeno-García AZ, Burgos A, García-Alonso FJ, Bustamante-Balén M, Martínez-Bauer E, Peñas B, Pellise M; EndoCAR group, Spanish Gastroenterological Association and the Spanish Digestive Endoscopy Society. Accuracy of the Narrow-Band Imaging International Colorectal Endoscopic Classification System in Identification of Deep Invasion in Colorectal Polyps. Gastroenterology. 2019;156:75-87. [PubMed] [DOI] [Cited in This Article: ] |
| 149. | Klenske E, Zopf S, Neufert C, Nägel A, Siebler J, Gschossmann J, Mühldorfer S, Pfeifer L, Fischer S, Vitali F, Iacucci M, Ghosh S, Rath MG, Klare P, Tontini GE, Neurath MF, Rath T. I-scan optical enhancement for the in vivo prediction of diminutive colorectal polyp histology: Results from a prospective three-phased multicentre trial. PLoS One. 2018;13:e0197520. [PubMed] [DOI] [Cited in This Article: ] |
| 150. | Puig I, Kaltenbach T. Optical Diagnosis for Colorectal Polyps: A Useful Technique Now or in the Future? Gut Liver. 2018;12:385-392. [PubMed] [DOI] [Cited in This Article: ] |
| 151. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [PubMed] [DOI] [Cited in This Article: ] |
| 152. | Zhang Y, Chen HY, Zhou XL, Pan WS, Zhou XX, Pan HH. Diagnostic efficacy of the Japan Narrow-band-imaging Expert Team and Pit pattern classifications for colorectal lesions: A meta-analysis. World J Gastroenterol. 2020;26:6279-6294. [PubMed] [DOI] [Cited in This Article: ] |
| 153. | Sugimoto S, Yabana T, Nomura T, Hayashi S, Okuda N, Temma T, Hashimoto Y, Ito T, Takami M, Oyamada J, Kamei A. Can Non-expert Physicians Use the Japan Narrow-band Imaging Expert Team Classification to Diagnose Colonic Polyps Effectively? J Anus Rectum Colon. 2020;4:100-107. [PubMed] [DOI] [Cited in This Article: ] |
| 154. | Murakami T, Sakamoto N, Fukushima H, Shibuya T, Yao T, Nagahara A. Usefulness of the Japan narrow-band imaging expert team classification system for the diagnosis of sessile serrated lesion with dysplasia/carcinoma. Surg Endosc. 2021;35:4528-4538. [PubMed] [DOI] [Cited in This Article: ] |
| 155. | Komeda Y, Kashida H, Sakurai T, Asakuma Y, Tribonias G, Nagai T, Kono M, Minaga K, Takenaka M, Arizumi T, Hagiwara S, Matsui S, Watanabe T, Nishida N, Chikugo T, Chiba Y, Kudo M. Magnifying Narrow Band Imaging (NBI) for the Diagnosis of Localized Colorectal Lesions Using the Japan NBI Expert Team (JNET) Classification. Oncology. 2017;93 Suppl 1:49-54. [PubMed] [DOI] [Cited in This Article: ] |
| 156. | Sumimoto K, Tanaka S, Shigita K, Hirano D, Tamaru Y, Ninomiya Y, Asayama N, Hayashi N, Oka S, Arihiro K, Yoshihara M, Chayama K. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85:816-821. [PubMed] [DOI] [Cited in This Article: ] |
| 157. | Hosotani K, Imai K, Hotta K, Ito S, Kishida Y, Yabuuchi Y, Yoshida M, Kawata N, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Ono H. Diagnostic performance for T1 cancer in colorectal lesions ≥10 mm by optical characterization using magnifying narrow-band imaging combined with magnifying chromoendoscopy; implications for optimized stratification by Japan Narrow-band Imaging Expert Team classification. Dig Endosc. 2021;33:425-432. [PubMed] [DOI] [Cited in This Article: ] |
| 158. | Hirata D, Kashida H, Iwatate M, Tochio T, Teramoto A, Sano Y, Kudo M. Effective use of the Japan Narrow Band Imaging Expert Team classification based on diagnostic performance and confidence level. World J Clin Cases. 2019;7:2658-2665. [PubMed] [DOI] [Cited in This Article: ] |
