Published online Dec 7, 2015. doi: 10.3748/wjg.v21.i45.12896
Peer-review started: April 19, 2015
First decision: July 14, 2015
Revised: August 5, 2015
Accepted: October 13, 2015
Article in press: October 13, 2015
Published online: December 7, 2015
AIM: To summarize the current knowledge about the potential relationship between hepatitis C virus (HCV) infection and the risk of several extra-liver cancers.
METHODS: We performed a systematic review of the literature, according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) Statement. We extracted the pertinent articles, published in MEDLINE and the Cochrane Library, using the following search terms: neoplasm/cancer/malignancy/tumor/carcinoma/adeno-carcinoma and non-Hodgkin lymphomas, kidney/renal-, cholangio-, pancreatic-, thyroid-, breast-,oral-, skin-, prostate-, lung-, colon-, stomach-, haematologic. Case series, case-series with control-group, case-control, cohort-studies as well as meta-analyses, written in English were collected. Some of the main characteristics of retrieved trials, which were designed to investigate the prevalence of HCV infection in each type of the above-mentioned human malignancies were summarised. A main table was defined and included a short description in the text for each of these tumours, whether at least five studies about a specific neoplasm, meeting inclusion criteria, were available in literature. According to these criteria, we created the following sections and the corresponding tables and we indicated the number of included or excluded articles, as well as of meta-analyses and reviews: (1) HCV and haematopoietic malignancies; (2) HCV and cholangiocarcinoma; (3) HCV and pancreatic cancer; (4) HCV and breast cancer; (5) HCV and kidney cancer; (6) HCV and skin or oral cancer; and (7) HCV and thyroid cancer.
RESULTS: According to available data, a clear correlation between regions of HCV prevalence and risk of extra-liver cancers has emerged only for a very small group of types and histological subtypes of malignancies. In particular, HCV infection has been associated with: (1) a higher incidence of some B-cell Non-Hodgkin-Lymphoma types, in countries, where an elevated prevalence of this pathogen is detectable, accounting to a percentage of about 10%; (2) an increased risk of intra-hepatic cholangiocarcinoma; and (3) a correlation between HCV prevalence and pancreatic cancer (PAC) incidence.
CONCLUSION: To date no definitive conclusions may be obtained from the analysis of relationship between HCV and extra-hepatic cancers. Further studies, recruiting an adequate number of patients are required to confirm or deny this association.
Core tip: Hepatitis C virus (HCV) is an oncogenic virus and a well-known risk factor for hepatocellular carcinoma. Some reports suggested that its infection is associated with development of cholangiocarcinoma and some types of lymphomas, but a comprehensive assessment of the possible role of HCV in extrahepatic carcinogenesis has not been yet performed. Aim of this review is to focus on HCV infection association with extra-liver neoplasms, as lymphomas, pancreatic cancer and breast-, renal-, oral- and thyroid-cancers. Our results strongly support the need of additional studies to ensure a precise estimate of the effect of HCV on these different types of extra-hepatic cancers.
- Citation: Fiorino S, Bacchi-Reggiani L, de Biase D, Fornelli A, Masetti M, Tura A, Grizzi F, Zanello M, Mastrangelo L, Lombardi R, Acquaviva G, di Tommaso L, Bondi A, Visani M, Sabbatani S, Pontoriero L, Fabbri C, Cuppini A, Pession A, Jovine E. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J Gastroenterol 2015; 21(45): 12896-12953
- URL: https://www.wjgnet.com/1007-9327/full/v21/i45/12896.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i45.12896
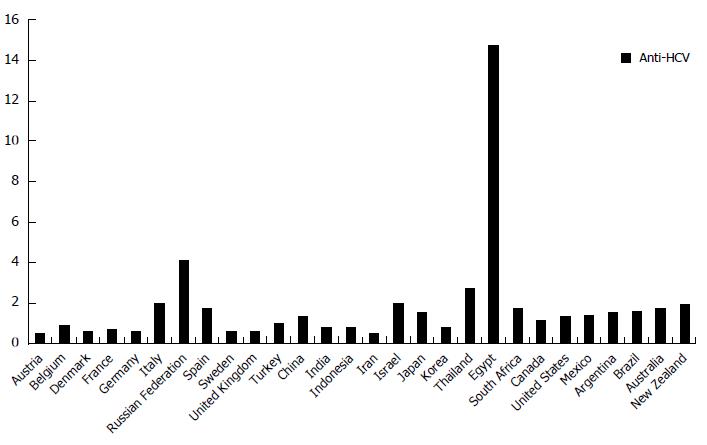
Hepatitis C virus (HCV) is a major global health problem, because it represents a very important cause of mortality, morbidity and resource utilization. Although remarkable differences are detectable in the world, depending on geographical areas and ethnicity, it is estimated that the prevalence of HCV infection is about 2% worldwide (Figure 1)[1]. Approximately 180 million people carriers this pathogen persistently[2]. HCV chronic infection can lead to a necro-inflammatory liver disease, with different pattern of severity and course. This condition is associated with an increased risk of cirrhosis, liver failure and hepatocellular carcinoma[3]. Although liver is the main target for HCV, it is now well-known that this pathogen may induce extra-hepatic pathological conditions, including mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, Sjögren’s syndrome, thyroiditis, a high prevalence of autoantibodies[4] as well as Central and Peripheral Nervous System demyelinating disorders[5]. Several of these manifestations are thought to be caused by the host immune response to this micro-organism and not by a direct viral cytopathic effect. In particular, chronic antigenic stimulation by HCV promotes B-lymphocyte clonal expansion, with the production and release of monoclonal and polyclonal antibodies and generation of immune complexes[6]. Their deposition in small vessels and glomerular capillary walls induces complement activation and, as consequence, tissue injury[7]. In addition, several studies have shown that HCV may infect organs and tissues other than the liver. In particular, presence of antigens, genome and/or replicative sequences of HCV have been detected in several extra-hepatic localizations, such as peripheral blood cells (i.e., neutrophils, T- and B-lymphocytes)[8,9] or kidney[10], skin[11,12], oral mucosa[13], salivary glands[14] and pancreas tissues as well as, in a small number of cases, from heart, gallbladder, intestine and adrenal glands tissues[15,16].
Although HCV antigens and replicative forms have been detected in various extra-hepatic sites, the possible role on the onset of malignancies in these organs is still under investigation. Some evidences have recently suggested the possibility that this pathogen may be associated with the development of a wide spectrum of hematologic or solid cancers, such as non-Hodgkin lymphomas, biliary duct-, bladder-, renal-, pancreatic-, thyroid-, breast- and prostate-carcinomas. Here we summarize the current knowledge about the potential link between HCV infection and risk of these malignancies and we performed a systematic review of the literature that reports the prevalence of HCV infection in patients, suffering from above mentioned malignancies.
See supplementary Material and Methods for further information.
A systematic computer-based search of published articles, according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) Statement, issued in 2009, was conducted through Ovid interface, in order to identify relevant studies on the potential association between HCV infection and malignancies other than hepatocellular carcinoma (HCC). The literature review was performed in February 2015. The following electronic databases were used: MEDLINE (1950 to February, 2015) and the Cochrane Library (until the fourth quarter of 2014) for all relevant articles. The search strategy and the search terms were developed with the support of a professional research librarian. The search text words were identified by means of controlled vocabulary, such as the National Library of Medicine’s MESH (Medical Subject Headings) and Keywords. In our review, assessing the possible association between HCV infection and risk of malignancies other than HCC, we focused on the following malignancies: (1) lymphomas and, in particular, non-Hodgkin lymphomas; (2) biliary ducts-/gallbladder-; (3) renal-/kidney-; (4) pancreatic-; (5) thyroid-; (6) breast-; (7) lung-; (8) stomach-; (9) colon-; (10) skin-/oral-; (11) bladder-; and (12) prostate-carcinomas. The inclusion criteria for our analysis were: (1) study designs by considering data from all published case series, case-control-, hospital-based case-control-, population-based case-control- as well as cohort-studies; (2) articles which were reported in English, as peer-reviewed, full-text publications, whereas papers that were not published as full reports, such as conference abstracts, case reports, and editorials were excluded; (3) clinical series or studies evaluating histological specimens, that included at least 15 patients, therefore reports with fewer than 15 subjects were not considered; and (4) papers describing the type of tests used to assess HCV presence; in particular, in all studies virus search was performed by means of second- or third-generation enzyme-linked immunosorbent assay (ELISA) or recombinant immunoblot assay (RIBA) for confirmation as well as in a large part of available trials HCV-RNA presence was also tested.
Data extraction: Two authors (Masetti M and Bacchi-Reggiani L), independently and in a parallel manner, performed the literature search, identified and screened relevant articles, based on title or title and abstract. If a study was considered potentially eligible by either of the 2 reviewers, the full article of this research was collected for further assessment. Other two authors (Zanello M and Mastrangelo L) independently extracted and tabulated all relevant data from included studies by means of a standardized flow path, according to the Cochrane handbook section 7.3a checklist of domains. The following information was obtained from each study, by means of a predefined data extraction form, including: first author’s name, study design, inclusion and exclusion criteria, year of publication, country of origin, ethnicity, matching criteria, number of cases and controls, diagnostic methods to detect each malignancy, HCV detection assays. The accuracy of data collection was checked by Tura A and any disagreements concerning the results were settled by consensus between all authors. With the purpose to prevent multiple inclusions of the same data, we searched the presence of possible duplicates, examining the first author’s name as well as the place and the period of subjects’ enrolment. When different versions of the same study were detected, only the most recent one was considered.
The search of MEDLINE and Cochrane Library produced the following citations: (1) haematopoietic malignancies: 1424; (2) biliary ducts-/cholangio-: 616; (3) renal-/kidney-: 891; (4) pancreatic-: 244; (5) thyroid-: 126; (6) breast-: 180; (7) lung-: 247; (8) stomach-: 141; (9) colon-: 115; (10) skin-/oral-: 598; (11) bladder-: 150; and (12) prostate-carcinomas: 43.
After a preliminary review of the titles and/or abstracts with the exclusion of non-pertinent articles, we obtained these results: (1) haematopoietic malignancies, including lymphomas/non-Hodgkin lymphomas: 126; (2) biliary ducts-/cholangio-: 48; (3) renal-/kidney-: 10; (4) pancreatic-: 15; (5) thyroid-: 11; (6) breast-: 8; (7) lung-: 3; (8) stomach-: 2; (9) colon-: 5; (10) skin-/oral-: 11; (11) bladder-: 3; and (12) prostate-carcinomas: 5.
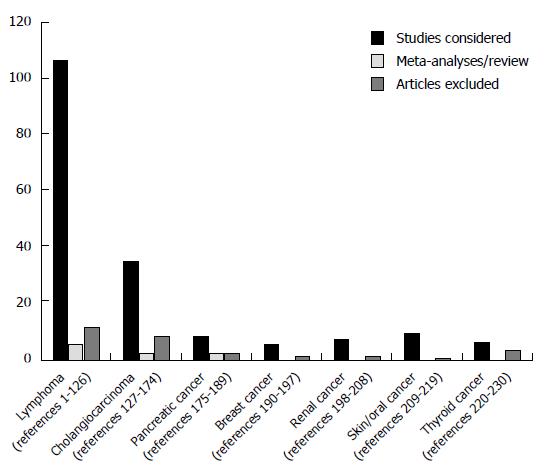
We screened the potentially relevant studies and, in accordance with predefined criteria, we identified and considered in our systematic review the following number of studies: (1) haematopoietic malignancies: 108 articles considered, 6 reviews/meta-analyses, 12 papers excluded[17-146]; (2) biliary ducts/cholangiocarcinoma: 36 articles considered, 3 reviews/meta-analyses, 9 papers excluded[147-195]; (3) renal/kidney: 8 articles considered, 2 papers excluded[118,146,196-203]; (4) pancreatic: 9 articles considered, 3 reviews/meta-analyses, 3 papers excluded[118,146,170,197,204-214]; (5) thyroid: 7 articles considered, 4 papers excluded[52,73,118,146,196,197,215-217]; (6) breast: 6 articles considered, 2 papers excluded[117,118,146,196,197,202,218,219]; (7) lung: 2 articles meeting inclusion criteria, 1 not[146,196,197]; (8) stomach: 2 articles meeting inclusion criteria[146,220]; (9) colon: 3 articles meeting inclusion criteria, 2 not[118,146,196,197,202]; (10) skin/oral: 10 articles considered, 1 paper excluded[118,146,197,221-228]; (11) bladder: 3 articles meeting inclusion criteria[118,146,197]; and (12) prostate-carcinomas: 4 articles meeting inclusion criteria, 1 not[118,146,196,197,202] (Tables 1, 2, 3, 4, 5, 6 and 7). A limited number of identified studies were formally designed as “cohort-“ or “case-control” trials, adequately reporting inclusion criteria for the control group, such as “odds ratios” after adjustment for the most important confounding factors, or showing that cases and controls were matched by sex and age. Whether these data were not indicated, but an acceptable series of healthy subjects or patients with different diseases were recruited for comparison and were described, we defined the considered study, as: “case series with control group”.
| Author/Journal/Publication year | Study design | Diagnosis | HCV positive HM/total HM | Control source | HCV positive controls/total controls | Percentage of HCV-positive cases with 95%CI | Main conclusions |
| Akdogan M Turk J Gastroenterol 1998 | Case series study with control group Period: NR | All lymphomas: NHLs: 30 HL: 18 NHLs NHL classification: Working Formulation | (1) NHL: 4/30 (13.3%) (2) Patients with Hodgkin Lymphoma | (2) Healthy blood donors | (1) 17/9488 (0.8%) | 13.3 (3.8-30.7) | Increased prevalence of HCV persistent infection in patients with NHL, but not in patients with HL, in comparison with general population |
| Amin J J Hepatol 2006 | Community-based cohort-study Period: 1990-2002 | NHLs Cohort of HCV positive patients: 75834, Cohort of HBV/HCV positive patients: 2604 Incidence of LNHs observed in the study cohort was compared to expected incidence derived from New South Wales population cancer rates by calculating standardised incidence ratios | Individuals with HCV infection: 75834 LNH cases detected: 33 | Incidence observed in the study cohort was compared to expected incidence derived from NSW population cancer rates by calculating standardised incidence ratios (SIR) | SIR: 0.9 (0.6-1.2) | 0.04 (0.03-0.05) | In HCV infection group no increased overall risk of NHL-cell lymphoma, but, a number of B-cell NHLs (diffuse NHL, immunoproliferative malignancies and chronic lymphocytic leukaemias) had SIRs greater than one |
| Anderson LA Epidemiol Biomarkers Prev 2008 | Population-based nested case-control study of hematopoietic malignancies Period: 1993 and 2002 | Subjects with hematopoietic malignancies identified, using SEER-Medicare data. SEER program: a cancer surveillance program supported by the National Cancer Institute and covering about 25% of United States population NHL classification: World Health Organization classification Myeloproliferative malignancies classification: acute- and chronic myeloid leukaemia, myelodysplastic syndrome, chronic myeloproliferative disease | 195/61464 (0.3%) cases with Hemato-poietic malignancies identified NHLs: 103/33940 (0.3%) DLBCL: 34/10144 (0.3%) BL: 2/197 (1.5%) MZL: 12/1908 (0.6%) FL: 19/4491 (0.4%) CLL: 23/10170 (0.2%) LL: 2/1148 HL: 3/1155 (0.3%) PCM: 31/9995 (0.3%) Myeloid neoplasm: 47/11945 (0.4%) AML: 23/6068 (0.4%) CML: 1/1528 (0.1%) MS: 18/3084 (0.6%) CMD: 1/1346 (0.1%) | Controls were identified by means of Medicare, a federally funded program administered by the Centres for Medicare and Medicaid Services, For each included case, two controls were selected at random from the 5% random sample of Medicare beneficiaries | 264/122531 (0.2%) population-based controls identified | 0.3 (0.2-0.4) | Association between HCV and elevated risk of NHLs and acute myeloid leukemia. HCV may induce lymphoproliferative malignancies through chronic immune stimulation |
| Arcaini L Clinical Lymphoma, Myeloma & Leukemia 2011 | Case series study with control group Period: NR | Splenic MZLs NHL classification: World Health Organization classification | 25/92 Splenic MZL patients (27.2%) | Patients (122) with WMc 66/122 subjects with HCV markers | 6/66 WMc patients (9%) | 27.2 (18.1-36.2) | Despite similar outcomes among SMZL and WM, SMZL appears as a disease with distinct clinical and histologic characteristics, and a peculiar association with HCV infection |
| Arican A Med Oncol 2000 | Case series Period: February-October 1997 | NHLs Low-grade: 12 (27%) Intermediate grade: 24 (55%) High-grade: 8 (18%) NHL classification: Working Formulation | 2/44 (4.5%) | NR | NR | 4.5 (0-10.7) | No association between HCV chronic infection and NHL development in this study. The prevalence of HCV infection reported to be 0.3%-1.5% in healthy Turkish-blood donors in previous studies be 1.5% in healthy Turkish-blood donors in previous |
| Aviles A Med Oncol 2003 | Case-control study Period: January 1997-December 1999 | B-cell NHLs: 416 Diffuse large cell: 236 Follicular: 97 Marginal B-cell zone: 83 NHL classification: World Health Organization classification | B-cell NHLs 2/416 (0.5%) | Group 1: 682 first-degree relatives (spouses, children, fathers, and brothers of the patient) living in the neighboring area of the patient. Group 2: 832 healthy blood donors, donating during the same period of time at the central blood bank. Group 3: Neoplastic disease group, with 408 patients with solid tumors, breast cancer:127 colon cancer: 94 gastric cancer; 79 lung cancer: 98 Group 4: 353 patients with HCV-positive related chronic liver disease | Prevalence of HCV equal to: (1) 0 among first-degree relatives of patients (2) 0.12 (0.02-0.88) among healthy blood donors (3) 0.56, (0.28-0.75). among patients with solid tumors (4) No patients with HCV chronic liver disease developed malignant lymphoma in a median follow-up of 7.9 yr | 0.5 (0-1.1) | Association between HCV infection and development of malignant lymphoma represents an hazardous observation, the close association reported in areas with a higher prevalence of HCV infection has to be considered with caution, because other epidemiological factors have not been considered, such as a high prevalence of HCV infection compared to other areas |
| Bauduer F Hematol Cell Ther 1999 | Case series Period: January 1995-June 1998 | NHLs: 136 subjects B-cell-NHLs: 110 patients NHL classification: Revised European American Lymphoma (REAL) histological scheme | 2/136 (1.5%) | NR | NR | 1.5 (0-3.4) | No evidence of relationship between HCV and NHLs |
| Besson C J Clin Oncol 2006 | Case control Period: March 1993-June 2002 | B-NHL (DLBCL) NHL classification: Working Formulation | 26/5586 (0.5%) | (1) HCV negative patients with DLCL enrolled in the present study (2) individuals with DLCL randomly chosen among HCV-negative patients included in the GELA program | (1) 5586 (2) 35 | 0.5 (0.29-0.64) | HCV-positive patients with DLBCL differ from other patients both at presentation and during chemotherapy. Specific protocols evaluating antiviral therapy should be designed for these patients |
| Bianco E Haematologica 2004 | Italian multi-center case-control study Period: January 1998 -February 2001 | All lymphomas: 637 HD: 157 CLL: 100 ALL: 54 MM: 107 AML: 140 CML: 49 T-NHL: 30 NHL classification for T-NHLs: REAL/WHO classification | 44/637 (6.9%) HD: 5/157 (3.2%) CLL: 9/100 (9%) ALL: 4/54 (7.6%) MM: 5/107 (4.7%) AML: 11/140 (7.9%) CML: 6/49 (12.2%) T-NHL 4/30 (13.8%) | Patients from other departments of the same hospitals: the departments of dentistry, dermatology, general surgery, gynecology, internal medicine, ophthalmology, orthopedics, otorhinolaryngology, and traumatology | 22/396 (5.6%) | 6.9 (4.9-8.8) | Possible association of HCV infection not only with B-NHL but also with some other lymphoid and myeloid malignancies, however no definitive significant results, due to the absence of large groups of patients to confirm this assumption |
| Bronowicki JP Hepatology 2003 | Case records Data obtained from the hepatology gastroenterology, hematology, internal medicine and pathology departments of 64 French hospitals Period: 1992-1999 | All PLL: 31 cases, 27/31 patients with a B-cell lymphoma: -DLBCL: 22, -BL: 1, -EMZBL of mucosa-associated lymphoid tissue type: 3, unclassified, small B-cell lymphoma:1, T-cell lymphomas: 4 NHL classification: World Health Organization classification | HCV-test available for 28 subjects, HCV test available in 23 patients with B-cell PLL 1 HCV positive patient with peripheral T-cell lymphoma 5/23 (21.7%) | NR | NR | 21.7 (7.5- 43.7) | This study confirms the rarity of PLL and demonstrates an increased prevalence of HCV infection |
| Cavanna L Haematologica 1995 | Case- control study Period: 1985-1990 | All LPDs: 300 patients Anti-HCV positive patients 57/300 (19.7%) NHLs: 150; HL: 20 CLL: 40 Plasma cell discrasias: 90 | NHL: 38/150 (25.3%) HL: 2/20 (10%) CLL: 2/40 (5%) Plasma cell discrasias: 15/90(16%) | Blood donors | 53/3108 (1.7%) | 25.3 (18.3-32.3) | High prevalence of anti-HCV antibodies among patients with lymphoprolipherative disorders as compared with the control group of healthy blood donors |
| Caviglia GP J Gastroenterol Hepatol 2014 | Cohort study Period: January 2006 -December 2013 | 1313 patients with chronic HCV hepatitis 121 patients with extra-hepatic manifestations: B-NHL: 41/1323 (3.1%) MCS: 25/1323 (1.9%) MGUS: 55/1323 (4.2%) NHL classification: World Health Organization classification | B-cell NHL: 41 MZL: 15 (36.6%), had DLBCL: 10 (24.4%), FL: 4 (9.8%) LPL; 1 (2.4%), MM: 1 (2.4%), CLL: 1 (2.4%) and B-NHL not otherwise specified: 9 (22%) | Controls selected on the basis of the absence of extra-hepatic manifestation of HCV infection | 130 HCV positive subjects without extrahepatic manifestation | 3.1 (2.2-4) | Cirrhosis is an additional risk factor for the development of lymphoproliferative disorders in patients with chronic HCV infection |
| Chindamo MC Oncol Rep 2002 | Case series with control group Period: May 1995 -September 1998 | All lymphomas: 207 -HL: 67 -B-NHL: 87 -T-NHL: 22 -CLL: 31 NHL classification: Revised European American Lymphoma (REAL) histological scheme | B-cell NHL: 8/87 (9.2%) | (1) Blood donors (2) Other haematological malignancies (Hodgkin’s disease and chronic lymphocytic leukaemia) | (1) 472/39371 (1.2%) (2) 2/98 (2%) | 9.2 (3.1-15.2) | Association between HCV infection and NHLs |
| Chuang SS J Clin Pathol 2010 | Case-control study Period: January 2004 -December 2008 | All malignancies: 346 -HL: 25 (3HCV+) -B-NHL: 321 (DLBCL, FC CLL, MZL, BL, others) -T- or NK/T-cell NHL: 55 NHL classification: World Health Organization classification | All NHL: 35/321 (11%) B-cell NHL: 34/266 (12.8%) (3/38 with HBV coinfection) | Healthy Taiwanese subjects | 15/824 (1.8%) | 12.8 (8.7-16.8) | The incidence of HCV infection among lymphoma patients in Taiwan was significantly higher than that for healthy controls Non-MALT (nodal and splenic) MZL was the only group significantly associated with HCV |
| Cocco P Int J Hematol 2008 | Case-control study Period: -February 1999 - October 2002 - January 2002 - July 2003 | All malignancies (277): -HL: 13 -NHL: 264 (DLBCL, FC CLL, MZL, MM, T-cell NHL, others) NHL classification: World Health Organization classification | (1) All B cell- NHL: 20/237 (8.4%) (2) NHLs (excluding CLL and MM): 15/177 (8.5%) | Randomly selected controls from population registrars | 9/217 (4.1%) | (1) 8.4 (4.9-11.9) (2) 8.5 (4.3-12.5) | Acute or chronic hepatitis C is associated with a consistent risk increase in all lymphoma subtypes, but follicular lymphoma |
| Collier JD Hepatology 1999 | Case series with control group Period: February 1997 and May 1997 | B-cell NHLs: 100 NHL classification: Working Formulation | 1/100 (1%) | In-Hospital patients with nonhematologic malignancies, treated at the Princess Margaret Hospital | 1/100 (1%) | 1 (0-3) | No association between hepatitis C and B-cell lymphoma |
| Cowgill KD Int J Epidemiol 2004 | Case-control study Period: October 1999- and January 2003 | B-cell NHL: 220 NHL classification: NR | Total: 106/220 (48.1%) (1) anti-HCV+/RNA- 12/220 (5.4%) (2) anti-HCV+/RNA+ 94/220 (42.7%) | In-Hospital patients with fractures, treated at the Kasr El-Aini Orthopaedic Hospital, | Total: 80/222 (36%) (1) anti-HCV+/RNA-28/222 (12.6%) (2) anti-HCV+/RNA+ 52/222 (23.4%) | 48.2 (41.5-54.7) | Strong association between chronic HCV infection and risk of developing NHL, persisting after adjustment in multivariate models and after several sensitivity analyses |
| Cucuianu A Br J Haematol 1999 | Case series with control group Period: December 1997 and March 1999 | All B-cell NHL: 68 NHL classification: Working Formulation | 20/68 (29.5%) | Non-hospitalized Romanian individuals | 46/943 (4.9%) | 9.1 (5.3-12.9) | Detection of high prevalence (29.5%) of anti-HCV in patients with NHL, especially in low-grade types |
| De Renzo A Haematologica 2002 | Case-control Period: NR | All LPDs: 227 -B-cell LPds: 127 -HL 100 NHL classification: Revised European American Lymphoma (REAL) histological scheme | B-cell LPds: 22/127 (17.3%) B-NHL 12/61(19.7%) MM 4/48 (8.3%) WM 4/9 44.4%) CLL 2/9 (22.2%) | A group of occasional blood donors from the same geographical area, studied as healthy controls | -HL 2/100 (2%) -Controls: 2/110 (1.8%) | 19.7 (9.7-29.6) | Detection, in Southern Italy, of a higher prevalence of HCV infection in patients suffering from B-LPD in comparison with healthy subjects, particularly in patients with B-cell-NHL, CLL and WMc |
| De Renzo A Euro J Haematology 2008 | Case series Period: 1990-2005 | All NHLs patients observed: 550 Primary hepatic lymphomas (PHL): 6 Primary splenic Lymphomas (PSL): 19 NHL classification: World Health Organization classification | PHL: 4/6 PSL: 13/19 | NR | NR | PHL 66.7 (22.3-95.7) PSL 68.4 (43.5-87.4) | High prevalence of HCV infection among patients with rare haematologic malignancies (PHL and PSL ), favourable outcome of these subjects |
| De Rosa G Am J Hematol 1997 | Case series with control group Period: November 1994 -November 1995 | All Lympho-prolipherative Disorders (315): (1) No-B LPD: 52 HD: 43 (1 HCV+) T-NHL: 9 (2) B LPD: 272, including; NHL- B-cell lymphoma, CLL, HCL, MGUS, WMc, MM, ( 59 HCV+) NHL classification: Working Formulation | B-cell NHL: 21/91 (23.1%) | (1) Patients with Hodgkin Lymphoma (2) Healthy blood donors | (1) 1/43 (2.3%) 0/9 (2) 30/1568 (1.9%) | 23.1 (14.4-33.7) | Detection of a higher prevalence of anti-HCV antibodies patients with B-Lymphoprolipherative disorders, as compared to the normal population and to patients with a non-B-lymphoprolipherative disorders |
| De Vita S Br J Cancer 1998 | Case-control study Period: January 1994-June 1997 | All malignancies 84 NHLs NHL classification: Working Formulation | 20/84 (23.8%) | Controls recruited at Aviano, with cancers in: ovary: 13 uterus:14, colon-rectum:13, pancreas:10, lung: 8, stomach: 6, oesophagus: 4 other sites: 5 HCC: 27 | Controls: 3/73 (4.1%) HCC: 11/27 (40.7%) | 23.8 (15.2-34.3) | Detection of a higher than expected prevalence of HCV infection in B-cell NHL patients |
