Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10851
Revised: February 11, 2014
Accepted: April 21, 2014
Published online: August 21, 2014
The development of non invasive biomarkers of disease has become a major focus of interest in nonalcoholic fatty liver disease (NAFLD). The large prevalence of the disease and the invasive nature of the investigation means that screening with liver biopsy is impractical. In addition to screening, the differentiation of those with simple steatosis vs steatohepatitis and fibrosis is clinically important as the prognosis of each differs. Serum biomarkers may be a combination of simple markers derived from large data sets or direct markers of disease activity. Serum markers of inflammation, apoptosis and oxidative stress in addition to fibrosis have been extensively studied in patients with NAFLD. Other techniques such as transient elastography, magnetic resonance elastography and acoustic radiation force imaging are becoming more established as noninvasive methods of detecting fibrosis in a variety of chronic liver conditions in addition to NAFLD. Newer high throughput methods such as proteomics and glycomics allow the nonhypothesis-driven identification of novel markers and may also potentially contribute to our understanding of the pathogenesis of the condition. This review addresses some of the methodological issues which need to be considered in the search for the ideal biomarker. It is likely that a combination of serum biomarkers and techniques such as transient elastography may provide the optimal diagnostic discrimination however this remains to be proven in large studies.
Core tip: The search for non invasive biomarkers is a major focus of interest in the field of nonalcoholic fatty liver disease (NAFLD). Though the diagnosis of NAFLD is still a histological one, the dramatic rise in prevalence and the spectrum of severity mean that liver biopsy has become impractical for all. Both serum biomarkers of inflammation and fibrosis and assessment of fibrosis using techniques such as transient elastography may have a role to play. Newer techniques (the “omics”) may not only lead to novel biomarkers but also allow better understanding of the pathophysiology of the condition.
- Citation: Fitzpatrick E, Dhawan A. Noninvasive biomarkers in non-alcoholic fatty liver disease: Current status and a glimpse of the future. World J Gastroenterol 2014; 20(31): 10851-10863
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10851
Ultimately 10% to 28% of nonalcoholic steatohepatitis (NASH) patients develop cirrhosis and hepatocellular carcinoma[1-3]. The criterion standard for diagnosis and assessing progression of disease is liver histology, though this has inherent limitations. Still, the decision “if or when” to perform and repeat a liver biopsy in patients with nonalcoholic fatty liver disease (NAFLD) remains controversial. The prevalence of the condition is such that the resources needed to perform liver biopsy on every patient with NAFLD would be enormous. Liver biopsy often requires admission to hospital and sedation. Risks include bleeding and very rarely death[4]. For the same reason, repeated biopsy is not a suitable tool for regularly monitoring progression of disease or response to treatment. In addition, biopsy samples only 1/50000 of the liver, raising the possibility of sampling error[5].
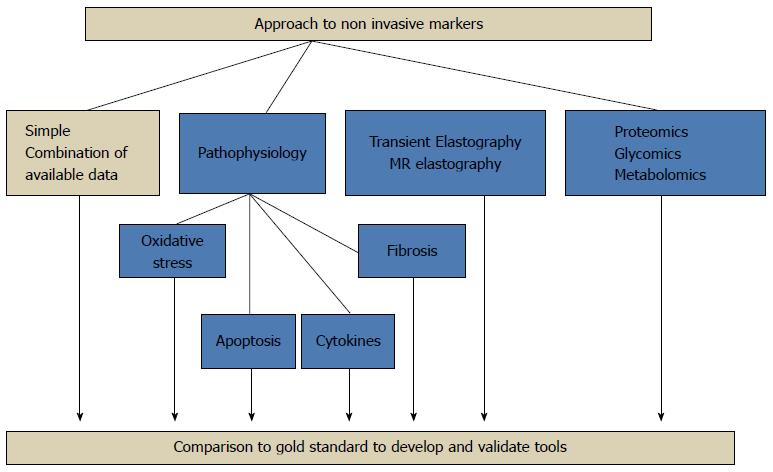
There has been much focus on the development and validation of noninvasive biomarkers of NAFLD in recent years. There is an urgent need for a less invasive method than biopsy of screening the population, stratifying disease severity and following disease progression. This is particularly relevant in the paediatric population. Many markers of inflammation, hepatocyte apoptosis, fibrosis and oxidative stress are under investigation. The European Association for the Study of the Liver special topic conference on NAFLD called for a renewed focus on noninvasive biomarkers of disease[6]. In common with all biomarkers which are “biological markers of disease presence and progression”[7], important characteristics include; sufficient sensitivity to identify those with disease, specificity to exclude those without disease, cost-effectiveness, ease of use and reproducibility. There are several different approaches to the identification of biomarkers: the first is the use of clinical or biochemical markers that have been derived from large association studies. The second is the use of algorithms including markers of extracellular matrix turnover in the case of fibrosis and inflammation/cell death in the case of inflammatory change. The third is the non-hypothesis driven new-technology based approach such as microarray techniques, proteomics and glycomics[8,9] (Figure 1).
The pathophysiology and evolution of the particular pathological condition is an important consideration in the development and evaluation of biomarkers. In the case of NAFLD; there are two potential targets. The first is the differentiation of simple steatosis from steatohepatitis. This is important as the prognosis of those with simple steatosis is different from those with NASH[10]. The second issue is the identification of fibrosis stage. This is the main determinant of prognosis and knowing the extent of fibrosis is useful in making treatment decisions, in patient selection for treatment studies and in monitoring progression/regression. Most longitudinal cohort studies in NAFLD have shown that prognosis is determined by stage and rate of progression of fibrosis rather than the presence of necro-inflammation[1,2,11]. Clinical importance lies with being able to differentiate between no/minimal fibrosis (F0/F1), significant fibrosis (F2), severe fibrosis (F3) and cirrhosis (F4).
Important issues to be considered in the design and validation of any noninvasive markers include the inherent limitations of liver biopsy as the criterion standard and the differences in prevalence of different disease stages (spectrum bias).
Variations in size of biopsy tissue, number of portal tracts and fragmentation will all influence accuracy of liver biopsy in determining the true stage of fibrosis as described previously[12,13]. In the case of NAFLD the degree of steatosis and inflammation is assessed separately to fibrosis and scoring systems such as the NASH activity score is used to distinguish simple steatosis from steatohepatitis. Both intra and interobserver variability may also significantly affect the score[14]. Thus, the ability of noninvasive biomarkers to differentiate between fibrosis stages is limited by the criterion standard.
Some of these issues in terms of scoring variability may be overcome using techniques such as collagen proportionate area quantification, however the limitations of a short or nonrepresentative biopsy remain.
The ideal outcome measure for any noninvasive biomarker is disease outcome over time, such as has been reported by Parkes et al[15]. Long-term outcomes (morbidity/mortality/need for transplantation) are the optimal measures, though are not feasible in shorter term studies.
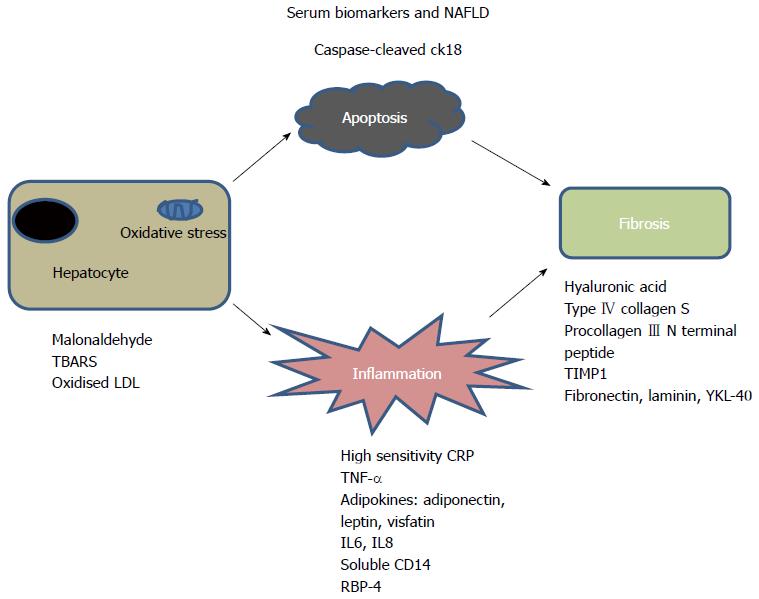
Large adult series have suggested scoring systems using age, BMI, insulin resistance, aspartate aminotransferase/alanine aminotransferase (AST/ALT), platelet count and albumin to differentiate mild from severe disease[16-19] (Table 1). These simple markers are neither sensitive nor specific enough in isolation[20,21]. A growing understanding of the pathophysiology of the disease has allowed the investigation of more specific, mechanism-based biomarkers. These biomarkers focus on the specific pathways involved in the progression of the disease process: hepatocyte apoptosis, oxidative stress, inflammation and fibrosis[8,22,23] (Figure 2).
| Biomarkers | Study description | Results | Ref. |
| Simple markers | Adults: 97 obese patients undergoing bariatric surgery, 35 had NASH | Algorithm using AST and presence of T2DM, AUC of 0.82 for prediction of NASH | [55] |
| Adults: 80 NAFLD; 39 SS, 41 NASH | Score using age, gender, AST, BMI, Hyaluronic acid, AST: ALT ratio. AUROC for NASH of 0.76 | [56] | |
| Adults: 200 patients undergoing bariatric surgery. 64 had NASH | AUROC for NASH: 0.8 using a score composed of Hypertension, Diabetes, AST > 27, ALT > 27, Sleep apnoea, non-black race | [53] | |
| Adults: 80 NAFLD; 39 SS, 41 NASH | Score using age, gender, AST, BMI, Hyaluronic acid, AST: ALT ratio. AUROC for NASH of 0.76 | [56] | |
| Inflammation | Adults: 57 NASH, 17 SS, 10 controls | AUROC NASH with HOMA-IR and Adiponectin/Leptin ratio: 0.82 | [32] |
| Adults: 26 NASH, 19 SS; 38 obese, 12 controls | TNF-α, IL8, Age, ALT higher in NAFLD; TNF-α predictor | [27] | |
| Adults: 20 NAFLD, 30 obese | Insulin resistance, ferritin, glutathione peroxidase, higher in NAFLD than obese | [23] | |
| Adults: 80 NASH, 29 simple steatosis | Lower Adiponectin, higher TNF-α, higher IR in NASH vs controls | [30] | |
| Lower Adiponectin, higher HOMA-IR in NASH vs SS | |||
| Paediatric: 36 training and 36 validation NAFLD | AUROC for Adiponectin/HOMA-IR as predictors of NASH: 0.79 AUROC for NASH using TNF-α was 0.91, Leptin: 0.8 combined: 0.96 | [29] | |
| Adults: 23 NASH, 21 SS, 18 controls | IL6 and TNF-α, TNFR1 higher in those with NASH vs rest TNF-α, CCL2/MCP-1 higher and Adiponectin lower in NASH | [35] | |
| Adults: 22 SS, 25 NASH, 30 controls | [28] | ||
| Adults: 28 NAFLD, 33 controls, 30 obese | Resistin linked to NAFLD severity, but not adiponectin, leptin or IR | [33] | |
| Paediatric: 59 NAFLD | RBP-4 levels inverse relationship with NASH | [57] | |
| Algorithms | |||
| NASH test | 257 patients (17% NASH) and 383 controls | AUROC 0.79 for NASH. 13 variables: Age, Sex, Weight Height, TG, cholesterol, α2-macroglobulin, ApoA1, Haptoglobin, AST, ALT, γGT, bilirubin | [58] |
| NASH Diagnostics | Adults: 101 NAFLD, 69 test, (32% NASH) 32 validation | AUROC 0.91 for prediction of NASH. Sensitivity 96%, specificity70% with combination of CK18-M65, CK18-M30, resistin and adiponectin | [44] |
| NAFIC score | Adult Japanese patients with NAFLD | AUROC for NASH in test group 0.85. AUROC for NASH in validation group 0.78. Variables: Ferritin, fasting insulin, type IV collagen S | [59] |
| 177 test group (95 NASH), 442 validation group | |||
| Nice model | Adults: 454 obese, 310 test, 154 validation | Model: AUROC for prediction of NASH 0.88 in test and 0.83 in validation set Algorithm: CK18-M30, ALT, presence of MS Combination of Insulin resistance, Hypertension and ALT gives sensitivity of 80% and specificity of 89% in prediction of NASH | [60] |
| HAIR | Adults: 105 obese patients undergoing bariatric surgery, including 26 with NASH | [50] |
Generic markers of inflammation such as ferritin and high sensitivity C-reactive protein show an association with NASH[24-26]. Adipokines and other cytokines have been shown to correlate well with presence and severity of the disease[27]. In particular, high serum levels of tumor necrosis factor-α (TNF-α) and low levels of adiponectin are associated with greater degree of liver damage[27-30]. Other adipocytokines; visfatin and leptin may be useful predictors of disease though there is inconsistent evidence[28,31]. Lemoine et al[32] found that the adiponectin: leptin ratio in combination with homeostasis model assessment insulin resistance index gave an area under the receiver operating characteristic (AUROC) curve of 0.82 for prediction of disease. Resistin was shown by Pagano et al[33] to correlate to severity of NASH in a study of 91 patients, but in another study was found to be lower in children with NASH vs simple steatosis[34]. Interleukin (IL)6 and IL8 have also been studied and found to have an AUROC of 0.8 for the prediction of NASH[35,36]. The results of circulating levels of adipokines as predictors of disease are inconsistent however and may not be sensitive or specific enough to act as robust biomarkers in isolation.