| 159. | Bisschops R, Hassan C, Bhandari P, Coron E, Neumann H, Pech O, Correale L, Repici A. BASIC (BLI Adenoma Serrated International Classification) classification for colorectal polyp characterization with blue light imaging. Endoscopy. 2018;50:211-220. [PubMed] [DOI] [Cited in This Article: ] |
| 160. | Rondonotti E, Hassan C, Andrealli A, Paggi S, Amato A, Scaramella L, Repici A, Radaelli F. Clinical Validation of BASIC Classification for the Resect and Discard Strategy for Diminutive Colorectal Polyps. Clin Gastroenterol Hepatol. 2020;18:2357-2365.e4. [PubMed] [DOI] [Cited in This Article: ] |
| 161. | IJspeert JE, Bastiaansen BA, van Leerdam ME, Meijer GA, van Eeden S, Sanduleanu S, Schoon EJ, Bisseling TM, Spaander MC, van Lelyveld N, Bargeman M, Wang J, Dekker E; Dutch Workgroup serrAted polypS & Polyposis (WASP). Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2016;65:963-970. [PubMed] [DOI] [Cited in This Article: ] |
| 162. | Lee J, Bae JH, Chung SJ, Kang HY, Kang SJ, Kwak MS, Seo JY, Song JH, Yang SY, Yang JI, Lim SH, Yim JY, Lim JH, Chung GE, Jin EH, Choi JM, Han YM, Kim JS. Impact of comprehensive optical diagnosis training using Workgroup serrAted polypS and Polyposis classification on detection of adenoma and sessile serrated lesion. Dig Endosc. 2021;. [DOI] [Cited in This Article: ] |
| 163. | Sano Y, Horimatsu T, Fu KI, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesionsusing a Narrow-Band Imaging system. Digestive Endoscopy. 2006;18:S44-S51. [DOI] [Cited in This Article: ] |
| 164. | Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano's capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc. 2011;23 Suppl 1:112-115. [PubMed] [DOI] [Cited in This Article: ] |
| 165. | Katagiri A, Fu KI, Sano Y, Ikematsu H, Horimatsu T, Kaneko K, Muto M, Yoshida S. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:1269-1274. [PubMed] [DOI] [Cited in This Article: ] |
| 166. | Singh R, Jayanna M, Navadgi S, Ruszkiewicz A, Saito Y, Uedo N. Narrow-band imaging with dual focus magnification in differentiating colorectal neoplasia. Dig Endosc. 2013;25 Suppl 2:16-20. [PubMed] [DOI] [Cited in This Article: ] |
| 167. | Singh R, Nordeen N, Mei SL, Kaffes A, Tam W, Saito Y. West meets East: preliminary results of narrow band imaging with optical magnification in the diagnosis of colorectal lesions: a multicenter Australian study using the modified Sano's classification. Dig Endosc. 2011;23 Suppl 1:126-130. [PubMed] [DOI] [Cited in This Article: ] |
| 168. | Zorron Cheng Tao Pu L, Yamamura T, Nakamura M, Koay DSC, Ovenden A, Edwards S, Burt AD, Hirooka Y, Fujishiro M, Singh R. Comparison of different virtual chromoendoscopy classification systems for the characterization of colorectal lesions. JGH Open. 2020;4:818-826. [PubMed] [DOI] [Cited in This Article: ] |
| 169. | Pu LZCT, Cheong KL, Koay DSC, Yeap SP, Ovenden A, Raju M, Ruszkiewicz A, Chiu PW, Lau JY, Singh R. Randomised controlled trial comparing modified Sano's and narrow band imaging international colorectal endoscopic classifications for colorectal lesions. World J Gastrointest Endosc. 2018;10:210-218. [PubMed] [DOI] [Cited in This Article: ] |
| 170. | Lopez-Ceron M, Sanabria E, Pellise M. Colonic polyps: is it useful to characterize them with advanced endoscopy? World J Gastroenterol. 2014;20:8449-8457. [PubMed] [DOI] [Cited in This Article: ] |
| 171. | Kato S, Fu KI, Sano Y, Fujii T, Saito Y, Matsuda T, Koba I, Yoshida S, Fujimori T. Magnifying colonoscopy as a non-biopsy technique for differential diagnosis of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2006;12:1416-1420. [PubMed] [DOI] [Cited in This Article: ] |