| Duberg AS Hepatology 2005 | Nationwide cohort of HCV-infected persons Cancer Registry used to identify all incident cancers diagnosed in the cohort malignant NHL Period: 1990-2000 | All malignancies: Patients with B-Cell NHLs, after exclusion of patients with HIV coinfection: 16 CLL: 4 MM:7 ALL: 1 HL: 1 NHL classification: NR | B-Cell NHLs: 16 in 27150 HCV positive patients included in the cohort, HCV infection diagnosis made to the Swedish Institute for Infectious Disease Control (SMI) | NR | NR | 0.06 (0.04-0.1) | A significantly increased risk of NHL and MM observed in this study, although an underestimation of the risk may have been caused by the delayed diagnosis of HCV |
| Ellenrieder VJ Hepatol 1998 | Case series Period: 1991- 1995 | B-cell NHLs: Low-grade B-cell NHL: 55 High- low-grade B-cell: 14 NHL classification: Kiel Classification | 3/69 (4.3%) CLL: 1/14 CC: 0/4 CB: 1/14 CCBC: 1/19 IC = 0/18 | NR | NR | 4.3 (0.9-12.2) | No aetiological role of HCV in the development of NHL in German |
| El-Serag HB Hepatology 2002 | Cohort study Period: 1992- 1999 | Identification of LNHs cases by means of ICD-9-CM diagnosis codes NHL classification: NR | 421/34204 (1.23%) | 34204 HCV positive patients and 136816 randomly selected patients without HCV (controls) | 1669/136816 (1.22%) | 1.23 (1.1-1.3) | Significant high association between HCV infection and NHL, after adjustment for age |
| Engels EA Int J Cancer 2004 | Case-control study Period: July 1998-June 2000 | All NHL subtypes: (1) 32/813 (3.9%) (2) Low-grade B-cell NHL 18/411 (4.4%) (3) Intermediate-and high-grade B-cell NHL 8/275 (2.9%) (4) T-cell NHL 2/50 (4.0%) (5) other/unknown 4/77 (5.2%) NHL classification: Revised European American Lymphoma (REAL) histological scheme | 26/686 (3.8%) | Eligible cases and controls sampled from individuals 20-74 yr old, prospectively identified by using Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute (NCI) | 14/684 (2.1%) | 3.8 (2.3-5.2) | Detection of an association between HCV infection and NHL in the United States. HCV infection may be a cause of NHL |
| Ferri C Br J Haematol 1994 | Case series with control group Period: NR | B-cell NHL: 50 NHL classification: Working Formulation | B-cell NHL: 17/50 (34%) | (1) Patients with Hodgkin Lymphoma (2) Healthy subjects (3) anti-HCV negative patients with type B or delta chronic active hepatitis | 1/30 (3%) 30 15 HCV prevalence in in the healthy Italian population: 1.3% | 34 (20.8-47.1) | Presence of HCV infection in a substantial number of unselected NHL patients, particularly in comparison with HCV prevalence in control groups and in healthy Italian population |
| Franceschi S Cancer Epidemiol Biomarkers Prev 2011 | Nested case-control study Period: standardized lifestyle and personal history questionnaires collected between 1991 and 2000. Vital status followed up to 2004 and 2006 | All lymphomas: 1023 cases NHL: 739 MM: 238 HL: 46 HCV positive: 12/1023 (1.17%) NHL classification: World Health Organization classification | B-cell NHLs: 628/1023 (61.4%) Number of HCV positive patients in B-NHLs not reported 9/730 HCV positive patients in all NHLs 14/1454 HCV positive in controls HL: 2/46 (4.3%) MM: 1/238 (0.4%) | Lymphoid tissue Malignancies classified according to the second revision of the International Classification of Diseases for Oncology (ICD-O-2) and to the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Third Edition | 18/2028 controls (0.9%) | 61.4 (58.4-64.3) | The present study neither weakened nor strengthened the evidence of an association between HCV and NHL or other lymphoid tissue malignancies |
| Gentile G Cancer Epidemiol Biomarkers Prev 1996 | Hospital-based case-control study of risk factors for acute leukemias Period: 1 November 1986 - 31 March 1990 | All acute leukemias: 430 Diagnosis performed by means of: French-American-British classification of bone marrow aspirates for acute leukemias and RAEB, whereas diagnosis for CML was based on typical dinical and cytogenetic laboratory features | All acute leukemias: 27/430 (6.3%): AML: 15/172 (8.7%) ALL: 5/67 (7.5%) CML: 2/125 (1.6%) RAEB: 5/66 (7.6%) | Controls recruited in the region of the three hospitals (Rome, Bologna, Pavia) during the study period among outpatients without hematobogical malignancies who were seen in the same hospitals at which cases had been identified | 44/857 (5.1%) | 6.3 (3.9-8.5) | Association between acute leukemias, RAEB, and CML Possible association between hepatitis B virus, AML, RAEB, and CML, but further confirmation required |
| Genvresse I Ann Hematol 2000 | Case series Period: 1995-2000 | All lymphomas: 119 (1) B-NHLs: 105 (2) T-NHLs: 14 NHL classification: REAL histological scheme | (1) 2/105 (1.9%) (2) 0/14 | NR | NR | 1.9 (0-4.5) | Possible HCV involvement in NHLs development via a continuous antigenic stimulation, leading to a B-cell clonal expansion |
| Germanidis G Blood 1999 | Case series with control group Period: January 1994 -July 1997 | B-NHL: 201 HD: 94 NHL classification: Revised European American Lymphoma (REAL) histological scheme | B-NHL: 4/201 (2%) | Hematologic malignancies different from B-cell NHL (HD) | 1/94 (1.1%) | 2 (0-3.9) | No existence of a significant relationship between HCV infection and B-NHL in France |
| Giordano TP JAMA 2007 | Cohort study Period: 1997-2004 | Identification of LNH cases by means of ICD-9-CM diagnosis codes NHL classification: NHL (200, 202.0-202.2, 202.8), WMc (273.0, 273.3), HL (201), MM (203.0-203.1, 238.6), ALL (204.0), CLL (204.1) AnLLs 205.0, 206.0), CML (205.1), other leukemia (204.2, 204.8-204.9, 205.2, 205.8-205.9, 206.1-206.2, 206.8-206.9, 207.8, 208.0-208.2, 208.88.9), MGUS (273.1, 273.2) | HCV- positive cohort: 146394 patients During follow-up, 813 patients in HCV-infected cohort (0.5%) had a HIV diagnosis NHL: 319 HL: 65 MM: 95 CCL:69 ALL: 27 WMc: 67 CML: 30 | Inpatients records from more than 150 United States Veterans Affairs (VA) hospitals in the Patients’ treatment file and outpatients records from any VA facility in the Output Clinic File | HCV- negative cohort: 572293 patients. During follow-up, 35696 uninfected HCV patients (6.2%) had a recorded HCV diagnosis and 1539 patients (0.3%) a HIV diagnosis NHL: 1040 HL: 295 MM: 431 CCL:343 ALL; 184 WMc: 98 CML: 163 | 0.2 (0.19-0.25) | An increased risk of: (1) non-Hodgkin lymphoma overall (20%-30%), (2) Waldenström macroglobulinemia, a low-grade lymphoma (3-fold higher risk), (3) cryoglobulinemia, in subjects with HCV infection. An etiological role for HCV, in causing lymphoproliferation and non-Hodgkin lymphoma, supported by these results |
| Goldman L Cancer Causes Control 2009 | Case-control study Period: October 1999 -March 2004 | All lymphomas: 139/296 (47% ) -T-NHL: 8/24 (34.8%) -DLBCL:79/146 (54.9%) -MZL: 14/24 (58.3%) -CLL: 24/58 (41.4%) -FC: 9/23 (40.9%) -MCL: 5/16 (31.3%) NHL classification: World Health Organization classification | B-NHL: 131/272 (48.2%) | Cancer-free subjects, sampled from the Kasr El Aini Faculty of Medicine Orthopaedic Hospital in Cairo | 283/786 (37.4%) | 48.2 (42.2-54.1) | HCV is a risk factor for diffuse large B cell, marginal zone, and follicular lymphomas in Egypt |
| Guida M Leukemia 2002 | Case-control study Period: September 1999-October 2001 | All Lymphomas: 12/60 (20%) MM: 5/60 B-NHL: 55/60 NHL classification: Working Formulation | B-NHL: 12/55 (21.8%) | Control patients with non-hematological malignancies recruited from the Surgery Department the Oncology Institute of Bari (Italy) | 9/63 (14.2%) | 21.8 (10.9-32.7) | Moderate increase of prevalence of HCV infection among patients with B cell lymphoproliferative disorders in a very homogeneous population of southern Italy |
| Hanley J Lancet 1996 | Case series Period: NR | All LPDs: 72. B-cell NHLs: 38 MM: 24 MGUS: 10 NHL classification: Working Formulation | 0/72 | NR | NR | 0 (0-4.9) | No association between chronic HCV infection and risk of NHLs development |
| Harakati MS Saudi Med J 2000 | Case series with control group Period | B-cell NHLs: 56 patients NHL classification | B-NHL: 12/56 (21.4%) | (1) Blood donors and general medical patients) (2) Other hematologic malignancies other than B-cell NHL | (1) 3/104 (3%), (2) 2/41 (5%) | 21.4 (10.6-32.7) | Higher prevalence of Hepatitis C virus infection in Saudi Arab patients with B-cell non-Hodgkin’s lymphoma than in the control groups |
| Hausfater P Am J Hematol 2001 | Prospective controlled study Period: June to September 1998 | All LPD: 394 B-NHL: 164 HD: 34 CLL: 107 MM: 54 WMc:12 NHL classification: NR | B-NHL: 3/164 (1.8%) | (1) In-Hospital patients without cancers (2) Nonmalignant hematological diseases (3) Hematological malignancies other than B-cell NHL | (1) 3/694 (0.43%) (2) 8/224 (3.6%) (3) 9/425 (2.1%) | 1.8 (0-3.8) | No increased prevalence of HCV infection in patients admitted to the Hematology department for B-NHL. No major pathophysiologic role of HCV in lymphoproliferative disorders in Paris |
| Hwang JP J Oncol Pract 2014 | Cohort-study Period: January 2004 -April 2011 | Patients’data, obtained from four institutional sources: Tumor registry: to assess patients’ demographic characteristics Pharmacy informatics: to evaluate chemotherapy drugs and dates administered. Patient accounts: to identify study patients’ International Classification of Diseases (ninth edition; ICD-9) codes Laboratory informatics: to determine HCV antibody (anti-HCV) and ALT test dates and results | 141877 patients with cancer, who were newly registered at MD Anderson Cancer during the study period. Patients considered in the study: 16, 773. HCV screened subjects: 1628/16773 (9.7%) with NHLs, 1400 patients with anti-HCV test 42 NHLs antiHCV-positive (3%) | NR | NR | 3 (2.1-3.9) | HCV screening rates were low, even among patients with risk factors, and the groups with the highest rates of screening did not match the groups with the highest rates of a positive test result |
| Imai Y Hepatology 2002 | Cohort study Period: February 1992 -July 1992 | NHLs: 187 B-cell NHLs: 156 T-cell NHL:31 NHL classification: World Health Organization classification | 21/156 (13.5%) | Use of screening data of 197600 first-time voluntary blood donors to the Osaka Red Cross Blood Center | Expected numbers of anti- HCV-positive patients with NHL categorized by gender and phenotype in general population: 4.64 | 13.5 (8.1-18.8) | A significantly higher frequency of HCV infection in B cell NHL in comparison with that in birth cohort- and sex-matched blood donors; chronic HCV infection may be associated with B-cell NHL in Japan |
| Isikdogan A Leuk Lymphoma 2003 | Case series with control group Period: December 1997-September 2001 | NHLs: 119 High-grade NHLs: 10 Intermediate-grade: 64 Low-grade: 45 NHL classification: Working formulation | 0/119 | Subjects admitted as outpatients at Internal Medicine of Dicle Univeristy, Diyarbakir, without history of haematological disordres, during the same period | 117 | 0 (0-3) | No relationship between HCV and NHLs in the Southeastern Anatolia of Turkey |
| Iwata H Haematologica 2004 | Hospital-based case control Study Period: 1995-2001 | All NHLs: 145, 140 with anti-HCV test NHL classification: World Health Organization classification | 16/140 (11.4%) | Randomly selected controls from patients admitted to the (1) orthopedics (290 patients, 286 with anti-HCV markers) or (2) ear, nose and throat (284 patients, 282 with anti-HCV markers) departments of the hospital | (1) 9//286 (3.1%) (2) 20/282 (7%) | 11.4 (6.1-16.7) | Significant association between HCV infection, and malignant lymphoma by multivariate analysis |
| Izumi T Blood 1996 | Case series Period: 1992-1997 | All lymphomas: 83 patients, B-cell NHLs: 54 Non-B-cell NHLs: 20 HLs: 9 NHL classification: NR | B-cell NHLs: 12/54 (22.2%) Non-B-cell NHLs: 0/20 HLs: 0/9 | NR | NR | 22.2 (11.2-33.3) | Direct causal relationship between the occurrence of PHSL and chronic HCV infection |
| Izumi T Leukemia 1997 | Case series Period: NR | B-cell LPDs: 50 patients B-cell NHLs: 25 MM: 21 WMc: 4 NHL classification: NR | 4/25 (16%) | NR | NR | 16 (4.5-36.1) | Association between HCv infection and B-cell NHLs |
| Karavattathayyil SJ Am J Clin Pathol 2000 | Case series Period: January 1993-December 1996 | Patients with B-cell NHLs: 31 NHL classification: World Health Organization classification | Positive HCV-RNA strands: 8/31 (25.8%) Negative HCV-RNA strands: 6/31 (19.4%) | (1) T-cell NHLs: 2 cases (2) HL: 2 cases (3) Patients with lymph nodes removed for reasons other than lymphoma: 28 | 0/32 | Positive HCV-RNA strands: 25.8 (10.4-41.2) Negative HCV-RNA strands 19.4 (5.4-33.2) | Presence of HCV infection in a significant percentage of paraffin-embedded tissue from B-cell NHLs patients, compared with control subjects; detection of negative-strand RNA suggests HCV replication in these tissues, excluding the possibility of contamination with viral RNA or blood |
| Kashyap A Ann Intern Med 1998 | Case series with control group Period: February 1992-December 1995 | All NHLs: 312 36 HCV positive patients NHL classification: NR | NHLs: 36/312 (11.5%) | (1) Healthy United States blood donors (2) Black and Hispanic patient population at City of Hope National Medical Center | (1) (0.4%) (2) approximately 25% | 11.5 (8-15.1) | Prevalence of HCV positivity is still much higher than expected, even after adjustment for differences in patient demographic characteristics |
| Kaya H Clin Lab Haematol 2002 | Case-control study Period: NR | All NHLs: 70 patients Low-grade NHLs: 22, Intermediate- grade NHLs: 17 high-grade NHLs: 31 NHL classification: Working Formulation | 1/70 (1.4%) | Healthy-subjects admitted at Departments of Haematology, Ataturk University, Erzurum | 1/70 (1.4%) | 1.4 (0-4.2) | No aetiologic role of HCV in NHL development |
| Kim JH Jpn J Cancer Res 2002 | Case-control study Period: January 1997 -December 1998 | NHLs: 233 patients 214 patients with anti-HCV positivity NHL classification: Working Formulation | 7/214 (3.3%) | Control groups comprised patients with (1) non-hematological malignancy (control group 1) and subjects with (2) non-malignant conditions (control group 2) diagnosed at Seoul National University Hospital during the same period. For each case, four controls selected | (1) 7/426 (1.6%) (2) 12/439 (2.7%) | 3.3 (0.8-5.6) | No association between NHL and HCV infection |
| King PD Clin Lab Haematol 1998 | Case series series with control group Period: June 1995-May 1997 | All lymphomas: 93 patients. NHLs: 73 patients HL: 20 patients 438 HCV positive patients NHL classification: Working Formulation | 1/73 (1.4%) | Patients with HL admitted at Department of Gastroenterology, University of Missouri Hospital | 0/20 1/438 (0.22%) patients developed NHL | 1.4 (0-4) | No association between NHL and HCV infection |
| Kocabaş E Eur J Epidemiol 1997 | Case series with control group Period: October 1993-March1994 | 137 Children with malignancies: Acute leukemia: 48 Lymphoma: 51 Solid tumours: 38 NHL classification: | 8/137 children were anti HCV positive, 129 patients were anti- HCV negative, but 7/129 were HCV-RNA positive | Children admitted, at Balcah Hospital, Adana, during the same period with diseases other than malignancies | 1/45 | 5.8 (1.9-9.7) | HCV infection is common among Turkish children with different types of cancer |
| Kuniyoshi M J Gastroenterol Hepatol 2001 | Case-control Study Period:January 1990-March 1998 | NHLs 348 patients 20/348 (8.1%) HCV positive patients with NHLs NHL classification: Working Formulation | B-cell NHLs: 15/348 (4.3%) | 1658234 blood donors, representing general population in the area (Fukuoka, Japan) | 11922/1658234 (0.72%) | 4.3 (2.1-6.4) | Involvement of HCV infection in the development of a subgroup of NHL, in males |
| Luppi M Ann Oncol 1998 | Case series Period: January 1989-August 1993 | B-cell NHLs: 157 patients NHL classification: Revised European American Lymphoma (REAL) histological scheme | 35/157 (22.3%) HCV positive B-cell NHLs: LDBCL 8/35 (23%) FC: 14/35 (40%) LPL: 2/35 (6%) 122/157 (67.7%) HCV negative B-cell NHLs | NR | NR | 22.3 (15.8-28.8) | Association of HCV infection with the malignant proliferation of defined B-cell subsets other than the immunoglobulin Mk B-cell subset involved in the pathogenesis of mixed cryoglobulinemia type II and associated lymphoplasmacytoid lymphoma type |
| Markovic Hepato-Gastroenterology 1999 | Case-series Period: January 1991-April 1996 | All lymphomas: 305 patients NHLs: 300 patients HL: 5 patients 181 patients with anti-HCV test NHL classification: NR | 3/181 (1.6%) | NR | NR | 1.7 (0-3.5) | No association between HCV infection and non-Hodgkin’s lymphomas, because of low HCV prevalence in Slovenia |
| Mazzaro C Br J Haematol 1996 | Case-series with control group Period: NR | All lymphomas: 199 patients Low-grade NHLs: 105 (52.7%) Intermediate grade NHLs: 48 (24.1%) High-grade: 39 (19.6%) MALT: 5 (2.5%) T-cell NHLs: 2 (1%) NHL classification: Working Formulation | 57/199 (28.6%) Low-grade NHLs: 40/110 (36.47%) Intermediate grade NHLs: 6/48 (12.5%) High-grade: 9/39 (23.1%) | (1) Patients with other haematological malignancies, including HL (21 patients), CLL (41), myelodysplastic syndrome (72), plasma cell myeloma (19); (2) general population of two towns in the same geographical area (Cormons and Campogalliano) in the cohort study called Dyonisos project | (1) 5/153 (3.1%) (2) 199/6917 (2.9%) | 28.6 (22.4-34.9) | Important role of HCV in the development of low-grade non-Hodgkin’s lymphomas |
| McColl MD Leuk Lymphoma 1997 | Case series Period: NR | B-Cell NHL: 72 patients Low-grade: 41 Intermediate-grade: 23 High grade: 8 NHL classification: Working Formulation | 0/72 | Patients with CLL, recruited at two Hospital in the West of Scotland | 0/38 | 0 (0-9.2) | Possible role of HCV infection in the aetiology of certain subgroups of NHLs, although this effect may be regional |
| Mele A Blood 2003 | Multicenter case- study with control group Period: 1998-2001 | B-Cell NHL: 400 patients NHL classification: REAL/World Health Organization classifications | 70/400 (17.5%) Aggressive B-NHL: 43/230 (18.7%) Indolent NHL:27/170 (15.9%) | Patients recruited n other departments of the same Hospitals: the departments of dentistry, dermatology, general surgery, gynecology, internal medicine, ophthalmology, orthopedics, otorhinolaryngology and traumatology | 22/396 (5.6%) | 17.5 (13.8-21.2) | Detection of an association between HCV and B-NHL |
| Mizorogi F Intern Med 2000 | Case series with control group Period: January 1993-December 1998 | Patients with LPDs: 161, subdivided into 2 groups: (1) patients with B-cell LPDs, including B-cell-NHLs: 100 MM: 17 CLL: 4 (2) patients with non B-cell LPDs: 38 NHL classification: Working Formulation | B-cell NHLs: 17/100 (17%) | Subjects with miscellaneous diseases other than liver diseases or LPDs, used as controls | nonB-cell LPDs: 0/25 34/516 (6.6%) | 17 (9.6-24.3) | Higher prevalence of HCV infection in patients with B-cell NHL than in those with non-B-cell NHL and the control group, frequent primary liver involvement and liver-related causes of death in HCV-positive patients with B-cell NHL |
| Montella M Leuk Res 2001 | Case-control study Period: January 1997 and December 1999 | -B-cell-NHLs: 101 -HL: 63 -T-cell NHLs: 10 -MM: 41 NHL classification: Working Formulation/REAL | 25/101 (24.8%) | Controls: patients with no history of malignant tumor, admitted to the National Cancer Institute and Cardarelli Hospital of Naples, in the same period | -Controls: 17/226 (8%) -HL: 6/63 (10%) -T-cell NHLs: 3/10 (30%) -MM: 13/41 (32%) | 24.8 (16.3-33.1) | Detection of a significant association between HCV infection and B-cell NHLs in the extranodal localization, and also indicate an association for the nodal seat |
| Morton LM Cancer Epidemiol Biomarkers Prev 2004 | Population-based case-control study of women in Connecticut The Yale Comprehensive Cancer Center’s Rapid Case Ascertainment Shared Resource (RCA), a part of the Connecticut Tumor Registry (CTR), a population-based tumor registry Period: 1995-2001 | All lymphomas: B cell 362 T cell 34 Others: 60 NHL classification: World Health Organization classification Incident cases of NHL identified by means of (ICD)-O: M-9590-9595, 9670-9687, 9690-9698, 9700-9723 | B cell 7/362 (1.9%) T cell 0/4 Others 1/60 (1.6%) Total: 8/464 (2%) | A population-based control group of female residents of Connecticut, aged 21-84, assembled using two methods:-Random digit dialing used to contact women less than 65 yr of age,-random selection from the files of the Centers for Medicare and Medicaid Services for women aged 65 yr and older | 5/534 (1%) | 1.9 (0.5-3.3) | Indirect HCV involvement in the development of B-NHL, this risk varying by B-NHL subtype among women |
| Musolino C Haematologica 1996 | Case series Period: NR | All-NHLs:24 HCV positive: 2 patients HCV-RNA positive: 5 patients NHL classification: Working Formulation | 5/24 HCV-RNA positive/NHLs | NR | NR | 20.8 (7.1-42.2) | Possible HCV involvement in NHL development |
| Musto P Blood 1996 | Case series with control group Period: NR | B-LPDs B-NHL: 150 HCL: 9 CLL: 41 MM: 90 WMc:13 MGUS: 47 NHL classification: NR | B-NHLs: 40/150 (26.7%) HCL:1/9 (11.1%) CLL: 8/41 (19.5%) MM: 10/90 (11.1%) WMc: 3/13 (23%) MGUS: 6/47 (12.8%) | Patients hospitalized for acute trauma | 25/466 (5.4%) | 26.7 (19.6-33.7) | A significantly higher prevalence of anti-HCV in patients with B-NHLs than in controls and independent of age |
| Nicolosi Guidicelli S Hematol Oncol 2012 | Case-control study Period: July 2001 to March 2002 | All lymphomas: 137 NHL classification: World Health Organization’s classification | 6/137 (4.4%) | Patients observed in Hospital Clinic, Barcelona and San Giovanni Hospital, Bellinzona, (ideally in traumatology and orthopaedic divisions | 7/125 (5.6%) | 4.4 ( 0.9-7.8) | Existence of marked geographic differences in the prevalence of HCV in NHL but no significant evidence for an association between HCV and B-cell NHLs |
| Nieters A Gastroenterology 2006 | European Multicenter Case-Control Study Period: 1998-2004 | Total Lymphomas: 1807 NHL classification: World Health Organization’s classification | 53/1807 (2.9%) | Controls drawn randomly from population registers of the study regions in Germany and Italy. In the remaining countries, controls recruited from the same hospital as cases | 41/1788 (2.3%) | 2.9 (2.1-3.7) | Positive association between HCV infection and B-cell lymphoma and a role of viral replication in lymphomagenesis |
| Ogino H Hepatol Res 1999 | Case-control sudy Period: 1991-1997 | All LPDs: 43 patients NHLs: 33 ALL: 10 NHL classification: Working Formulation | 4/33 (12.1%) | (1) 45 patients, undergoing colonscopy from July 1995 to June 1996 (2) 10599 healthy subjects, receiving a general medical check-up in Toyama prefecture from April 1996 to March 1997 | 2/45 (4.4%) | 12.1 (3.4-28.2) | High prevalence of HCV infection in patients with NHL in Toyama prefecture in Japan |
| Ohsawa M Int J Cancer 1999 | Cohort-study Period: 1957-1997 | Patients with HCV chronic infection, included in the present study: 2162 NHL classification: World Health Organization’s classification | Patients developing B-cell NHLs: 4/2162 During follow-up | Expected number of cases of NHLs in the sex-, age- and calendar year-matched general population: 1.90 | NR | 0.2 (0-0.3) | Chronic HCV infection moderately associated with increased risk of NHL |
| Okan V Int J Hematol 2008 | Case series with control group Period: NR | All Lymphomas: 334 NHL classification: World Health Organization’s classification | 9/334 (2.7%) MM: 1/67 (3.1%) CLL: 2/78 (2.5%) DLBCL: 4/67(6%) Follicular 0/9 Mantle: 1/11 (9%) Other: 0/26 T-cell lymphoid tumors: 1/16 (6.2%) HL: 0/60 | Controls recruited, using records from the University blood center in Gaziantep | 9/802 (1.1%) | 2.7 (0.9-4.4) 6 (0.3-11.6) | Higher HCV- seropositivity rate in patients with DLBCL in comparison with controls. No significant differences in the prevalence of HCV seroposity between patients with lymphoproliferative disorders and controls |
| Omland LH Int J Cancer 2012 | Cohort-study Period:1991-2006 Patients and subjects with HCV infection identified by means of: -Danish HCV cohort (DANVIR). -Civil registration system (CRS)-Danish cancer registry (DCR). -Danish national patient registry (DNPR) | 10 digit civil registration number assigned to all individuals in Denmark Analysis of the association between HCV and risk of NHL (ICD-10 codes: C82.0-85.9 and C96) NHL classification: Cancers classified according to the "International Classification of Diseases" 7th revision (ICD-7) for the period 1943-1977 and the 10th revision (ICD-10) for the period 1978-2006 | -11975 anti- HCV-positive patients LNH cases detected: 12 12/11975: 0.1% | Comparison cohort, which consisted of 6 age- and gender-matched individuals (without a HCV diagnosis) from the general population randomly selected from the CRS, on the day HCV-infection was diagnosed in the corresponding DANVIR cohort member | -71850 anti- HCV-positive patients LNH cases detected: 24 | 0.1 (0.04-0.15) | Possible increased risk of NHLs in patients with chronic HCV infection |
| Panovska I Br J Haematol 2000 | Case-series with control group Period: NR | B-cell-NHLs: 112 NHL classification: REAL histological scheme | 1/112 (0.9%) | Patients with other B-cell malignancies HL: 38 CLL: 43, ALL: 9 MM: 26 WMc: 1 Prevalence of HCV carriers in Republic of Macedonia within the general population is equal to 2.0% | 1/137 (0.72%) | 0.9 (0-2.6) | Low prevalence of HCV infection in patients with B-cell NHL from Macedonia and a lack of association between the two disorders |
| Park SC J Med Virol 2008 | Case-control study Period: January 1998- December 2001 | 235 patients with NHLs, B-cell subtypes: 168 T-cell subtypes: 57 not identified subtypes: 10 NHL classification: NR | 5/235 (2.1%) No information about number of patients with HCV infection and B-NHL cases | Patients with advanced gastric cancer diagnosed at the Korea Cancer Center Hospital | 7/235 (3%) | 2.1 (0.3-3.9) | No association between HCV infection and non-Hodgkin’s lymphoma |
| Paydas S Br J Cancer 1999 | Case series Period: NR | LPDs: 228 patients NHL: 98 CLL: 38 MM: 47 HD: 36 ALL: 9 NHL classification: NR | NHL: 9/98 (9.2%) CLL: 4/38 (10.5%) MM: 5/47 (10.6%) HD: 7/36 (19.4%) ALL: 1/9 (11.1%) | NR | NR | 9.2 (3.4-14.9) | HCV infection as a causative and/or contributing factor in lymphoproliferation in this study |
| Pellicelli World J Gastroenterology 2011 | Case-series Period: January 2008 -January 2009 | 125 patients with B-cell NHLs NHL classification: World Health Organization’s classification | 24/125 (19.2%) | NR | NR | 19.2 (12.3-26.1) | HCV genotypes and duration of HCV infection differed between B-NHL subtypes. Indolent lymphomas can be managed with antiviral treatment, while DLBCL is not affected by the HCV infection |
| Pioltelli P Lancet 1996 | Case-series with control groups Period: January-June 1995 | All Lymphomas: 204 NHLs: 126 HL: 78 28HCV positive lymphomas NHL classification: Working Formulation | 26/126 (20.6%) | (1) HL (2) candidated blood donors (3) elderly people | (1) 2/78 (2) 9/832 (3) 9/94 | 20.6 (13.5-27.7) | High prevalence of HCV infection in NHLs, in the absence of an increased risk for HCV infection and of a clinical history of MC |