Markers of apoptosis/cell death have been shown to be very useful in differentiating simple steatosis from NASH[37]. The extrinsic (death receptor mediated) and intrinsic (organelle initiated) cell death pathways convene at the mitochondria with permeabilisation of the mitochondrial outer membrane and release of proteins from the mitochondrial inner membrane into the cytosol[38]. Activation of caspase 3 results in cleavage of cytokeratin 18 (CK18) which is a major intermediate filament in hepatocytes. CK18-M30 fragments have recently been shown by a number of studies to correlate well with severity of NASH[39-42]. A two step approach using CK-18 and FGF21 further improves accuracy in diagnosing NASH in one study[43]. CK18-M65 levels (antibodies which recognise uncleaved CK18) are used as biomarkers of total cell death[44] and in one study had equal AUROC to CK18 M30 (0.8) in detecting NASH. Changes in the biomarkers also correlated with histological progression[45].
Markers of oxidative stress including lipid peroxidation products, may also be useful biomarkers of disease. However these substances are relatively volatile and not always easily measured in serum. The relative importance of mitochondrial, peroxisomal, CYP450, Nitric oxygen synthetase and myeloperoxidase pathways is not yet known[46]. Malonaldehyde, thiobarbituric acid reactive substances (TBARS) and oxidised low density lipoprotein (LDL) have all been measured as markers of oxidative stress in patients with NASH but with some conflicting results[47,48]. The interaction of molecules such as oxidized LDL and TBARS with stellate cells may be important in promoting fibrosis[49].
A number of predictive models to differentiate either NAFLD from obese controls or simple steatosis from NASH have been developed and validated. Tools include the HAIR score (Hypertension, ALT, insulin resistance) which gives an AUROC of 0.9[50], and the NashTest® (consisting of 13 variables including weight, triglycerides, glucose, α2-macroglobulin and apolipoprotein A) which has an AUROC of 0.79 for differentiation of NASH from simple steatosis[51]. When the NashTest® is combined with the SteatoTest® (10 variables including simple blood tests, age, gender and BMI)[52] and the Fibrotest® into what is known as the Fibromax® panel, the diagnostic accuracy improves further[52]. Campos describes a NASH clinical scoring system using AST, hypertension, presence of type 2 diabetes, ALT, obstructive sleep apnoea and non-black ethnicity. This system has an AUROC of 0.75 for diagnosis of NASH[53]. NASH diagnostics uses a combination of CK 18-M30 and M65 levels with adiponectin and resistin values to give an AUROC of 0.91 in the test and 0.73 in the validation groups. A recent meta-analysis has evaluated the performance of the NashTest® and ActiTest® for the diagnosis of NASH in 494 obese patients with a prevalence of NASH of 17.2%. The weighted AUROC was significant for the diagnosis of NASH at 0.84 (0.82-0.86, P < 0.0001)[54].
It is the severity and rate of progression of fibrosis rather than inflammation per se that determines outcome in the majority of cases[55,56]. The importance of staging disease in the context of fibrosis across liver disease in general is thus manifold. Firstly in the development of treatment decision algorithms; this is particularly relevant in adult viral hepatitis. Secondly functional tests may be even better than biopsy or measurement of hepatic vein pressure gradient in predicting outcome and thus planning appropriate follow up and services[57,58]. Finally the diagnosis of cirrhosis is important so that surveillance for varices and hepatocellular carcinoma may be instigated. These issues are clearly applicable across the spectrum of chronic liver disease, not alone NAFLD[59,60].
Noninvasive markers of fibrosis may consist of simple bedside tests or indices which have been studied in large cohorts of patients with liver disease. These include the AST to platelet ratio index[61], the AST to ALT ratio[62], FIB-4[63] and the Forn’s index[64]. These tools have also been validated in the NAFLD population with AUROC between 0.67-0.86 for differentiation of severity of fibrosis[65-67]. Algorithms of simple markers derived from logistic regression analysis of large cohorts with NAFLD are also described. The BAAT score (consisting of BMI, ALT, age and triglyceride levels) has an AUROC of 0.86 for prediction of no fibrosis, 0.75 for F2, 0.92 for F3 and 0.81 for cirrhosis in NAFLD[68]. The BARD score (BMI, AST/ALT ratio, diabetes) was developed in a cohort of 827 patients with NAFLD and was found to be useful in excluding patients without advanced NAFLD[18,69]. Other panels of markers specific for NAFLD include the NAFLD fibrosis score (incorporating presence of diabetes, AST, ALT, BMI, platelets and albumin) giving an AUROC of 0.88 for advanced fibrosis[16]. This was validated by Shah et al[65] with an AUROC for advanced fibrosis of 0.77 and by McPherson et al[66] with an AUROC of 0.84. It has also been validated in Chinese[70] and bariatric surgery cohorts[71]. In a recent meta-analysis the AUROC for the NAFLD fibrosis score was found to be 0.85 with a pooled sensitivity of 90% and specificity of 97%[25].
Fibrometer™ incorporating age, weight, fasting glucose, AST, ALT, ferritin and platelets has been validated in a NAFLD population[67]. The test demonstrates an AUROC of 0.94 for significant fibrosis, 0.9 for severe fibrosis and 0.9 for cirrhosis.