| 172. | Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series. Eur J Gastroenterol Hepatol. 2011;23:903-911. [PubMed] [DOI] [Cited in This Article: ] |
| 173. | Fu KI, Sano Y, Kato S, Fujii T, Nagashima F, Yoshino T, Okuno T, Yoshida S, Fujimori T. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: a prospective study. Endoscopy. 2004;36:1089-1093. [PubMed] [DOI] [Cited in This Article: ] |
| 174. | McCarty TR, Aihara H. Predicting depth of invasion for JNET Type 2B colorectal lesions: Is there a role for magnifying chromoendoscopy? Dig Endosc. 2021;33:344-346. [PubMed] [DOI] [Cited in This Article: ] |
| 175. | Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, Pellisé M, Antonelli G, Bustamante Balen M, Coron E, Cortas G, Iacucci M, Yuichi M, Longcroft-Wheaton G, Mouzyka S, Pilonis N, Puig I, van Hooft JE, Dekker E. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:1155-1179. [PubMed] [DOI] [Cited in This Article: ] |
| 176. | Sidhu M, Shahidi N, Vosko S, van Hattem WA, Tate DJ, Bourke MJ. Incremental benefit of dye-based chromoendoscopy to predict the risk of submucosal invasive cancer in large nonpedunculated colorectal polyps. Gastrointest Endosc. 2022;95:527-534.e2. [PubMed] [DOI] [Cited in This Article: ] |
| 177. | Popoutchi P, Mota FL, Averbach M, de Menezes MS, Coudry RA. Acetic acid spray contribution in the endoscopic diagnosis of serrated polyposis syndrome. VideoGIE. 2018;3:65-67. [PubMed] [DOI] [Cited in This Article: ] |
| 178. | Wiessner JR, Brown H, Haller B, Abdelhafez M, Poszler A, Schmid RM, von Delius S, Klare P. Near focus NBI endoscopy plus acetic acid for optical polyp characterization in the colorectum - A proof of principle study. Scand J Gastroenterol. 2019;54:377-383. [PubMed] [DOI] [Cited in This Article: ] |
| 179. | Dolz-Abadía C, Vilella-Martorell A. [Submucosal chromoendoscopy. A technique that highlights epithelia and differentiates histological components, and renders colon polypectomy easier and safer]. Rev Esp Enferm Dig. 2015;107:430-435. [PubMed] [DOI] [Cited in This Article: ] |
| 180. | Sakamoto T, Matsuda T, Aoki T, Nakajima T, Saito Y. Time saving with narrow-band imaging for distinguishing between neoplastic and non-neoplastic small colorectal lesions. J Gastroenterol Hepatol. 2012;27:351-355. [PubMed] [DOI] [Cited in This Article: ] |
| 181. | Repici A, Hassan C, Radaelli F, Occhipinti P, De Angelis C, Romeo F, Paggi S, Saettone S, Cisarò F, Spaander M, Sharma P, Kuipers EJ. Accuracy of narrow-band imaging in predicting colonoscopy surveillance intervals and histology of distal diminutive polyps: results from a multicenter, prospective trial. Gastrointest Endosc. 2013;78:106-114. [PubMed] [DOI] [Cited in This Article: ] |
| 182. | Paggi S, Rondonotti E, Amato A, Fuccio L, Andrealli A, Spinzi G, Radaelli F. Narrow-band imaging in the prediction of surveillance intervals after polypectomy in community practice. Endoscopy. 2015;47:808-814. [PubMed] [DOI] [Cited in This Article: ] |
| 183. | Rastogi A, Rao DS, Gupta N, Grisolano SW, Buckles DC, Sidorenko E, Bonino J, Matsuda T, Dekker E, Kaltenbach T, Singh R, Wani S, Sharma P, Olyaee MS, Bansal A, East JE. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: a video-based study. Gastrointest Endosc. 2014;79:390-398. [PubMed] [DOI] [Cited in This Article: ] |
| 184. | Ikematsu H, Matsuda T, Emura F, Saito Y, Uraoka T, Fu KI, Kaneko K, Ochiai A, Fujimori T, Sano Y. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33. [PubMed] [DOI] [Cited in This Article: ] |