| Pioltelli P Am J Hematol 2000 | Case-control study Period: 01/01/96-30/06/97 | Patients with B-cell NHLs: 300 NHL classification: Working Formulation (WF) and REAL histological scheme | 48/300 (16%) | Individuals consecutively recruited during routine visits at medicine, surgery, or traumatology departments during the recruitment period of the study population (1) Patients with internal and surgical diseases (2) Patients with solid neoplasm (3) Patients with autoimmune disorders | (1) 51/600 (2) 15/247 (3) 6/122 | 16 (11.8-20.1) | The prevalence of HCV infection is higher in patients with NHLs than in non-neoplastic people and in patients with non-lymphoproliferative malignancies or receiving immunosuppressive treatment, but the small difference among these groups, the identical genotype pattern between NHL and controls do not support the hypothesis that HCV plays a role in lymphomagenesis |
| Pivetti S Br J Haematol 1996 | Case-series with control group Period: NR | Patients with LPDs: 167 patients (30 HCV positive) HL: 30 NHLs: 47 CLL: 29 MM: 18 MGUS: 31 WMc: 12 NHL classification: NR | 7/47 (14.9%) | (1) Patients with connective tissue diseases (2) Patients with idiopathic thrombocytopenic purpura | (1) 26/100 (26%) (2) 12/33 (36.4%) | 14.9 (4.7-25) | HCV may link lymphoid malignancies and autoimmune diseases by skewing the activity of the immune system toward the production of autoAbs |
| Pozzato G Blood 1994 | Case series Period: NR | 31 patients with MC. 12 patients/31 with low-grade NHLs 26/31 HCV positive NHL classification: Working Formulation | 10/12 patients with low-grade NHLs were anti-HCV positive | NR | NR | 83.3 (51.6-97.9) | HCV associated with a high prevalence of low-grade non-Hodgkin's lymphomas |
| Prati D Br J Haematol 1999 | Case series Period: January 1989 -August 1998 | Primary cutaneous B-cell NHL. NHL classification: European Organisation for Research and Therapy of Cancer (EORTC) | 1/34 (2.9%) | NR | NR | 2.9 (0-8.6) | Primary cutaneous B-cell NHL might represent a distinctive group among B-cell NHLs |
| Rabkin CS Blood 2002 | Cohort study Period: June 1959 and September 1966 | All LPDs: 95 B-cell NHL: 57 MM: 24 HL: 14. NHL classification: Tumors classified according to the International Classification of Diseases for Oncology, second edition, as NHL (histologic classifications 9590 through 9642 and 9670 through 9698), multiple myeloma (9730 through 9732), or Hodgkin disease (9650 through 9667) | 4/95 (4.2%) 0/95 at RIBA 0/95 at HCV-RNA | Study subjects (48 420 individuals) recruited from the Child Health and Development Study (CHDS) cohort established in 1959 at the Kaiser Foundation Health Plan, Oakland, CA | 1/48.420 at ELISA 0/48420 at RIBA | 4.2 (0.1-8.2) | Not substantial role of chronic HCV infection in the etiology of B-cell neoplasia |
| Ramos-Casals M J Rheumatol 2004 | Case series Period: 1994-2000 | NHL classification: World Health Organization’s classification | 6/98 | NR | NR | 6.1 (1.3-10.8) | Description concerning a triple association of HCV infection, autoimmune diseases and NHLs |
| Salem AK Gulf J Oncol 2009 | Case series with control-group Period: January 2005-January 2007 | All NHLs: 192 patients NHL classification: NR | 29/192 (15.1%) | Patients checked for HCV infection with several acute medical conditions and coming from different parts of the country | 814/20329 (4%) | 15.1 (10-20.1) | Higher prevalence of HCV infection among Yemeni patients with NHL than among persons in the control group |
| Salem Z Eur J Epidemiol 2003 | Case-series with control group Period: NR | B-cell NHL: 35 patients. NHL classification: NR | 0/35 | (1) Patients with different malignancies (malignant myeloproliferative disorders: 12, malignant lymphoproliferative disorders: 28, non haematological cancers: 23 patients) (2) Healthy blood donors and patients without malignant conditions, attending General Medicine of American university, Beirut | (1) 0/63 (2) 0/220 | 0 (0 -10) | No association between HCV infection and B-cell NHLs development in Lebanese patients |
| Sansonno D Blood 1996 | Case series Period: January 1991 to December 1995 | 12 HCV-positive patients with MC and 2 HCV-positive patients with reactive lympho-adenopaties NHL classification: Working Formulation | 3/12 (25%) | NR | NR | 25 (0.5-49.5) | These data emphasize that lymphoid organs may be a site of HCV infection. The demonstration of HCV-related proteins in a nonmalignant condition, namely HRL, indicates that HCV infection precedes the neoplastic transformation and possibly plays a major role in lymphomagenesis in MC |
| Schöllkopf C Int J Cancer 2008 | Nation-wide Danish-Swedish case-control study (Scandinavian Lymphoma Etiology study, SCALE) Period: The SCALE study population includes the entire Danish population between June 1, 2000 - August 30, 2002, and the Swedish population between October 1, 1999-April 15, 2002 | All lymphomas: 2819 NHLs: 2353 HL: 466 NHL classification: World Health Organization’s classification | HCV positive NHLs: 57 (2.4%) HL: 6 (1%) at III G ELISA test,only NHLs: 7/2353 (0.7%) HL: 0 positive at ELISA test and positive or intermediate at RIBA test for anti-HCV antibodies | Controls randomly sampled from the entire Danish and Swedish populations using continuously updated, computerized population registers | 21/1856 (1%) | 2.4 (1.8-3) | Positive association between HCV and risk of NHL, in particular of B-cell origin |
| Seve P Eur J Gastroenterol Hepatol 2004 | Cross-sectional study Period: January 1997-December 1998 | B-NHL:212 patients BL 6 DLBCL 109 FC 31 LL 7 LPL 5 MALT 17 MCL 21 MZL16 NHL classification: Revised European American Lymphoma (REAL) classification | (1) 6/212 (2.8%) (2) MALT 3/17 | Transfusion patients from surgical emergency, internal medicine pneumology, endocrinology, gastroeterology, nephrology, oncology, general surgery, orthopaedics, rheumatology, obstetrics and gynaecology, and intensive care wards | 20/974 (2.05%) | (1) 2.8 (0.6-5) (2) 17.6 (3.8-43.4) | Possible association between HCV and MALT lymphoma |
| Shariff S Ann Oncol 1999 | Case series with control group Period: 1996 and part of 1997 | patients with B-cell NHL NHL classification: Working Formulation/Revised European American Lymphoma (REAL) classification | 2/88 (2.3) | (1) patients with a T-cell NHL (2) second control group, including health-care workers, recruited between 1995 and 1997 | 0/37 11/1085 (1%) | 2.3 (0-5.3) | Chronic HCV infection as a risk factor for B-cell NHL in certain populations or with certain genotypes of the virus, no significant association in British Columbia |
| Shirin H Isr Med Assoc J 2002 | Case control group Period: May 1997 -September 1999 | B-NHL (DLCL FC CLL) NHL classification: Revised European American Lymphoma (REAL) classification | Total: 212 patients Lymphoproliferative disorders: 10/128 (7.8%) | (1) Patients with Myeloprolipherative and myelodisplastic disorders: (2) Israeli blood donors | (1) 1/84 (1.1%) (2) HCV prevalence equal to 0.64% | 7.8 (3.1-12.4) | Significant association between HCV infection and diffuse large B cell lymphoma |
| Silvestri F Bood 1996 | Case series with control group Period: NR | 537 unsekected patients with LPDs B-cell NHLs: 311 T-cell NHLs: 57 MM: 78 HL:88 ALL: 23 NHL classification: Kiel classification/Revised European American Lymphoma (REAL) classification | 29/311 (9%) | NR | T-cell NHLs: 2/57 (4%) MM: 3/78 (4%) HL:0/88 ALL: 1/23 (4%) | 9 (6-12.5) | High prevalence of HCV infection in patients with B-cell NHL |
| Silvestri F Haematologica 1997 | Case series Period: NR | B-cell NHLs NHL classification: Revised European American Lymphoma (REAL) classification | 42/470 (8.9%) 21/22 (95.4%) B cell-NHLs patients with cryo-globulinemia 21/448 (4.6%) B cell-NHLs patients without cryo-globulinemia | NR | NR | 8.9 (6.3-11.5) | Close association between HCV infection and B-cell NHLs |
| Singer IO Leuk Lymphoma 1997 | Case-series with control group Period: NR | All Lymphomas: 50 unselected patients B-cell NHLs: 31 T-cell NHLs: 6 HL: 13 NHL classification: Working Formulation | 0/31 | No information about control groups | 0/19 | 0 (0-11.2) | No evidence supporting an association between HCV infection and LNH development |
| Sonmez M Tumori 2007 | Case-control study Period: 2002-2005 | B-cell NHLs: 109 DLBCL: 71 Small-cell LL: 38 NHL classification: World Health Organization’s classification | 3/109 (2.8%) Low grade: 1/38 (2.6%) High grade: 2/71 (2.9%) | Patients selected from orthopedics, general surgery, urology, ophthalmology, otorhino-laryngology clinics with irrelevant diseases | 28/551 (5.1%) | 2.8 (0-5) | No difference in the incidence of HCV infection between NHL- and control- group |
| Spinelli JJ Int J Cancer 2008 | Population-based case-control study Period: March 2000 and February 2004 | All-NHL cases:795, from the Greater Vancouver Regional District (GVRD) and the Capital Regional District (CRD), including the city of Victoria, enrolled from the BC Cancer Registry NHL classification: World Health Organizations classification | NHLs: 19/795 (2.4%) B-cell NHLs:18/717 (2.5%) T-cell NHLs: 1/78 | Controls selected from the Client Registry of the BC Ministry of Health | 5/697 (0.7%) | 2.4 (1.3-3.4) | HCV infection contributes to increase NHL risk |
| Swart A BMJ Open 2012 | Cohort-study Period: 1 January 1993-31 December 2007 | Individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people as opioid substitution therapy. Solid cancers classified according to the International Classification of Diseases (ICD), 10th revision, haematopoietic neoplasms and Kaposi sarcomas classified according to the ICD for Oncology, 3rd edition | Patients considered in the study: 29613 Subjects with HCV infection alone: 14892 Observed number of LNH in HCV-positive cohort: 75 | Calculation of expected number of incident LNHs | Expected number of LNH: 49.6 | 0.5 (0.4-0.6) | Association between HCV infection and LNHs |
| Takai S Eur J Haematol 2005 | Case series Period: January 1996 to September 200 | All haematological malignancies: 601 NHL: 218 DLBCL: 110 FCL: 100 MCL: 3 PTCL: 5 Acute Leukemia: 246 AML: 193 ALL: 53 Adult T-cell Leukaemia: 13 MM:124 | 37/601 patients were anti-HCV positive NHL: 22/218 (10.1%) DLBCL: 13/110 (11.8%) FCL: 8/100 (8%) MCL: 1/3 (33%) PTCL:1/5 (20%) AML: 5/193 (2.6%) ALL: 2/53 (1.8%) adult T-cell Leukaemia: 0/13 MM: 8/124 | NR | NR | NHLs: 10.1 (6.1-14.1) DLBCL: 11.8 (5.8-17.8) FCL: 8 (2.7-13.3) MCL: 33 (0-86.2) PTCL:20 (0-63) AML: 2.6 (0.4-4.8) ALL: 1.8 (0-8.9) MM: 6.5 (2.2-10.8) | High prevalence of HCV infection in NHL Possible role of HCV in the pathogenesis of NHs |
| Takeshita M Histopathology 2006 | Case series with control group Period: NR | All-Lymphomas: 537 (1) HL: 18 -B-NHL: 400 (DLBCL, FC CLL, MALT, PCM, MCL, MZL, BL, others) -T-cell NHL: 96 -NK/T-cell NHL: 23 NHL classification: World Health Organization’s classification | B-cell NHL 45/400 (11.3%) Primary Effusion Lymph: 3/6 (50%) BL: 1/7 (14.3%) DLCL:28/161 (17.4%) FL: 3/47 (6.4%) MALTOMA: 5/52 (9.6%) MM: 4/81 (4.9%) CLL, SMZL, Mantle cell Lymp: 0 | (1) Other haematological malignancies (2) Blood donors | (1) HL: 1/18 (5.6%) T-cell NHL: 5/96 (5.2%) NK-Tcell Lymphomas: 2/23 (8.7%) (2) 396/15567 (2.5%) | 11.3 (8.1-14.3) | HCV infection may play a role in lymphomagenesis of splenic and gastric DLBCL |
| Talamini R Int J Cancer 2004 | Case-control study Period: January 1999 -July 2002 | Total NHL: 225 cases 44/225 HCV positive patients NHL classification: International Classification of Diseases for Oncology, updated to include categories in the Revised European-American Lymphoma (REAL)/World Heath Organization classification | 44/225 HCV positive patients 40/225 (17.8%) patients with B-cell NHLs (1) Low-grade B-cell: 24 (2) Intermediate- and high-grade B-cell: 16 (3) T-cell: 2 (4) Unknown:2 | Patients with a wide spectrum of acute conditions admitted at National Cancer Institute, Aviano; the “Santa Maria degli Angeli” General Hospital, Pordenone; the “Pascale” National Cancer Institute, Naples and 4 general hospitals, Naples | 45/504 (8.9%) | 17.8 (12.8-22.8) | HCV infection associated with an increased NHL risk |
| Teng CJ Clinics 2011 | Case series Period: 2003-2008 | MM: 155 patients 30 patients with chronic hepatitis MM diagnosis: International Myeloma Working Group | 14/155 (9%) 1/155 with HBV/HCV co-infection | NR | NR | 9 (4.5-13.5) | High prevalence of cytogenetic abnormalities in patients with HCV chronic hepatitis |
| Thalen DJ Br J Haematol 1997 | Case series Period: NR | NHLs: 115 patients B-cell NHLs: 99/115 (86%) T-cell NHLs: 15 (13%) Unclassified: 1 (1%) NHL classification: Working Formulation | B-cell-NHLs: 0/99 T cell NHLs: 0/15 | NR | NR | 0 (0-3.7) | No association between HCV infection and B-cell NHLs in the study |
| Timuragaoğlu A Haematologia 1999 | Case series with control group Period: NR | NHLs: 48 patients NHL classification: Working Formulation | Anti HCV positive: 0/48 HCV-RNA positive: 3/35 (8.6%) | Patients with various haematological disorders (MM, HL, acute myeloblastic leukaemia, acute lymphoblastic leukaemia, chronic myelogenous leucemia, idiopathic thrombocytopenic purpura, myelodysplastic syndrome) | 0/28 | 8.6 (1.8-23.1) | Association between HCV infection and B-cell NHLs in the study |
| Tkoub EM Blood 1998 | Case series with control group Period: NR | 46 patients with gastric MALT: High grade: 21 Low-grade 25 37/46 patients with Helicobacter Pylori NHL classification: NR | 1/46 (2.2%) | Patients with gastroduodenal disease: 84 with duodenal ulcer 43 with gastric ulcer 38 with dispepsia | 4/165 (2.4%) | 2.2 (0-6.3) | No link between HCV infection and gastric MALT in France |
| Tursi A Am J Gastroenterol 2002 | Case series Period: NR | 25 HCV positive patients with gastric MALT. -20/25 (80%) with grade 2-5/25 (20%) with grade 3. 18/25 patients with Helicobacter Pylori NHL classification: World Health Organization’s classification | NR | NR | NR | MALT grade 2: 80 (59.3-93.2) MALT grade 3: 20 (6.8-40.7) | HCV may colonize gastric MALT, allowing the development of a grade of acquired MALT, this represents the first step toward a MALT lymphoma |
| Udomsakdi-Auewarakul C Blood 2000 | Case series Period: NR | All malignancies: 130 Intermediate- to high-grade NHL: 98 Low-grade NHL 32 patients NHL classification: Working Formulation | 2/98 (2%) 1/32 (3.1%) | NR | NR | 2.(0-4.8) | No association between HCV infection and NHLs in this study from Thailand, where HCV infection is highly prevalent |
| Vajdic CM Cancer Epidemiol Biomarkers Prev 2006 | Population-based case-control study Period: January 2000 and August 2001 | Total Lymphomas:694 -B-cell NHLs: 659 (95%) -T-cell NHLs: 28 (4%) -Undetermined: 7 (1%) NHL classification: World Health Organization’s classification | NHLs: 3/694 (0.4%) | Potential participants (both cases and controls) received a letter to inviting their participation in research about the development of NHL | 2/694 (0.3%) | 0.4 (0-0.9) | No strong evidence for an association between any infection and non-Hodgkin lymphoma risk in immunocompetent people, but increased risk between HCV infection and non-Hodgkin lymphoma in subjects with injecting drug use |
| Vallisa D Am J Med 1999 | Case-control study Period: 1990-1996 | B-cell-NHLs: 175 patients NHL classification: Working Formulation/Revised European American Lymphoma clasification | 65/175 (37.1%) | Subjects without lymphoma selected from: (1) inpatients (175) (2) outpatients (175) cared at Civil Hospital, Piacenza, subdivided into 2 groups | (1) 17/175 (10%) (2) 15/175 (9%) | 37.1 (30-44.3) | Possible HCV role as an etiologic agent in non-Hodgkin’s B-cell lymphoma |
| Varma S Hepatol Int 2011 | Case-control study Period: NR | B-NHLs: 57 patients High-grade disease (DLBCL): 44 (77.2%) Intermediate-disease (FC): 6 (10.5%) Low grade disease: (small lymphocytic): 7 (12.3%) NHL classification: World Health Organizations classification | 1/57 (1.7%) | Patients with non-hematological conditions admitted to Departments of Ophthalmology, Otorhinolaryngology, Dermatology, and Internal Medicine in the Hematology Clinic, Institute of Medical Education and Research, Chandigarh | 2/171 (1.2%) | 1.7 ( 0-5.1) | No significant association between HCV infection and NHL in Northern India |
| Veneri D Am J Hematol 2007 | Case series Period: January 1995 -December 2006 | 947 patients with lymphoproliferative disorders: DLBCL: 361 MM: 139 B-cell MZL: 62 HL: 103 B-CLL: 186 FL. 96 NHL classification: World Health Organization’s classification | 55/947 patients were HCV positive DLBCL: 27/361 (7.5%) MM: 1/139 (0.7%) B-cell MZL: 15/62 (24.2%) HL: 4/103 (3.9%) B-CLL: 4/186 (2.1%) FL: 4/96 (4.2%) | NR | NR | DLBCL: 7.5 (4.7-10.2) B-cell MZL: 24.2 (13.5-34.8) | Confirmed association between a subset of B-cell lymphomas and HCV infection |
| Yamac K Eur J Epidemiol 2000 | Case series Period: August 1996-June 1998, | All Lymphomas: 92 NHLs: 73 HL 19 NHL classification: Revised European American Lymphoma clasification | 1/92 (1.1%) | NR | NR | 1.1 (0-3.2) | No significant association between HCV and NHL in the study |
| Yenice N Turk J Gastroenterol 2003 | Case series with control group | All Lymphomas: 134 B cell NHLs: 84 HLs: 50 | B-cell NHLs: 6/84 (7.1%) HLs: 1/50 (2%) | Healthy blood donors | 1/100 (1%) | 7.1 (1.6-12.6) | HCV may play a role in the development of B-cell non-Hodgkin lymphoma, but not in Hodgkin lymphoma |
| Yoshikawa M J Clin Gastroenterol 1997 | Case series with control group Periood: NR | All Lymphomas: 100 B-NHLs: 55 T-NHLs: 10 HL: 5 MM: 25 B-CLL: 2 MGUS: 3 NHL classification: Working Formulation | B-NHLs: 9/55 (16.4%) MM:5/25 (20%) MGUS: 1/3 (33.3%) | Patients with any cancer in digestive organs except liver enrolled at Nara Medical University | 1/25 (4%) | 16.4 (6.5-26.1) | High rates of HCV infection detected in B-NHL and MM |
| Yu SC Kaohsiung J Med Sci 2013 | Case series Period: 1988-2011 | All lymphomas: 74 patients: -B-cell lymphomas: 69 -T-cell lymphomas: 3 -Lymphoblastic Lymphoma: 1 -Unspecified high-grade lymphoma: 1 41/74 patients with serology for HCV infection NHL classification: World Health Organization’s classification | Patients with B-cell-NHL and with serology for HCV infection: 39 Patients with B-cell-NHL and HCV positive 10/39 (25.6%) | NR | NR | 25.6 (11.9-39.3) | High HCV sero-prevalence in patients with early-stage DLBCL suggests a role of HCV in the pathogenesis of primary DLBCL |
| Zucca E Haematologica 2000 | Case series Period: 1990 and 1995 | B-cell NHLs: 180 Anti-Helicobacter antibodies detected in 81/180 (45%) patients. NHL classification: REAL histological scheme | 17/180 (9.4%) Gastric lymphoma: 2 Non gastric lymphoma: 15 | A survey of 5424 subjects new blood donors from the same area tested between 1992 and 1997 (Swiss Red Cross Transfusional Medicine Service for Canton Ticino) | 49/5424 (0.9%) | 9.4 (5.1-13.7) | High prevalence of HCV infection detected in NHL lymphoma patients and associated with a shorter time to lymphoma progression. HCV infection not correlated with primary gastric presentation or with MALT-type histology |
| Zuckerman E Ann Intern Med 1997 | Controlled, cross-sectional study. Period: October 1994 and May 1996 | B-cell NHLs: 120 patients NHL classification: Working Formulation | B-cell NHLs 26/120 (22%) | (1) Patients with hematologic malignancies other than B-cell NHLs; (2) Patients without hematologic malignancies, attending the general medicine clinic at LAC-USC and with: systemic hypertension or ischemic heart disease: 69 diabetes mellitus: 35 primary hypothyroidism: 10 | 268 patients 7/154 (4.5%) (2) 6/114 (5%) | 21.7 (14.3-29) | Increased prevalence of HCV infection among patients from the United States with B-cell lymphoma, but uncertain generalizability to other populations, because of high number of patients, belonging to Hispanic ethnicity |
| Author/Journal/Publication year | Study design study period | CCA diagnosis | HCV positive colangiocarcinoma (n)/total colangiocarcinoma cases (n) | Total patients enrolled and control source | HCV positive controls (n)/controls (n) | Percentage of HCV-positive cases with 95%CI | Main conclusion |
| (A) | |||||||
| Abdel Wahab M 2007 | Case series Period: Januiary 1995-October 2004 | Histologic confirmation/CT/MRI/ERCP/PTD | 440 patients with hilar cholangiocarcinoma 238 anti-HCV positive patients 238/440 (54%) | NR | NR | 54.1 (49.4-58.7) | Liver cirrhosis and HCV may be risk factors For hilar cholangiocarcinoma in Egypt |
| Barusrux S Asian Pacific J Cancer Prev 2012 | Case series with control group Period: NR | Histologic confirmation | 8/295 (2.7%) | Total patients: 6120 Controls randomly selected from people in 4 provinces in Thailand, representing 4 geographically distinct areas and thus, populations in the North, North-east, South and Center of the country, respectively | 125/5825 (2.15%) HCV-Ab prevalence in Thailand ranging from 1.5% to 2.15%. Sunanchaikarn S, Theamboonlers A, Chongsrisawat V et al (2007). Seroepidemiology and genotypes of hepatitis C virus in Thailand. Asian Pac J Allergy, 25, 175-182 | 2.7 (0.8-4.5) | No significant association between CAA and HCV in northeast Thailand, with prevalence of HCV infection comparable among CCA and general population |
| Chantajitr S J Hepatobiliary Pancreat Surg 2006 | Case series with control group Period: 2000-2004 | Histologic confirmation | HCC-CCA = 25 15 patients with test for anti- HCV 2/15 (13.3%) | Total patients: 75. 50 individuals, diagnosed with HCC at Ramathibodi Hospital | HCC = 50 32 patients with test for anti- HCV 1/32 (3.1%) | 13.3 (1.6-40.5) | No significant differences in presence of hepatitis C virus (HCV) antibody (13% vs 3%) as etiologic risk factor between HCC-CC and HCC patients |
| Donato F Cancer Causes and control 2001 | Hospital-based case-control study Period: January 1, 1995-July 31, 2000 | Histologic confirmation | 6/24 (25%) | Total individuals: 848. Subjects unaffected by liver diseases or malignant neoplasms, admitted to the Department of Ophtalmology, Dermatology, Urology, Surgery, Cardiology, Internal Medicine in the two main Hospitals in Brescia, enrolled as controls | 50/824 (6%) | 25 (7.7-42.3) | HCV as possible risk factor for ICC in Western countries |
| El-Serag H Hepatology 2009 | Cohort study Period: October 1, 1988, and September 30, 2004 | Identification of PAC cases by means of ICD-9-CM diagnosis codes (157.0, 157.1, 157.2, 157.3, 157.8, 157.9) Identification of HCV infected subjects by means of ICD-9-CM diagnosis codes (070.41, 070.44, 070.51, 070.54 and V02.62) | HCV-infected cohort: 146394 patients ICC = 14 ECC = 15 | 718687 patients (146394 HCV-infected cohort, 572293 HCV-uninfected cohort) , ICC: 37 and ECC: 75 (14 ICC and 15 ECC in HCV infected patients, 23 ICC and 60 ECC in HCV uninfected subjects) | HCV-uninfected cohort: 572293 patients ICC = 23 ECC = 60 | ICC: 0.01 (0-0.15) ECC: 0.01 (0-0.15) | A more than twofold elevated risk of ICC in patients with HCV infection, absence of an association with ECC |
| Hai S Dig Surg 2005 | Case series with control group Period: January 1997 - December 2002 | Histologic confirmation | 19/50 (38%) | Total patients: 50 Subjects admitted to the Osaka City University Hospital or the Osaka City General Hospital | 31/50 (62%) | 38 (24.5-51.4) | Possibility to detect a small ICC or a hepatocellular carcinoma by means of a follow-up for patients with chronic HCV by imaging series at regular intervals |
| Hsing AW Int J Cancer 2008 | Population- based case-control study Period: June 1997 - May 2001, | Histologic confirmation or by means of ERCP | 3/234 (2%) with gallbladder cancers 2/134 (1.5%) with extrahepatic bile duct cancers 1/49 (2%) with Ampulla of Vater carcinomas | Total patients: 1696 Controls represented by biliary stone case patients and by healthy subjects without a history of cancer, randomly selected from all permanent residents listed in the Shanghai Resident Registry | 2/301 (0.7%) patients with gallbladder stones, 5/216 (2.3%) with bile duct stones and 15/762 (2%) healthy individuals | 1.5 (0-3.5) | Low prevalence of HCV infection in this population (2%), therefore limited ability to detect an association with biliary diseases |
| Kobayashi M Cancer 2000 | Case series with control group Period: 1980-1997 | Cirrhosis confirmation by means of liver biopsy, peritoneoscopy, or both | 14/600 (2.3%) developed CCA 11/14 patients with CCA 3/14 patients with CCA-HCC | 600 HCV positive patients in follow-up between 1980 to 1997 | 206/600 (34.3%) patients developed HCC in the same period | 2.3 (1.1-3.5) | HCV-related cirrhosis as a major risk factor for primary CCA in Japanese patients |
| Kuper H Soz Praventivmed 2001 | Case-control study Period: January 1995-December 1998 | Histologic confirmation | 0/6 with CCA | Total subjects: 699 Controls represented by patients with injuries or eye, ear, nose and throat conditions admitted to three teaching Hospitals in Athens | 52/333 (16%) with HCC 1/360 (0.3%) controls | 0 (0-45.9) | No HCV positivity in CCA patients |
| Lee CH Br J Cancer 2009 | Case-control study Period: 1991-2005 | Histologic confirmation | 21/160 (13.1%) | Individuals generally surveyed for any disease Chang Gung Memorial Hospital at the Lin-Ko Medical Center | 10/160 (6.3%) | 13.1 (7.9-18.3) | HCV-associated ICC and HCC shared common disease process for carcinogenesis and, possibly, both arose from the hepatic progenitor cells |
| Lee TY Am J Gastroenterol 2008 | Hospital-based case-control study Period: 2000- 2004 | Histologic confirmation | 12/622 (1.9%) | Total subjects:3110 2488 healthy controls selected from 192655 individuals undergoing routine health examinations at the health promotion center at Asan Medical Center, Seoul | 47/2488 (1.9%) | 1.9 (0.8-3) | No significant association between ICC and HCV |
| Lee WS Surg Today 2006 | Case series with control group Period: November 1994- December 2003 | Histologic confirmation | ICC = 3/79 (3.8%) HCC-CCA = 4/33 (12.1%) | Total patients: 952, subjects, undergoing surgical resection at Samsung Medical Center, because of: HCC-CCA = 33 ICC = 79 HCC = 832 | HCC = 61/832 (6.5%) | 3.8 (0-8) | Significantly poorer survival rates of patients with transitional type HCC-CCA in comparison with HCC after hepatic resection |
| Matsumoto K Intern Med 2014 | Case series with control group Period: NR | Histologic confirmation | 145 patients undergoing surgical resection because of ICC: 50 ECC: 95 (1) ECC: 7/95 (7.4%) (2) ICC: 10/50 (20%) | General Japanese population (individuals ≥ 20 yr of age) | HCV-Ab prevalence equal to 1.2% in the Japanese individuals ≥ 20 yr of age | (1) 7.4 (2.1-12.6) (2) 20 (8.9-31) | HCV infection as a possible risk factor for the development of CCA. Surveillance of ICC and ECC required in HCV carriers |