The HAIR algorithm combines presence of systemic hypertension, elevated ALT and insulin resistance and has a sensitivity of 80% and specificity of 89% for NASH in patients undergoing bariatric surgery[50]. The FIB-4 score has an AUROC of 0.8 for advanced fibrosis in 541 patietns with NAFLD[65].
Other biomarkers measure the degree of extracellular matrix (ECM) turnover. Using such ECM markers is a more direct method of assessing fibrogenic activity, and will tend to measure a dynamic process rather than a static one. Hyaluronic acid is one of the most validated markers of fibrosis in liver disease, synthesised by stellate cells and metabolised by sinusoidal endothelial cells[72,73]. Hyaluronic acid was found to be an accurate marker of fibrosis in NAFLD[74,75].
Combinations of both clinical markers and ECM turnover include the FibroTest®[54,76,77], an algorithm of 13 markers derived from regression analysis including haptoglobin, α2-macroglobulin, apolipoprotein A1, bilirubin, γ-glutamyl transpeptidase, age and gender. It has an AUROC of 0.84 for advanced fibrosis in NAFLD[78].
The European Liver Fibrosis test (ELF)™ combining hyaluronic acid, procollagen III N-terminal peptide and TIMP1 was first derived by Rosenberg et al[79] in a cohort of over 1000 patients with chronic liver disease including NAFLD and has since been validated in other NAFLD cohorts with the addition of several simple markers to improve accuracy[80]. Importantly this test has been shown to correlate well with outcome[15].
Table 2 summarises previous studies investigating serum biomarkers of fibrosis in NAFLD[81-84].
| Biomarkers | Cohort | Results | Ref. |
| FibroTest®: α2macroglobulin, Apolipoprotein A1, Haptoglobin, γGT, Bilirubin | 267 patients | AUROC ≥ F2 0.8, ≥ F3 0.88 | [81] |
| NAFLD Fibrosis score: Age, BMI, Hyperglycaemia, Platelets, Albumin, AST/ALT | 733 patients | AUROC ≥ F3 0.88 | [16] |
| 331 patients | AUROC ≥ F3 0.82 | [71] | |
| 162 patients | AUROC ≥ F3 0.64 | [70] | |
| 91 patients | AUROC ≥ F3 0.89 | [80] | |
| 92 patients | AUROC ≥ F3 0.74 | [18] | |
| 235 patients | AUROC ≥ F2 0.88 | [67] | |
| 138 patients | AUROC ≥ F3 0.68 | [69] | |
| 246 patients | AUROC ≥ F2 0.62, ≥ F3 0.75 | [82] | |
| 588 patients | AUROC ≥ F3 0.85 | [59] | |
| 541 patients | AUROC ≥ F3 0.77 | [65] | |
| 145 patients | AUROC ≥ F3 0.81 | [66] | |
| BARD: | 827 patients | AUROC ≥ F3 0.81 | [18] |
| BMI, AST:ALT ratio, DM | 246 patients | AUROC ≥ F2 0.59, ≥ F3 0.64 | [82] |
| 138 patients | AUROC ≥ F3 0.67 | [69] | |
| 541 patients | AUROC ≥ F3 0.7 | [65] | |
| 145 patients | AUROC ≥ F3 0.77 | [66] | |
| ELFTM | 192 patients | AUROC ≥ F1 0.76, ≥ F2 0.82, ≥ F3 0.9 | [80] |
| Hyaluronic acid, P3NP, TIMP1 | 91 patients (plus simple markers) | AUROC ≥ F1 0.84, ≥ F2 0.93, ≥ F3 0.98 | [80] |
| 121 paediatric patients | AUROC ≥ F1 0.92, ≥ F2 0.98, ≥ F3 0.99 | [83] | |
| FibroMeterTM: | 235 patients | AUROC ≥ F2 0.94, ≥ F3 0.94 | [67] |
| APRI | 111 patients | AUROC advanced fibrosis 0.85 | [84] |
| 541 patients | AUROC ≥ F3 0.73 | [65] | |
| 145 patients | AUROC ≥ F3 0.67 | [66] | |
| 235 patients | AUROC ≥ F3 0.87 | [67] | |
| AST:ALT ratio | 541 patients | AUROC ≥ F3 0.74 | [65] |
| 145 patients | AUROC ≥ F3 0.83 | [66] | |
| BAAT: | 93 patients | AUROC ≥ F1 0.86, ≥ F2 0.9 | [68] |
| BMI Age ALT Triglycerides | |||
| FIB-4: | 541 patients | AUROC ≥ F3 0.8 | [65] |
| Age, AST, platelets, ALT | 145 patients | AUROC ≥ F3 0.86 | [66] |
Biomarkers of NAS and fibrosis have also been reported by a few paediatric studies as referenced below. These studies are relatively limited by the size of the cohorts involved and are mostly validation of adult biomarkers.
The following studies report predictors of NAFLD using routine clinical parameters in cohorts of obese children. Sartorio et al[85] reported a multivariate analysis of 267 obese children and found that BMI Z-score, ALT, uric acid, glucose and insulin were useful predictors of NAFLD. Mandato reported insulin resistance, ferritin, C-reactive protein and glutathione peroxidase as good discriminators of those with NAFLD from those without in a cohort of obese children[23]. Neither of these studies used a histological diagnosis of NAFLD.
Adipocytokines have been investigated in a number of studies. Manco et al[29] found that TNF-α and leptin were significantly different in groups of children with NAS ≥ 5 and NAS < 5[29,86]. Louthan et al[87] also used an adipocytokine profile to discriminate steatohepatitis. Other markers include retinal binding protein-4[57] and Fetiun A[88], both of which have been shown to reliably distinguish NASH from simple steatosis/simple obese controls in paediatric studies.
Alisi et al[89] investigated both endotoxin and plasminogen activator inhibitor 1 (PAI-1) levels in serum of 40 children with NAFLD and 9 controls and with multivariate analysis found that endotoxin (P < 0.0001) and PAI-1 (P = 0.009) were significantly higher in patients with a histological score of NAS ≥ 5. Our group has also reported that the CK18-M30 fragment level is a good discriminator of NASH vs simple steatosis[90] following on from the validation of the marker in a large group of adult patients with NAFLD[91].
As with adult studies, the noninvasive diagnosis of fibrosis (rather than necro-inflammatory change) in NAFLD is considered separately. It is important to acknowledge that the different distribution of fibrosis in paediatric patients may affect the validity of applying measures derived from adult cohorts to this population.
Iacobellis et al[19] reported a cohort of 69 children with NAFLD, 60% of whom had fibrosis. They found that BMI was the only significant predictor of fibrosis with multivariable analysis of simple clinical parameters. BMI had an odds ratio of 5.85 for predicting presence of fibrosis. Manco et al[92] found waist circumference as a significant predictor of fibrosis in a cohort of 197 children with NAFLD (OR = 2.4, 95%CI: 1.04-5.54). In both these studies the number of children in the F2-F4 groups was small.
Nobili et al[93] developed and internally validated the pediatric NAFLD fibrosis index (PNFI) in 136 children with NAFLD. Logistic regression analysis of gender, age, BMI, waist circumference, ALT, AST, γGT, albumin, prothrombin time, glucose, insulin, cholesterol and triglyceridesa were used to develop a predictive model called the paediatric NAFLD with an AUROC for detection of fibrosis was 0.85. Again this study was limited in view of small numbers in fibrosis groups F2-F4.
The ELF™ test was evaluated by Nobili et al[83] in 122 children with NAFLD. Simple markers including age, waist circumference and triglycerides were added to improve diagnostic accuracy. Excellent AUROC for any (0.92), significant (0.98) and advanced (0.99) disease were achieved. In this cohort 37 (30%) had no fibrosis, 58 (48%) scored as F1, 9 (7%) as F2, and 8 (6.5%) as F3-F4. Alkhouri et al[94] developed this further and validated both the PNFI and ELF™ in a cohort of 111 children with NAFLD (69% with fibrosis). The area under the curve for presence of fibrosis was 0.76 for PNFI, 0.92 for ELF™ and when the two indices were combined: 0.94. The major issue in both studies was the skew towards no or minimal disease, potentially overestimating the accuracy of the test.
Ultrasound (US) has a high sensitivity and specificity for diagnosis of steatosis > 30%, but is not good at detecting fibrosis. Because of the low cost, the absence of radiation exposure and the wide availability, US is often used in screening for NAFLD. The accumulation of fat causes the liver to appear hyperechoic compared with the kidney. This finding is nonspecific and does not differentiate fat from other substances such as glycogen. When compared with histological findings, the sensitivity of US to detect fat infiltration below 30% of the liver is low[95]. Computed tomography (CT) is rarely used for the assessment of NAFLD in children because of its ionizing radiation exposure. Magnetic resonance imaging (MRI) and spectroscopy are the imaging techniques with the greatest accuracy to determine hepatic fat content in studies of both adults and children[96-99]. Aside from liver fat, however, other features of NASH cannot be assessed. Other methods include MR elastography which visualises and measures propagating shear waves and has a high sensitivity (> 85%) and specificity (> 90%) for fibrosis[100]. Cost of this technique may be preclusive however.
For diagnosis of NASH, Iijima et al[101] have reported on the use of contrast ultrasound with Levovist with an AUC of 1.0. The decreased accumulation of micro-bubbles with advancing degree of fibrosis is unique to NAFLD.
Two recent reports have examined the use of acoustic radiation force-based shear stiffness in NAFLD, an ultrasound based investigation which correlates well with the stage of fibrosis in the condition[102,103].
Transient elastography (Fibroscan®) has been shown to be a useful method for detection of liver fibrosis. This technique uses both ultrasound (5 MHz) and low frequency (50 Hz) elastic waves with a propagation velocity directly related to the stiffness of the liver; i.e., the stiffer the medium, the faster the wave. The low frequency vibrations are transmitted to the skin by placement of the probe at the intercostal space where a liver biopsy would be performed. A shear wave is induced which propagates into the liver. The wave passes through tissue 2.5-6.5 cm below skin surface, (in those 0 to 7 years a modified probe which can measure 2.5-5.5 cm is used). A pulse-echo acquisition is then used to measure the propagating wave’s velocity which is proportional to tissue stiffness represented by the equation for Young’s elastic modulus E (3pv2) (p = density, v = shear velocity). Machine based software determines whether each measurement is successful or not. Requirements for accurate evaluation of liver stiffness include an interquartile range of +/- 30% of the median value and ratio of successful measurements to the total no of acquisitions > 60%.
Transient elastography (TE) has been well validated and was the subject of a recent systematic review of 50 studies which concluded that Fibroscan® has excellent diagnostic capability across different liver diseases for cirrhosis[104]. There was some variability for diagnosis of lesser degrees of fibrosis.
In NAFLD, a number of studies have demonstrated the efficacy of TE in distinguishing severity of fibrosis. In a study of 246 adults with NAFLD, TE had an AUROC of 0.84, 0.93 and 0.95 in distinguishing significant fibrosis, severe fibrosis and cirrhosis respectively[82]. A Japanese study demonstrated similar results[105]. A recent report of 52 children with NAFLD has shown an AUROC of 0.977, 0.992 and 1 for distinguishing any, significant and severe fibrosis[106]. Feasibility and reproducibility of transient elastography is an issue when patients have a BMI > 30[107,108]. An XL probe is now available for better accuracy in this scenario[108,109] demonstrating reliable measurements in 73% using the XL probe vs 50% with the S probe[108].
This is a technology similar to TE in which a region in the liver is targeted and using real-time B-mode ultrasound imaging, the measured shear wave speed is observed at several locations and quantified. Tracking beams are applied adjacent to the push pulse path until the passing shear wave front is detected. The time between the generation of the shear wave and the detection of the peak is used to compute shear wave velocity. Again, this should be proportionate to stiffness of the tissue. This technique has the relative advantage of being able to select an appropriate area for analysis. It is emerging as an effective tool for differentiation of no/mild fibrosis from more severe fibrosis in patients with NAFLD[108,110] with an AUROC of 0.9 in one study[111].
MR may be useful in detection of steatosis as above however the differentiation of patients with advanced disease from those with simple steatosis requires assessment of fibrosis. Similarly to transient elastography, MR elastography (MRE) may be a useful tool in this regard. Kim et al[112] report a comparison of MRE to 6 laboratory based models of fibrosis in 142 patients with liver biopsy-confirmed NAFLD. The cut off for advanced fibrosis in this cohort was 4.15 kPa with an AUROC of 0.954, a sensitivity of 0.85 and specificity of 0.929. They found that MRE could potentially be a useful tool but did not meet the sensitivity or specificity of the NAFLD fibrosis score or the FIB-4 score.
Chen et al[113] studied 58 patients with NAFLD and found that liver stiffness using a threshold of ≥ 2.74 kPA could differentiate patients with NASH from simple steatosis with a sensitivity of 94% and a sensitivity of 73% (AUROC 0.94).
The use of relatively new, high throughput techniques such as proteomics, glycomics and microarray studies in the derivation of panels of biomarkers associated with a disease may also give an insight into pathophysiology of the condition.
Younossi et al[114] found 34 different expression of genes in those with NASH vs controls. Four were confirmed using real time reverse transcription PCR. Sreekumar et al[115] found 16 genes expressed differently in NASH-associated cirrhosis vs other aetiologies; mainly genes which were involved in the anti-oxidant response as well as fat and carbohydrate metabolism. Yoneda et al[116] performed a microarray analysis of NASH vs simple steatosis and found expression of 27 genes at higher levels in NASH. The upregulated gene sets included those responsible for the platelet derived growth factor, hepatic nuclear factor 3 and the smad4 pathways.
Proteomic studies use pattern recognition with subtraction. Several previous studies have reported different protein peaks in the serum of those with NASH vs simple steatosis[117,118]. Two important proteomic studies using liver tissue and serum respectively of adult patients with and without NAFLD revealed an increased expression of lumican, (a keratan sulphate proteoglycan involved in collagen cross-linking and epithelial-mesenchymal transition) in patients with NASH vs normal and simple steatosis[119,120]. Yu et al[121] used proteomics to demonstrate that higher baseline haemoglobin values were associated with the development of NAFLD in a prospective study of 6944 subjects.
Glycosylation is the post-translational modification of secreted proteins with carbohydrate moieties conveying structural diversity and with a possible role in protein folding and in cell to cell interaction including migration, solubility and receptor attachment[122,123]. Changes in glycosylation serve as a particularly good marker of liver dysfunction for a number of reasons. Most glycoproteins in serum (aside from IgG) are made in the liver. Thus, the N-glycome profile will reflect any changes in either the liver or B cell function. In addition, both the asialoglycoprotein receptor and the mannose/O-linked beta-N-acetylglucosamine receptor in liver are important in clearing aberrantly glycosylated proteins from the serum. In the presence of architectural disarray, these receptors are decreased in number and thus there is a build-up of glycoproteins in serum[124]. With a systems biology approach to the analysis using high-throughput technology, serum N-glycomics may prove to be valuable biomarkers of disease.
Previously reported glycomic analysis of liver disease include the development of the GlycoCirrhotest[125], the GlycoFibrotest[126], and the GlycoHCC test[127] which can predict the presence of cirrhosis, fibrosis and hepatocellular carcinoma respectively due to difference in N-glycome patterns. Two recent studies have investigated the potential of Glycomics in non-invasive evaluation of NAFLD[128-130].
Glycomics was also demonstrated to have a tole n biomarker discovery in paediatric NAFLD[131].
In view of the high prevalence of NAFLD in the population, in both adults and children, and the fact that up to a one third will develop end stage liver disease and/or hepatocellular carcinoma, it is important that we develop noninvasive methods to diagnose and monitor this liver condition. A differentiation needs to be made between those with advance disease/or are at risk of developing advanced disease from those who have simple steatosis and are unlikely to progress. Liver biopsy is not a practical tool for this mass screening though the disease is still defined histologically. Noninvasive biomarkers either in blood or imaging techniques show promise in this context and in many centres are used routinely. It is possible that a combination of blood biomarkers with methods such as transient elastography or acoustic radiation force impulse may yield the highest diagnostic discrimination. New techniques such as proteomics and glycomics may not only allow development of novel markers but also allow us a better insight into the pathophysiology of the condition.
P- Reviewer: Corrales FJ, Sinakos E, Vassalle C S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] [Cited in This Article: ] |
| 2. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Kim KM, Choi WB, Park SH, Yu E, Lee SG, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Diagnosis of hepatic steatosis and fibrosis by transient elastography in asymptomatic healthy individuals: a prospective study of living related potential liver donors. J Gastroenterol. 2007;42:382-388. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Cadranel JF. [Good clinical practice guidelines for fine needle aspiration biopsy of the liver: past, present and future]. Gastroenterol Clin Biol. 2002;26:823-824. [PubMed] [Cited in This Article: ] |
| 5. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [PubMed] [Cited in This Article: ] |
| 6. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054-1061. [PubMed] [Cited in This Article: ] |
| 8. | Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582-589. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Miller MH, Ferguson MA, Dillon JF. Systematic review of performance of non-invasive biomarkers in the evaluation of non-alcoholic fatty liver disease. Liver Int. 2011;31:461-473. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375-378. [PubMed] [Cited in This Article: ] |
| 11. | Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373-375. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Poynard T, Halfon P, Castera L, Charlotte F, Le Bail B, Munteanu M, Messous D, Ratziu V, Benhamou Y, Bourlière M. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther. 2007;25:733-739. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, Alexander G, Ramage J, Dusheiko G. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245-1251. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Guha IN, Parkes J, Roderick PR, Harris S, Rosenberg WM. Non-invasive markers associated with liver fibrosis in non-alcoholic fatty liver disease. Gut. 2006;55:1650-1660. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441-1447. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Iacobellis A, Marcellini M, Andriulli A, Perri F, Leandro G, Devito R, Nobili V. Non invasive evaluation of liver fibrosis in paediatric patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2006;12:7821-7825. [PubMed] [Cited in This Article: ] |
| 20. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792-798. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis. 2008;40:371-378. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Mandato C, Lucariello S, Licenziati MR, Franzese A, Spagnuolo MI, Ficarella R, Pacilio M, Amitrano M, Capuano G, Meli R. Metabolic, hormonal, oxidative, and inflammatory factors in pediatric obesity-related liver disease. J Pediatr. 2005;147:62-66. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Targher G. Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. J Hepatol. 2006;45:879-881; author reply 881-882. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [PubMed] [Cited in This Article: ] |
| 26. | Hui JM, Farrell GC, Kench JG, George J. High sensitivity C-reactive protein values do not reliably predict the severity of histological changes in NAFLD. Hepatology. 2004;39:1458-1459. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412-421. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167-1174. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954-960. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46-54. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Le D, Marks D, Lyle E, Corless CL, Diggs BS, Jobe BA, Kay T, Deveney CW, Wolfe BM, Roberts CT. Serum leptin levels, hepatic leptin receptor transcription, and clinical predictors of non-alcoholic steatohepatitis in obese bariatric surgery patients. Surg Endosc. 2007;21:1593-1599. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, Bastard JP, Poupon R, Housset C, Capeau J. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431-1438. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Pagano C, Soardo G, Pilon C, Milocco C, Basan L, Milan G, Donnini D, Faggian D, Mussap M, Plebani M. Increased serum resistin in nonalcoholic fatty liver disease is related to liver disease severity and not to insulin resistance. J Clin Endocrinol Metab. 2006;91:1081-1086. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Fitzpatrick E, Dew TK, Quaglia A, Sherwood RA, Mitry RR, Dhawan A. Analysis of adipokine concentrations in paediatric non-alcoholic fatty liver disease. Pediatr Obes. 2012;7:471-479. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39-45. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372-1379. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [PubMed] [Cited in This Article: ] |
| 38. | Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708-1717. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, Feldstein AE. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249-1254. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N, Afendy A, Younoszai Z, Goodman Z, Baranova A. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21:431-439. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013;108:1526-1531. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Shen J, Chan HL, Wong GL, Choi PC, Chan AW, Chan HY, Chim AM, Yeung DK, Chan FK, Woo J. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012;56:1363-1370. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, Rafiq N, Goodman Z, Chandhoke V, Baranova A. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes Surg. 2008;18:1430-1437. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Shen J, Chan HL, Wong GL, Chan AW, Choi PC, Chan HY, Chim AM, Yeung DK, Yu J, Chu WC. Assessment of non-alcoholic fatty liver disease using serum total cell death and apoptosis markers. Aliment Pharmacol Ther. 2012;36:1057-1066. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:46-53. [PubMed] [Cited in This Article: ] |
| 47. | Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850-855. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497-1502. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121-138. [PubMed] [Cited in This Article: ] |
| 50. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [PubMed] [Cited in This Article: ] |
| 51. | Felice MS, Hammermuller E, De Dávila MT, Ciocca ME, Fraquelli LE, Lorusso AM, Sackmann-Muriel F. Acute lymphoblastic leukemia presenting as acute hepatic failure in childhood. Leuk Lymphoma. 2000;38:633-637. [PubMed] [Cited in This Article: ] |
| 52. | Munteanu M, Ratziu V, Morra R, Messous D, Imbert-Bismut F, Poynard T. Noninvasive biomarkers for the screening of fibrosis, steatosis and steatohepatitis in patients with metabolic risk factors: FibroTest-FibroMax experience. J Gastrointestin Liver Dis. 2008;17:187-191. [PubMed] [Cited in This Article: ] |
| 53. | Campos GM, Bambha K, Vittinghoff E, Rabl C, Posselt AM, Ciovica R, Tiwari U, Ferrel L, Pabst M, Bass NM. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916-1923. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Poynard T, Lassailly G, Diaz E, Clement K, Caïazzo R, Tordjman J, Munteanu M, Perazzo H, Demol B, Callafe R. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS One. 2012;7:e30325. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399-408. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26:151-156. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Nobili V, Alkhouri N, Alisi A, Ottino S, Lopez R, Manco M, Feldstein AE. Retinol-binding protein 4: a promising circulating marker of liver damage in pediatric nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:575-579. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257-268. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M, Amor IB, Staccini-Myx A, Saint-Paul MC, Berthier F. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther. 2010;32:1315-1322. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Fraquelli M, Bardella MT, Peracchi M, Cesana BM, Bianchi PA, Conte D. Gallbladder emptying and somatostatin and cholecystokinin plasma levels in celiac disease. Am J Gastroenterol. 1999;94:1866-1870. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734-739. [PubMed] [Cited in This Article: ] |
| 63. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Santangelo A, Peracchi M, Conte D, Fraquelli M, Porrini M. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br J Nutr. 1998;80:521-527. [PubMed] [Cited in This Article: ] |
| 65. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Calès P, Lainé F, Boursier J, Deugnier Y, Moal V, Oberti F, Hunault G, Rousselet MC, Hubert I, Laafi J. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. 2009;50:165-173. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. [PubMed] [Cited in This Article: ] |
| 69. | Ruffillo G, Fassio E, Alvarez E, Landeira G, Longo C, Domínguez N, Gualano G. Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:160-163. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Wong VW, Wong GL, Chim AM, Tse AM, Tsang SW, Hui AY, Choi PC, Chan AW, So WY, Chan FK. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008;103:1682-1688. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Qureshi K, Clements RH, Abrams GA. The utility of the “NAFLD fibrosis score” in morbidly obese subjects with NAFLD. Obes Surg. 2008;18:264-270. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Piperno A, Sampietro M, Pietrangelo A, Arosio C, Lupica L, Montosi G, Vergani A, Fraquelli M, Girelli D, Pasquero P. Heterogeneity of hemochromatosis in Italy. Gastroenterology. 1998;114:996-1002. [PubMed] [Cited in This Article: ] |
| 73. | Hartley JL, Brown RM, Tybulewicz A, Hayes P, Wilson DC, Gillett P, McKiernan P. Hyaluronic acid predicts hepatic fibrosis in children with hepatic disease. J Pediatr Gastroenterol Nutr. 2006;43:217-221. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Kaneda H, Hashimoto E, Yatsuji S, Tokushige K, Shiratori K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2006;21:1459-1465. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779-786. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Colli A, Cocciolo M, Mumoli N, Cattalini N, Fraquelli M, Conte D. Hepatic artery resistance in alcoholic liver disease. Hepatology. 1998;28:1182-1186. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Zubizarreta P, Felice MS, Alfaro E, Fraquelli L, Casak S, Quinteros R, Cygler A, Gallego M, Pérez LE, Sackmann-Muriel F. Acute myelogenous leukemia in Down’s syndrome: report of a single pediatric institution using a BFM treatment strategy. Leuk Res. 1998;22:465-472. [PubMed] [Cited in This Article: ] |
| 78. | Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] [Cited in This Article: ] |
| 80. | Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD, Aithal GP. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455-460. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, Pinzani M, Rosenberg WM. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160-167. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Kruger FC, Daniels CR, Kidd M, Swart G, Brundyn K, van Rensburg C, Kotze M. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011;101:477-480. [PubMed] [Cited in This Article: ] |
| 85. | Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, Bedogni G. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61:877-883. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Nobili V, Manco M, Ciampalini P, Diciommo V, Devito R, Piemonte F, Comparcola D, Guidi R, Marcellini M. Leptin, free leptin index, insulin resistance and liver fibrosis in children with non-alcoholic fatty liver disease. Eur J Endocrinol. 2006;155:735-743. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005;147:835-838. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:4479-4485. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Alisi A, Manco M, Devito R, Piemonte F, Nobili V. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2010;50:645-649. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr. 2010;51:500-506. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Manco M, Bedogni G, Marcellini M, Devito R, Ciampalini P, Sartorelli MR, Comparcola D, Piemonte F, Nobili V. Waist circumference correlates with liver fibrosis in children with non-alcoholic steatohepatitis. Gut. 2008;57:1283-1287. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Alkhouri N, Carter-Kent C, Lopez R, Rosenberg WM, Pinzani M, Bedogni G, Feldstein AE, Nobili V. A combination of the pediatric NAFLD fibrosis index and enhanced liver fibrosis test identifies children with fibrosis. Clin Gastroenterol Hepatol. 2011;9:150-155. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [PubMed] [Cited in This Article: ] |
| 96. | Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, Stevens WR. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619-625. [PubMed] [Cited in This Article: ] |
| 97. | Radetti G, Kleon W, Stuefer J, Pittschieler K. Non-alcoholic fatty liver disease in obese children evaluated by magnetic resonance imaging. Acta Paediatr. 2006;95:833-837. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287-4294. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Pacifico L, Celestre M, Anania C, Paolantonio P, Chiesa C, Laghi A. MRI and ultrasound for hepatic fat quantification: relationships to clinical and metabolic characteristics of pediatric nonalcoholic fatty liver disease. Acta Paediatr. 2007;96:542-547. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332-342. [PubMed] [Cited in This Article: ] |
| 101. | Iijima H, Moriyasu F, Tsuchiya K, Suzuki S, Yoshida M, Shimizu M, Sasaki S, Nishiguchi S, Maeyama S. Decrease in accumulation of ultrasound contrast microbubbles in non-alcoholic steatohepatitis. Hepatol Res. 2007;37:722-730. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [PubMed] [DOI] [Cited in This Article: ] |
| 103. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [PubMed] [Cited in This Article: ] |
| 104. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330-1331. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, Marcellini M, Pinzani M. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442-448. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325-e331. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int. 2010;30:1043-1048. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | Guzmán-Aroca F, Frutos-Bernal MD, Bas A, Luján-Mompeán JA, Reus M, Berná-Serna Jde D, Parrilla P. Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging. Eur Radiol. 2012;22:2525-2532. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis. 2011;12:10-16. [PubMed] [Cited in This Article: ] |
| 112. | Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411-419. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Younossi ZM, Gorreta F, Ong JP, Schlauch K, Del Giacco L, Elariny H, Van Meter A, Younoszai A, Goodman Z, Baranova A. Hepatic gene expression in patients with obesity-related non-alcoholic steatohepatitis. Liver Int. 2005;25:760-771. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Sreekumar R, Rosado B, Rasmussen D, Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology. 2003;38:244-251. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Yoneda M, Endo H, Mawatari H, Nozaki Y, Fujita K, Akiyama T, Higurashi T, Uchiyama T, Yoneda K, Takahashi H. Gene expression profiling of non-alcoholic steatohepatitis using gene set enrichment analysis. Hepatol Res. 2008;38:1204-1212. [PubMed] [DOI] [Cited in This Article: ] |
| 117. | Younossi ZM, Baranova A, Ziegler K, Del Giacco L, Schlauch K, Born TL, Elariny H, Gorreta F, VanMeter A, Younoszai A. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology. 2005;42:665-674. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Trak-Smayra V, Dargere D, Noun R, Albuquerque M, Yaghi C, Gannagé-Yared MH, Bedossa P, Paradis V. Serum proteomic profiling of obese patients: correlation with liver pathology and evolution after bariatric surgery. Gut. 2009;58:825-832. [PubMed] [DOI] [Cited in This Article: ] |
| 119. | Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W. Cell-mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl. 2008;14:688-694. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634. [PubMed] [Cited in This Article: ] |
| 121. | Yu C, Xu C, Xu L, Yu J, Miao M, Li Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J Hepatol. 2012;56:241-247. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Blomme B, Van Steenkiste C, Callewaert N, Van Vlierberghe H. Alteration of protein glycosylation in liver diseases. J Hepatol. 2009;50:592-603. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, Kitazume S, Taniguchi N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008;99:1304-1310. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Burgess JB, Baenziger JU, Brown WR. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology. 1992;15:702-706. [PubMed] [Cited in This Article: ] |
| 125. | Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429-434. [PubMed] [Cited in This Article: ] |
| 126. | Vanderschaeghe D, Laroy W, Sablon E, Halfon P, Van Hecke A, Delanghe J, Callewaert N. GlycoFibroTest is a highly performant liver fibrosis biomarker derived from DNA sequencer-based serum protein glycomics. Mol Cell Proteomics. 2009;8:986-994. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Liu XE, Desmyter L, Gao CF, Laroy W, Dewaele S, Vanhooren V, Wang L, Zhuang H, Callewaert N, Libert C. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426-1435. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421-429. [PubMed] [Cited in This Article: ] |
| 129. | Chen C, Schmilovitz-Weiss H, Liu XE, Pappo O, Halpern M, Sulkes J, Braun M, Cohen M, Barak N, Tur-Kaspa R. Serum protein N-glycans profiling for the discovery of potential biomarkers for nonalcoholic steatohepatitis. J Proteome Res. 2009;8:463-470. [PubMed] [DOI] [Cited in This Article: ] |
| 130. | Blomme B, Francque S, Trépo E, Libbrecht L, Vanderschaeghe D, Verrijken A, Pattyn P, Nieuwenhove YV, Putte DV, Geerts A. N-glycan based biomarker distinguishing non-alcoholic steatohepatitis from steatosis independently of fibrosis. Dig Liver Dis. 2012;44:315-322. [PubMed] [Cited in This Article: ] |
| 131. | Blomme B, Fitzpatrick E, Quaglia A, De Bruyne R, Dhawan A, Van Vlierberghe H. Serum protein N-glycosylation in paediatric non-alcoholic fatty liver disease. Pediatr Obes. 2012;7:165-173. [PubMed] [Cited in This Article: ] |