| 185. | Tate DJ, Jayanna M, Awadie H, Desomer L, Lee R, Heitman SJ, Sidhu M, Goodrick K, Burgess NG, Mahajan H, McLeod D, Bourke MJ. A standardized imaging protocol for the endoscopic prediction of dysplasia within sessile serrated polyps (with video). Gastrointest Endosc. 2018;87:222-231.e2. [PubMed] [DOI] [Cited in This Article: ] |
| 186. | Chino A, Osumi H, Kishihara T, Morishige K, Ishikawa H, Tamegai Y, Igarashi M. Advantages of magnifying narrow-band imaging for diagnosing colorectal cancer coexisting with sessile serrated adenoma/polyp. Dig Endosc. 2016;28 Suppl 1:53-59. [PubMed] [DOI] [Cited in This Article: ] |
| 187. | Yoshida N, Naito Y, Inada Y, Kugai M, Inoue K, Uchiyama K, Handa O, Takagi T, Konishi H, Yagi N, Morimoto Y, Wakabayashi N, Yanagisawa A, Yoshikawa T. The detection of surface patterns by flexible spectral imaging color enhancement without magnification for diagnosis of colorectal polyps. Int J Colorectal Dis. 2012;27:605-611. [PubMed] [DOI] [Cited in This Article: ] |
| 188. | Akarsu C, Sahbaz NA, Dural AC, Kones O, Binboga S, Kabuli HA, Gumusoglu AY, Alis H. FICE in Predicting Colorectal Flat Lesion Histology. JSLS. 2017;21. [PubMed] [DOI] [Cited in This Article: ] |
| 189. | Kim YS, Kim D, Chung SJ, Park MJ, Shin CS, Cho SH, Kim JS, Song IS. Differentiating small polyp histologies using real-time screening colonoscopy with Fuji Intelligent Color Enhancement. Clin Gastroenterol Hepatol. 2011;9:744-749.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 190. | Longcroft-Wheaton G, Brown J, Cowlishaw D, Higgins B, Bhandari P. High-definition vs. standard-definition colonoscopy in the characterization of small colonic polyps: results from a randomized trial. Endoscopy. 2012;44:905-910. [PubMed] [DOI] [Cited in This Article: ] |
| 191. | Akarsu C, Sahbaz NA, Dural AC, Unsal MG, Kones O, Kocatas A, Halicioglu I, Alis H. FICE vs Narrow Band Imaging for In Vivo Histologic Diagnosis of Polyps. JSLS. 2016;20. [PubMed] [DOI] [Cited in This Article: ] |
| 192. | Basford PJ, Longcroft-Wheaton G, Higgins B, Bhandari P. High-definition endoscopy with i-Scan for evaluation of small colon polyps: the HiSCOPE study. Gastrointest Endosc. 2014;79:111-118. [PubMed] [DOI] [Cited in This Article: ] |
| 193. | Guo CG, Ji R, Li YQ. Accuracy of i-Scan for Optical Diagnosis of Colonic Polyps: A Meta-Analysis. PLoS One. 2015;10:e0126237. [PubMed] [DOI] [Cited in This Article: ] |
| 194. | Pigò F, Bertani H, Manno M, Mirante V, Caruso A, Barbera C, Manta R, Bassotti G, Olivetti G, Conigliaro RL. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis. 2013;28:399-406. [PubMed] [DOI] [Cited in This Article: ] |
| 195. | Lee JS, Jeon SW, Kwon YH. Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study. Clin Endosc. 2021;54:881-887. [PubMed] [DOI] [Cited in This Article: ] |
| 196. | Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011;74:603-609. [PubMed] [DOI] [Cited in This Article: ] |
| 197. | Wu CH, Chen TH, Hsu CM, Su MY, Chiu CT, Wu RC, Lai CC. Linked-color imaging combined with the NICE classification system for optical diagnosis of colon polyps: new image-enhanced endoscopic technology for pathological prediction. Ther Clin Risk Manag. 2017;13:1317-1321. [PubMed] [DOI] [Cited in This Article: ] |
| 198. | Rondonotti E, Paggi S, Amato A, Mogavero G, Andrealli A, Conforti FS, Conte D, Spinzi G, Radaelli F. Blue-light imaging compared with high-definition white light for real-time histology prediction of colorectal polyps less than 1 centimeter: a prospective randomized study. Gastrointest Endosc. 2019;89:554-564.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 199. | Yoshida N, Yagi N, Inada Y, Kugai M, Okayama T, Kamada K, Katada K, Uchiyama K, Ishikawa T, Handa O, Takagi T, Konishi H, Kokura S, Yanagisawa A, Naito Y. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc. 2014;26:250-258. [PubMed] [DOI] [Cited in This Article: ] |
| 200. | Hassan C, Bisschops R, Bhandari P, Coron E, Neumann H, Pech O, Correale L, Repici A. Predictive rules for optical diagnosis of < 10-mm colorectal polyps based on a dedicated software. Endoscopy. 2020;52:52-60. [PubMed] [DOI] [Cited in This Article: ] |
| 201. | Ito R, Ikematsu H, Murano T, Shinmura K, Kojima M, Kumahara K, Furue Y, Sunakawa H, Minamide T, Sato D, Yamamoto Y, Takashima K, Yoda Y, Hori K, Yano T. Diagnostic ability of Japan Narrow-Band Imaging Expert Team classification for colorectal lesions by magnifying endoscopy with blue laser imaging versus narrow-band imaging. Endosc Int Open. 2021;9:E271-E277. [PubMed] [DOI] [Cited in This Article: ] |
| 202. | Sato R, Fujiya M, Watari J, Ueno N, Moriichi K, Kashima S, Maeda S, Ando K, Kawabata H, Sugiyama R, Nomura Y, Nata T, Itabashi K, Inaba Y, Okamoto K, Mizukami Y, Saitoh Y, Kohgo Y. The diagnostic accuracy of high-resolution endoscopy, autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma. Endoscopy. 2011;43:862-868. [PubMed] [DOI] [Cited in This Article: ] |
| 203. | Ignjatovic A, East JE, Guenther T, Hoare J, Morris J, Ragunath K, Shonde A, Simmons J, Suzuki N, Thomas-Gibson S, Saunders BP. What is the most reliable imaging modality for small colonic polyp characterization? Endoscopy. 2011;43:94-99. [PubMed] [DOI] [Cited in This Article: ] |
| 204. | Lv X, Wang C, Xie Y. Comparison of diagnostic efficacy between AFI, NBI, and AFI combined with NBI for colonic cancers: A meta-analysis. Saudi J Gastroenterol. 2017;23:82-90. [PubMed] [DOI] [Cited in This Article: ] |
| 205. | Jin EH, Lee D, Bae JH, Kang HY, Kwak MS, Seo JY, Yang JI, Yang SY, Lim SH, Yim JY, Lim JH, Chung GE, Chung SJ, Choi JM, Han YM, Kang SJ, Lee J, Chan Kim H, Kim JS. Improved Accuracy in Optical Diagnosis of Colorectal Polyps Using Convolutional Neural Networks with Visual Explanations. Gastroenterology. 2020;158:2169-2179.e8. [PubMed] [DOI] [Cited in This Article: ] |
| 206. | Sánchez-Montes C, Sánchez FJ, Bernal J, Córdova H, López-Cerón M, Cuatrecasas M, Rodríguez de Miguel C, García-Rodríguez A, Garcés-Durán R, Pellisé M, Llach J, Fernández-Esparrach G. Computer-aided prediction of polyp histology on white light colonoscopy using surface pattern analysis. Endoscopy. 2019;51:261-265. [PubMed] [DOI] [Cited in This Article: ] |
| 207. | Xu Y, Ding W, Wang Y, Tan Y, Xi C, Ye N, Wu D, Xu X. Comparison of diagnostic performance between convolutional neural networks and human endoscopists for diagnosis of colorectal polyp: A systematic review and meta-analysis. PLoS One. 2021;16:e0246892. [PubMed] [DOI] [Cited in This Article: ] |
| 208. | Zachariah R, Samarasena J, Luba D, Duh E, Dao T, Requa J, Ninh A, Karnes W. Prediction of Polyp Pathology Using Convolutional Neural Networks Achieves "Resect and Discard" Thresholds. Am J Gastroenterol. 2020;115:138-144. [PubMed] [DOI] [Cited in This Article: ] |
| 209. | Kominami Y, Yoshida S, Tanaka S, Sanomura Y, Hirakawa T, Raytchev B, Tamaki T, Koide T, Kaneda K, Chayama K. Computer-aided diagnosis of colorectal polyp histology by using a real-time image recognition system and narrow-band imaging magnifying colonoscopy. Gastrointest Endosc. 2016;83:643-649. [PubMed] [DOI] [Cited in This Article: ] |
| 210. | Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HH, Tseng VS. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology. 2018;154:568-575. [PubMed] [DOI] [Cited in This Article: ] |
| 211. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [PubMed] [DOI] [Cited in This Article: ] |
| 212. | Renner J, Phlipsen H, Haller B, Navarro-Avila F, Saint-Hill-Febles Y, Mateus D, Ponchon T, Poszler A, Abdelhafez M, Schmid RM, von Delius S, Klare P. Optical classification of neoplastic colorectal polyps - a computer-assisted approach (the COACH study). Scand J Gastroenterol. 2018;53:1100-1106. [PubMed] [DOI] [Cited in This Article: ] |
| 213. | Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, Iqbal N, Chandelier F, Rex DK. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94-100. [PubMed] [DOI] [Cited in This Article: ] |
| 214. | Min M, Su S, He W, Bi Y, Ma Z, Liu Y. Computer-aided diagnosis of colorectal polyps using linked color imaging colonoscopy to predict histology. Sci Rep. 2019;9:2881. [PubMed] [DOI] [Cited in This Article: ] |
| 215. | Horiuchi H, Tamai N, Kamba S, Inomata H, Ohya TR, Sumiyama K. Real-time computer-aided diagnosis of diminutive rectosigmoid polyps using an auto-fluorescence imaging system and novel color intensity analysis software. Scand J Gastroenterol. 2019;54:800-805. [PubMed] [DOI] [Cited in This Article: ] |
| 216. | Ozawa T, Ishihara S, Fujishiro M, Kumagai Y, Shichijo S, Tada T. Automated endoscopic detection and classification of colorectal polyps using convolutional neural networks. Therap Adv Gastroenterol. 2020;13:1756284820910659. [PubMed] [DOI] [Cited in This Article: ] |
| 217. | Rodriguez-Diaz E, Baffy G, Lo WK, Mashimo H, Vidyarthi G, Mohapatra SS, Singh SK. Real-time artificial intelligence-based histologic classification of colorectal polyps with augmented visualization. Gastrointest Endosc. 2021;93:662-670. [PubMed] [DOI] [Cited in This Article: ] |
| 218. | van der Zander QEW, Schreuder RM, Fonollà R, Scheeve T, van der Sommen F, Winkens B, Aepli P, Hayee B, Pischel AB, Stefanovic M, Subramaniam S, Bhandari P, de With PHN, Masclee AAM, Schoon EJ. Optical diagnosis of colorectal polyp images using a newly developed computer-aided diagnosis system (CADx) compared with intuitive optical diagnosis. Endoscopy. 2021;53:1219-1226. [PubMed] [DOI] [Cited in This Article: ] |
| 219. | Sakamoto T, Nakashima H, Nakamura K, Nagahama R, Saito Y. Performance of Computer-Aided Detection and Diagnosis of Colorectal Polyps Compares to That of Experienced Endoscopists. Dig Dis Sci. 2022;67:3976-3983. [PubMed] [DOI] [Cited in This Article: ] |
| 220. | Yoshida N, Inoue K, Tomita Y, Kobayashi R, Hashimoto H, Sugino S, Hirose R, Dohi O, Yasuda H, Morinaga Y, Inada Y, Murakami T, Zhu X, Itoh Y. An analysis about the function of a new artificial intelligence, CAD EYE with the lesion recognition and diagnosis for colorectal polyps in clinical practice. Int J Colorectal Dis. 2021;36:2237-2245. [PubMed] [DOI] [Cited in This Article: ] |
| 221. | Lu Z, Xu Y, Yao L, Zhou W, Gong W, Yang G, Guo M, Zhang B, Huang X, He C, Zhou R, Deng Y, Yu H. Real-time automated diagnosis of colorectal cancer invasion depth using a deep learning model with multimodal data (with video). Gastrointest Endosc. 2022;95:1186-1194.e3. [PubMed] [DOI] [Cited in This Article: ] |
| 222. | Lui TKL, Wong KKY, Mak LLY, Ko MKL, Tsao SKK, Leung WK. Endoscopic prediction of deeply submucosal invasive carcinoma with use of artificial intelligence. Endosc Int Open. 2019;7:E514-E520. [PubMed] [DOI] [Cited in This Article: ] |
| 223. | Barua I, Mori Y, Bretthauer M. Colorectal polyp characterization with endocytoscopy: Ready for widespread implementation with artificial intelligence? Best Pract Res Clin Gastroenterol. 2021;52-53:101721. [PubMed] [DOI] [Cited in This Article: ] |
| 224. | Singh R, Sathananthan D, Tam W, Ruszkiewicz A. Endocytoscopy for Diagnosis of Gastrointestinal Neoplasia: The Expert's Approach. Video J and Encyclope of GI Endosc. 2013;1:18-19. [DOI] [Cited in This Article: ] |
| 225. | Takamaru H, Wu SYS, Saito Y. Endocytoscopy: technology and clinical application in the lower GI tract. Transl Gastroenterol Hepatol. 2020;5:40. [PubMed] [DOI] [Cited in This Article: ] |
| 226. | Kudo T, Kudo SE, Mori Y, Wakamura K, Misawa M, Hayashi T, Miyachi H, Katagiri A, Ishida F, Inoue H. Classification of nuclear morphology in endocytoscopy of colorectal neoplasms. Gastrointest Endosc. 2017;85:628-638. [PubMed] [DOI] [Cited in This Article: ] |
| 227. | Nakamura H, Kudo SE, Misawa M, Kataoka S, Wakamura K, Hayashi T, Kudo T, Mori Y, Takeda K, Ichimasa K, Miyachi H, Katagiri A, Ishida F, Inoue H. Evaluation of microvascular findings of deeply invasive colorectal cancer by endocytoscopy with narrow-band imaging. Endosc Int Open. 2016;4:E1280-E1285. [PubMed] [DOI] [Cited in This Article: ] |
| 228. | Kudo SE, Mori Y, Wakamura K, Ikehara N, Ichimasa K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Hayashi T, Miyachi H, Inoue H, Hamatani S. Endocytoscopy can provide additional diagnostic ability to magnifying chromoendoscopy for colorectal neoplasms. J Gastroenterol Hepatol. 2014;29:83-90. [PubMed] [DOI] [Cited in This Article: ] |
| 229. | Kudo T, Kudo SE, Wakamura K, Mori Y, Misawa M, Hayashi T, Kutsukawa M, Ichimasa K, Miyachi H, Ishida F, Inoue H. Diagnostic performance of endocytoscopy for evaluating the invasion depth of different morphological types of colorectal tumors. Dig Endosc. 2015;27:754-761. [PubMed] [DOI] [Cited in This Article: ] |
| 230. | Misawa M, Kudo SE, Mori Y, Nakamura H, Kataoka S, Maeda Y, Kudo T, Hayashi T, Wakamura K, Miyachi H, Katagiri A, Baba T, Ishida F, Inoue H, Nimura Y, Mori K. Characterization of Colorectal Lesions Using a Computer-Aided Diagnostic System for Narrow-Band Imaging Endocytoscopy. Gastroenterology. 2016;150:1531-1532.e3. [PubMed] [DOI] [Cited in This Article: ] |
| 231. | Kudo SE, Misawa M, Mori Y, Hotta K, Ohtsuka K, Ikematsu H, Saito Y, Takeda K, Nakamura H, Ichimasa K, Ishigaki T, Toyoshima N, Kudo T, Hayashi T, Wakamura K, Baba T, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Artificial Intelligence-assisted System Improves Endoscopic Identification of Colorectal Neoplasms. Clin Gastroenterol Hepatol. 2020;18:1874-1881.e2. [PubMed] [DOI] [Cited in This Article: ] |
| 232. | König TT, Goedeke J, Muensterer OJ. Multiphoton microscopy in surgical oncology- a systematic review and guide for clinical translatability. Surg Oncol. 2019;31:119-131. [PubMed] [DOI] [Cited in This Article: ] |
| 233. | Terradillos E, Saratxaga CL, Mattana S, Cicchi R, Pavone FS, Andraka N, Glover BJ, Arbide N, Velasco J, Etxezarraga MC, Picon A. Analysis on the Characterization of Multiphoton Microscopy Images for Malignant Neoplastic Colon Lesion Detection under Deep Learning Methods. J Pathol Inform. 2021;12:27. [PubMed] [DOI] [Cited in This Article: ] |