| Mohammad-Alizadeh AH Asian Pac J Cancer Prev 2012 | Case series with control group Period: 2004-2011 | Histologic confirmation ERCP MRCP | CCA: 43/283 (15.2%) No distinction between HCV and number of ICC and ECC cases | Total subjects: 566 Patients with the primary or final diagnosis of CAA, admitted to gastroenterology ward of a tertiary academic center in Tehran-Iran | Gallstones 72/283 (25.4%), diabetes 70/283 (24.6%), HBV infection 52/283 (18.3%), primary sclerosing cholangitis 16/283 (5.6%) smoking 120/283 (42.3%) | 15.2 (11-19.3) | In current study smoking, opiate and alcohol use as the most common risk factors in CCA patients, chronic hepatitis C infection and cirrhosis represent further risk factors |
| Nuzzo G Updates Surg 2010 | Case series with control group Period: 1997- 2008 | Histologic confirmation | 8/55 (14.5%) (2 patients with HBV coinfection), undergoing surgical resection at Policlinico Gemelli, Rome | Total subjects: 55 | 47/55 (76.5%) | 14.5 (5.2-23.8) | ICC associated with chronic HCV infection in 14.5% of patients |
| Perumal V Human Pathology 2006 | Case series with control group Period: NR | Histologic confirmation | 2/11 (18.2%) | 10 liver specimens from anti-HCV negative individuals and 13 liver specimens from individuals who were negative for HBV surface antigen by serologic testing, used as negative controls HCV RNA-positive liver tissues from HCV positive cases used as positive controls for HCV RNA detection, at Johns Hopkins Hospital, Baltimore | Total subjects: 21 | 18.2 (2.2-51.8) | Possible etiologic role of HCV in some cases of ICC |
| Portolani N Annals of Surgical Oncology 2008 | Case series with control group Period: 1990-2006 | Histologic confirmation or typical findings on ultrasound, CT-, MRI- examination | ICC = 33 patients undergoing resection and 16 not resected 6/33 (18.1%) | Total subjects: 51 Patients diagnosed with ICC-HCC at the Surgical Clinic of Brescia University, Italy | ICC-HCC = 18 patients undergoing resection 11/18 (61.1%) | 18.1 (5-31.3) | HCV infection and cirrhosis as a risk condition for ICC and combined HCC-ICC |
| Qu Z Asia-Pacific Journal of Clinical Oncology 2012 | Case series with control group Period: January 1990 - June 2001 | Histologic confirmation of ECC | ECC: 305, 139 with test for anti- HCV ECC: 6/139 (4.3%) | Total subjects: 353 Patients with BBD with cholelithiasis or acute cholangitis, undergoing surgical intervention selected as controls at Tianjin Nankai Hospital, Tianjin Third Central Hospital, Tianjin Medical University General Hospital and The Second Hospital of Tianjin Medical University hospitals in the corresponding time period | BBD:480, 214 with test for anti-HCV BBD:12/214 (5.6%) | 4.3 (0.9-7.6) | No association between chronic HCV infection and ECC |
| Shaib YH Gastroenterology 2005 | Hospital-Based Case-Control Study Period: 1993-1999 | Histologic confirmation HCV defined by using ICD-9 codes for HCV (ICD-9 codes 070.41, 070.44, 070.51, 070.54, and V02.62) or for unspecified hepatitis (ICD-9 codes 070.9, 571.4, 571.8, and 571.9) | Data obtained from the National Cancer Institute (NCI)’s Surveillance, Epidemiology and End Results program SEER-Medicare database, linking SEER registry information with Medicare claims data, it is a program of the NCI to collect population-based cancer incidence and survival data, including population-based cancer registries in 5 states and 6 metropolitan areas ( about 14% of the United States population). ICC cases: 625 (3) HCV-specific codes: 5/625 (0.8%) (1) HCV (including unspecified hepatitis):35/625 (5.6%) | Controls included in the study derived from the 5% random sample of Medicare-enrolled beneficiaries with no cancer of any type residing in the geographic regions of SEER registries | 90834 controls (1) HCV (including unspecified hepatitis): 940 (1%) (3) HCV-specific codes: 161 (0.2%) | 0.8 (0.1-1.4) | Chronic HCV infection as possible risk factors for ICC |
| Shaib YH Am J Gastroenterol 2007 | Hospital-Based Case-control Study Period: 1992-2002 | Histologic confirmation | 246 patients undergoing surgical resection because of ICC: 5/83 (6%) ECC: 6/163 (3.7%) | Total patients: 482 Controls randomly selected from an existing database of healthy individuals at M.D. Anderson | 2/236 (0.8%) | ICC: 6 (0.9-11.1) ECC: 3.7 (0.8-6.5) | Chronic HCV infection as possible risk factors for ICC but not ECC |
| Shin RH Int J Epidemiol 1996 | Case-control study Period: August 1990-August 1993 | Histologic confirmation or typical findings on ultrasound, CT-, MRI- examination | 41 patients with CCAs 203 patients with HCC (1) 29/41 patients with tests for antiHCV/HBV status. 4/29 (13.8%) HCV positive (2) 128/203 patients with test for antiHCV/HBV status 17/128 (13.3%) HCV positive | (1) Inpatients without liver disease, systemic disease, and malignant disorders from the Departments of Ophthalmology or Otorhinolaryngology (2) healthy people who had visited the Non-Communicable Disease Control Center All subjects were visited at the Tnje University Pusan Paik Hospital | (3) 203 (4) 203 394/406 subjects with tests for anti-HCV status. 23/394 (6.6%) HCV positive | (1) 13.8 (1.2-26.3) (2) 13.3 (7.4-19.1) | No association between chronic HCV infection and CCA |
| Songsivilai S Trans R Soc Trop Med Hyg 1996 | Case series with control group Period: July 1993 - June 1995 | Histologic confirmation | 0/30 | Total subjects: 110 Patients with HCC, undergoing surgical resection at Siriraj Hospital, Mahidol University, Bangkok | 9/80 (11.2%) | 0 (0-11.6) | No association between chronic HCV infection and CCA |
| Srivatanakul P Asian Pacific J Cancer Prev 2010 | Case-control study Period: September 1999 -2001 | Histology, or typical findings on ultrasound examination with an elevated titre (≥ 40 units/mL) of CA 19-9 and normal level of alpha-fetoprotein (AFP < 20 ng/mL) | 7/103 (6.8%) | Total subjects: 206 Community hospitals in Nakhon Phanom Province and Nakhon Phanom Provincial Hospital | 0/103 | 6.8 (1.9-11.6) | Possible role of HCV infection in the development of CCA in northeast Thailand |
| Taguchi J J Gatroenterol Hepatol 1996 | Case series with control group Period: January 1988-July 1995 | Histologic confirmation | 14/20 (70%) | Total subjects: 367 HCC-CCA: 23/367, 20 patients with anti-HCV markers | 6/20 (30%) | 70 (49.9-90) | HCC-CCA associated with chronic HCV infection in 70% of patients |
| Tanaka M J Viral Hepat 2010 | Cohort study Period: 1991-1993 | ICC cases identified by the ICD-10 code (C22.1). diagnosis of ICC was based on histological examination and/or combined clinical, radiological (echography, CT and endoscopic retrograde cholangio-pancreatography) and laboratory findings | ICC: 11 cases 1/11 (9.1%) | 154814 study subjects voluntary blood donors | 1927/154814 (1.2%) | 9.1 (0.2-41.3) | No association between HCV infection and ICC development |
| Tomimatsu M Cancer 1993 | Case series with control group Period: January 1985 - December 1990 | Histologic confirmation | (1) CCA: Anti-HCV +: 4/13 (30.8%) HBsAg+: 3/13 (23.1%) Anti-HCV-/HBsAg-: 6/13 (46.1%) (2) CCA-HCC: Anti-HCV +: 5/7 (71.4%) HBsAg+: 1/7 (14.3%) Anti-HCV- /HBsAg-: 1/7 (14.3%) | Total subjects: 141 Patients with HCC, undergoing surgical resection at the Institute of Gastroenterology of Tokyo Women’s Medical College | Anti-HCV +: 85/121 (70.3%), Anti-HCV+ /HBsAg+: 5/121 (4.1%) HBsAg+: 16/121 (13.2%) HBsAg-/anti-HCV -: 15/121 (12.4%) | (1) 30.8 (9-61.4) (2) 71.4 (29-96.3) | The anti-HCV-positive rate was high in combined HCC-CC as well as in HCC |
| Uenishi T Journal of Surgical Oncology 2014 | Case series with control group Period: January 2000 - December 2011 | Histological confirmation | 33/90 (36.7%) | Total subjects: 90 Patients enrolled at Hirakata and Osaka University Hospital | 57/90 (63.4%) | 36.7 (26.7-46.6) | HCC-related death often occurred in patients undergoing curative resection for HCV-related ICC. HCV as adverse prognostic factor after curative resection for mass-forming ICC |
| Yamamoto M Cancer 1998 | Case-series Period: February 1990 - March 1996 | Histologic confirmation | 50 patients with ICC Anti-HCV positive: 16/50 (32%) HBsAg+/Anti-HCV positive: 1 (2%) | NR | NR | 32 (19-44.9) | Minute nodular ICC appears to be related to hepatitis viral infection and could be detected at an early stage, similar to hepatocellular carcinoma, by following up cases of chronic hepatitis or cirrhosis |
| Yamamoto S Cancer Sci 2004 | Hospital case-control based study Period: January 1991 - December 2002 | Histologic confirmation | 18/50 (36%) | Total subjects: 255 Control patients enrolled at the two major medical centers of Osaka City | 7/205 (3%) | 36 (22.7-49.3) | HCV infection as a possible etiology of ICC in Japan |
| Yano Y Jpn J Clin Oncol 2003 | Case-control study Period: January 1978 - December 1998 | Histologic confirmation | HCV alone: (1) HCC-CCA = 10/26 (38.5%) (2) CCA = 5/53 (9.4%) HCV + HBV: 1/53 (2%) | Total subjects: 1172 Patients with HCC, undergoing surgical resection at the Department of Surgery, National Cancer Center Hospital, Tokyo | HCV alone: HCC = 526/1093 (48%) HCV + HBV: 16/1093 (1%) | (1) 38.5 (19.8-57.1) (2) 9.4 (1.5-17.3) | HCC-CCA represents a variant of ordinary HCC with cholangiocellular features, rather than an intermediate disease entity between HCC and CCA |
| Wahab A M Hepatogastro-enterology 2007 | Case series Period: January 1995 - October 2004 | Histologic confirmation or typical findings on CT, ERCP, MRI and PTD | Total patients: 440238/440 (54.1%) | NR | NR | 54.1 (49.4-58.7) | HCV chronic infection as possible risk factor for hilar CCA in Egypt |
| Welzel TM Clin Gastroenterol Hepatol 2007 | Population-based case-control study Period: 1993-1999 | Identification of CAA cases from the Surveillance, Epidemiology and End Results-Medicare databases by means of ICD-9-CM diagnosis codes: (C22.0, C22.1, C24.0, 8010, 8020, 8041, 8070, 8140, 8144, 8160, 8161, 8260, 8310, 8480, 8490, 8560). Identification of HCV infection by means of ICD-9-CM diagnosis codes 070.41, 070.44, 070.51, 070.54 and 070.7 | (1) ICC = 5/535 (0.9%) (2) ECC = 5/549 (0.9%) | 102782 cancer-free controls identified using the Surveillance, Epidemiology and End Results-Medicare databases | 142/102782 | ICC: 0.9 (0.1-1.7) ECC: 0.9 (0.1-1.7) | Association between HCV infection and ICC |
| Zhou HQ Hepatobiliary Pancreat Dis Int 2007 | Case-series Period: January 1996 - November 2005 | Histologic confirmation | (1) HCC: 132 patients Anti-HCV positive: 26/132 (19.7%) (2) CCA: 44 patients Anti-HCV positive: 4/44 (9.1%) (3) HCC-CCA: 15 anti-HCV positive: 3/15 (20%) | NR | NR | (1) 19.7 (12.9-26.4) (2) 9.1 (0.6-17.5) (3) 20 (4.3-48) | Percentage of cHCC-CC patients with serum anti-HCV antibodies were similar to those of HCC patients but different from CC patients |
| Zhou YM World J Gastroenterol 2008 | Hospital-based-case control Study Period: February 2004 - May 2006 | Histologic confirmation | 9/312 (2.9%) | Total patients: 750 Controls were selected from patients who were unaffected by liver diseases in the Changhai Hospital of the Second Military Medical University | 6/438 (1.4%) | 2.9 (0.9-4.7) | No significant difference between cases and controls in the prevalence of anti-HCV seropositivity |
| (B) | |||||||
| Torbenson M Am J Surg Pathol 2007 | Review of liver explants with control group from 3 transplant centers Period: 1995 -2005 | Histologic confirmation in explanted livers | (1) HCV alone = 10/511 (2%) (2) HCV + alcohol = 4/85 (5%) | 1058 total liver explants Control groups included: (1) alcohol cirrhosis, (2) chronic hepatitis B infection, (3) nonviral causes of cirrhosis such as cryptogenic cirrhosis, (4) noncirrhotic livers that were transplanted for fulminant liver failure | (1) Alcohol cirrhosis = 5/ 112 (4%) (2) HBVchronic hepatitis = 0/67 (0%) (3) Cirrhosis from nonviral and non alcohol causes = 0/149 (0%) (4) Noncirrhotic =/134 (0%) | (1) 2 (0.7-3.1) (2) 4.7 (0.2-9.2) | Dysplasia detectable within the intrahepatic bile ducts in chronic HCV cirrhosis; chronic HCV, alone or in association with alcohol, as major risk factor for ICC |
| Wu TT Cancer 2009 | Review of liver explants with control group at Mayo Clinic Rochester, Minnesota Period: 1995 - 2007 | Histologic confirmation in explanted livers | (1) Alcohol-related and HCV-related cirrhosis: 24/26 (92%) (2) HCV-related cirrhosis: 27/44 (61%) | 244 total liver explants Causes: 94 alcohol-related cirrhosis, 44 HCV-related cirrhosis, 26 alcohol- and HCV-related cirrhosis, 28 massive hepatic necrosis, 24 correction of metabolic conditions, 16 primary or metastatic tumors, 8 nodular regenerative hyperplasia, 2 subacute Budd- Chiari syndrome, 2 liver failure during the first week after transplantation | Noncirrhotic 27/80 (34%) alcohol-related cirrhosis 86/94 (91%) | (1) 92.3 (74.9-99) (2) 61.4 (46.9-75.7) | Epidemiologic role of HCV and alcohol in the development of CCA |
| First author/Journal/Publication year | Study design/study period | PAC diagnosis | HCV positive PAC (n)/total PAC cases (n) | Control source | HCV positive controls (n)/controls (n) | Percentage of HCV-positive cases with 95%CI | Main conclusions |
| Amin J J Hepatol 2006 | Community-based cohort-study Period: 1990-2002 | Identification of pancreatic cancer cases by means of ICD-10- diagnosis codes | -Individuals with HCV infection: 75834 PAC detected: 17/75834 (0.02%) | Incidence observed in the study cohort was compared to expected incidence derived from NSW population cancer rates by calculating standardised incidence ratios | SIRs: 1.4 (0.8-2.2) | 0.02 (0.01-0.03) | No evidence supporting an association between HCV infection and PAC development |
| Chang MC World J Gastroenterol 2014 | Case-control study Period: 2000-2013 | Histological or citological | 22/585 (3.8%) | Controls were individuals recruited from a free screening program in a community located in Northern Taiwan | 45/1716 (2.6%) | 3.8 (2.2-5.3) | HCV infection not associated with higher risk of PAC development, after adjustement for age, sex, diabetes and smoking (independent risk factors for PAC) |
| El Serag Hepatology 2009 | Cohort study Cohort: 718687 patients PAC detected: 617 Period: 1988-2004 | Identification of PAC cases by means of ICD-9-CM diagnosis codes (157.0, 157.1, 157.2, 157.3, 157.8, 157.9) Identification of HCV infected subjects by means of ICD-9-CM diagnosis codes (070.41, 070.44, 070.51, 070.54 and V02.62) | 146394 patients in HCV-infected cohort PAC detected:140/146,394 (0.09%) | Sources included inpatients records from more than 150 of USA Veterans Affairs (VA) hospitals in the Patients treatment file and outpatients records from any VA facility in the Output Clinic File | 572293 patients in HCV-uninfected cohort PAC detected: 477 | 0.09 (0.08-0.11) | Higher risk of PAC in patiens of HCV-infected cohort , but this association was attenuated after adjustement for alcohol use, pancreatitis, choledocholitiasis, cholelithiasis or primary sclerosing cholangitis |
| Hassan MM J Clin Oncol 2008 | Hospital-based case-control study Period: 2000-2007 | Histological confirmation | 6/474 (1.5%) | Community-based (healthy genetically unrelated family members of patients with cancer other than pancreatic, GI, lung or head cancers) | 9/872 (1%) | 0.8 (0.02-1.6) | HCV infection not associated with higher risk of PAC development |
| Huang J Br J Cancer 2013 | Retrospective Nationwide cohort study 197208 participants: Period: 1990-2006 | Identification of PAC cases from the Swedish Cancer Register (International Classification of Disease ICD-7: 157) and from the Cause of Death Register (ICD-9: 157; ICD-10: C25) | Individuals in HCV reference cohort: 39442 PAC detected: 34/39442 (0.09%) | Control population obtained from the national surveillance database at the Swedish Institute for Infectious Disease Control. The expected numbers of calculated PAC from the observed person-time in each 5-yr age group by sex and the corresponding Swedish population incidence rates. | Expected number of PAC: 16.5 | 0.09 (0.05-0.11) | Statistically significant increased risk of PAC development |
| Omland LH Clinical Epidemiology 2010 | Cohort-study Period: 1994 - 2003 | Patients and subjects with HCV infection identified by means of: -The Danish National Hospital Registry (DNHR) -The Danish Cancer Registry People listed in DNHR with at least one diagnosis of acute or chronic HCV infection (ICD-10 B17.1 and 18.2) were included Cancer diagnoses based on the Danish version of the international classification of diseases, 8th revision (ICD-8) until Dec 31, 1993, and 10th version (ICD-10) thereafter | 4349 patients with HCV infection in the DNHR 4/4349 PAC detected (0.1%) | The expected number of cases of cancer after a diagnosis of HCV infection using Danish incidence rates of first cancer diagnoses according to sex, age, and year of diagnosis in 1-yr intervals was calculated | Expected number of PAC: 1.01 | 0.1 (0-0.18) | Association between HCV infection and higher risk of PAC development |
| Qiwen Ben Pancreas 2012 | Double-centre ongoing hospital-based case-control study. Period: January 1, 2004- August 31, 2008 January 1, 2003- Octobet 31, 2009 | Histological or citological confirmation | 14/943 (1.5%) | Patients admitted to the same Hospitals (Ruijin Hospital and Changai Hospital, Shangai for any acute conditions) | 12/1128 (1.1%) | 1.5 (0.7-2.2) | No higher HCV prevalence in patients with PAC in comparison with controls |
| Swart A BMJ Open 2012 | Cohort-study Patients considered in the study: 29613 1 January 1993 - 31 December 2007 | Individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people as opioid substitution therapy. Solid cancers classified according to the International Classification of Diseases (ICD), 10th revision, haematopoietic neoplasms and Kaposi sarcomas classified according to the ICD for Oncology, 3rd edition | Subjects with HCV infection alone: 14892 Observed number of PAC in HCV-positive cohort: 20/14892 (0.1%) | Calculation of expected number of incident PAC | Expected number of PAC: 7.12 | 0.13 (0.08-0.21) | Increased risk of PAC in patients with HCV infection |
| Woo SM J Korean Med Sci 2013 | Case-control study Period: 2001-2011 | Histological or radiological/clinical confirmation | 753 patients with PAC 724/753 with available anti-HCV test 21/724 (2.8%) | Individuals subjected to routine health examination in the Cancer Screening Cohort | 36/3012 (1.2%) | 2.9 (1.7-4.1) | Seropositivity for anti-HCV, infection, may increase the risk of developing PC in Korea |
| Author/Journal/Publication year | Country | Study design/study period | Diagnosis | Sample size (HCV positive breast cancer cases) | Control source | HCV positive controls/controls | Matching factors | Percentage of HCV-positive cases with 95%CI | Main conclusions |
| Amin J J Hepatol 2006 | Australia | Community-based cohort-study Period: 1990-2002 | Patients’data obtained from: -New South Wales (NSW) Australia Health Department’s Notifiable Diseases Database (NDD) for notification of newly diagnosed HCV infection -NSW Central Cancer Registry (CCR) for notification of incident cancer cases -National Death Index (NDI) database, containing records of all deaths in Australia since 1980 Identification of breast cancer cases by means of ICD-10-diagnosis codes | Individuals with HCV infection: 75834 Breast cancers detected: 50 50/75834 | Incidence observed in the study cohort was compared to expected incidence derived from NSW population cancer rates by calculating standardised incidence ratios | SIR: 0.3 (0.4-0.5) | NR | (0.05-0.09) | No evidence supporting an association between HCV infection and breast cancer development |
| Hwang JP J Oncol Pract 2014 | United States | Cohort-study Period: January 2004 - April 2011 | Patients’data, obtained from four institutional sources: Tumor registry: to assess patients’ demographic characteristics Pharmacy informatics: to evaluate chemotherapy drugs and dates administered. Patient accounts: to identify study patients’ International Classification of Diseases (ninth edition; ICD-9) codes Laboratory informatics: to determine HCV antibody (anti-HCV) and ALT test dates and results | 141877 patients with cancer, who were newly registered at MD Anderson Cancer during the study period. Patients considered in the study: 16773. HCV screened subjects: 2330/16773 (13.9%) HCV screened females: 1038 HCV-positive patients with cancers: 35/2330 (1.5%) HCV-positive females with cancers: 12 (1) HCV-positive females with breast cancers: 3/12 (2) HCV-negative females with breast cancer: 102/1026 | NR | NR | NR | (1) 25 (5.5-57.2) (2) 9.9 (8.1-11.8) | HCV screening rates were low, even among patients with risk factors, and the groups with the highest rates of screening did not match the groups with the highest rates of a positive test result |
| Larrey D World J Gastroenterol 2010 | France | Case serie with control gorup Period: NR | Females with history of HCV-related chronic infection, observed in Liver Unit of Montpellier School of Medicine, France, for chronic liver diseases in several occasions for a period longer than 1 yr. Chronic hepatitis proved by liver biopsy and/or biological markers of inflammation and fibrosis | 17/294 (5.8%) | Females sequentially and prospectively seen during the same period with chronic liver disease over 1 yr, with well defined clinical, radiological and histological characteristics [chronic- HBV, alcoholic-liver disease, auto-immune hepatitis, hemochromatosis, non alcoholic fatty liver disease (NAFLD), cholangitis] | 5/107 (4.7%) | NR | 5.8 (3.1-8.4) | Chronic HCV infection is not a strong promoter of breast carcinoma in adult females of any age |
| Omland LH Clinical Epidemiology 2010 | Denmark | Cohort-study Period: 1994-2003 | Patients and subjects with HCV infection identified by means of: The Danish National Hospital Registry (DNHR) -The Danish Cancer Registry People listed in DNHR with at least one diagnosis of acute or chronic HCV infection (ICD-10 B17.1 and 18.2) were included Cancer diagnoses based on the Danish version of the international classification of diseases, 8th revision (ICD-8) until Dec 31, 1993, and 10th version (ICD-10) thereafter | 4349 patients with HCV infection in the DNHR 2 breast cancer detected 2/4349 (0.05%) | The expected number of cases of cancer after a diagnosis of HCV infection using Danish incidence rates of first cancer diagnoses according to sex, age, and year of diagnosis in 1-yr intervals was calculated | Expected number of breast cancers 8.05 | NR | 0.05 (0-0.1) | No association between HCV infection and higher risk of breast cancer development |
| Su FH BMC Cancer 2011 | Taiwan | Population-based study Period: 2000-2008 | Data retrieved from National Health Insurance Research Database (NHIRD), which is maintained by the National Health Research Institute (NHRI), Taiwan. Newly diagnosed breast cancer identified from the registry for Catastrophic Illness Patients Database (ICD-9-CM code 174 and 175). Identification of HCV infected subjects by means of ICD-9-CM diagnosis codes (ICD-9- CM 070.41, 070.44, 070.51, 070.54, and V02.62) | 56/1958 (2.9%) | Randomly selected and matched individuals without a history of breast cancer (control to patient ratio was 4:1) | 178/7832 (2.3%) | age- and sex | 2.9 (2.1-3.5) | HCV infection associated with early onset risk of breast cancer in areas endemic for HCV |
| Swart A BMJ Open 2012 | Australia | Cohort-study 1 January 1993 - 31 December 2007 | Individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people as opioid substitution therapy. Solid cancers classified according to the International Classification of Diseases (ICD), 10th revision, haematopoietic neoplasms and Kaposi sarcomas classified according to the ICD for Oncology, 3rd edition | Patients considered in the study: 29613 Subjects with HCV infection alone: 14892 Observed number of breast cancer in HCV-positive cohort: 48 48/14892 (0.03%) | Calculation of expected number of incident breast cancer | Expected number of breast cancers: 101 | NR | 0.03 (0.02-0.04) | No evidence supporting an association between HCV infection and breast cancer development |
| Author/Journal/Publication year | Country | Study design/study period | Diagnosis | Sample size (HCV positive RCC cases) | Control source | HCV positive controls/controls | Matching factors | Percentage of HCV-positive cases with 95%CI | Main conclusions |
| Amin J J Hepatol 2006 | Australia | Community-based cohort-study Period: 1990-2002 | Identification of renal cancer cases by means of ICD-10- diagnosis codes | Individuals with HCV infection: 75834 RCC detected: 19 19/75834 | Incidence observed in the study cohort was compared to expected incidence derived from NSW population cancer rates by calculating standardised incidence ratios | SIR: 0.9 (0.6-1.4) | NR | 0.02 (0.01-0.03) | No evidence supporting an association between HCV infection and kidney cancer development |
| Budakoğlu B Med Oncol 2012 | Turkey | Case series with control group 2005-2010 | Histological confirmation | 15/903 (1.7%) | Data collected in previous prevalence studies in healthy subjects in three different geographical areas of the Turkey, used as control group | 81/5267 (1.5%) | NR | 1.7 (0.8-2.4) | No higher frequency of HCV positivity in RCC patients in comparison with healthy people |
| Gonzalez HC Dig Dis and Sci 2015 | United States | Case series with control group January 2011 - August 2013 | Histological confirmation | Anti-HCV positive: 11/140 (7.9%) (2) HCV-RNA positive: 9/140 (6.4%) | Consecutive individuals newly diagnosed with colon cancer. The control group recruited simultaneously and from the same health care system (Henry Ford Health System in Detroit, Michigan) | Anti-HCV positive: 1/100 (1%) HCV-RNA positive: 0/100 | NR | (1) 7.9 (3.4-12.3) (2) 2.3 (10.5) | Increased risk of RCC in subjects with HCV chronic infection |
| Gordon SC Cancer Epidemiol Biomarkers Prev 2010 | United States | Cohort study Period: 1997-2006 | Use of administrative data from Henry Ford Hospital, an integrated healthcare delivery system serving southeastern Michigan. Cancer diagnosis codes in administrative databases [International Classification of Diseases, 9th ed., Clinical Modification (ICD-9-CM) codes in the range of 140 through 208.9] | 72487 patients tested for anti-HCV 3057/72487 anti-HCV positive patients 17/3057 (0.6%) with RCC | Control cohort of patients who tested negative for anti-HCV | 64006/72487 anti-HCV negative patients 177/64006 (0.3%) with RCC | NR | 0.6 (0.3-0.8) | Chronic infection with HCV confers an increased and independent risk for developing RCC |
| Hofmann JN Eur J Cancer Prev 2011 | Sweden | Nationwide register-based cohort- study Period: 1990-2008 | HCV diagnosis extracted from the national surveillance database at the Swedish Institute for Infectious Disease Control (SMI). Cancer diagnoses were coded using the seventh revision of the International Classification of Diseases (ICD-7) (ICD-7 codes 180.0 and 180.9) | 43000 Lag period after HCV notification (1) None: 38, Expected: 27.1 (2) Three months 33 Expected: 26.5 (3) One year: 29 Expected: 24.9 | A non-HCV-infected cohort selected from the general population | 215000 | Year of birth, sex, and county of residence in Sweden, five subjects never diagnosed with HCV infection were matched to each HCV-infected subject | (1) 0.06 (0.09-0.21) (2) 0.05 (0.08-0.21) (3) 0.05 (0.07-0.21) | In the cohort of HCV-infected subjects, no increased risk of developing kidney cancer but an enhanced risk of non-cancer chronic kidney disease, particularly among women |
| Malaguarnera M Eur J Int Medicine (2006) | Italy | Case-control study Period: NR | All cancer patients: 236 HCV diagnosis performed with II G ELISA test. Cancers diagnosed at Garibaldi Hospital | 15 patients with RCC 8/15 (53%) HCV positive patients | Elderly volunteers evaluated at Garibaldi Hospital, Catania | 30/300 (10%) | Age, sex and previous blood transfusions | 53.3 (26.5-78.7) | High prevalence of anti-HCV antibodies in patients with renal cancer |
| Omland LH Clinical Epidemiology 2010 | Denmark | Cohort-study Period: 1994-2003 | Patients and subjects with HCV infection identified by means of: -The Danish National Hospital Registry (DNHR) -The Danish Cancer Registry People listed in DNHR with at least one diagnosis of acute or chronic HCV infection (ICD-10 B17.1 and 18.2) were included Cancer diagnoses based on the Danish version of the international classification of diseases, 8th revision (ICD-8) until Dec 31, 1993, and 10th version (ICD-10) thereafter | 4349 patients with HCV infection in the DNHR 4 renal cancer detected 4/4349 | The expected number of cases of cancer after a diagnosis of HCV infection using Danish incidence rates of first cancer diagnoses according to sex, age, and year of diagnosis in 1-year intervals was calculated | Expected number of kidney cancers: 1.11 | NR | 0.1 (0-0.2) | Association between HCV infection and higher risk of renal cancer development |
| Swart A BMJ Open 2012 | Australia | Cohort-study 1 January 1993 - 31 December 2007 | Individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people as opioid substitution therapy. Solid cancers classified according to the International Classification of Diseases (ICD), 10th revision, haematopoietic neoplasms and Kaposi sarcomas classified according to the ICD for Oncology, 3rd edition | Patients considered in the study: 29613 Subjects with HCV infection alone: 14892 Observed number of RCCs in HCV-positive cohort: 20 20/14892 | Calculation of expected number of incident RCCs | Expected number of RCCs: 18.1 | NR | 0.1 (0.08-0.20) | No evidence supporting a strong association between HCV infection and RCC development |
| Author/Journal/Publication year | Study design/study period | Diagnosis | Sample size (cases/controls) | Control source | HCV positive controls/controls | Percentage of HCV-positive cases with 95%CI | Main conclusions |
| Amin J J Hepatol 2006 | Community-based cohort-study Period: 1990-2002 | Identification of skin/oral cancer cases by means of ICD-10- diagnosis codes | Individuals with HCV infection: 75834 Skin/oral cancer: 19 including , mouth (7 cases), tongue (6 cases), tonsil (6 cases) no skin cancers described | Incidence observed in the study cohort was compared to expected incidence derived from NSW population cancer rates by calculating standardised incidence ratios | SIR: Mouth: 1.5 (0.7-3.2) Tongue: 1.1 (0.5-2.4) Tonsil: 2.1 (1-4.8) | 0.02 (0.01-0.03) | No evidence supporting an association between HCV infection and skin/oral cancer development, low increased risk for tonsil cancer |
| Eftekharian A Eur Arch Otorhinolaryngol 2012 | Case-series 107 patients with SCCHN Period: October 2008-June2010 | Histological confirmation: SCCHN | 1/107 (0.9%) | NR | NR | 0.9 (0-2.7) | HCV at least in Iran not a risk factor for SCCHN |
| Gandolfo S Oral Oncol 2004 | Case-series 402 patients with OLP Patients with available HCV test: 357 HCV positive patients: 69/357 (19.3%) Period: January 1988 - July 1999 | During the follow-up period: 9 patients developed an oral squamous cell carcinoma Histological confirmation: OSCC | HCV positive patients with OSCC: 4/9 (44.5%) | NR | NR | 44.5 (11.9-76.9) | Possible increased risk for OSCC in HCV-related infection in patients oral lichen planus (OLP) |
| Nagao Y J Oral Pathol Med 1995 | Case-series 100 patients with oral cancer enrolled Period: January 1989-October 1993 | Histological confirmation: Different histotypes | 24/100 (24%) | Patients with non-malignant disease receiving dental treatment at the Department of Oral Surgery of the Kurume University Patients with gastric cancer | (1) 11/104 (10.6%); (2) 12/113 (10.6%) | 24 (15.6-32.3) | HCV causing pathologic changes in the oral cavity, with HCV involved in cancerization |
| Nagao Y J Oral Pathol Med 2000 | Biopsies of 36 patients, including: (1) OLP: 19; (2) Oral cancer: 17 Period: NR | Histological confirmation: Well-differentiated SCCHN | (1) 14/19 (73.7%); (2) 7/17 (41. 2%) | Biopsies of 10 patients, including: (3) Non-malignant disease with HCV (4) Non-malignant disease without HCV | (3): 6 (4): 4 | (1) 73.7 (53.8-93.4); (2) 41.2 (17.8-64.5) | HCV causing pathologic changes in the oral cavity, with HCV involved in cancerization |
| Nobles J Laringoscope 2004 | Case-series 100 patients with SCCHN enrolled. Period: June 1991-December 2002 | Histological confirmation: SCCHN | 21/100 (21%) | NR | NR | 21 (13-28.9) | A large number of patients (21%) with SCCHN, included in this study, coinfected with HCV. This prevalence is significantly increased when compared with the general population (1.4%) or the population at VA hospitals (9.9%) |
| Omland LH Clinical Epidemiology 2010 | Cohort-study Period: 1994-2003 | Patients and subjects with HCV infection identified by means of: -The Danish National Hospital Registry (DNHR) -The Danish Cancer Registry People listed in DNHR with at least one diagnosis of acute or chronic HCV infection (ICD-10 B17.1 and 18.2) were included Cancer diagnoses based on the Danish version of the international classification of diseases, 8th revision (ICD-8) until Dec 31, 1993, and 10th version (ICD-10) thereafter | 4349 patients with HCV infection in the DNHR 4 oropharyngeal cancers detected | The expected number of cases of cancer after a diagnosis of HCV infection using Danish incidence rates of first cancer diagnoses according to sex, age, and year of diagnosis in 1-yr intervals was calculated | Expected number of oropharyngeal cancers: 1.73 | 0.1 (0-0.2) | No sssociation between HCV infection and higher risk of oropharyngeal cancer development |
| Su FH PlosOne 2012 | Nationwide Population-Based Cohort Study HCV positive patients: 5311 HCV and HBV positive patients: 3519 Period: 1996-2008 | Data obtained from the Taiwan National Health Insurance Research Database (NHIRD). HCV cases identified by means of ICD-9-CM diagnosis codes (ICD-9-CM: 070.41, 070.44, 070.51, 070.54, V02.62) | (1) 21/5311; (2) 9/3519 | Controls identified by means of a systematic random sampling method to select 4 insured people without viral hepatitis for every insured person with viral hepatitis during the same period | 147/84796 | (1) 0.4 (0.2-0.5); (2) 0.3 (0.09-0.4) | HCV infection is a risk factor for oral cavity cancer. In addition, subjects with HCV infection tend to be at early onset risk for oral cavity malignancy |
| Swart A BMJ Open 2012 | Cohort-study 1 January 1993 - 31 December 2007 | Individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people as opioid substitution therapy. Solid cancers classified according to the International Classification of Diseases (ICD), 10th revision, haematopoietic neoplasms and Kaposi sarcomas classified according to the ICD for Oncology, 3rd edition | Patients considered in the study: 29613 Subjects with HCV infection alone: 14892 Observed number of following cancer in HCV-positive cohort: (1) Tonsil: 10; (2) Mouth:8; (3) Salivary gland: 4; (4) Tongue: 9 Total: 31 | Calculation of expected number of incident tonsil/mouth/salivary gland/tongue cancers | Expected number of oral cancers: Tonsil: 2.96 Mouth; 3.54 Salivary gland: 2.75 Tongue:5.35 | 0.2 (0.1-0.3) | Possible association between HCV and tonsil/mouth cancers. No association between HCV infection and tongue/salivary cancers |
| Takata Y Oral Diseases 2002 | Case series Patients with anti-HCV antibodies: 2613 HCV positive patients: 151/2613 (5.8%) Period: January 1989 -December 1998 | Histological confirmation Histotype not reported | 25/245 (10.2%) | NR | NR | 10.2 (6.4-13.9) | High HCV antibody prevalence in patients with oral cancer. Possible no important association between oral cancer and HCV infection, with increased prevalence, depending on higher age of anti-HCV positive patients |
| Author/Journal/Publication year | Study design/study period | Diagnosis | Sample size | Control source | Controls | Percentage of HCV-positive cases with95%CI | Main conclusions |
| Amin J J Hepatol 2006 | Community-based cohort-study Period: 1990-2002 | Identification of thyroid cancer cases by means of ICD-10- diagnosis codes | Individuals with HCV infection: 75834 Thyroid cancers detected: 9 | Incidence observed in the study cohort was compared to expected incidence derived from NSW population cancer rates by calculating standardised incidence ratios | SIR: 0.3 (0.2-0.7) | 0.01 (0-0.02) | No evidence supporting an association between HCV infection and thyroid cancer development |
| Antonelli A Clin Exp Rheumat 2002 | Case-control study Period: 1999-2001 | FNA PTC | 94 patients with HCV-associated MC Patients with PTC and HCV-associated MC/patients with HCV-associated MC: 2/94 (2.1%) | Control group obtained from a sample (2401 individuals) of the general population, 5 controls were randomly associated with each MC patient | 0/470 | 2.1 (0-5) | Possible association between HCV-related MC and thyroid cancer, careful monitoring of the thyroid opportune, during the clinical follow-up of HCV- associated MC patients |
| Antonelli A Thyroid 2007 | Case-control study Period: January 1995 - December 2001 | FNA PTC | 308 HCV positive patients PTC and HCV positive cases/all HCV positive cases: 6/308 (1.9%) | (1) subjects from an iodine deficient area; (2) subjects from an iodine-sufficient area | PTC cases/all HCV negative controls: (1) 0/616; (2) 1/616 | 1.9 (0.4-3.4) | High prevalence of thyroid papillary cancer in HCV+ patients, overall in presence of thyroid autoimmunity; careful thyroid monitoring is indicated during the follow-up of these patients |
| Giordano TP JAMA 2007 | Cohort study Period: 1997-2004 | Identification of HCV infected subjects by means of ICD-9-CM , diagnosis codes of HCV infection (070.41, 70.44, 070.51, 070.54, V02.62) Identification of thyroid cancer by menas of ICD-9-CM diagnosis codes: 193 | HCV-positive cohort: 146394 patients During follow-up, 813 patients in HCV-infected cohort (0.5%) had a HIV diagnosis. 46 patients developed thyroid cancer | Inpatients records from more than 150 United States Veterans Affairs (VA) hospitals in the Patients’ treatment file and outpatients records from any VA facility in the Output Clinic File | HCV- negative cohort: 572293 patients. During follow-up, 35696 uninfected HCV patients (6.2%) had a recorded HCV diagnosis and 1539 patients (0.3%) a HIV diagnosis 274 patients developed thyroid cancer | 0.03 (0.02-0.04) | No increased, risk for thyroid cancer in HCV-positive cohort |
| Montella M Oncol Rep 2003 | Case-control study Period 1997-1999 | Histological confirmation PTC | HCV positive PTC cases/all PTC cases: 16/130 (12.3%) | Control group including subjects, operated for benign diseases. Cases and controls selected from the hospital tumor registry | 242 controls and 311 surgical procedures. HCV positive controls/total controls 18/311 | 12.3 (6.6-17.9) | Association between HCV and thyroid cancer. This malignancy more readily detectable in countries with a high prevalence of HCV |
| Omland LH Clinical Epidemiology 2010 | Cohort-study Period: 1994-2003 | Patients and subjects with HCV infection identified by means of: -The Danish National Hospital Registry (DNHR) -The Danish Cancer Registry People listed in DNHR with at least one diagnosis of acute or chronic HCV infection (ICD-10 B17.1 and 18.2) were included Cancer diagnoses based on the Danish version of the international classification of diseases, 8th revision (ICD-8) until Dec 31, 1993, and 10th version (ICD-10) thereafter | 4349 patients with HCV infection in the DNHR 1 thyroid cancer detected | The expected number of cases of cancer after a diagnosis of HCV infection using Danish incidence rates of first cancer diagnoses according to sex, age, and year of diagnosis in 1-yr intervals was calculated | Expected number of thyroid cancers: 0.46 | 0.02 (0-0.06) | No association between HCV infection and higher risk of thyroid cancer development |
| Swart A BMJ Open 2012 | Cohort-study 1 January 1993 - 31 December 2007 | Individuals registered on the Pharmaceutical Drugs of Addiction System, a record of all NSW Health Department authorities that administer methadone or buprenorphine to opioid-dependent people as opioid substitution therapy. Solid cancers classified according to the International Classification of Diseases (ICD), 10th revision, haematopoietic neoplasms and Kaposi sarcomas classified according to the ICD for Oncology, 3rd edition | Patients considered in the study: 29613 Subjects with HCV infection alone: 14892 Observed number of thyroid cancer in HCV-positive cohort: 48 | Calculation of expected number of incident thyroid cancer | Expected number of thyroid cancers: 34.4 | 0.3 (0.2-0.4) | No evidence supporting an association between HCV infection and thyroid cancer development |
On the basis of our results, we summarised some of the main characteristics of retrieved trials, which were designed to investigate the prevalence of HCV infection in each type of the above- mentioned malignancies. In particular, we created a main table and included a short description in the text for each of these tumours, whether at least five studies, evaluating this parameter and meeting inclusion criteria, were available in literature. In each of these tables, we reported the following data of studies considered: first author’s name, study design, year of publication, country of origin, matching criteria, number of cases and controls, diagnostic methods to detect each malignancy, percentage of HCV-positive cases with 95% confidence intervals (CIs) and main conclusions. CIs for each proportion were calculated according to normal distribution or binomial distribution as appropriate. Accordingly to these pre-definite criteria, we created the following sections and the corresponding Tables: (1) HCV and haematopoietic malignancies (Table 1); (2) HCV and cholangiocarcinoma (Table 2); (3) HCV and pancreatic cancer (Table 3); (4) HCV and breast cancer (Table 4); (5) HCV and kidney cancer (Table 5); (6) HCV and skin or oral cancer (Table 6); and (7) HCV and thyroid cancer (Table 7). Furthermore, we created additional tables, reporting both the studies not considered in our systematic review and the meta-analyses, assessing the association between HCV infection and risk of each human malignancy (see Tables 1-7). A summary of number of studies and meta-analyses is shown in Figure 2. Age-standardized incidence rates of each malignancy per 100000 person-years are reported for sex and for different countries in Figure 3[229].
The analysis revealed that most of available studies assessed the prevalence of this pathogen in patients with non-hodgkin-lymphomas (NHLs) and, in particular, in subjects with B-lymphocyte NHLs. However, the aim, design, inclusion criteria and definition of controls widely varied among the identified trials. In particular, available studies assessed the prevalence of HCV infection either in B-, T-, NK-, subtypes of NHLs or in B-cell NHLs alone or in NHLs together with different lymphoprolipherative disorders or in lymphoprolipherative malignancies other than NHLs.
When we examined the large series of these trials, evaluating NHLs, it was necessary to take into account some problems of comparability, such as: type of NHL classification; ASSAYS used to detect HCV infection; and aim of selected studies.
Depending on year of publication, different classifications for NHLs have been used, including: Working Formulation, REAL or WHO classifications. In addition, only in some articles a detailed description of nodal or extra-nodal NHLs has been performed as well as a few studies were identifiable as formal case-control, reporting an adequate description of control groups characteristics, such as matching factors (i.e., age, gender, birthplace and performance status), odds ratios adjusted for potential confounding factors. Furthermore, the sensitivity and specificity of diagnostic tests, used to detect antibodies anti-HCV or viral genome, largely differed among the trials considered in our systematic review (Table 1). In some studies the presence of anti-HCV antibodies was assayed by second-generation-, in others by third-generation-immune-enzymatic screening tests or by confirmatory tests, such as second-/third-generation-RIBA assays or by search of viral genome by means of different polymerase chain reaction (PCR)-based techniques. Furthermore, some hematologic malignancies, such as chronic lymphocytic leukemia have been classified into the category of NHLs in several trials, whereas they have been considered as entities distinct from NHLs in other reports. However, to date even if a large methodological heterogeneity exists among all these studies, the number of trials performed worldwide to assess the potential effect of HCV infection on the risk of NHLs is wide. Therefore, the available data, collected in peoples of various ethnicities as well as in populations of different geographical areas may provide a valid representation of the real situation in a large number of distinct countries. In particular, according to the results of available studies and meta-analyses, an association between HCV infection and B-lymphocyte NHLs development has emerged, with an assessed moderate risk for lymphoma development and odds ratios ranging between 2 and 3 on average. Nevertheless, this estimation differs largely, not only depending on the histological types considered but also on the geographical location and race of populations included in the different trials. An increased risk of NHLs has been described in studies performed in countries, where an elevated HCV prevalence is detectable, including Italy and Spain in Southern Europe, Japan and Taiwan in Asia as well as in Egypt and in southern United States areas. In these regions the percentage of HCV-associated NHLs can also reach a value equal to 10%. On the other hand, the association between HCV and NHLs development has not been confirmed in countries with low viral prevalence, such as countries of Northern Europe (United Kingdom, German, France, Denmark) or North America (Canada, Northern and United States regions). According to the results of a large European multicenter case-control-study as well as of available meta-analyses some subtypes of B-lymphocyte NHLs have resulted to be more frequently associated with diffuse large B-cell lymphoma (DLBCL) with an OR equal to 2.24, marginal zone lymphoma (MZL) with an OR equal to 2.47, and lymphoplasmocytic lymphoma (LPL) with an OR equal to 2.57[129]. In an additional large population-based trial in United States, an enhanced risk for follicular lymphoma (OR = 1.88), Burkitt’s lymphoma (OR = 5.21), DLBCL (OR = 1.52) and MZL (OR = 2.20) was reported[119]. However, other than above mentioned lymphoprolipherative diseases, no clear relationship has emerged, concerning HCV infection and haematological malignancies. In particular, even if some trials reported a higher prevalence of HCV in patients with Multiple Myeloma[36,76,133], this observation has not been confirmed in further reports[24,119,126]. In addition, no statistically significant association has been found between anti-HCV sero-positivity and Hodgkin Lymphoma risk. To date, no studies have evaluated whether some risk factors, such as smoking habit, alcohol use or diabetes may act in cooperation with this virus and increase the risk of lymphoprolipherative disorders. The results of our research, concerning the possible association between HCV infection and hematopoietic malignancies, studies not considered, as well as meta-analyses are summarised in Tables 1, 8 and 9. Figure 3A shows the age-standardized incidence rates of main haematopoietic malignancies per 100000 person-years.
| Studies (First author/Journal/Year of publication) | Study title | Main findings for exclusion | Study conclusion |
| Arcaini L Cancer 2004 | Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles | Duplicate | High HCV seroprevalence in patients with MZL |
| Catassi C Rec Prog Med 1998 | High prevalence of hepatitis C virus (HCV) infection in patients with non-Hodgkin lymphoma at the onset: preliminary results of a multicentre Italian study | Full-text in Italian | Possible causative role of the HCV in lymphomagenesis |
| Dal Maso L Haematologica 2004 | Hepatitis B and C viruses and Hodgkin lymphoma: a case-control study from northern and southern Italy | Evaluation of the association between HCV infection and HL risk | No role of HCV in the etiology of HL |
| de Sanjose S Int J Cancer 2004 | Role of hepatitis C virus infection in malignant lymphoma in Spain | Duplicate | HCV infection is associated with an increased risk of lymphoma in Spain |
| Domingo JM Med Clin (Barc) 2001 | Hepatitis C virus infection in patients with non Hodgkin's lymphoma | Full-text in Spanish | Possible association between HCV infection and NHLs |
| Ferri C JAMA 1994 | Non-Hodgkin’s lymphoma: possible role of hepatitis C virus | Duplicate | Possible role of HCV infection in B-cell NHLs development |
| Ferri C QJM 1996 | Chronic hepatitis C and B-cell non-Hodgkin's lymphoma | Duplicate | Possible role of HCV infection in B-cell NHLs development |
| Gasztonyi B Orv Hetil 2000 | Hepatitis C virus infection and B-cell non-Hodgkin's lymphoma | Full-text in Humgarian | HCV might have an aetiological role in the lymphoproliferation leading to B-cell NHL |
| Grudeva-Popova J BUON 2013 | Non-Hodgkin lymphomas and carrier state of viral hepatitis B and C | Incomplete data concerning the association between HCV infection and NHLs | Hepatitis virus carrier state did not alter significantly the clinical course and disease prognosis |
| Izumi T Leukemia 1997 | B cell malignancy and hepatitis C virus infection | Duplicate | Association between persistent HCV infection and the occurrence of B- cell malignancy |
| Montella M Liver 2001 | HCV and cancer: a case-control study in a high-endemic area | Duplicate | Expected increases not only in liver cancer, but also in tumors associated with the immune system |
| Sánchez Ruiz AC Med Clin (Barc) 2001 | Prevalence of hepatitis C virus infection in patients with non-Hodgkin's lymphoma | Full-text in Spanish | Higher prevalence of HCV in our B-NHL patients |
| First author/Country | Title | Number of studies considered | Main conclusion | Matching factors considered |
| Gisbert JP, 2003 | Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis | 23 | HCV prevalence in patients with B-NHL is approximately 15%, higher than that reported not only in general population (1.5%) but also in patients with other hematologic malignancies (2.9%), suggesting a role of HCV in the etiology of B-NHL | Age, sex, smoking, race, when available |
| Matsuo K, 2004 | Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies | 23 | Strongly positive association between anti-HCV seropositive test subjects and risk of NHL. Individualists with anti-HCV positive test have approximately five times higher risk of NHL. This association is consistent regardless of the endemic status of HCV, as well as subgroup analysis for B-/T-NHL | Age and sex, when available |
| Negri E, 2004 | B-cell non-Hodgkin’s lymphoma and hepatitis C virus infection: a systematic review | 15 | A high HCV prevalence in B-NHL was found in southern and eastern Europe, Japan and the southern United States, but not in central and northern Europe, Canada, northern United States, or a few Asian countries. The odds ratio of B-NHL for HCV infection was relatively weak, ranging from 2 to 4 in most studies. Thus, even if the observed association were causal, the percentage of cases of B-NHL attributable to HCV infection would be relatively low (10%) also in countries with a high prevalence of HCV infection in the general population, and extremely low in other countries | Age and sex, whether available |
| Dal Maso L, 2006 | Hepatitis C Virus and Risk of Lymphoma and Other Lymphoid Neoplasms: A Meta-analysis of Epidemiologic Studies | 18 | The pooled relative risks (RR) were consistently increased for all major B-NHL subtypes, T-NHL, and primary sites of NHL presentation. The etiologic fraction of NHL attributable to HCV varies greatly by country, and may be upward of 10% in areas,where HCV prevalence is high .Associations weaker than with NHL were found between HCV infection and Hodgkin’s lymphoma | Age and sex, when available |
| de Sanjose S, 2008 | Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium | 7 | The results of the present study confirm the association between HCV infection and NHL and specific B-NHL subtypes (diffuse large B-cell lymphoma, marginal zone lymphoma and lymphoplasmacytic lymphoma). This research has sufficient statistical power to confirm these associations in populations with low HCV prevalence | Age, sex, county of residence, study site, geographic area, when available |
| Libra M, 2010 | Extrahepatic disorders of HCV infection: A distinct entity of B-cell neoplasia? | 18 Review of Italian studies | The results of the study confirm the association between HCV infection and NHL and specific B-NHL subtypes. The higher prevalence of anti-HCV Abs was observed among lymphoplasmacytoid/lymphoplasmacytic/immunocytoma histotype whereas the lowest was among small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL). Overall, these studies strongly support the notion that HCV-associated lymphomas may be a distinct entity and further characterization of the mechanisms by which HCV infection contributes to B-cell NHL development may improve its diagnosis, classification and treatment | NR |
Colangiocarcinoma (CCAs) arises from the biliary tract and can be classified into two major types with respect to location: intrahepatic cholangiocarcinoma (ICCA) and extrahepatic cholangiocarcinoma (ECCA). The latter form of malignancy may be further divided into ductal or peri-hilar cancers. The second-order bile ducts and the cystic duct represent the point of distinction between ICCAs and peri-hilar CCAs as well as respectively. The importance of this distinct classification reflects differences in clinical presentation, natural history and treatment of intra- and extra-hepatic cholangiocarcinomas.
Overall, a large series of trials, have been performed in different populations and geographical areas. Some of these studies have been carried out both in areas with an elevated prevalence of cholangiocarcinoma, including China, Korea, Japan and Egypt, and in regions with low prevalence of this cancer, including Italy, United States and Denmark. Therefore, these reports may be considered as a rather representative estimation of the real epidemiological burden of this tumour worldwide. In our systematic review we identified 36 studies. The aim, design, inclusion criteria and definition of controls widely varied among the identified trials and some of these have not distinguished among ICCs from ECCs. In particular, 6 have been carried out in United States, 3 in China, 2 in Korea, 9 in Japan, 4 in Thailand, 3 in Italy, 1 in Egypt, 1 in Iran, 1 in Greece, although a low number of studies Northern Europe. However, from the identified studies HCV infection has emerged as risk for ICC, but not for ECC, in a large number of distinct countries worldwide, even if, in some regions in Southern-East Asia, other factors are potentially involved in CCAs development. In these areas, further infectious agents, such as Opisthorchis viverrini and Clonorchis sinensis, represent risk factors for CCAs. In available meta-analyses, the presence of HCV was associated with a statistically significant increased risk of ICC incidence, with an OR ranging from 3.42 (95%CI: 1.96-5.99) to 4.84 (95%CI: 2.41-9.71)[168,190]. The results of our review, concerning the possible association between HCV infection and CCA risk, studies not considered, as well as meta-analyses are summarised in Tables 2, 10 and 11. Age-standardized incidence rates of ICC malignancies per 100000 person-years is reported in Figure 3B.
| Studies (First author/Journal/Year of publication) | Study title | Main findings | Study conclusion |
| Choi D J Hepatology 2006 | Cholangiocarcinoma and Clonorchis sinesis infection: A case-control study in Korea | Assessment of Clonorchis sinensis role in the risk of developing CCA, including extrahepatic CCA | HCV infection detected in 1/51 (2%) patients in CCA group and 1/51 (2%) in control gorup |
| Jarnagin WR Cancer 2002 | Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors | No distinction between HBV/HCV infected patients | The demographic and clinical features of patients with combined tumors were most similar to those of patients with CC. Most important, combined tumors were not found to be associated with chronic liver disease |
| Liu X Zhonghua Wai Ke Za Zhi 2002 | Pathogenesis of hilar cholangiocarcinoma and infection of hepatitis virus | Full-text in Chinese | HCV-core protein may play an important role in the pathogenesis of hilar cholangiocarcinoma. HCV-infected patients with hilar cholangiocarcinoma infected may have a high grade malignancy and a poor prognosis |
| Lu H Chin Med J (Engl) 2000 | Detection of hepatitis C virus RNA sequences in cholangiocarcinomas in Chinese and American patients | Only 12 patients included | High rate of HCV-RNA detection in CCA cases, mainly in Chinese patients as compared to United States subjects |
| Shirakawa H Hokkaido Igaku Zasshi 1996 | Analysis of hepatitis C virus (HCV) genotypes in hepatocellular carcinoma | Full-text in Japanese | 2/11 HCV seropositive Japanese patients with cholangiocarcinoma |
| Tao LY Liver International 2009 | Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China | Shortage of information on HCV infection | No possible assessment of significant association between HCV infection and ICC or ECC |
| Yin F Chin Med J (Engl) 1998 | Detection of hepatitis C virus RNA sequences in hepatic portal cholangiocarcinoma tissue by reverse transcription polymerase chain reaction | Only 6 patients included | High rate of HCV-RNA detection in CCA cases |
| Zhang H Zhonghua Bing Li Xue Za Zhi 1996 | Detection of hepatitis B virus DNA and hepatitis C virus RNA in human hepatocellular carcinoma by polymerase chain reaction | Full-text in Chinese | HCV may play an important role in hepatic carcinogenesis because of its high positive rate |
| Zou SQ Zhonghua Wai Ke Za Zhi 2003 | The retrospective analysis of HBV and HCV infection in cholangiocarcinoma | Full-text in Chinese | The HCV infection is associated with hilar cholangiocarcinoma, in particular with the proximal bile duct. The hilar cholangiocarcinoma in HCV-infected patients presents higher malignant degree and a poor prognosis |
| First author | Title | Number of studies considered | Main conclusion | Matching factors considered |
| Shin HR, 2010 | Epidemiology of cholangiocarcinoma: An update focusing on risk factors | 11 | HCV infection is associated with an increased risk for CCA, but its possible role in development of this malignancy requires further investigation | NR |
| Palmer WC, 2012 | Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma | 8 | HCV infection is associated with an increased risk for intrahepatic cholangiocarcinoma | NR |
| Zhou Yanming, 2012 | Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis | 16 | HCV infection is associated with an increased risk of ICC | NR |
Until few years ago, although it was well-known that several viruses, including HCV, may infect pancreas and cause the acute inflammation of this organ[230], no studies had been specifically designed to investigate the possible role of HCV in the PDAC development. Different viral and host factors have contributed to make the study of the pancreas extremely hard, including the localization of this organ in retro-peritoneum, the small size of precursor malignant lesions, the difficulty to identify HCV antigens and/or genome in normal and/or cancerous pancreatic tissue specimens. Nevertheless, since 2008, this topic has gained a progressive interest, and an increasing number of case-control-, cohort-studies and meta-analyses with different design have been carried out with this purpose. To date the number of available studies, concerning the association between HCV and PAC risk, is still limited, but among these trials, some have suggested that HCV infection represents a risk factor for PDAC, whereas others have not confirmed this association[205-207]. Two trials have been carried out in Chinese population, 2 in United States, 2 in Australia and 1 in Sweden and 1 in Korea. Among these studies, the largest-sized one has been performed in United States. On the basis of these reports, up to now, three meta-analyses have been carried out. One of these reported no statistically significant relationship between anti-HCV positivity and PDAC risk because of the small number of studies available, although a borderline value was detected in this comparison (RR = 1.16 with 95%CI ranging from 0.99 to 1.3)[211], but the others found an increased risk of this malignancy in HCV-infected patients, in comparison to controls (RR = 1.21 with 95%CI ranging from 1.02 to 1.44 or RR = 1.26 with 95%CI ranging from 1.03 to 1.5)[204,212]. However, none of these studies, available to date, included the Swedish-, the Korean-, the Australian-reports as well as the last Chinese case-control research. The majority of these trials have been published only recently, in particular, between the end of 2013 and the beginning of 2014, therefore their results were not included in the described meta-analyses. They showed that HCV infection is associated with an increased risk to develop PDAC. The characteristics and number of studies included in the reported meta-analyses vary among the 3 identified meta-analyses, depending on the different selection criteria used by each author. Furthermore, although most studies included as matching factors smoking habit, alcohol use or diabetes, no research has assessed whether these variables may act in cooperation with HCV and increase the risk of PAC. The results of our review, concerning the possible association between HCV infection and PAC risk, studies not considered, as well as meta-analyses are summarised in Tables 3, 12 and 13. Age-standardized incidence rates of pancreatic cancer per 100000 person-years is shown in Figure 3C.
| Studies (First author/Journal/Year of publication) | Study title | Main findings | Study conclusion |
| (A) | |||
| Hong SG, 2010 Korean J Hepatol 2010; 16: 1 | The relationship between hepatitis B virus infection and the incidence of pancreatic cancer: a retrospective case-control study | Full-text in Korean | Absence of significant association between anti-HCV positivity and pancreatic cancer |
| Xu P, 2011 Cancer (Chinese J) 2011; 31: 653-657 (52) | Risk factors for pancreatic cancer: a case-control study | Full-text in Chinese | Absence of significant relationship between anti-HCV positivity and pancreatic cancer |
| (B) | |||
| Fang Zhu Asian Pacific J Cancer Prev 2011 | Chronic hepatitis virus infection and pancreatic cancer: a case-control study in southern China | No detailed description of number of patients with HCV-infection in case- and control group | Increased prevalence of anti-HCV antibodies in patients with pancreatic cancer |
| First author/Country | Title | Number of studies considered | Main conclusion | Adjustment for diabetes, alcohol, cigarette |
| Fiorino S, 2013 Italy | Association between hepatitis B or hepatitis C virus infection and risk of pancreatic adenocarcinoma development: a systematic review and meta-analysis | 3 studies available for assessment of HCV infection and PAC risk | No statistically significant relationship between anti-HCV positivity and PAC risk, although a borderline value was detected in this comparison (RR = 1.16 (95%CI: 0.99-1.3) | Y Y Y |
| Xing S, 2013 China | Chronic hepatitis virus infection increases the risk of pancreatic cancer: a meta-analysis | 7 studies included for assessment of HCV infection and PAC risk | Higher PAC risk in anti-HCV positive patients: RR = 1.21 (95%CI: 1.02-1.44) | Y Y Y |
| Xu JH, 2013 China | Hepatitis B or hepatitis C virus infection and risk of pancreatic cancer: a meta-analysis of observational studies | 5 studies available for assessment of HCV infection and PAC risk | Higher risk of pancreatic cancer in subjects with past-exposure to HCV: RR = 1.26 (95%CI: 1.03-1.5) | Y Y Y |
On the basis of the observation that the wide geographical differences in the age-standardized incidence of breast cancer[229] cannot be entirely explained by variations in known risk factors among countries, it was hypothesized, since 1997, that a relationship might exist between this malignancy and late exposure to a common virus[231]. It has been reported that some viruses may be involved in the occurrence of this malignancy[232], but, in particular, in 1999 an anecdotal report suggested, for the first time, the HCV might play a role in the development of some solid tumors other than liver, including breast cancer. Since then, some studies investigated the possible involvement of HCV in breast carcinogenesis. Among the 6 trials identified in our review, 5 were cohort- and 1 case-control-studies. Two of them were performed in Australia, 1 in United States, 1 in Denmark, 1 in Taiwan and 1 in France, respectively. Two studies, 1 case-control performed in Taiwan and 1 cohort trials in France were designed with the primary aim to assess the potential relationship between HCV and breast cancer risk, whereas the others were not. Only the research carried out in Taiwan found an association with early onset risk of breast cancer.
The results of our review, concerning the possible association between HCV infection and renal cancer risk as well as studies not considered are summarised in Tables 4 and 14. To date, no meta-analysis has been published on this topic. Age-standardized incidence rates of breast cancer per 100000 person-years is reported in Figure 3D.
| Studies (First author/Journal/Year of publication) | Study title | Main findings for exclusion | Study conclusion |
| Bruno G Clin Ter 1999 | Hepatitis C virus: a high risk factor for a second primary malignancy besides hepatocellular carcinoma. Fact or fiction? | Very small number of patients considered in the present study | HCV could have played an important role not only in the development of HCC but of the second primary malignancy |
| Malaguarnera M Eur J Int Medicine 2006 | Hepatitis C virus in elderly cancer patients | No available information concerning number of HCV positive patients with breast cancer | No higher prevalence of breast cancer in patients with HCV infection |
Although some authors have reported, several years ago, that HCV is associated with an higher probability to develop chronic end-stage renal diseases[233] or that a relationship may exist between this virus and a major risk of kidney cancer, as reported in some case reports[234,235] or in very small series of patients[236], only in the last 5 years, the interest for this topic has progressively increased and adequately powered studies have been carried out in different geographical areas worldwide. To date 4 cohort-, 1 case-control- and 2 case series with control groups studies available in literature, have been considered in our systematic review. Two among cohort-trials were performed in Australia, 1 in United States and 1 in Sweden, whereas the case-control trial in Italy. One case series study was carried out in United States[199] and one in Turkey[198]. The results of these reports are not univocal. In particular, only 1 of these cohort studies as well as 1 case series with control group research and case-control study have detected an association between HCV infection and risk of kidney malignancy[196,199,200], whereas the remaining articles did not. However, it has to be underlined that 2 of these cohort studies were designed to assess the incidence for all cancer types following HCV infection and not specifically the renal one [118,146]. In addition, a significant relationship between renal carcinoma and viral hepatitis has been described in a further research, but it has not been considered, because no data on the type viral hepatitis infection were reported[203]. The results of our review, concerning the possible association between HCV infection and renal cancer risk as well as studies not considered are summarised in Tables 5 and 15. To date, no meta-analysis has been published on this topic. Age-standardized incidence rates of renal cancer per 100000 person-years is reported in Figure 3E.
| Studies (First author/Journal/Year of publication) | Study title | Main findings for exclusion | Study conclusion |
| Macleod LC J Urol 2013 | Risk Factors for Renal Cell Carcinoma in the VITAL Study | No data on the type of viral hepatitis infection reported in VITAL study | A significant association of RCC with viral hepatitis |
| Bruno G Clin Ter 1999 | Hepatitis C virus: a high risk factor for a second primary malignancy besides hepatocellular carcinoma. Fact or fiction? | Very small number of patients considered in the present study | HCV could have played an important role not only in the development of HCC but of the second primary malignancy |
A relationship between HCV and oral squamous cell carcinoma (OSCC) was described, for the first time, in 1995[224] and then it was also reported in 1997, when a high prevalence of anti-HCV antibodies and of viral genome was showed in patients with head and neck squamous cell carcinomas[237]. Since then, cases of OSCC and verrucous carcinoma have been described in anti-HCV positive subjects with or without associated oral lichen planus (OLP)[238,239]. The prevalence of this inflammatory mucocutaneous condition varies largely among different geographical areas, with the highest rates observed in countries with HCV hyperendemia[240]. According to one study performed in 1557 patients with OLP, a more elevated HCV prevalence was observed in individuals, suffering from this disease, than that in the control group (1.9%, 0.4% respectively, P < 0.001)[241]. The association between HCV infection and OLP has been recently confirmed by three independent meta-analyses and it emerged across throughout the world. However, this relationship was most frequently detected in East- and South-East Asia as well as in South American- and Mediterranean-regions[242-244]. HCV might be involved in this type of process[245]. It has been suggested that OLP is a precancerous lesion, although the degree of risk of this disease for development of oral cancer is controversial[225,239]. Additional studies have shown a significant higher prevalence of HCV infection in patients with squamous cell carcinoma of head and neck (SCCHN) than that described in controls[225], whereas others did not[227]. To date, 10 studies, concerning relationship between HCV infection and oral/skin cancers have been published. Our findings, concerning the possible association between HCV infection and oral/skin cancer risk as well as studies not considered are summarised in Tables 6 and 16. To date, no meta-analysis has been published on this topic. Age-standardized incidence rates of oral-cancer per 100000 person-years is reported in Figure 3F.
| Studies (First author/Journal/Year ofpublication) | Study title | Main findings for exclusion | Study conclusion |
| Hunt J Laringoscope 2005 | Outcome Analysis of Patients with Squamous Cell Carcinoma of the Head and Neck and Hepatitis C Virus | Duplicate | HCV positive with SCCHN patients have not a worse outcome than their HCV negative counterparts |
The first description of an association between HCV and risk of thyroid cancer development dates back to 1999, when Antonelli et al[246] reported a high prevalence (2.2%) of thyroid cancer in a series of 139 patients with chronic hepatitis C infection in comparison to no case among 835 control subjects, who were long-term residents of an iodine-deficient area[246]. Since then some case-control studies have been carried out to assess this finding[73,74,215-217,247]. These trials have been performed in Italy and all have confirmed the previous above-mentioned assumption, although, some of these represent duplicates[73,74,215-217,247]. Afterwards, three cohort studies have been published to assess this subject in the last years, but no evidence supporting an association between HCV infection and thyroid cancer development has emerged from these reports. Two of these trials were designed to assess the incidence for all cancer types following HCV infection and not specifically the thyroid one[118,146]. Our findings, concerning the possible association between HCV infection and renal cancer risk as well as studies not considered are summarised in Tables 7 and 17. To date, no meta-analysis has been published on this topic. Age-standardized incidence rates of thyroid cancer per 100000 person-years is reported in Figure 3G.
| Studies (First author/Journal/Year of publication) | Study title | Main findings for exclusion | Study conclusion |
| Antonelli A JAMA 1999 | Thyroid cancer in patients with hepatitis C infection | Duplicate | Higher prevalence of thyroid cancer in a series of patients with chronic hepatitis C infection in comparison to no case in controls |
| Montella M Int J Cancer 2000 | Is HCV infection associated with thyroid cancer? A case-control study | Duplicate | Possible oncogenetic role of HCV for thyroid cancer, possible association more detectable in countries with a high prevalence of HCV |
| Montella M Liver 2001 | HCV and cancer: a case-control study in a high-endemic area | Duplicate | Expected increases not only in liver cancer, but also in tumors associated with the immune system |
| Malaguarnera M Eur J Int Medicine 2006 | HCV in elderly cancer patients | No available information concerning number of HCV positive patients with thyroid cancer | No higher prevalence of thyroid cancer in patients with HCV infection |
To our knowledge, this is the first study aimed to review systematically the prevalence of HCV infection in a wide spectrum of human malignancies, to summarise the retrieved data and to discuss the possible role of this pathogen in the genesis of the discussed tumours. Since several years the possible involvement of different viruses in human carcinogenesis has been reported, with increasing frequency, in a large series of epidemiological studies. Recently, the International Agency for Research on Cancer (IARC) has comprehensively assessed and confirmed the human carcinogenicity of 7 viral agents, including HCV. In particular, it has been recognised that HCV acts as an indirect carcinogen, by promoting and maintaining a state of chronic inflammation in infected sites[248]. This event is now a well-known condition that is involved in the process of hepatic carcinogenesis. However, even if liver is the main target of HCV, its tropism for this organ is not exclusive. In particular, viral antigens and genomes have been also detected in extra-hepatic tissues[249]. All these evidences have contributed to consider the possible role of this pathogen as a pro-cancerous agent also in organs other from liver. On the basis of this assumption, as it has been observed for the strong relationship between HCV and hepatocellular carcinoma (HCC) development, it is conceivable to think that a similar ecological correlation might exist between HCV infection and a major risk of some extra-hepatic cancers in regions where the prevalence of this pathogen is high in comparison with geographical areas with a lower one. However, to date, the possibility that this virus may be involved in the carcinogenesis of organs other than liver has been systematically investigated only for a very limited number of malignancies. In particular, available studies have been mainly focused on hematopoietic malignancies and on cholangiocarcinomas, also on the basis of some epidemiological observations, reporting a major risk of mixed cryoglobulinemia and monoclonal gammopathy in HCV positive patients[250] as well as of a higher incidence of cholangiocarcinoma and other types of human cancer other than liver in subjects with cirrhosis, irrespective of etiology[251]. In this study, enrolled patients suffered from alcoholic-, primary biliary-, and chronic-hepatitis-related cirrhosis, whereas in a group of patients the causes of this pathological condition were non-specified. Unfortunately, no detailed information was available, concerning the HCV status in the cohort of subjects with persistent viral hepatitis. Even if this observation was very interesting, it has not stimulated the achievement of studies investigating specifically the possible association between hepatitis viruses (in particular HCV) and human cancers other than HCC. Only in the last years this idea has gained interest an increasing number of trials have been designed and carried out with the purpose. Nevertheless, to date, few data are available yet and no final or univocal conclusions may be drawn. However, putting together the results of published studies on the possible association between HCV and risk of NHLs, cholangio-, pancreatic-, breast-, renal-, skin/oral- and thyroid-cancers, the reports concerning the estimates of prevalence of HCV infection worldwide as well as the tables on the age-standardized incidence rates of the mentioned malignancies per 100000 person-years in different geographical areas, some interesting consideration may be made (Figures 2 and 3). Actually, a clear correlation between regions of HCV prevalence and risk of extra-liver cancers has emerged only for a very small group of types and histological subtypes of malignancies. In particular, HCV infection has resulted to be associated with: (1) a higher incidence of some B-cell NHLs types, in countries, where an elevated prevalence of this pathogen is detectable, accounting to a percentage of about 10%. Furthermore, an additional factor potentially confirming the causal role between HCV and lymphomas, in particular B-cell NHLs, is represented by the observation of the regression of a significant percentage of low-grade B-cell NHLs after HCV eradication by means of an efficacious antiviral treatment. Early evidences, concerning this assumption, date back to the end of Twentieth Century with anecdotal reports and the beginning of the following Century, when further studies, enrolling a wider number of patients, were carried out for the first time[252]; and (2) an increased risk of intra-hepatic cholangiocarcinoma development in subjects with anti-HCV positivity, as reported by a large number of available studies with OR ranging from 3.42 to 4.84. According to an epidemiological view-point, it has to be considered the following evidence: liver flukes are associated with an increased risk both for ICC and ECC, but in endemic-nations (such as Taiwan, Singapore and Korea) and in other Eastern areas with low rates of this type of infection, the incidence of ICC is more elevated in comparison with ECC. On the other hand, in the western areas ECC incidence is higher than that of ICC. Taking into account HCV epidemiology, the prevalence of this virus presents a wide variability worldwide. More elevated percentages are detectable in developing areas, such as in South-Eastern-Asia and Egypt, intermediate ones in Southern Europe and the lowest ones in Northern Europe and America. Therefore, a correlation between HCV prevalence and ICC incidence seems to emerge from these observations.
Concerning the possible association between the infection caused by this hepato-tropic virus, surprisingly, although a higher risk of PADC in HCV-positive patients has been observed in some trials and it has been reported in the available meta-analyses, age-standardized rates of this cancer present interesting geographical variations. In particular, PADC incidence worldwide is 3-4 times higher in more economically developed countries as well as in northern area of the world, where HCV prevalence is lower in comparison with less developed countries. Different reasons may explain these results, including the accuracy of diagnostic methods used to diagnose pancreatic malignancies in distinct geographical regions worldwide and of data to assess the rates of incidence of PDAC. However, it is well-known that on the basis of a morphogenetic viewpoint, pancreas and liver share several characteristics in their embryological development, arising from common multi-potent cells of endoderm origin. Therefore, HCV might replicate also in pancreatic cells as it does in hepatocytes[253,254]. Furthermore, according to epidemiological studies, type 2 diabetes represents a risk factor for PAC[255,256] with chronic hyperglycemia and hyperinsulinemia as proposed pathogenetic mechanisms involved in the promotion of this type of malignancy. Both conditions may induce proliferation, decrease apoptosis and promote invasion ability of pancreatic cancer cells[257-259]. A recent and interesting systematic review and dose-response meta-analysis, assessing blood glucose concentration and risk of pancreatic cancer, has shown that every 0.56 mmol/L (10 mg/dL) increase in fasting blood glucose , a 14% enhancement in the rate of PADC occurs[260]. On the other hand, it is now accepted that HCV infection is associated with an increased risk of type 2 diabetes[261,262]. Therefore, HCV infection, promoting and diabetes might act in cooperation with hyperinsulinemia, hyperglycemia in the promotion of PAC. This consideration may contribute to explain the epidemiological evidence of a more elevated incidence of this cancer in the most economically developed countries, where high rates of obesity, metabolic syndrome and diabetes are observed, in comparison with less developed regions in the world.
On the other hand, up to now no definitive and univocal conclusions may be obtained from the analysis of relationship between HCV and breast-, renal-, skin/oral- and thyroid-cancers. Most of the available studies, in particular a large part of cohort trials, have not confirmed the existence of these associations. However, the lack of similar evidences may be only apparent and some elements potentially limiting the conclusions of these reports have to be taken into account. In particular, most of available data have been obtained from some retrospective cohort-trials. Although the large size and the lengthy follow-up of this type of research provides the statistical power to obtain adequate information on cancer risk in the investigated population, the retrospective nature of these studies has to be considered. The use of routinely collected administrative data, based on population registries may represent a potential limiting factor in these trials. Possible errors in diagnosis, in codes of cancers or infective diseases and in reported data may affect the hospitalization records in some case/control subjects as well as the possibility that in several countries worldwide only a part of general population is included in national cancer registry may influences the obtained results. In addition, some of these trials have evaluated the incidence of a wide spectrum of malignancies and they have been not designed to assess a specific type of human tumors. It has to be also underlined that, according to age-standardized incidence rates of these neoplasms, remarkable temporal changes in human cancer trends have been observed worldwide. The combined analysis of these figures, as performed some years ago for HCV-related liver cancer, has induced some authors to hypothesize a possible role of HCV in the development of these malignancies. For example, in Japan, Tanaka et al have examined temporal trends for HCC incidence rates in a period ranging from 1981 and 2003 in Osaka Prefecture. They provided an explanation in the context of HCV infection rates. According to these findings, they noted that in that span of time the incidence peak of HCC was detectable in men during their 50s, 60s and 70s of age in 1986, 1995 and 2000 and then it was progressively decreasing. He postulated that the observed trend was due to the restriction of virus transmission[263]. As previously suggested, it should be taken into account that an enhanced incidence of ICC has been observed in the most developed regions in the years, ranging from 1980 to 2000, has been considered as caused by the parallel increase of HCV infection in these areas. This hypothesis has been definitively shown in United States[264]. In addition, the relationship between HCV and risk of ICC has been assessed in patients with different degree of hepatic damage. It has been demonstrated that the probability of ICC development increases as the hepatic damage impairs[167]. Furthermore, a previous Italian research had suggested that HCV might have a role in thyroid cancer. This represents a rather rare malignancy, but its standardized incidence ratio has progressively increased between the end of ‘80s and the beginning of ‘90s in several well-developed countries, including Italy. In this country, in the same period of time an increasing prevalence of HCV infection was observed[247]. Unfortunately, no additional trials have been performed, with the purpose to assess the possible impact of HCV infection worldwide on the age-standardized incidence rates of the aforementioned malignancies in different geographical areas and to distinguish the potential contribution of this pathogen as pro-carcinogenic agent from other risk factors. Therefore this field of research still remains largely unexplored. In addition, it has to be taken into account that HCV represents a common cause of cirrhosis development and this condition itself is a well-known independent risk factor for the development of a wide spectrum of human malignancies different from liver. Several mechanisms may be responsible for carcinogenetic role of this pathogen. It may act indirectly as well as directly. Both these activities have been proposed for this virus and plausible evidences reported in literature. To date the existence of an indirect action of this microorganism is the most convincing pathogenic mechanism. In particular, as widely reported for liver, HCV might promote in extra-hepatic organs a persistent inflammation and induce the cancerous transformation, as a consequence of a progressive rearrangement of their structure. This event might play a role in the development of all the types of the aforementioned malignancies and not only involved in hepatic carcinogenesis. This process is characterized by the interaction and the cooperation among viral-related proteins, homing-cells (specific-tissue-cells, depending on the considered organ, endothelial cells, and stem cells), not homing cells (lymphomononuclear and polymorfonuclear specific and nonspecific cells), cytokines, costimulatory molecules and additional biological mediators (i.e., PGs and oxidants). This situation promotes a self-maintaining and amplifying loop, in which HCV stimulate, in turn, PGE2, enzymes (such as cyclo-oxygenase-2 or COX-2), growth factors, interleukins production and cellular signaling pathway function. The complex cooperation and interaction among these mediators is one of the main factors responsible for final outcome: viral control with recovery or its persistence in the infected-organs, with progressive development of a tissue necro-inflammatory process, potentially evolving toward malignant transformation. Presence of inflammation favours genetic instability in cells and increases the probability which further genetic and epigenetic alterations arise. Perturbance of homeostasis in adult-cells may re-modulate activity and expression of genes as well as of transcription factors that govern their regeneration and/or differentiation programs as well as the processes involved in energy production. In addition, alteration of function of some intracellular pathways, such as K-RAS, Hedgehog-, Jak-STAT-, Notch-and TGF-β signalling-cascades, may also play a key role in carcinogenesis. In particular, this events may be induced not only by inflammation itself, but also by the direct action of some viral proteins such as: core- and NS5A on the intracellular cascades pathways function. These viral elements may affect the levels of activities of the afore-mentioned signalling-cascades and contribute to deregulation of cell-cycle checkpoint controls. To date a few studies have been performed with the aim to specifically investigate the pathogenetic mechanisms, potentially involved in the development of extra-liver malignancies in anti-HCV positive patients. The majority of reports upon this topic concerns lymphoproliferative malignancies. A study has described an increased expression of genes associated with lymphomagenesis in peripheral blood B cells of chronic HCV positive patients[265].
Furthermore, it has been shown that the telomere deletion of 1p36.3 in B-cell NHLs is significantly more frequent in patients with HCV infection in comparison with anti-HCV negative individuals[266]. Deletion of the 1p36 genomic locus is associated with the loss of p73, a tumour suppressor gene, that may be inactivated both in lymphomas and in other human cancers[267,268]. On the basis of available studies, this topic is progressively acquiring an increasing importance, but to date only some definitive conclusions have been obtained, while a large number of questions still remain unanswered. Further well-designed trials, enrolling an adequate number of patients as well as focusing on populations of different geographical areas and involving larger series of patients are required to confirm or deny this association as well as to identify the pathogenetic mechanisms, potentially involved in HCV-associated human carcinogenesis.
The authors thank Dr. Simonetta Righi, Biblioteca Centralizzata, Policlinico S Orsola-Malpighi, Università di Bologna, Bologna, Italy, for her support in the search of scientific bibliography and Mr. Fabio Castellini, Dipartimento di Emergenza-Urgenza, Ospedale di Budrio, Budrio, Bologna, Italy, for his active support in clinical research.
Hepatitis C virus (HCV) is an oncogenic virus and a well-known risk factor for hepatocellular carcinoma. In the last years, some studies have shown that its antigens and replicative sequences are detectable also in organs other than liver. However, the significance of this feature is uncertain. Some reports and meta-analyses suggested that its infection is associated with development of cholangiocarcinoma and some types of lymphomas, but a comprehensive assessment of the possible role of HCV in extrahepatic carcinogenesis has not been yet performed.
Actually, a clear correlation between regions of HCV prevalence and risk of extra-liver cancers has emerged only for a very small group of types and histological subtypes of malignancies. In particular, HCV infection has resulted to be associated with a higher incidence of: (1) some B-cell NHLs types, in countries, where an elevated prevalence of this pathogen is detectable, accounting to a percentage of about 10%; (2) intra-hepatic-, but not extrahepatic-cholangiocarcinoma; and (3) pancreatic cancer development. No definitive and univocal conclusions may be obtained from the analysis of relationship between HCV and breast-, renal-, skin/oral- and thyroid-cancers, although a possible association between renal-, skin/oral- and thyroid-malignancies and HCV infection has been suggested by some studies. These results strongly supports the need of additional studies with large sample size to ensure a precise estimate of the effect of HCV on these different types of cancers to improve our knowledge on carcinogenetic potential of HCV for extra-hepatic organs and on possible pathogenetic mechanisms. Few published studies available on the association of HCV and some types of human malignancies, such as breast, kidney, oral/skin and thyroid cancers and mainly enrolling populations of Asian ethnicity. Substantial variation by different geographical areas in serum prevalence of HCV antibodies and genotypes.
This paper is very interesting because it clarifies a neglected aspect of HCV infection: the role of virus in pathogenesis of extrahepatic neoplasms. The data analysis is very accurate as well as the description of pathophysiological mechanisms of HCV-mediated cancerogenesis.
P- Reviewer: Xu R, Zocco MA S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1325] [Cited by in F6Publishing: 1311] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 2. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. [PubMed] [Cited in This Article: ] |
| 3. | Younossi ZM, Kanwal F, Saab S, Brown KA, El-Serag HB, Kim WR, Ahmed A, Kugelmas M, Gordon SC. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39:518-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204-2212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 50] [Reference Citation Analysis (0)] |
| 5. | Mariotto S, Ferrari S, Monaco S. HCV-related central and peripheral nervous system demyelinating disorders. Inflamm Allergy Drug Targets. 2014;13:299-304. [PubMed] [Cited in This Article: ] |
| 6. | Pawlotsky JM, Roudot-Thoraval F, Simmonds P, Mellor J, Ben Yahia MB, André C, Voisin MC, Intrator L, Zafrani ES, Duval J. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med. 1995;122:169-173. [PubMed] [Cited in This Article: ] |
| 7. | Ko HM, Hernandez-Prera JC, Zhu H, Dikman SH, Sidhu HK, Ward SC, Thung SN. Morphologic features of extrahepatic manifestations of hepatitis C virus infection. Clin Dev Immunol. 2012;2012:740138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Crovatto M, Pozzato G, Zorat F, Pussini E, Nascimben F, Baracetti S, Grando MG, Mazzaro C, Reitano M, Modolo ML. Peripheral blood neutrophils from hepatitis C virus-infected patients are replication sites of the virus. Haematologica. 2000;85:356-361. [PubMed] [Cited in This Article: ] |
| 9. | Zignego AL, Macchia D, Monti M, Thiers V, Mazzetti M, Foschi M, Maggi E, Romagnani S, Gentilini P, Bréchot C. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382-386. [PubMed] [Cited in This Article: ] |
| 10. | Sansonno D, Lauletta G, Montrone M, Grandaliano G, Schena FP, Dammacco F. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol. 2005;140:498-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Arrieta JJ, Rodriguez-Inigo E, Casqueiro M, Bartolomé J, Manzarbeitia F, Herrero M, Pardo M, Carreno V. Detection of hepatitis C virus replication by In situ hybridization in epithelial cells of anti-hepatitis C virus-positive patients with and without oral lichen planus. Hepatology. 2000;32:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Kurokawa M, Hidaka T, Sasaki H, Nishikata I, Morishita K, Setoyama M. Analysis of hepatitis C virus (HCV) RNA in the lesions of lichen planus in patients with chronic hepatitis C: detection of anti-genomic- as well as genomic-strand HCV RNAs in lichen planus lesions. J Dermatol Sci. 2003;32:65-70. [PubMed] [Cited in This Article: ] |
| 13. | Carrozzo M, Quadri R, Latorre P, Pentenero M, Paganin S, Bertolusso G, Gandolfo S, Negro F. Molecular evidence that the hepatitis C virus replicates in the oral mucosa. J Hepatol. 2002;37:364-369. [PubMed] [Cited in This Article: ] |
| 14. | Toussirot E, Le Huédé G, Mougin C, Balblanc JC, Bettinger D, Wendling D. Presence of hepatitis C virus RNA in the salivary glands of patients with Sjögren’s syndrome and hepatitis C virus infection. J Rheumatol. 2002;29:2382-2385. [PubMed] [Cited in This Article: ] |
| 15. | De Vita S, De Re V, Sansonno D, Sorrentino D, Corte RL, Pivetta B, Gasparotto D, Racanelli V, Marzotto A, Labombarda A. Gastric mucosa as an additional extrahepatic localization of hepatitis C virus: viral detection in gastric low-grade lymphoma associated with autoimmune disease and in chronic gastritis. Hepatology. 2000;31:182-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, Yang DL, Hao LJ. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805-811. [PubMed] [Cited in This Article: ] |
| 17. | Akdoğan M, Mert A, Tabak F, Ӧzdemir S, Sonsuz A, Sentŭrk H. Hepatitis C infection in non-Hodgkin’s lymphoma. Turk J Gastroenterol. 1998;1:73-75. [Cited in This Article: ] |
| 18. | Arcaini L, Paulli M, Boveri E, Vallisa D, Bernuzzi P, Orlandi E, Incardona P, Brusamolino E, Passamonti F, Burcheri S. Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles. Cancer. 2004;100:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Arcaini L, Varettoni M, Boveri E, Orlandi E, Rattotti S, Zibellini S, Merli M, Lucioni M, Rizzi S, Gotti M. Distinctive clinical and histological features of Waldenström’s macroglobulinemia and splenic marginal zone lymphoma. Clin Lymphoma Myeloma Leuk. 2011;11:103-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Arican A, Sengezer T, Bozdayi M, Bozkaya H, Uçgül E, Dinçol D, Uzunalimoğlu O. Prevalence of hepatitis-G virus and hepatitis-C virus infection in patients with non-Hodgkin’s lymphoma. Med Oncol. 2000;17:123-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Avilés A, Valdez L, Halabe J, Neri N, Nellen H, Huerta-Guzmán J, Nambo MJ. No association between lymphoma and hepatitis C virus. Med Oncol. 2003;20:165-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Bauduer F, Katsahian S, Blanchard Y, Oui B, Capdupuy C, Renoux M. Descriptive epidemiology of non-Hodgkin lymphomas in a southwestern French hematology center: absence of significant relationship with hepatitis C virus infection. Hematol Cell Ther. 1999;41:191-193. [PubMed] [Cited in This Article: ] |
| 23. | Besson C, Canioni D, Lepage E, Pol S, Morel P, Lederlin P, Van Hoof A, Tilly H, Gaulard P, Coiffier B. Characteristics and outcome of diffuse large B-cell lymphoma in hepatitis C virus-positive patients in LNH 93 and LNH 98 Groupe d’Etude des Lymphomes de l’Adulte programs. J Clin Oncol. 2006;24:953-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Bianco E, Marcucci F, Mele A, Musto P, Cotichini R, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Specchia G. Prevalence of hepatitis C virus infection in lymphoproliferative diseases other than B-cell non-Hodgkin’s lymphoma, and in myeloproliferative diseases: an Italian Multi-Center case-control study. Haematologica. 2004;89:70-76. [PubMed] [Cited in This Article: ] |
| 25. | Boffetta P, Armstrong B, Linet M, Kasten C, Cozen W, Hartge P. Consortia in cancer epidemiology: lessons from InterLymph. Cancer Epidemiol Biomarkers Prev. 2007;16:197-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 251] [Reference Citation Analysis (0)] |
| 26. | Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, Rousselet MC, Dharancy S, Gaulard P, Flejou JF. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. 2003;37:781-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Catassi C, Fabiani E, Coppa GV, Gabrielli A, Centurioni R, Leoni P, Barbato M, Viola F, Martelli M, De Renzo A. [High prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma at the onset. Preliminary results of an Italian multicenter study]. Recenti Prog Med. 1998;89:63-67. [PubMed] [Cited in This Article: ] |
| 28. | Chindamo MC, Spector N, Segadas JA, Pimenta G, Vanderborght B, Morais JC, Milito C, Moraes Coelho HS. Prevalence of hepatitis C infection in patients with non-Hodgkin’s lymphomas. Oncol Rep. 2002;9:657-659. [PubMed] [Cited in This Article: ] |
| 29. | Chuang SS, Liao YL, Chang ST, Hsieh YC, Kuo SY, Lu CL, Hwang WS, Lin IH, Tsao CJ, Huang WT. Hepatitis C virus infection is significantly associated with malignant lymphoma in Taiwan, particularly with nodal and splenic marginal zone lymphomas. J Clin Pathol. 2010;63:595-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Ohmuro H, Tomino Y, Tsushima Y, Shimizu M, Kuramoto T, Koide H. Elevation of serum IgA1 levels in patients with diabetic nephropathy. Nephron. 1993;63:355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Collier JD, Zanke B, Moore M, Kessler G, Krajden M, Shepherd F, Heathcote J. No association between hepatitis C and B-cell lymphoma. Hepatology. 1999;29:1259-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Cowgill KD, Loffredo CA, Eissa SA, Mokhtar N, Abdel-Hamid M, Fahmy A, Strickland GT. Case-control study of non-Hodgkin’s lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol. 2004;33:1034-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Cucuianu A, Patiu M, Duma M, Basarab C, Soritau O, Bojan A, Vasilache A, Mates M, Petrov L. Hepatitis B and C virus infection in Romanian non-Hodgkin’s lymphoma patients. Br J Haematol. 1999;107:353-356. [PubMed] [Cited in This Article: ] |
| 34. | Dal Maso L, Talamini R, Montella M, Crovatto M, Franceschi S. Hepatitis B and C viruses and Hodgkin lymphoma: a case-control study from Northern and Southern Italy. Haematologica. 2004;89:ELT17. [PubMed] [Cited in This Article: ] |
| 35. | De Renzo A, Persico E, de Marino F, di Giacomo Russo G, Notaro R, di Grazia C, Picardi M, Santoro L, Torella R, Rotoli B. High prevalence of hepatitis G virus infection in Hodgkin’s disease and B-cell lymphoproliferative disorders: absence of correlation with hepatitis C virus infection. Haematologica. 2002;87:714-718; discussion 718. [PubMed] [Cited in This Article: ] |
| 36. | De Rosa G, Gobbo ML, De Renzo A, Notaro R, Garofalo S, Grimaldi M, Apuzzo A, Chiurazzi F, Picardi M, Matarazzo M. High prevalence of hepatitis C virus infection in patients with B-cell lymphoproliferative disorders in Italy. Am J Hematol. 1997;55:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 37. | de Sanjose S, Nieters A, Goedert JJ, Domingo-Domenech E, Fernandez de Sevilla A, Bosch R, Herrera P, Domingo A, Petit J, Bosch X. Role of hepatitis C virus infection in malignant lymphoma in Spain. Int J Cancer. 2004;111:81-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | De Vita S, Zagonel V, Russo A, Rupolo M, Cannizzaro R, Chiara G, Boiocchi M, Carbone A, Franceschi S. Hepatitis C virus, non-Hodgkin’s lymphomas and hepatocellular carcinoma. Br J Cancer. 1998;77:2032-2035. [PubMed] [Cited in This Article: ] |
| 39. | Domingo JM, Romero MS, Palomera L, Gutiérrez M. [Hepatitis C virus infection in patients with non Hodgkin’s lymphoma]. Med Clin (Barc). 2001;117:638. [PubMed] [Cited in This Article: ] |
| 40. | Duberg AS, Nordström M, Törner A, Reichard O, Strauss R, Janzon R, Bäck E, Ekdahl K. Non-Hodgkin’s lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology. 2005;41:652-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Ellenrieder V, Weidenbach H, Frickhofen N, Michel D, Prümmer O, Klatt S, Bernas O, Mertens T, Adler G, Beckh K. HCV and HGV in B-cell non-Hodgkin’s lymphoma. J Hepatol. 1998;28:34-39. [PubMed] [Cited in This Article: ] |
| 42. | El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 43. | Engels EA, Chatterjee N, Cerhan JR, Davis S, Cozen W, Severson RK, Whitby D, Colt JS, Hartge P. Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. Int J Cancer. 2004;111:76-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longombardo G, Lombardini F, Greco F, Capochiani E, Mazzoni A. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol. 1994;88:392-394. [PubMed] [Cited in This Article: ] |
| 45. | Ferri C, La Civita L, Caracciolo F, Zignego AL. Non-Hodgkin’s lymphoma: possible role of hepatitis C virus. JAMA. 1994;272:355-356. [PubMed] [Cited in This Article: ] |
| 46. | Franceschi S, Lise M, Trépo C, Berthillon P, Chuang SC, Nieters A, Travis RC, Vermeulen R, Overvad K, Tjønneland A. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol Biomarkers Prev. 2011;20:208-214. [PubMed] [Cited in This Article: ] |
| 47. | Gasparotto D, De Re V, Boiocchi M. Hepatitis C virus, B-cell proliferation and lymphomas. Leuk Lymphoma. 2002;43:747-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Gasztonyi B, Pár A, Szomor A, Battyány I, Nagy A, Kereskai L, Losonczy H, Mózsik G. Hepatitis C virus infection associated with B-cell non-Hodgkin’s lymphoma in Hungarian patients. Br J Haematol. 2000;110:497-498. [PubMed] [Cited in This Article: ] |
| 49. | Gentile G, Mele A, Monarco B, Vitale A, Pulsoni A, Visani G, Castelli G, Rapicetta M, Verani P, Martino P. Hepatitis B and C viruses, human T-cell lymphotropic virus types I and II, and leukemias: a case-control study. The Italian Leukemia Study Group. Cancer Epidemiol Biomarkers Prev. 1996;5:227-230. [PubMed] [Cited in This Article: ] |
| 50. | Genvresse I, Späth-Schwalbe E, Meisel H, Kaufmann O, Krüger DH, Possinger K. Primary hepatic or splenic diffuse large B-cell lymphoma and hepatitis C virus infection: a non-fortuitous association? Ann Hematol. 2000;79:530-532. [PubMed] [Cited in This Article: ] |
| 51. | Germanidis G, Haioun C, Pourquier J, Gaulard P, Pawlotsky JM, Dhumeaux D, Reyes F. Hepatitis C virus infection in patients with overt B-cell non-Hodgkin’s lymphoma in a French center. Blood. 1999;93:1778-1779. [PubMed] [Cited in This Article: ] |
| 52. | Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, Engels EA. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 53. | Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125:1723-1732. [PubMed] [Cited in This Article: ] |
| 54. | Goldman L, Ezzat S, Mokhtar N, Abdel-Hamid A, Fowler N, Gouda I, Eissa SA, Abdel-Hamid M, Loffredo CA. Viral and non-viral risk factors for non-Hodgkin’s lymphoma in Egypt: heterogeneity by histological and immunological subtypes. Cancer Causes Control. 2009;20:981-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Grudeva-Popova J, Nenova I, Mateva N, Ananoshtev N, Popov V, Atanasova M. Non-Hodgkin lymphomas and carrier state of viral hepatitis B and C. J BUON. 2000;18:239-244. [PubMed] [Cited in This Article: ] |
| 56. | Guida M, D’Elia G, Benvestito S, Casamassima A, Micelli G, Quaranta M, Moschetta R, De Lena M, Lorusso V. Hepatitis C virus infection in patients with B-cell lymphoproliferative disorders. Leukemia. 2002;16:2162-2163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Hanley J, Jarvis L, Simmonds P, Parker A, Ludlam C. HCV and non-Hodgkin lymphoma. Lancet. 1996;347:1339. [PubMed] [Cited in This Article: ] |
| 58. | Harakati MS, Abualkhair OA, Al-Knawy BA. Hepatitis C Virus infection in Saudi Arab patients with B-cell non-Hodgkin’s lymphoma. Saudi Med J. 2000;21:755-758. [PubMed] [Cited in This Article: ] |
| 59. | Hausfater P, Cacoub P, Sterkers Y, Thibault V, Amoura Z, Nguyen L, Ghillani P, Leblond V, Piette JC. Hepatitis C virus infection and lymphoproliferative diseases: prospective study on 1,576 patients in France. Am J Hematol. 2001;67:168-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Imai Y, Ohsawa M, Tanaka H, Tamura S, Sugawara H, Kuyama J, Fukuda K, Yonezawa T, Matsuzawa Y. High prevalence of HCV infection in patients with B-cell non-Hodgkin’s lymphoma: comparison with birth cohort- and sex-matched blood donors in a Japanese population. Hepatology. 2002;35:974-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Iwata H, Matsuo K, Takeuchi K, Kishi Y, Murashige N, Kami M. High incidences of malignant lymphoma in patients infected with hepatitis B or hepatitis C virus. Haematologica. 2004;89:368-370. [PubMed] [Cited in This Article: ] |
| 62. | Izumi T, Sasaki R, Miura Y, Okamoto H. Primary hepatosplenic lymphoma: association with hepatitis C virus infection. Blood. 1996;87:5380-5381. [PubMed] [Cited in This Article: ] |
| 63. | Izumi T, Sasaki R, Tsunoda S, Akutsu M, Okamoto H, Miura Y. B cell malignancy and hepatitis C virus infection. Leukemia. 1997;11 Suppl 3:516-518. [PubMed] [Cited in This Article: ] |
| 64. | Karavattathayyil SJ, Kalkeri G, Liu HJ, Gaglio P, Garry RF, Krause JR, Dash S. Detection of hepatitis C virus RNA sequences in B-cell non-Hodgkin lymphoma. Am J Clin Pathol. 2000;113:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Kaya H, Polat MF, Erdem F, Gündogdu M. Prevalence of hepatitis C virus and hepatitis G virus in patients with non-Hodgkin’s lymphoma. Clin Lab Haematol. 2002;24:107-110. [PubMed] [Cited in This Article: ] |
| 66. | King PD, Wilkes JD, Diaz-Arias AA. Hepatitis C virus infection in non-Hodgkin’s lymphoma. Clin Lab Haematol. 1998;20:107-110. [PubMed] [Cited in This Article: ] |
| 67. | Kuniyoshi M, Nakamuta M, Sakai H, Enjoji M, Kinukawa N, Kotoh K, Fukutomi M, Yokota M, Nishi H, Iwamoto H. Prevalence of hepatitis B or C virus infections in patients with non-Hodgkin’s lymphoma. J Gastroenterol Hepatol. 2001;16:215-219. [PubMed] [Cited in This Article: ] |
| 68. | Luppi M, Longo G, Ferrari MG, Barozzi P, Marasca R, Morselli M, Valenti C, Mascia T, Vandelli L, Vallisa D. Clinico-pathological characterization of hepatitis C virus-related B-cell non-Hodgkin’s lymphomas without symptomatic cryoglobulinemia. Ann Oncol. 1998;9:495-498. [PubMed] [Cited in This Article: ] |
| 69. | Mazzaro C, Zagonel V, Monfardini S, Tulissi P, Pussini E, Fanni M, Sorio R, Bortolus R, Crovatto M, Santini G. Hepatitis C virus and non-Hodgkin’s lymphomas. Br J Haematol. 1996;94:544-550. [PubMed] [Cited in This Article: ] |
| 70. | McColl MD, Singer IO, Tait RC, McNeil IR, Cumming RL, Hogg RB. The role of hepatitis C virus in the aetiology of non-Hodgkins lymphoma--a regional association? Leuk Lymphoma. 1997;26:127-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Mele A, Pulsoni A, Bianco E, Musto P, Szklo A, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Liso V. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Mizorogi F, Hiramoto J, Nozato A, Takekuma Y, Nagayama K, Tanaka T, Takagi K. Hepatitis C virus infection in patients with B-cell non-Hodgkin’s lymphoma. Intern Med. 2000;39:112-117. [PubMed] [Cited in This Article: ] |
| 73. | Montella M, Crispo A, de Bellis G, Izzo F, Frigeri F, Ronga D, Spada O, Mettivier V, Tamburini M, Cuomo O. HCV and cancer: a case-control study in a high-endemic area. Liver. 2001;21:335-341. [PubMed] [Cited in This Article: ] |
| 74. | Montella M, Crispo A, Frigeri F, Ronga D, Tridente V, De Marco M, Fabbrocini G, Spada O, Mettivier V, Tamburini M. HCV and tumors correlated with immune system: a case-control study in an area of hyperendemicity. Leuk Res. 2001;25:775-781. [PubMed] [Cited in This Article: ] |
| 75. | Morton LM, Engels EA, Holford TR, Leaderer B, Zhang Y, Zahm SH, Boyle P, Zhang B, Flynn S, Tallini G. Hepatitis C virus and risk of non-Hodgkin lymphoma: a population-based case-control study among Connecticut women. Cancer Epidemiol Biomarkers Prev. 2004;13:425-430. [PubMed] [Cited in This Article: ] |
| 76. | Musto P, Dell’Olio M, Carotenuto M, Mangia A, Andriulli A. Hepatitis C virus infection: a new bridge between hematologists and gastroenterologists? Blood. 1996;88:752-754. [PubMed] [Cited in This Article: ] |
| 77. | Nicolosi Guidicelli S, Lopez-Guillermo A, Falcone U, Conconi A, Christinat A, Rodriguez-Abreu D, Grisanti S, Lobetti-Bodoni C, Piffaretti JC, Johnson PW. Hepatitis C virus and GBV-C virus prevalence among patients with B-cell lymphoma in different European regions: a case-control study of the International Extranodal Lymphoma Study Group. Hematol Oncol. 2012;30:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Nieters A, Kallinowski B, Brennan P, Ott M, Maynadié M, Benavente Y, Foretova L, Cocco PL, Staines A, Vornanen M. Hepatitis C and risk of lymphoma: results of the European multicenter case-control study EPILYMPH. Gastroenterology. 2006;131:1879-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Ogino H, Satomura Y, Unoura M, Yoshida T, Oguri H, Kaneko S, Kobayashi K. Hepatitis B, C and G virus infection in patients with lymphoproliferative disorders. Hepatol Res. 1999;14:187-194. [Cited in This Article: ] |
| 80. | Ohsawa M, Shingu N, Miwa H, Yoshihara H, Kubo M, Tsukuma H, Teshima H, Hashimoto M, Aozasa K. Risk of non-Hodgkin’s lymphoma in patients with hepatitis C virus infection. Int J Cancer. 1999;80:237-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 81. | Omland LH, Jepsen P, Krarup H, Christensen PB, Weis N, Nielsen L, Obel N, Sørensen HT, Stuver SO. Liver cancer and non-Hodgkin lymphoma in hepatitis C virus-infected patients: results from the DANVIR cohort study. Int J Cancer. 2012;130:2310-2317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Panovska I, Georgievski B, Stojanovic A, Cevreska L, Efremov DG. Low prevalence of chronic hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma patients from a population with a high prevalence of healthy hepatitis c virus carriers. Br J Haematol. 2000;109:249-250. [PubMed] [Cited in This Article: ] |
| 83. | Park SC, Jeong SH, Kim J, Han CJ, Kim YC, Choi KS, Cho JH, Lee M, Jung HH, Ki SS. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J Med Virol. 2008;80:960-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Paydas S, Kiliç B, Sahin B, Buğdayci R. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders in Southern Turkey. Br J Cancer. 1999;80:1303-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Pioltelli P, Gargantini L, Cassi E, Santoleri L, Bellati G, Magliano EM, Morra E. Hepatitis C virus in non-Hodgkin’s lymphoma. A reappraisal after a prospective case-control study of 300 patients. Lombart Study Group of HCV-Lymphoma. Am J Hematol. 2000;64:95-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 86. | Pioltelli P, Zehender G, Monti G, Monteverde A, Galli M. HCV and non-Hodgkin lymphoma. Lancet. 1996;347:624-625. [PubMed] [Cited in This Article: ] |
| 87. | Prati D, Zanella A, De Mattei C, Farma E, Boschetti C, Sirchia G, Venegoni L, Berti E. Chronic hepatitis c virus infection and primary cutaneous B-cell lymphoma. Br J Haematol. 1999;105:841. [PubMed] [Cited in This Article: ] |
| 88. | Rabkin CS, Tess BH, Christianson RE, Wright WE, Waters DJ, Alter HJ, Van Den Berg BJ. Prospective study of hepatitis C viral infection as a risk factor for subsequent B-cell neoplasia. Blood. 2002;99:4240-4242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Ramos-Casals M, Trejo O, García-Carrasco M, Cervera R, De La Red G, Gil V, López-Guillermo A, Ingelmo M, Font J. Triple association between hepatitis C virus infection, systemic autoimmune diseases, and B cell lymphoma. J Rheumatol. 2004;31:495-499. [PubMed] [Cited in This Article: ] |
| 90. | Salem Z, Nuwaiyri-Salti N, Ramlawi F, Ramia S. Hepatitis C virus infection in Lebanese patients with B-cell non-Hodgkin’s lymphoma. Eur J Epidemiol. 2003;18:251-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Sansonno D, De Vita S, Cornacchiulo V, Carbone A, Boiocchi M, Dammacco F. Detection and distribution of hepatitis C virus-related proteins in lymph nodes of patients with type II mixed cryoglobulinemia and neoplastic or non-neoplastic lymphoproliferation. Blood. 1996;88:4638-4645. [PubMed] [Cited in This Article: ] |
| 92. | Schöllkopf C, Smedby KE, Hjalgrim H, Rostgaard K, Panum I, Vinner L, Chang ET, Glimelius B, Porwit A, Sundström C. Hepatitis C infection and risk of malignant lymphoma. Int J Cancer. 2008;122:1885-1890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Sève P, Renaudier P, Sasco AJ, Dumontet C, Salles G, Coiffier B, Zoulim F, Broussolle C, Trépo C. Hepatitis C virus infection and B-cell non-Hodgkin’s lymphoma: a cross-sectional study in Lyon, France. Eur J Gastroenterol Hepatol. 2004;16:1361-1365. [PubMed] [Cited in This Article: ] |
| 94. | Shariff S, Yoshida EM, Gascoyne RD, Le N, Connors JM, Middleton PJ, Shenkier TN. Hepatitis C infection and B-cell non-Hodgkin’s lymphoma in British Columbia: a cross-sectional analysis. Ann Oncol. 1999;10:961-964. [PubMed] [Cited in This Article: ] |
| 95. | Shirin H, Davidovitz Y, Avni Y, Petchenko P, Krepel Z, Bruck R, Meytes D. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Isr Med Assoc J. 2002;4:24-27. [PubMed] [Cited in This Article: ] |
| 96. | Silvestri F, Barillari G, Fanin R, Pipan C, Falasca E, Salmaso F, Zaja F, Infanti L, Patriarca F, Botta GA. Hepatitis C virus infection among cryoglobulinemic and non-cryoglobulinemic B-cell non-Hodgkin’s lymphomas. Haematologica. 1997;82:314-317. [PubMed] [Cited in This Article: ] |
| 97. | Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, Russo D, Falasca E, Botta GA, Baccarani M. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296-4301. [PubMed] [Cited in This Article: ] |
| 98. | Singer IO, Cumming RL, Hogg RB. Is hepatitis C associated with non-Hodgkin’s lymphoma? Leuk Lymphoma. 1997;26:633-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Sonmez M, Bektas O, Yilmaz M, Durmus A, Akdogan E, Topbas M, Erturk M, Ovali E, Omay SB. The relation of lymphoma and hepatitis B virus/hepatitis C virus infections in the region of East Black Sea, Turkey. Tumori. 2002;93:536-539. [PubMed] [Cited in This Article: ] |
| 100. | Spinelli JJ, Lai AS, Krajden M, Andonov A, Gascoyne RD, Connors JM, Brooks-Wilson AR, Gallagher RP. Hepatitis C virus and risk of non-Hodgkin lymphoma in British Columbia, Canada. Int J Cancer. 2008;122:630-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Talamini R, Montella M, Crovatto M, Dal Maso L, Crispo A, Negri E, Spina M, Pinto A, Carbone A, Franceschi S. Non-Hodgkin’s lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | Thalen DJ, Raemaekers J, Galama J, Cooreman MP. Absence of hepatitis C virus infection in non-Hodgkin’s lymphoma. Br J Haematol. 1997;96:880-881. [PubMed] [Cited in This Article: ] |
| 103. | Timurağlu A, Colak D, Oğünç D, Karadoğan I, Undar L. Hepatitis C virus association with non-Hodgkin’s lymphoma. Haematologia (Budap). 1999;29:301-304. [PubMed] [Cited in This Article: ] |
| 104. | Tkoub EM, Haioun C, Pawlotsky JM, Dhumeaux D, Delchier JC. Chronic hepatitis C virus and gastric MALT lymphoma. Blood. 1998;91:360. [PubMed] [Cited in This Article: ] |
| 105. | Tursi A, Brandimante G, Chiarelli F, Spagnoli A, Torello M. Detection of HCV RNA in gastric mucosa-associated lymphoid tissue by in situ hybridization: evidence of a new extrahepatic localization of HCV with increased risk of gastric malt lymphoma. Am J Gastroenterol. 2002;97:1802-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Udomsakdi-Auewarakul C, Auewarakul P, Sukpanichnant S, Muangsup W. Hepatitis C virus infection in patients with non-Hodgkin lymphoma in Thailand. Blood. 2000;95:3640-3641. [PubMed] [Cited in This Article: ] |
| 107. | Vajdic CM, Grulich AE, Kaldor JM, Fritschi L, Benke G, Hughes AM, Kricker A, Turner JJ, Milliken S, Armstrong BK. Specific infections, infection-related behavior, and risk of non-Hodgkin lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2006;15:1102-1108. [PubMed] [Cited in This Article: ] |
| 108. | Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, Lazzaro A, Trabacchi E, Anselmi E, Arcari AL. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 109. | Varma S, Menon MC, Garg A, Malhotra P, Sharma A, Chawla YK, Dhiman RK. Hepatitis C virus infection among patients with non-Hodgkin’s lymphoma in northern India. Hepatol Int. 2011;5:688-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Yamac K, Aydemir S, Ozturk G, Fen T, Senol E. Hepatitis C infection in lymphoma patients in a Turkish center. Eur J Epidemiol. 2000;16:685. [PubMed] [Cited in This Article: ] |
| 111. | Yoshikawa M, Imazu H, Ueda S, Tamagawa T, Yoneda S, Yamane Y, Takaya A, Fukui H, Nakano H. Prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma and multiple myeloma. A report from Japan. J Clin Gastroenterol. 1997;25:713-714. [PubMed] [Cited in This Article: ] |
| 112. | Yu SC, Lin CW. Early-stage splenic diffuse large B-cell lymphoma is highly associated with hepatitis C virus infection. Kaohsiung J Med Sci. 2013;29:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Zucca E, Roggero E, Maggi-Solcà N, Conconi A, Bertoni F, Reilly I, Castelli D, Pedrinis E, Piffaretti JC, Cavalli F. Prevalence of Helicobacter pylori and hepatitis C virus infections among non-Hodgkin’s lymphoma patients in Southern Switzerland. Haematologica. 2000;85:147-153. [PubMed] [Cited in This Article: ] |
| 114. | Zuckerman E, Zuckerman T, Levine AM, Douer D, Gutekunst K, Mizokami M, Qian DG, Velankar M, Nathwani BN, Fong TL. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127:423-428. [PubMed] [Cited in This Article: ] |
| 115. | Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392. [PubMed] [Cited in This Article: ] |
| 116. | Herrinton LJ. Epidemiology of the Revised European-American Lymphoma Classification subtypes. Epidemiol Rev. 1998;20:187-203. [PubMed] [Cited in This Article: ] |
| 117. | Hwang JP, Suarez-Almazor ME, Torres HA, Palla SL, Huang DS, Fisch MJ, Lok AS. Hepatitis C virus screening in patients with cancer receiving chemotherapy. J Oncol Pract. 2014;10:e167-e174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 118. | Amin J, Dore GJ, O’Connell DL, Bartlett M, Tracey E, Kaldor JM, Law MG. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol. 2006;45:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 119. | Anderson LA, Pfeiffer R, Warren JL, Landgren O, Gadalla S, Berndt SI, Ricker W, Parsons R, Wheeler W, Engels EA. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17:3069-3075. [PubMed] [Cited in This Article: ] |
| 120. | Cavanna L, Sbolli G, Tanzi E, Romanò L, Civardi G, Buscarini E, Vallisa D, Berté R, Rossi A. High prevalence of antibodies to hepatitis C virus in patients with lymphoproliferative disorders. Haematologica. 1995;80:486-487. [PubMed] [Cited in This Article: ] |
| 121. | Ferri C, La Civita L, Monti M, Giannini C, Cecchetti R, Caracciolo F, Longombardo G, Lombardini F, Zignego AL. Chronic hepatitis C and B-cell non-Hodgkin’s lymphoma. QJM. 1996;89:117-122. [PubMed] [Cited in This Article: ] |
| 122. | Isikdogan A, Ayyildiz O, Dursun M, Tiftik N, Batun S, Muftuoglu E. Hepatitis C virus in patients with non-Hodgkin’s lymphoma in southeastern Anatolia region of Turkey: a prospective case-control study of 119 patients. Leuk Lymphoma. 2003;44:1745-1747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 123. | Kocabaş E, Aksaray N, Alhan E, Tanyeli A, Köksal F, Yarkin F. Hepatitis B and C virus infections in Turkish children with cancer. Eur J Epidemiol. 1997;13:869-873. [PubMed] [Cited in This Article: ] |
| 124. | Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K, Takahashi T, Oyama M. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Teng CJ, Liu HT, Liu CY, Hsih CH, Pai JT, Gau JP, Liu JH, Chiou TJ, Hsu HC, Chen PM. Chronic hepatitis virus infection in patients with multiple myeloma: clinical characteristics and outcomes. Clinics (Sao Paulo). 2011;66:2055-2061. [PubMed] [Cited in This Article: ] |
| 126. | Veneri D, Franchini M, Zanotti R, Frattini F, Randon F, Rinaldi M, Pizzolo G. Prevalence of hepatitis C virus infection among patients with lymphoproliferative disorders: a single center survey. Am J Hematol. 2007;82:1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 127. | Yenice N, Güllük F, Arican N, Türkmen S. HCV prevalence in Hodgkin and non-Hodgkin lymphoma cases. Turk J Gastroenterol. 2003;14:173-176. [PubMed] [Cited in This Article: ] |
| 128. | Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078-2085. [PubMed] [Cited in This Article: ] |
| 129. | de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM, Spinelli JJ, Zheng T, Zhang Y, Franceschi S. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 130. | Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745-752. [PubMed] [Cited in This Article: ] |
| 131. | Merli M, Visco C, Spina M, Luminari S, Ferretti VV, Gotti M, Rattotti S, Fiaccadori V, Rusconi C, Targhetta C. Outcome prediction of diffuse large B-cell lymphomas associated with hepatitis C virus infection: a study on behalf of the Fondazione Italiana Linfomi. Haematologica. 2014;99:489-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Pellicelli AM, Marignani M, Zoli V, Romano M, Morrone A, Nosotti L, Barbaro G, Picardi A, Gentilucci UV, Remotti D. Hepatitis C virus-related B cell subtypes in non Hodgkin’s lymphoma. World J Hepatol. 2011;3:278-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Takeshita M, Sakai H, Okamura S, Higaki K, Oshiro Y, Uike N, Yamamoto I, Shimamatsu K, Muranaka T. Prevalence of hepatitis C virus infection in cases of B-cell lymphoma in Japan. Histopathology. 2006;48:189-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Caviglia GP, Sciacca C, Abate ML, Olivero A, Rosso C, Touscoz GA, Ciancio A, Rizzetto M, Smedile A. Chronic hepatitis C virus infection and lymphoproliferative disorders: mixed cryoglobulinemia syndrome, monoclonal gammopathy of undetermined significance, and B-cell non-Hodgkin lymphoma. J Gastroenterol Hepatol. 2015;30:742-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | De Renzo A, Perna F, Persico M, Notaro R, Mainolfi C, de Sio I, Ciancia G, Picardi M, Del Vecchio L, Pane F. Excellent prognosis and prevalence of HCV infection of primary hepatic and splenic non-Hodgkin’s lymphoma. Eur J Haematol. 2008;81:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Kashyap A, Nademanee A, Molina A. Hepatitis C and B-cell lymphoma. Ann Intern Med. 1998;128:695. [PubMed] [Cited in This Article: ] |
| 137. | Kim JH, Bang YJ, Park BJ, Yoo T, Kim CW, Kim TY, Heo DS, Lee HS, Kim NK. Hepatitis B virus infection and B-cell non-Hodgkin’s lymphoma in a hepatitis B endemic area: a case-control study. Jpn J Cancer Res. 2002;93:471-477. [PubMed] [Cited in This Article: ] |
| 138. | Libra M, Polesel J, Russo AE, De Re V, Cinà D, Serraino D, Nicoletti F, Spandidos DA, Stivala F, Talamini R. Extrahepatic disorders of HCV infection: a distinct entity of B-cell neoplasia? Int J Oncol. 2010;36:1331-1340. [PubMed] [Cited in This Article: ] |
| 139. | Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology. 1999;46:2925-2930. [PubMed] [Cited in This Article: ] |
| 140. | Musolino C, Campo S, Pollicino T, Squadrito G, Spatari G, Raimondo G. Evaluation of hepatitis B and C virus infections in patients with non-Hodgkin’s lymphoma and without liver disease. Haematologica. 1996;81:162-164. [PubMed] [Cited in This Article: ] |
| 141. | Okan V, Yilmaz M, Bayram A, Kis C, Cifci S, Buyukhatipoglu H, Pehlivan M. Prevalence of hepatitis B and C viruses in patients with lymphoproliferative disorders. Int J Hematol. 2008;88:403-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 142. | Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, Sulfaro S, Franzin F, Tulissi P, Moretti M. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84:3047-3053. [PubMed] [Cited in This Article: ] |
| 143. | Pivetti S, Novarino A, Merico F, Bertero MT, Brunetto MR, Bonino F, Caligaris-Cappio F. High prevalence of autoimmune phenomena in hepatitis C virus antibody positive patients with lymphoproliferative and connective tissue disorders. Br J Haematol. 1996;95:204-211. [PubMed] [Cited in This Article: ] |
| 144. | Salem AK. Prevalence of HCV among Yemeni patients with non-Hodgkin’s lymphoma at AI-Thawra teaching hospital. Gulf J Oncolog. 2009;22-29. [PubMed] [Cited in This Article: ] |
| 145. | Sánchez Ruiz AC, Yebra Bango M, Portero F, Provencio Pulla M, Miralles Flores C, España Saz P. [Prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma]. Med Clin (Barc). 2001;116:333-334. [PubMed] [Cited in This Article: ] |
| 146. | Swart A, Burns L, Mao L, Grulich AE, Amin J, O’Connell DL, Meagher NS, Randall DA, Degenhardt L, Vajdic CM. The importance of blood-borne viruses in elevated cancer risk among opioid-dependent people: a population-based cohort study. BMJ Open. 2012;2:e001755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Abdel Wahab M, Mostafa M, Salah T, Fouud A, Kandeel T, Elshobary M, Abd Allah OF, Elghawalby N, Sultan A, Ezzat F. Epidemiology of hilar cholangiocarcinoma in Egypt: single center study. Hepatogastroenterology. 2007;54:1626-1631. [PubMed] [Cited in This Article: ] |
| 148. | Kuper H, Lagiou P, Mucci LA, Tamimi R, Benetou V, Trichopoulos D. Risk factors for cholangiocarcinoma in a low risk Caucasian population. Soz Praventivmed. 2001;46:182-185. [PubMed] [Cited in This Article: ] |
| 149. | Lu H, Ye MQ, Thung SN, Dash S, Gerber MA. Detection of hepatitis C virus RNA sequences in cholangiocarcinomas in Chinese and American patients. Chin Med J (Engl). 2000;113:1138-1141. [PubMed] [Cited in This Article: ] |
| 150. | Mohammad-Alizadeh AH, Ghobakhlou M, Shalmani HM, Zali MR. Cholangiocarcinoma: an-eight-year experience in a tertiary-center in Iran. Asian Pac J Cancer Prev. 2012;13:5381-5384. [PubMed] [Cited in This Article: ] |
| 151. | Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao KY, Chen XG, Yang YF, Su ZX, Chen B. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol. 2011;18:1258-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 152. | Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933-940. [PubMed] [Cited in This Article: ] |
| 153. | Songsivilai S, Dharakul T, Kanistanon D. Hepatitis C virus genotypes in patients with hepatocellular carcinoma and cholangiocarcinoma in Thailand. Trans R Soc Trop Med Hyg. 1996;90:505-507. [PubMed] [Cited in This Article: ] |
| 154. | Taguchi J, Nakashima O, Tanaka M, Hisaka T, Takazawa T, Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996;11:758-764. [PubMed] [Cited in This Article: ] |
| 155. | Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, Zhang SM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 156. | Tomimatsu M, Ishiguro N, Taniai M, Okuda H, Saito A, Obata H, Yamamoto M, Takasaki K, Nakano M. Hepatitis C virus antibody in patients with primary liver cancer (hepatocellular carcinoma, cholangiocarcinoma, and combined hepatocellular-cholangiocarcinoma) in Japan. Cancer. 1993;72:683-688. [PubMed] [Cited in This Article: ] |
| 157. | Uenishi T, Nagano H, Marubashi S, Hayashi M, Hirokawa F, Kaibori M, Matsui K, Kubo S. The long-term outcomes after curative resection for mass-forming intrahepatic cholangiocarcinoma associated with hepatitis C viral infection: a multicenter analysis by Osaka Hepatic Surgery Study Group. J Surg Oncol. 2014;110:176-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 158. | Wu TT, Levy M, Correa AM, Rosen CB, Abraham SC. Biliary intraepithelial neoplasia in patients without chronic biliary disease: analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer. 2009;115:4564-4575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 159. | Yamamoto M, Takasaki K, Nakano M, Saito A. Minute nodular intrahepatic cholangiocarcinoma. Cancer. 1998;82:2145-2149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 160. | Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T, Makuuchi M. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283-287. [PubMed] [Cited in This Article: ] |
| 161. | Yin F, Chen B. Detection of hepatitis C virus RNA sequences in hepatic portal cholangiocarcinoma tissue by reverse transcription polymerase chain reaction. Chin Med J (Engl). 1998;111:1068-1070. [PubMed] [Cited in This Article: ] |
| 162. | Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Jang KT, Lee NY, Kim S, Hong ST. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006;44:1066-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 163. | Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959-964. [PubMed] [Cited in This Article: ] |
| 164. | Parkin DM, Srivatanakul P, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L’Abbé KA, Wild CP. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991;48:323-328. [PubMed] [Cited in This Article: ] |
| 165. | Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620-626. [PubMed] [Cited in This Article: ] |
| 166. | Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 167. | Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592-595. [PubMed] [Cited in This Article: ] |
| 168. | Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632-635. [PubMed] [Cited in This Article: ] |
| 169. | Barusrux S, Nanok C, Puthisawas W, Pairojkul C, Poovorawan Y. Viral hepatitis B, C infection and genotype distribution among cholangiocarcinoma patients in northeast Thailand. Asian Pac J Cancer Prev. 2012;13 Suppl:83-87. [PubMed] [Cited in This Article: ] |
| 170. | El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 171. | Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 172. | Portolani N, Baiocchi GL, Coniglio A, Piardi T, Grazioli L, Benetti A, Ferrari Bravo A, Giulini SM. Intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma: a Western experience. Ann Surg Oncol. 2008;15:1880-1890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 173. | Qu Z, Cui N, Qin M, Wu X. Epidemiological survey of biomarkers of hepatitis virus in patients with extrahepatic cholangiocarcinomas. Asia Pac J Clin Oncol. 2012;8:83-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 174. | Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, Fiel MI, Schwartz M, Thung SN. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31:49-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 175. | Srivatanakul P, Honjo S, Kittiwatanachot P, Jedpiyawongse A, Khuhaprema T, Miwa M. Hepatitis viruses and risk of cholangiocarcinoma in northeast Thailand. Asian Pac J Cancer Prev. 2010;11:985-988. [PubMed] [Cited in This Article: ] |
| 176. | Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 384] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 177. | Hai S, Kubo S, Yamamoto S, Uenishi T, Tanaka H, Shuto T, Takemura S, Yamazaki O, Hirohashi K. Clinicopathologic characteristics of hepatitis C virus-associated intrahepatic cholangiocarcinoma. Dig Surg. 2005;22:432-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 178. | Nuzzo G, Giuliante F, Ardito F, De Rose AM, Vellone M, Clemente G, Chiarla C, Giovannini I. Intrahepatic cholangiocarcinoma: prognostic factors after liver resection. Updates Surg. 2010;62:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 179. | Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat. 2010;17:742-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 180. | Torbenson M, Yeh MM, Abraham SC. Bile duct dysplasia in the setting of chronic hepatitis C and alcohol cirrhosis. Am J Surg Pathol. 2007;31:1410-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 181. | Zuo HQ, Yan LN, Zeng Y, Yang JY, Luo HZ, Liu JW, Zhou LX. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:161-165. [PubMed] [Cited in This Article: ] |
| 182. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 409] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 183. | Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (2)] |
| 184. | Zhou Y, Zhao Y, Li B, Huang J, Wu L, Xu D, Yang J, He J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 185. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 186. | Liu X, Zou S, Qiu F. Pathogenesis of hilar cholangiocarcinoma and infection of hepatitis virus. Zhonghua Wai Ke Za Zhi. 2002;40:420-422. [PubMed] [Cited in This Article: ] |
| 187. | Shirakawa H. Analysis of hepatitis C virus (HCV) genotypes in hepatocellular carcinoma. Hokkaido Igaku Zasshi. 1996;71:677-688. [PubMed] [Cited in This Article: ] |
| 188. | Zhang H, Tang Y, Lu X. [Detection of hepatitis B virus DNA and hepatitis C virus RNA in human hepatocellular carcinoma by polymerase chain reaction]. Zhonghua Bing Li Xue Za Zhi. 1996;25:70-72. [PubMed] [Cited in This Article: ] |
| 189. | Zou SQ, Liu XF, Guo RX, Li CL, Zhou XS, Zhu XG, Huang ZQ. The retrospective analysis of HBV and HCV infection in cholangiocarcinoma. Zhonghua Wai Ke Za Zhi. 2003;41:417-419. [PubMed] [Cited in This Article: ] |
| 190. | Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 353] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 191. | Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg. 2006;13:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 192. | Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, Ortiz-Conde BA, Goedert JJ, Fraumeni JF, O’Brien TR. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008;122:1849-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 193. | Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471-2477. [PubMed] [Cited in This Article: ] |
| 194. | Matsumoto K, Onoyama T, Kawata S, Takeda Y, Harada K, Ikebuchi Y, Ueki M, Miura N, Yashima K, Koda M. Hepatitis B and C virus infection is a risk factor for the development of cholangiocarcinoma. Intern Med. 2014;53:651-654. [PubMed] [Cited in This Article: ] |
| 195. | Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37:1211-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 196. | Malaguarnera M, Gargante MP, Risino C, Ranno S, Berretta M, Cannizzaro MA, Costanzo M, Fricia T, Rampello E, Romano M. Hepatitis C virus in elderly cancer patients. Eur J Intern Med. 2006;17:325-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 197. | Omland LH, Farkas DK, Jepsen P, Obel N, Pedersen L. Hepatitis C virus infection and risk of cancer: a population-based cohort study. Clin Epidemiol. 2010;2:179-186. [PubMed] [Cited in This Article: ] |
| 198. | Budakoğlu B, Aksoy S, Arslan Ç, Üyetürk Ü, Babacan NA, Özcan MF, Yıldız R, Öven BB, Özdemir NY, Dizdar Ö. Frequency of HCV infection in renal cell carcinoma patients. Med Oncol. 2012;29:1892-1895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 199. | Gonzalez HC, Lamerato L, Rogers CG, Gordon SC. Chronic hepatitis C infection as a risk factor for renal cell carcinoma. Dig Dis Sci. 2015;60:1820-1824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 200. | Gordon SC, Moonka D, Brown KA, Rogers C, Huang MA, Bhatt N, Lamerato L. Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol Biomarkers Prev. 2010;19:1066-1073. [PubMed] [Cited in This Article: ] |
| 201. | Hofmann JN, Törner A, Chow WH, Ye W, Purdue MP, Duberg AS. Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: a nationwide register-based cohort study in Sweden. Eur J Cancer Prev. 2011;20:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 202. | Bruno G, Andreozzi P, Graf U, Santangelo G. Hepatitis C virus: a high risk factor for a second primary malignancy besides hepatocellular carcinoma. Fact or fiction? Clin Ter. 1999;150:413-418. [PubMed] [Cited in This Article: ] |
| 203. | Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, White E. Risk factors for renal cell carcinoma in the VITAL study. J Urol. 2013;190:1657-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 204. | Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19:4234-4241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 205. | Ben Q, Li Z, Liu C, Cai Q, Yuan Y, Wang K, Xiao L, Gao J, Zhang H. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas. 2012;41:435-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 206. | Chang MC, Chen CH, Liang JD, Tien YW, Hsu C, Wong JM, Chang YT. Hepatitis B and C viruses are not risks for pancreatic adenocarcinoma. World J Gastroenterol. 2014;20:5060-5065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 207. | Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26:4557-4562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 208. | Huang J, Magnusson M, Törner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer. 2013;109:2917-2923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 209. | Woo SM, Joo J, Lee WJ, Park SJ, Han SS, Kim TH, Koh YH, Kim HB, Hong EK. Risk of pancreatic cancer in relation to ABO blood group and hepatitis C virus infection in Korea: a case-control study. J Korean Med Sci. 2013;28:247-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 210. | Zhu F, Li HR, Du GN, Chen JH, Cai SR. Chronic hepatitis B virus infection and pancreatic cancer: a case-control study in southern China. Asian Pac J Cancer Prev. 2011;12:1405-1408. [PubMed] [Cited in This Article: ] |
| 211. | Fiorino S, Chili E, Bacchi-Reggiani L, Masetti M, Deleonardi G, Grondona AG, Silvestri T, Magrini E, Zanini N, Cuppini A. Association between hepatitis B or hepatitis C virus infection and risk of pancreatic adenocarcinoma development: a systematic review and meta-analysis. Pancreatology. 2013;13:147-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 212. | Xing S, Li ZW, Tian YF, Zhang LM, Li MQ, Zhou P. Chronic hepatitis virus infection increases the risk of pancreatic cancer: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2013;12:575-583. [PubMed] [Cited in This Article: ] |
| 213. | Hong SG, Kim JH, Lee YS, Yoon E, Lee HJ, Hwang JK, Jung ES, Joo MK, Jung YK, Yeon JE. The relationship between hepatitis B virus infection and the incidence of pancreatic cancer: a retrospective case-control study. Korean J Hepatol. 2010;16:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 214. | Xu P, Huang Q, Liu C, Xie F, Shao F, Zhu C. Risk factors for pancreatic cancer: a case-control study. Aizheng. 2011;31:653-657. [Cited in This Article: ] |
| 215. | Antonelli A, Ferri C, Fallahi P, Nesti C, Zignego AL, Maccheroni M. Thyroid cancer in HCV-related mixed cryoglobulinemia patients. Clin Exp Rheumatol. 2002;20:693-696. [PubMed] [Cited in This Article: ] |
| 216. | Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, Barani L, Marchi S, Ferrannini E. Thyroid cancer in HCV-related chronic hepatitis patients: a case-control study. Thyroid. 2007;17:447-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 217. | Montella M, Pezzullo L, Crispo A, Izzo F, Amore A, Marone U, Tamburini M, Ronga D, Chiofalo MG, Chiappetta G. Risk of thyroid cancer and high prevalence of hepatitis C virus. Oncol Rep. 2003;10:133-136. [PubMed] [Cited in This Article: ] |
| 218. | Larrey D, Bozonnat MC, Kain I, Pageaux GP, Assenat E. Is chronic hepatitis C virus infection a risk factor for breast cancer? World J Gastroenterol. 2010;16:3687-3691. [PubMed] [Cited in This Article: ] |
| 219. | Su FH, Chang SN, Chen PC, Sung FC, Su CT, Yeh CC. Association between chronic viral hepatitis infection and breast cancer risk: a nationwide population-based case-control study. BMC Cancer. 2011;11:495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 220. | Amin J, Gidding H, Gilbert G, Backhouse J, Kaldor J, Dore G, Burgess M. Hepatitis C prevalence--a nationwide serosurvey. Commun Dis Intell Q Rep. 2004;28:517-521. [PubMed] [Cited in This Article: ] |
| 221. | Eftekharian A, Khajavi M, Shokoofi S, Rahmani Z, Gachkar L, Gerami H, Rajati M, Khademi B. Hepatitis C virus in patients with squamous cell carcinoma of the head and neck in Iran: is there any relation? Eur Arch Otorhinolaryngol. 2012;269:2571-2573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 222. | Gandolfo S, Richiardi L, Carrozzo M, Broccoletti R, Carbone M, Pagano M, Vestita C, Rosso S, Merletti F. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: a follow-up study in an Italian population. Oral Oncol. 2004;40:77-83. [PubMed] [Cited in This Article: ] |
| 223. | Nagao Y, Sata M, Noguchi S, Seno’o T, Kinoshita M, Kameyama T, Ueno T. Detection of hepatitis C virus RNA in oral lichen planus and oral cancer tissues. J Oral Pathol Med. 2000;29:259-266. [PubMed] [Cited in This Article: ] |
| 224. | Nagao Y, Sata M, Tanikawa K, Itoh K, Kameyama T. High prevalence of hepatitis C virus antibody and RNA in patients with oral cancer. J Oral Pathol Med. 1995;24:354-360. [PubMed] [Cited in This Article: ] |
| 225. | Nobles J, Wold C, Fazekas-May M, Gilbert J, Friedlander PL. Prevalence and epidemiology of hepatitis C virus in patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2004;114:2119-2122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 226. | Su FH, Chang SN, Chen PC, Sung FC, Huang SF, Chiou HY, Su CT, Lin CC, Yeh CC. Positive association between hepatitis C infection and oral cavity cancer: a nationwide population-based cohort study in Taiwan. PLoS One. 2012;7:e48109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 227. | Takata Y, Takahashi T, Fukuda J. Prevalence of hepatitis virus infection in association with oral diseases requiring surgery. Oral Dis. 2002;8:95-99. [PubMed] [Cited in This Article: ] |
| 228. | Hunt J, Hagan J, Nobles J, Wold C, Fazekas-May M, Gilbert J, Friedlander PL. Outcome analysis of patients with squamous cell carcinoma of the head and neck and hepatitis C virus. Laryngoscope. 2005;115:1882-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 229. | International Agency for Research on Cancer. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. : World Health Organization 2012; . [Cited in This Article: ] |
| 230. | Alvares-Da-Silva MR, Francisconi CF, Waechter FL. Acute hepatitis C complicated by pancreatitis: another extrahepatic manifestation of hepatitis C virus? J Viral Hepat. 2000;7:84-86. [PubMed] [Cited in This Article: ] |
| 231. | Richardson A. Is breast cancer caused by late exposure to a common virus? Med Hypotheses. 1997;48:491-497. [PubMed] [Cited in This Article: ] |
| 232. | Yasui Y, Potter JD, Stanford JL, Rossing MA, Winget MD, Bronner M, Daling J. Breast cancer risk and “delayed” primary Epstein-Barr virus infection. Cancer Epidemiol Biomarkers Prev. 2001;10:9-16. [PubMed] [Cited in This Article: ] |
| 233. | Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O’Hare AM. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 234. | Fayek S, Moore D, Bortecen KH, Yeh H, Markmann JF, Olthoff KM, Shaked A. Liver transplantation in the setting of extra-hepatic malignancy: two case reports. Transplant Proc. 2007;39:3512-3514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 235. | Garcia JH, Coelho GR, Cavalcante FP, Valença JT, Brasil IR, Cesar-Borges G, Costa PE, Viana CF, Rocha TD, Vasconcelos JB. Synchronous hepatocellular carcinoma and renal cell carcinoma in a liver transplant recipient: a case report. Transplantation. 2007;84:1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 236. | Di Micco B, Di Micco P. HCV and renal cell carcinoma: a new insight between HCV and oncogenesis? Report of five cases. Experimental Oncology. 2003;25:77-78. [Cited in This Article: ] |
| 237. | Nagao Y, Sata M, Itoh K, Chiba I, Komiyama K, Yanoma S, Eura M, Tanikawa K, Kameyama T. High prevalence of hepatitis C virus antibody and RNA in patients with head and neck squamous cell carcinoma. Hepatology Research. 1997;7:206-212. [Cited in This Article: ] |
| 238. | Carrozzo M, Carbone M, Gandolfo S, Valente G, Colombatto P, Ghisetti V. An atypical verrucous carcinoma of the tongue arising in a patient with oral lichen planus associated with hepatitis C virus infection. Oral Oncol. 1997;33:220-225. [PubMed] [Cited in This Article: ] |
| 239. | Lo Muzio L, Mignogna MD, Favia G, Procaccini M, Testa NF, Bucci E. The possible association between oral lichen planus and oral squamous cell carcinoma: a clinical evaluation on 14 cases and a review of the literature. Oral Oncol. 1998;34:239-246. [PubMed] [Cited in This Article: ] |
| 240. | Campisi G, Fedele S, Lo Russo L, Di Fede O, Aricò P, Craxì A, Mignogna MD. HCV infection and oral lichen planus: a weak association when HCV is endemic. J Viral Hepat. 2004;11:465-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 241. | Birkenfeld S, Dreiher J, Weitzman D, Cohen AD. A study on the association with hepatitis B and hepatitis C in 1557 patients with lichen planus. J Eur Acad Dermatol Venereol. 2011;25:436-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 242. | Lodi G, Olsen I, Piattelli A, D’Amico E, Artese L, Porter SR. Antibodies to epithelial components in oral lichen planus (OLP) associated with hepatitis C virus (HCV) infection. J Oral Pathol Med. 1997;26:36-39. [PubMed] [Cited in This Article: ] |
| 243. | Petti S, Rabiei M, De Luca M, Scully C. The magnitude of the association between hepatitis C virus infection and oral lichen planus: meta-analysis and case control study. Odontology. 2011;99:168-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 244. | Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol. 2009;145:1040-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 245. | Carrozzo M, Gandolfo S. Oral diseases possibly associated with hepatitis C virus. Crit Rev Oral Biol Med. 2003;14:115-127. [PubMed] [Cited in This Article: ] |
| 246. | Antonelli A, Ferri C, Fallahi P. Thyroid cancer in patients with hepatitis C infection. JAMA. 1999;281:1588. [PubMed] [Cited in This Article: ] |
| 247. | Montella M, Crispo A, Pezzullo L, Izzo F, Fabbrocini G, Ronga D, Tamburini M. Is hepatitis C virus infection associated with thyroid cancer? A case-control study. Int J Cancer. 2000;87:611-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 248. | Chen CJ, Hsu WL, Yang HI, Lee MH, Chen HC, Chien YC, You SL. Epidemiology of virus infection and human cancer. Recent Results Cancer Res. 2014;193:11-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 249. | Fiorino S, Bacchi-Reggiani L, Pontoriero L, Gallo C, Chili E, Masetti M, Zanini N, Grondona A, Silvestri T, Deleonardi G. Pancreatic carcinoma development: new etiological and pathogenetic evidence. Italian J Med. 2013;7:242-252. [Cited in This Article: ] |
| 250. | Andreone P, Zignego AL, Cursaro C, Gramenzi A, Gherlinzoni F, Fiorino S, Giannini C, Boni P, Sabattini E, Pileri S. Prevalence of monoclonal gammopathies in patients with hepatitis C virus infection. Ann Intern Med. 1998;129:294-298. [PubMed] [Cited in This Article: ] |
| 251. | Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 217] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 252. | Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59:169-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 253. | Zaret KS. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet. 2008;9:329-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 254. | Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707-1710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 255. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 737] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 256. | Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 257. | Chow YW, Pietranico R, Mukerji A. Studies of oxygen binding energy to hemoglobin molecule. Biochem Biophys Res Commun. 1975;66:1424-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 258. | Han L, Ma Q, Li J, Liu H, Li W, Ma G, Xu Q, Zhou S, Wu E. High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PLoS One. 2011;6:e27074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 259. | Li J, Ma Q, Liu H, Guo K, Li F, Li W, Han L, Wang F, Wu E. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS One. 2011;6:e17385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 260. | Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ. 2015;349:g7371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 261. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 262. | Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18:1642-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 106] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 263. | Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, Hijioka T, Katayama K, Yabuuchi I, Yoshihara H. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148:820-826. [PubMed] [Cited in This Article: ] |
| 264. | McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 265. | Ito M, Murakami K, Suzuki T, Mochida K, Suzuki M, Ikebuchi K, Yamaguchi K, Mizuochi T. Enhanced expression of lymphomagenesis-related genes in peripheral blood B cells of chronic hepatitis C patients. Clin Immunol. 2010;135:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 266. | Mosad E, Said Abd El-Rahman Allam M, Moustafa HM, Mohammed AE, El kebeer AM, Abdel-Moneim SS. Telomeric 1p36.3 deletion and Ki-67 expression in B-Non-Hodgkin’s Lymphoma patients associated with chronic hepatitis C virus infection. J Viral Hepat. 2014;21:950-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 267. | Smedley D, Sidhar S, Birdsall S, Bennett D, Herlyn M, Cooper C, Shipley J. Characterization of chromosome 1 abnormalities in malignant melanomas. Genes Chromosomes Cancer. 2000;28:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 268. | Stoffel A, Filippa D, Rao PH. The p73 locus is commonly deleted in non-Hodgkin’s lymphomas. Leuk Res. 2004;28:1341-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |