Published online Jun 14, 2018. doi: 10.3748/wjg.v24.i22.2373
Peer-review started: April 13, 2018
First decision: April 27, 2018
Revised: May 16, 2018
Accepted: May 26, 2018
Article in press: May 28, 2018
Published online: June 14, 2018
Helicobacter pylori (Hp) is a major human pathogen causing chronic, progressive gastric mucosal damage and is linked to gastric atrophy and cancer. Hp-positive individuals constitute the major reservoir for transmission of infection. There is no ideal treatment for Hp. Hp infection is not cured by a single antibiotic, and sometimes, a combined treatment with three or more antibiotics is ineffective. Atrophic gastritis (AG) is a chronic disease whose main features are atrophy and/or intestinal metaplasia of the gastric glands, which arise from long-standing Hp infection. AG is reportedly linked to an increased risk for gastric cancer, particularly when extensive intestinal metaplasia is present. Active or past Hp infection may be detected by conventional methods in about two-thirds of AG patients. By immunoblotting of sera against Hp whole-cell protein lysates, a previous exposure to Hp infection is detected in all AG patients. According to guidelines, AG patients with Hp positivity should receive eradication treatment. The goals of treatment are as follows: (1) Cure of infection, resolution of inflammation and normalization of gastric functions; (2) possible reversal of atrophic and metaplastic changes of the gastric mucosa; and (3) prevention of gastric cancer. An ideal antibiotic regimen for Hp should achieve eradication rates of approximately 90%, and complex multidrug regimens are required to reach this goal. Amongst the factors associated with treatment failure are high bacterial load, high gastric acidity, Hp strain, smoking, low compliance, overweight, and increasing antibiotic resistance. AG, when involving the corporal mucosa, is linked to reduced gastric acid secretion. At a non-acidic intra-gastric pH, the efficacy of the common treatment regimens combining proton pump inhibitors with one or more antibiotics may not be the same as that observed in patients with Hp gastritis in an acid-producing stomach. Although the efficacy of these therapeutic regimens has been thoroughly tested in subjects with Hp infection, there is a paucity of evidence in the subgroup of patients with AG. Bismuth-based therapy may be an attractive treatment in the specific setting of AG, and specific studies on the efficacy of bismuth-based therapies are needed in patients with AG.
Core tip: Atrophic gastritis (AG) may arise from long-standing Helicobacter pylori (Hp) infection and is linked to increased gastric cancer risk. According to guidelines, Hp-positive AG patients should receive eradication treatment. The goals of treatment are as follows: (1) Cure of infection, (2) possible reversal of atrophic/metaplastic changes, and (3) prevention of gastric cancer. When involving the corporal mucosa, AG is linked to reduced acid secretion. At a non-acidic intra-gastric pH, the efficacy of common treatment regimens may not be the same as those observed in an acid-producing stomach. There is a paucity of evidence of efficacy of eradication regimens in AG patients. Bismuth-based therapies may be promising.
- Citation: Lahner E, Carabotti M, Annibale B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J Gastroenterol 2018; 24(22): 2373-2380
- URL: https://www.wjgnet.com/1007-9327/full/v24/i22/2373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i22.2373
Helicobacter pylori (Hp), a gram-negative bacterium inhabiting the luminal surface of the gastric epithelium first isolated in 1982, is thought to have infected humans for more than 50000 years. Hp is the most infectious human pathogen, affecting approximately 50% of the population. In Northern Europe and North America, about one-third of adults have this bacterium, whereas in Southern and Eastern Europe, South America, and Asia, more than half of people are estimated to be infected. Hp infection occurs commonly in developing countries, whereas the infection rates are decreasing in developed countries, potentially indicating that socioeconomic status and living standards might play roles in the distribution of the infection[1].
As elegantly summarized by De Francesco et al[2], there is still no ideal treatment against Hp, and the “therapeutic battle” continues, probably for several reasons; Hp is a peculiar bacterium characterized by some particular features: (1) This organism, notwithstanding its pathogenic nature, has been present in the stomach of humans for many thousands years; (2) it is one of the very few bacteria able to survive in acidic gastric juice, as it has developed its ecological niche between the mucus and the epithelial layer; (3) its chronic presence in the human stomach may lead to benign and malignant disorders in the upper gastrointestinal tract and even to some disorders outside of the gastrointestinal tract; (4) although gram-negative, Hp is sensitive to penicillin, which generally works better on the wall of gram-positive bacteria; (5) Hp infection is not cured by a single antibiotic, and sometimes, a combined treatment with three or more antibiotics is ineffective; and (6) since Hp is able to evade several immune defence mechanisms, no effective vaccine has been developed.
Atrophic gastritis (AG) is a chronic disease whose main features are atrophy and/or intestinal metaplasia of the gastric glands. When the oxyntic mucosa is involved, atrophy leads to a lack of both gastric acid and intrinsic factor production as well as to cobalamin or iron malabsorption and eventually anaemia[3,4]. AG is a complex condition that may arise from long-standing Hp infection or in the context of autoimmune gastritis[5], and it is reportedly linked to an increased risk for gastric neoplasias such as intestinal-type adenocarcinoma and type 1 gastric carcinoids, in particular when extensive intestinal metaplasia is present[6]. In a meta-analysis, the ratios of the AG incidence in Hp-positive patients to that in Hp-negative ones ranged from 2.4 to 7.6, with a summary estimate of 5 (95%CI: 3.1-8.3)[7], thus suggesting a strong relationship between incidence of AG and Hp infection.
Ongoing Hp infection has been linked to an increased risk of gastric cancer, though the data are conflicting on whether the treatment of Hp infection prevents gastric cancer. Hp infection has also been identified as a decisive pathogenetic factor of gastric MALT lymphoma, and Hp eradication is the treatment of choice in all MALT lymphoma patients infected by the bacterium[1,8]. Hp has been classified as a class I carcinogen by the World Health Organization and the International Agency for Research on Cancer Consensus Group in 1994[8]. The Uemura study on 1526 Japanese subjects showed that gastric cancer developed in 2.9% of 1246 Hp-infected patients over 7.8 years, whereas gastric cancer was not observed in 280 uninfected control subjects or in a subgroup of 253 individuals who received Hp eradication therapy early during follow-up[9]. The greatest benefit of cure of Hp infection on gastric cancer risk in asymptomatic adults has been reported in regions with the highest incidence of gastric cancer; reported relative risks for regions of low, intermediate and high incidence of gastric cancer were 0.80, 0.49, and 0.45, respectively[10]. A recent study of nearly 39000 asymptomatic subjects showed that the cumulative incidence of gastric cancer was significantly higher in the non-eradication group (hazard ratio 4.1) compared to the eradication group or to the Hp-negative group[11], thus supporting the positive effect of Hp eradication on the prevention of gastric cancer in this setting.
A meta-analysis found that antral and body gastric atrophy could regress after eradicating Hp (pooled OR 0.5 and 0.2, P < 0.01), but this effect was not observed for intestinal metaplasia[12], suggesting that cure of Hp infection may have a beneficial long-term effect on gastric atrophy. Another meta-analysis reported that eradication of H. pylori is effective only in a subset of patients in whom intestinal metaplasia or dysplasia are absent[13]. Nevertheless, the reported predictors for body atrophy reversal were an absence of intestinal metaplasia (HR = 2.4, 95%CI: 1.2-4.8), mild atrophy (HR = 2.14, 95%CI: 1.12-4.1), and moderate-severe inflammation before treatment (HR = 5.3; 95%CI: 1.64-17.3)[14].
Given the important link between AG and gastric cancer, between gastric cancer and Hp infection, and between Hp infection and AG, the treatment of Hp infection in patients with AG is an important issue that we wish to focus on in this review.
AG, especially when associated with intestinal metaplasia, is a linked to a higher risk for gastric cancer, thus representing a precancerous condition. The eventual development of the intestinal-type gastric adenocarcinoma is the end result of an inflammation-metaplasia-dysplasia-carcinoma sequence, the so-called Correa cascade[15]. One important determinant of gastric cancer risk is how the precancerous changes in the gastric mucosa are distributed within the stomach. The oxyntic gland atrophy and/or the intestinal metaplasia distributed in a multifocal pattern, including the lesser curvature of the corpus and fundus (multifocal atrophic gastritis), reported as the “extensive” phenotype and has been associated with a higher risk of gastric cancer. The idea of ‘gastritis of the carcinoma phenotype’ proposes that corpus-predominant gastritis increases the risk of gastric cancer[16], probably due to changes in the intra-gastric milieu, such as increased pH, reduced ascorbic acid and the scavenging of nitrites, perhaps due to dysbiosis of the gastric microbiota[17,18] and probably also due to bacteria other than Hp, such as Lachnospiraceae, Lactobacillaceae, and Streptococcaceae[19].
Notwithstanding a growing body of evidence, the composition of a healthy gastric microbiota remains undefined, and the relationship between Hp and other gastric bacteria or other microorganisms is yet to be clarified. Some evidence shows that Hp decreases the diversity of the gastric microbiota, suggesting its predominance over other microorganisms. Therefore, Hp may represent the main but not the sole microbial trigger of gastric diseases, and microbes other than Hp may play a role in the occurrence of long-term complications of Hp infection[20]. Therefore, it might be speculated that the antibiotic treatment given for eradicating Hp may in some cases be beneficial for gastric cancer risk because it is efficacious in eliminating bacteria other than Hp.
Previous work tried to quantify the risk of gastric neoplasms in AG patients. A progression rate of AG to gastric cancer up to 2% yearly has been observed at follow-up periods up to 16 years[21,22]. A systematic review showed, in AG patients with pernicious anaemia, an estimated 7-fold relative risk of gastric cancer[23]. A very recent systematic review reported widely ranging annual incidence rates of gastric cancer in patients with gastric atrophy (0.53 to 15.24 per 1000 person years) and intestinal metaplasia (0.38 to 17.08 per 1000 person years)[24]. The clinical relevance of AG is supported by European guidelines recommending endoscopic-histologic surveillance with 3-year intervals in patients with moderate to severe extensive AG[25]. Similarly, the Kyoto guidelines on the management of Hp gastritis recommend surveillance of these patients[26].
AG is viewed as the first important step in the pathogenesis of gastric cancer, which probably develops in a multistep process beginning from chronic gastritis and going forward through AG, intestinal metaplasia, and dysplasia[15]. It is accepted that this sequence is usually triggered by Hp infection and is synergistically influenced by a variety of genetic and environmental factors. Amongst Hp-positive patients, only up to 2% of subjects will develop gastric cancer, supporting the idea that the final effects of Hp infection could be affected by its prevalence as well as environmental, bacterial, and host factors[8,27].
Amongst a prospective cohort of patients with AG involving the corporal mucosa, 22.6% and 52.7% of patients were Hp-positive as diagnosed, respectively, by histology of gastric biopsies and by anti-Hp IgG antibodies assessed by ELISA serology[28]. This result implied that there was active or past Hp infection in about two-thirds of these patients. A further study, investigating AG patients for previous exposure to Hp infection by immunoblotting of sera against Hp whole-cell protein lysates, observed that all the AG patients classified as Hp-negative by histology and conventional ELISA serology showed an immunoblotting seroreactivity, including in each case either cagA or vacA[29]; the concomitant seroreactivity against cagA and vacA was highly prevalent in the Hp-negative AG patients, similar to those with positive histologic infection (77.4% vs 86.2%) and with positive ELISA serology (vs 61.5%). These data suggest that immunoblotting is able to prove a previous exposure to Hp infection in virtually all patients with AG, making plausible a hidden role of the infection in this condition. In clinical practice, the presence of Hp infection in AG patients may be difficult to show, as non-invasive tests such as the urea breath test or the stool antigen test may be falsely negative, and the most reliable test seems to be the presence of active infection (acute inflammatory infiltration) on histological evaluation of gastric biopsies combined with Hp IgG serology[30].
As mentioned above, the outcome of Hp infection is highly strain- and host-dependent, which implies that multiple interplaying factors should be taken into consideration[31]. Amongst strain-dependent factors, a very recent systematic review showed that Hp strains positive for the virulence factors vacA, s1m1, and cagA can significantly increase the risk of gastric cancer, and these bacterial genetic markers may be used for risk stratification between different populations[32].
Corpus-predominant AG is considered one of the outcomes of Hp infection that puts patients at higher risk for gastric cancer[9]. A previous study using immunoproteome technology to identify Hp antigens showed that sera from AG (40.5% ± 2%) and gastric cancer patients (25.9% ± 1.8%) showed a significantly higher and stronger mean immunoreactivity vs Hp antigens compared to peptic ulcer patients (11.2% ± 1.3%). That method differentially recognized 17 Hp antigens[33]. These data suggest that patients with gastric cancer and those with AG, its precursor condition, may display a common serological immunorecognition pattern of Hp antigens, confirming the link between the infection and these conditions.
Hp is a major human pathogen causing chronic and progressive gastric mucosal damage, and it is aetiologically related to gastric atrophy and gastric cancer. Hp-positive individuals constitute the major reservoir for transmission of the infection[8,34]. According to main guidelines and consensus statements[26,35,36], all Hp-positive individuals should receive eradication treatment unless competing considerations are present. This recommendation implies that all patients with AG and positivity to Hp should receive eradication treatment. The possible goals of treatment of Hp infection in AG patients are as follows: (1) Cure of infection, resolution of related mucosal inflammation and normalization of gastric functions (acid secretion); (2) possible reversal of atrophic and metaplastic changes of the gastric mucosa and preventing the lesions reaching the so-called point of no return, beyond which the reversibility of histological changes is virtually considered not possible anymore; and (3) finally, prevention or risk reduction of gastric cancer, as current evidence is consistent with the notion that cure of Hp infection may stop the progression of damage and may reduce the Hp-related events increasing genetic instability in the gastric mucosa[10,37,38].
The potential benefits of cure of Hp infection for a single individual, including in terms of cancer risk reduction, depend on the degree and extent of atrophic damage that has already occurred at the time of eradication and the eventual reversibility of that damage[26,35]. Amongst the several approaches to stratify the risk, the validated histological staging systems, such as operative link for gastritis assessment (OLGA) and operative link for gastric intestinal metaplasia assessment (OLGIM), may be mentioned[39,40]. In geographical areas with a sufficiently high expertise, endoscopic scoring systems such as that of Kimura and Takemoto can be applied, but histological confirmation is still recommended[41].
The reversibility of AG after Hp eradication remains a controversial issue. A recent meta-analysis of 12 studies reported that eradication was linked with a significant reduction in AG in the corpus (P = 0.006) but not in the antrum (P = 0.06); furthermore, there was evidence for a significant effect on intestinal metaplasia neither in the corpus (P = 0.42) nor in the antrum (P = 0.76)[42]. Two other meta-analyses observed consistent findings[12,13], showing significant improvement of gastric atrophy after cure of Hp infection, whereas improvement was not shown for intestinal metaplasia. A very recent long-term follow-up study reported that AG and intestinal metaplasia in the antrum and corpus improved only in the Hp-cured patients compared to baseline[43]. These data support the idea that Hp eradication may be a prevention strategy for gastric cancer through resolution/improvement of precancerous lesions.
Since the 1990s, in different countries, national and international guidelines for the management of patients with Hp infection have been published. These guidelines generally comprise first-line therapy recommendations, which vary by country or region[26,35,44-51].
An ideal antibiotic regimen for Hp should achieve eradication rates of approximately 90%, and complex multidrug regimens are required to reach this goal. Amongst factors associated with treatment failure are high bacterial load, high gastric acidity, Hp strain, smoking, and low compliance. However, the increasing antibiotic resistance, particularly against clarithromycin, seems to be played a major role in poor outcomes. To limit the problem of resistance, a combination of drugs with no significant resistance would be necessary[2,35,44]. Decreasing eradication rates with standard therapies have prompted recent changes in recommended first-line therapies. Proposed treatment regimens are mainly bismuth-based triple therapies in Eastern guidelines, mainly concomitant and bismuth-based therapy in Western guidelines, and less commonly sequential or hybrid regimens[2]. However, these recommendations refer to chronic Hp gastritis, without taking into consideration the peculiar condition of AG.
In particular, AG, when involving the corporal mucosa, is notably linked to reduced gastric acid secretion and consequent hypochlorhydria. In this particular intra-gastric microenvironment with a non-acidic intra-gastric pH, the efficacy of the common treatment regimens using the combination of a proton pump inhibitor with one or more antibiotics may not be the same as observed in patients with Hp gastritis in an acid-producing stomach. Though the efficacy of these therapeutic regimens has been largely tested in subjects with Hp infection[52-54], there is a paucity of evidence in the subgroup of patients with AG. From some studies, albeit not designed for this aim, eradication rates of AG patients can be extrapolated, and they have ranged between 71% and 86%. In a previous study in which 192 patients with Hp-positive AG were treated with bismuth-based triple regimens, an overall eradication rate of 70.8% was achieved[14]. In another study, of 57 patients with intestinal metaplasia receiving standard triple therapy, the infection was successfully cured in 49 patients (eradication rate 85.9%)[55]. Less recent Japanese studies achieved eradication rates of 82.2% and 70.5%[56,57]. Table 1 summarizes the eradication rates reported in previous studies.
| Reference | Countries | Patients, n | Treatment regimen | Cured patients |
| Sánchez Cuén et al[55], 2016 | Mexico | 57 | Omeprazole (40 mg), amoxicillin (1 g), and clarithromycin (500 mg), twice daily for two wk | 49 (85.9) |
| Vannella et al[14], 2011 | Italy | 192 | Dicitrate bismuthate (120 mg qds) for 4 wk, plus amoxicillin (1 g tds), and metronidazole (250 mg tds) during the first 2 wk | 136 (70.8) |
| Kamada et al[56], 2003 | Japan | 45 | Omeprazole (20 mg), amoxicillin (1500 mg) and clarithromycin (600 mg) for 1 wk | 35 (82.2) |
| Ohkusa et al[57], 2001 | Japan | 163 | Proton-pump inhibitor and antibiotic therapy for 1 wk | 115 (70.5) |
Bismuth-based therapy may be an attractive treatment in the specific setting of AG. Bismuth has been used for centuries in medicine. From a gastroenterological perspective, bismuth salts have been used to treat peptic ulcer disease, dyspepsia, parasitic infections, microscopic colitis, and infectious diarrhoea[58]. Soon after the discovery of Hp, Marshall highlighted that some antimicrobial compounds (e.g., bismuth salts and metronidazole) had been used to treat peptic ulcer disease in the past with some success. These results led to a renewed interest in bismuth compounds, largely because bismuth was found to inhibit the growth of Hp and effective in eradicating the bacterium[59]. In 1995, two articles, independently and at the same time, showed that adding PPI to bismuth-based triple therapy increased treatment efficacy[60,61]. This combination mainly remained a rescue therapy during the following ten years, when the PPI-clarithromycin-based triple therapy was the standard therapy[62]. Bismuth has an established history in the treatment of Hp. Colloidal bismuth subcitrate has potent anti-Hp activity (MIC 4-32 μg/mL), and in vitro resistance has not been detected. Further, bismuth increases eradication when included in double, triple, and quadruple regimens[2].
In AG patients, in whom acid secretion is generally impaired, treatment with PPI may not make sense at all. Therefore, in this specific setting, bismuth-based therapy may represent a more promising treatment option, especially in the recent galenic formulation, bismuth subcitrate potassium, metronidazole, and tetracycline (BMT, sold under licence as Pylera®). In particular, this formula consists of 140 mg of bismuth subcitrate potassium (equivalent to Bi2O3), 125 mg of metronidazole and 125 mg of tetracycline hydrochloride given as a three-in-one capsule four times daily for ten days[63]. Generally, this formula is associated with 20 mg of omeprazole twice daily, which in hypochlorhydric AG patients is not indicated or even useless. One advantage of this three-in-one treatment is that it should allow us to standardize the doses of molecular antimicrobials, which is not always possible when the compounds are taken separately. Undeniably, 14 d triple therapy with bismuth salts, tetracycline and metronidazole was the first therapy to achieve consistently high Hp eradication rates[2,63]. The bismuth-based quadruple therapy is included among the recommended first-line therapies in the current European, United States, Canadian and Chinese guidelines[35,36,48,49]. Several studies have investigated the efficacy of bismuth-containing quadruple therapy. A previous systematic review showed that the triple capsule Pylera® achieved eradication rates ranging between 84% and 97%. Eradication rates were similar for clarithromycin- and metronidazole-resistant strains. Eradication rates with an omeprazole, bismuth, metronidazole and tetracycline regimen appeared comparable between metronidazole-resistant and -sensitive strains. This effect was not seen with the use of triple therapy in cases of clarithromycin resistance. Previous clinical trials did not report any serious side effects from bismuth-based regimens, and compliance was similar to standard triple therapy[63].
The safety of bismuth administration for Hp eradication has been confirmed in a systematic review of 35 randomized clinical trials with a total of 4763 patients, 2435 of whom were treated with bismuth salts[64]: no serious adverse event was reported with the bismuth therapy. There was also no statistically significant difference in the total number of adverse events between those receiving bismuth salts and other regimens or in the individual adverse events, that is, abdominal pain, diarrhoea, dizziness, headache, metallic taste, nausea or vomiting. A very recent Italian study compared 10 d sequential and bismuth-based quadruple therapies for first-line Hp treatment in 495 patients, achieving similarly high eradication rates (92% vs 91%) as first-line treatments for Hp infection in clinical practice[65]. Unfortunately, patients were not stratified according to pattern of gastritis.
Though bismuth salts represent a promising treatment in the setting of AG with a rationale based on the specific pharmacological, bacteriostatic properties of bismuth salts, the data on the efficacy and safety of bismuth-based regimens without use of PPIs in this specific setting are lacking and are urgently needed.
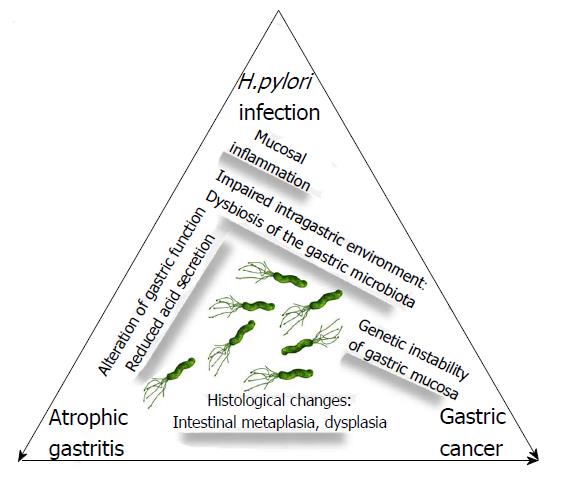
It should be kept in mind that patients with AG have a higher risk for gastric neoplasms and need endoscopic surveillance based on the extent and degree of these pre-neoplastic mucosal alterations as staged by OLGA/OLGIM. This outcome may have a negative impact on the quality of life of these patients. A recent paper showed that the quality of life (SF-8) scores on both the mental component summary and the physical component summary significantly improved after the eradication of Hp, irrespective of the symptoms, especially in patients who had an impaired quality of life before the eradication[66]. This improvement may represent a further reason to search for and to treat Hp infection in the specific setting of patients with AG, in whom the timely cure of Hp infection may lead to reversal of pre-neoplastic changes, restoration of gastric function, and elimination or reduction of gastric cancer risk, as schematically illustrated in Figure 1.
In the particular setting of AG, which is generally associated with a non-acidic intragastric pH, the efficacy of the common treatment regimens using proton pump inhibitors with one or more antibiotics may not be the same as those observed in patients with Hp gastritis in an acid-producing stomach. Although the efficacy of these therapy regimens has been thoroughly tested in subjects with Hp infection, there is a paucity of evidence in the subgroup of patients with AG. Bismuth-based therapy may be an attractive treatment in the specific setting of AG, and specific studies on the efficacy of bismuth-based therapies are needed in patients with AG.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dinç T, Savarino V, Une C S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19 Suppl 1:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 312] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 2. | De Francesco V, Bellesia A, Ridola L, Manta R, Zullo A. First-line therapies for Helicobacter pylori eradication: a critical reappraisal of updated guidelines. Ann Gastroenterol. 2017;30:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Rugge M, Capelle LG, Cappellesso R, Nitti D, Kuipers EJ. Precancerous lesions in the stomach: from biology to clinical patient management. Best Pract Res Clin Gastroenterol. 2013;27:205-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10:529-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 5. | Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol. 2009;15:5121-5128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 164] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (2)] |
| 6. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 505] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 7. | Adamu MA, Weck MN, Gao L, Brenner H. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Infection with Helicobactor pylori: IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, vol 61. Schistosomes, Liver Flukes, and Helicobactor pylori. Lyon, International Agency for Research on Cancer. 1994;177-241. [Cited in This Article: ] |
| 9. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3126] [Cited by in F6Publishing: 3020] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 10. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 552] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 11. | Bae SE, Choi KD, Choe J, Kim SO, Na HK, Choi JY, Ahn JY, Jung KW, Lee J, Kim DH. The effect of eradication of Helicobacter pylori on gastric cancer prevention in healthy asymptomatic populations. Helicobacter. 2018;23:e12464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12 Suppl 2:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Vannella L, Lahner E, Bordi C, Pilozzi E, Di Giulio E, Corleto VD, Osborn J, Delle Fave G, Annibale B. Reversal of atrophic body gastritis after H. pylori eradication at long-term follow-up. Dig Liver Dis. 2011;43:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] [Cited in This Article: ] |
| 16. | Meining A, Morgner A, Miehlke S, Bayerdörffer E, Stolte M. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis? Best Pract Res Clin Gastroenterol. 2001;15:983-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Dias-Jácome E, Libânio D, Borges-Canha M, Galaghar A, Pimentel-Nunes P. Gastric microbiota and carcinogenesis: the role of non-Helicobacter pylori bacteria - A systematic review. Rev Esp Enferm Dig. 2016;108:530-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Castaño-Rodríguez N, Goh KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7:15957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 19. | Lam SY, Yu J, Wong SH, Peppelenbosch MP, Fuhler GM. The gastrointestinal microbiota and its role in oncogenesis. Best Pract Res Clin Gastroenterol. 2017;31:607-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31:579-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, Guilherme M, Barbosa J, Lomba-Viana H, Silva R, Moreira-Dias L. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol. 2004;57:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Vannella L, Lahner E, Osborn J, Bordi C, Miglione M, Delle Fave G, Annibale B. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment Pharmacol Ther. 2010;31:1042-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 24. | Spence AD, Cardwell CR, McMenamin ÚC, Hicks BM, Johnston BT, Murray LJ, Coleman HG. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: a systematic review. BMC Gastroenterol. 2017;17:157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 451] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 26. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 899] [Cited by in F6Publishing: 948] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 27. | Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 28. | Annibale B, Negrini R, Caruana P, Lahner E, Grossi C, Bordi C, Delle Fave G. Two-thirds of atrophic body gastritis patients have evidence of Helicobacter pylori infection. Helicobacter. 2001;6:225-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Annibale B, Lahner E, Santucci A, Vaira D, Pasquali A, Severi C, Mini R, Figura N, Delle Fave G. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis. Helicobacter. 2007;12:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Lahner E, Vaira D, Figura N, Pilozzi E, Pasquali A, Severi C, Perna F, Delle Fave G, Annibale B. Role of noninvasive tests (C-urea breath test and stool antigen test) as additional tools in diagnosis of Helicobacter pylori infection in patients with atrophic body gastritis. Helicobacter. 2004;9:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475-2487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 32. | Pormohammad A, Ghotaslou R, Leylabadlo HE, Nasiri MJ, Dabiri H, Hashemi A. Risk of gastric cancer in association with Helicobacter pylori different virulence factors: A systematic review and meta-analysis. Microb Pathog. 2018;118:214-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Lahner E, Bernardini G, Possenti S, Renzone G, Scaloni A, Santucci A, Annibale B. Immunoproteomics of Helicobacter pylori infection in patients with atrophic body gastritis, a predisposing condition for gastric cancer. Int J Med Microbiol. 2011;301:125-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Díaz P, Valenzuela Valderrama M, Bravo J, Quest AFG. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front Microbiol. 2018;9:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 35. | Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1745] [Article Influence: 249.3] [Reference Citation Analysis (1)] |
| 36. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 744] [Cited by in F6Publishing: 847] [Article Influence: 121.0] [Reference Citation Analysis (1)] |
| 37. | Ushijima T, Hattori N. Molecular pathways: involvement of Helicobacter pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin Cancer Res. 2012;18:923-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Zabaleta J. MicroRNA: A Bridge from H. pylori Infection to Gastritis and Gastric Cancer Development. Front Genet. 2012;3:294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 40. | Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, Genta RM, Graham DY, Hattori T, Malfertheiner P. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 197] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 41. | Kimura K, Satoh K, Ido K, Taniguchi Y, Takimoto T, Takemoto T. Gastritis in the Japanese stomach. Scand J Gastroenterol Suppl. 1996;214:17-20; discussion 21-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, Zhang G. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 43. | Hwang YJ, Kim N, Lee HS, Lee JB, Choi YJ, Yoon H, Shin CM, Park YS, Lee DH. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther. 2018;47:380-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 90] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 44. | Zagari RM, Romano M, Ojetti V, Stockbrugger R, Gullini S, Annibale B, Farinati F, Ierardi E, Maconi G, Rugge M. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report 2015. Dig Liver Dis. 2015;47:903-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 45. | National Institute for Health and Care Excellence (NICE) guidelines: Gastro-eesophageal reflux disease and dyspepsia: investigation and management (CG184). NICE 2014: 1-41. Available from: www.nice.org.uk/guidance/cg184/resources/gastrooesophageal-reflux-disease-and-dyspepsia-inadults-investigation-and-management-pdf-35109812699845. [Cited in This Article: ] |
| 46. | Gisbert JP, Molina-Infante J, Amador J, Bermejo F, Bujanda L, Calvet X, Castro-Fernández M, Cuadrado-Lavín A, Elizalde JI, Gene E. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol Hepatol. 2016;39:697-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51-69.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 544] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 48. | Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K; Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 50. | Yaxley J, Chakravarty B. Helicobacter pylori eradication - an update on the latest therapies. Aust Fam Physician. 2014;43:301-305 [PMID 24791773]. [Cited in This Article: ] |
| 51. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 52. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (95)] |
| 53. | Yeo YH, Shiu SI, Ho HJ, Zou B, Lin JT, Wu MS, Liou JM, Wu CY; Taiwan Gastrointestinal Disease and Helicobacter Consortium. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta-analysis. Gut. 2018;67:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 54. | Xin Y, Manson J, Govan L, Harbour R, Bennison J, Watson E, Wu O. Pharmacological regimens for eradication of Helicobacter pylori: an overview of systematic reviews and network meta-analysis. BMC Gastroenterol. 2016;16:80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Sánchez Cuén JA, Irineo Cabrales AB, Bernal Magaña G, Peraza Garay F. Regression of gastric intestinal metaplasia after the eradication of Helicobacter pylori infection in a hospital in Mexico. Rev Esp Enferm Dig. 2016;108:770-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Kamada T, Haruma K, Hata J, Kusunoki H, Sasaki A, Ito M, Tanaka S, Yoshihara M. The long-term effect of Helicobacter pylori eradication therapy on symptoms in dyspeptic patients with fundic atrophic gastritis. Aliment Pharmacol Ther. 2003;18:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Ohkusa T, Fujiki K, Takashimizu I, Kumagai J, Tanizawa T, Eishi Y, Yokoyama T, Watanabe M. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann Intern Med. 2001;134:380-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Tillman LA, Drake FM, Dixon JS, Wood JR. Review article: safety of bismuth in the treatment of gastrointestinal diseases. Aliment Pharmacol Ther. 1996;10:459-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol. 2007;21:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | de Boer WA, Driessen WM, Jansz AR, Tytgat GN. Quadruple therapy compared with dual therapy for eradication of Helicobacter pylori in ulcer patients: results of a randomized prospective single-centre study. Eur J Gastroenterol Hepatol. 1995;7:1189-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Borody TJ, Andrews P, Fracchia G, Brandl S, Shortis NP, Bae H. Omeprazole enhances efficacy of triple therapy in eradicating Helicobacter pylori. Gut. 1995;37:477-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Malfertheiner P, Mégraud F, O’Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ. Current European concepts in the management of Helicobacter pylori infection--the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9:1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Saleem A, Qasim A, O’Connor HJ, O’Morain CA. Pylera for the eradication of Helicobacter pylori infection. Expert Rev Anti Infect Ther. 2009;7:793-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008;14:7361-7370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 85] [Cited by in F6Publishing: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Fiorini G, Zullo A, Saracino IM, Gatta L, Pavoni M, Vaira D. Pylera and sequential therapy for first-line Helicobacter pylori eradication: a culture-based study in real clinical practice. Eur J Gastroenterol Hepatol. 2018;30:621-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Taguchi H, Kanmura S, Maeda T, Iwaya H, Arima S, Sasaki F, Nasu Y, Tanoue S, Hashimoto S, Ido A. Helicobacter pylori eradication improves the quality of life regardless of the treatment outcome: A multicenter prospective cohort study. Medicine (Baltimore). 2017;96:e9507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |