INTRODUCTION
Maintenance of cholesterol homeostasis in the body requires accurate metabolic cross-talk between processes that govern de novo cholesterol synthesis and turnover to adequately cope with (large) fluctuations in dietary cholesterol intake. Imbalance may lead to elevated plasma cholesterol levels and increased risk for cardiovascular diseases (CVD), the main cause of death in Western society. A multitude of epidemiological studies has shown the direct link between high plasma cholesterol, particularly of low density lipoprotein (LDL) cholesterol, and risk for CVD. Treatment of high plasma cholesterol has been focused for many years on interference with cholesterol synthesis by application of statins. Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-controlling enzyme in the cholesterol biosynthesis pathway[1]. Inhibition of cholesterol synthesis leads to reduced production of very low density lipoprotein (VLDL) particles by the liver and particularly, up-regulation of LDL receptor activity. Both processes contribute to lowering of plasma LDL-cholesterol levels[2]. Large clinical trials have established the beneficial effects of statin treatment[3]. However, a relative large number of hypercholesterolaemic patients do not adequately respond to statin therapy or remain at risk for CVD despite substantial reductions in LDL cholesterol[4,5]. Consequently, alternative strategies are currently actively pursued, particularly high density lipoprotein (HDL)-raising approaches. These approaches are considered particularly promising, as data from epidemiological studies indicate that every 1 mg/dL increase in HDL cholesterol reduces CVD risk by 2%-3%[6,7]. In addition, strategies aiming at interference with intestinal cholesterol metabolism are gaining interest. A major development has been the introduction of ezetimibe, a potent inhibitor of intestinal cholesterol absorption that reduces plasma LDL-cholesterol by approximately 20% in mildly hypercholesteroleamic patients[8]. Likewise, phytosterol/stanol (esters)-enriched functional foods have successfully been introduced for lowering of plasma cholesterol levels through interference with cholesterol absorption[9].
Recently obtained insights in intestinal cholesterol trafficking may open even more promising avenues for further developments. It appears that the intestine actively excretes cholesterol and thereby, significantly contributes to fecal sterol excretion. In addition, it appears that the intestine is an important source of HDL-cholesterol, also known as “good” cholesterol. Thus, the intestine is an attractive target for new therapeutic strategies aimed to alter plasma cholesterol profiles and to reduce the risk for CVD. This review summarizes the important new findings regarding the mechanism(s) of intestinal cholesterol absorption, with specific focus on newly identified transporter proteins, the novel concept of direct intestinal cholesterol secretion and the role of the intestine in HDL biogenesis.
SOME BASIC FEATURES OF CHOLESTEROL
Cholesterol is essential for mammalian life as a structural component of cellular membranes, influencing membrane organization and thereby membrane properties[10]. Cholesterol is the precursor molecule of steroid hormones and therefore, essential for metabolic control. In the liver, cholesterol can be converted into bile salts, which represents the major pathway for cholesterol metabolism in quantitative sense. Bile salts are amphipathic molecules that facilitate the absorption of dietary cholesterol, fats and fat-soluble vitamins in the small intestine. Recently, it has become clear that bile salts are able to regulate gene expression through activation of the nuclear receptor, the farnesoid X receptor (FXR)[11-13]. Cholesterol or more correctly, oxidized cholesterol acts as a ligand for the nuclear liver X receptor (LXR or NRH2 or NRH3) and directly contributes to regulation of expression of genes involved in cholesterol, lipid, and glucose metabolism. Accumulation of free cholesterol rather than cholesterylesters, has been shown to induce apoptosis in macrophages by activating the Fas pathway[14]. Thus, cholesterol is a key component in cellular and whole-body physiology and cholesterol homeostasis is tightly regulated at a variety of levels.
Body cholesterol derives from two sources, i.e., de novo biosynthesis and diet. Cholesterol is synthesized from two-carbon acetyl-CoA moieties. The rate-controlling enzyme in the synthetic pathway is HMG-CoA reductase, a highly regulated enzyme that catalyses the conversion of HMG-CoA into mevalonate. Cholesterol itself regulates feed-back inhibition of HMG-CoA reductase activity, as accumulation of sterols in the endoplasmic reticulum (ER) membrane triggers HMG-CoA reductase to bind to Insig proteins, which leads to ubiquitination and degradation of HMG-CoA reductase[15,16]. In addition, cholesterol regulates the gene expression of HMG-CoA reductase indirectly by blocking the activation of the transcription factor, sterol regulatory element-binding protein 2 (SREBP2). Under low-cholesterol conditions, SREBP2 in the ER binds to the SREBP cleavage activating protein (SCAP), which escorts SREBP2 to the Golgi. In the Golgi, SREBP2 is cleaved to generate its transcriptionally active form, which activates transcription of the HMG-CoA reductase encoding gene. Upon accumulation of sterols in the ER-membrane, binding of cholesterol to the sterol-sensing domain of SCAP causes a conformation change, which induces binding of SCAP to the ER anchor protein Insig, preventing exit of SCAP-SREBP2 complexes to the Golgi thereby preventing activation of SREBP2[17] .
The contribution of the two sources to the total pool of cholesterol differs between species and prevailing diet composition, but the total cholesterol pool is similar in rodents and humans when expressed on the basis of body weight[18]. Cholesterol synthesis in the liver is highly sensitive to the amount of (dietary) cholesterol that reaches the liver from the intestine via the chylomicron-remnant pathway[19]. The Western-type human diet provides approximately 400 mg of cholesterol per day. On top of this, the liver secretes approximately 1 gram of cholesterol into bile per day[20]. Intestinal cholesterol absorption efficiency in humans is highly variable, ranging from 15% to 85% in healthy subjects[21]. After uptake by enterocytes, cholesterol is packed with triglycerides into chylomicrons and secreted into the lymph. In the circulation, the triglycerides are rapidly hydrolyzed and free fatty acids are taken up by the peripheral tissues. Cholesterol-enriched chylomicron remnants are subsequently cleared by the liver. Since chylomicron remnants, which contain most of the cholesterol that is being absorbed from the intestine, are rapidly taken up by the liver, interference with the absorption process directly influences hepatic cholesterol synthesis.
The healthy liver is perfectly equipped for handling large amounts of cholesterol. When relatively large amounts of cholesterol reach the liver, de novo synthesis and LDL uptake are rapidly down-regulated. In addition, the liver can dispose excess cholesterol molecules in several ways. A rapid response involves esterification of cholesterol by Acyl CoA cholesterol acyltransferase (ACAT) 2 for storage as cholesterylesters in cytoplasmic lipid droplets. Cholesterylester can be hydrolyzed when necessary and this esterification/hydrolysis cycle provides cells with short-term buffering capacity for cholesterol. The liver, like the intestine, is able to produce and secrete VLDL particles, which consist of a neutral lipid core composed of cholesterylesters and triacylglycerols and a monolayer surface containing phospholipids, free cholesterol, and a variety of apolipoproteins. Finally, cholesterol can be converted into bile acids by the hepatocytes, followed by their secretion into the bile along with significant amounts of free cholesterol and phosphatidylcholine. In humans, cholesterol lost via the feces consists of approximately 50% acidic (= bile acids) and 50% neutral sterols, emphasizing the point that conversion into bile acids represents a major pathway for cholesterol elimination.
Peripheral cells, e.g., macrophages, muscle and fat cells, are not able to form lipoproteins or to metabolize cholesterol extensively. Therefore, these cell types depend massively on efflux pathways for removal of their excess cholesterol. It is generally assumed that HDL is the primary acceptor for cholesterol efflux from cells. HDL cholesterol can subsequently be taken up by the liver for further processing. This pathway is generally referred to as the reverse cholesterol transport (RCT) pathway. The RCT pathway is particularly important for removal of excess cholesterol from macrophages, as accumulation of esterified cholesterol in these cells is considered a primary step in the development of atherosclerosis. Several epidemiological studies have shown that plasma HDL is an independent, negative risk factor for the development of CVD. The common hypothesis is that high HDL cholesterol levels decrease the risk for CVD by removing the excess of cholesterol from the macrophages and enhancing RCT. Recent work, however, indicates that this is an oversimplification and that current concepts of RCT require re-definition[22]. In addition, the anti-inflammatory and anti-oxidant features of molecules rather than cholesterol associated with the HDL particles, like paraoxonase, platelet activating factor-acetylhydrolase or lysophospholipids, are becoming increasingly apparent[23-25].
TOWARDS UNDERSTANDING OF INTESTINAL CHOLESTEROL ABSORPTION
In the past years, insight in regulation of cholesterol absorption has greatly increased by identification of transporter proteins involved. In addition, unraveling of molecular regulation of their expression is progressing. Yet, it should be realized that besides transporter proteins, the presence of bile acids in the intestinal lumen is an essential prerequisite for absorption to occur[26]. Micellar solubilization of (dietary/biliary) cholesterol is necessary for its absorption as exemplified by the fact that fractional cholesterol absorption is virtually zero in bile-diverted rats and Cyp7a1-deficient mice with a strongly diminished bile acid pool size[26].
Identification of novel proteins involved in cholesterol absorption
Cholesterol absorption has long been considered a merely passive process, despite the fact that the process is clearly selective since dietary cholesterol is absorbed with a relative high efficiency whereas structurally similar phytosterols are not. Several candidate intestinal cholesterol transporters have been proposed during the past couple of years, e.g., SR-B1[27] and aminopeptidase N[28], but their role (if any) has remained elusive so far. The recent identification of the Niemann-Pick C1 like 1 (NPC1L1) protein as a crucial molecule involved in cholesterol uptake by enterocytes[29] and of Abcg5 and Abcg8 proteins as (intestinal) cholesterol efflux transporters[30-32], has provided definite proof that cholesterol absorption is a protein-mediated, selective and active process.
The identification of NPC1L1 is strongly facilitated by the discovery of a powerful cholesterol absorption inhibitor named ezetimibe[33]. Ezetimibe and analogs comprise a new class of sterol absorption inhibitors that reduce diet-induced hypercholesterolemia in mice, hamsters, rats, rabbits, dogs, monkeys and humans[8,33-37]. Using a bioinformatics approach, Altmann et al[29] have identified the NPC1L1 protein as a putative cholesterol transporter in intestinal cells. NPC1L1 is expressed in the intestine at the brush border membrane and Npc1l1- deficient mice show a 69% reduction in fractional cholesterol absorption. Importantly, treatment with ezetimibe could not further reduce fractional cholesterol absorption efficiency in these mice, indicating that NPC1L1 at least is involved in a pathway targeted by ezetimibe[29]. In support of this, recent studies have shown that ezetimibe glucuronide, the active molecule, indeed binds to cells expressing NPC1L1[38]. Using intestinal brush border membrane (BBM) fractions, the authors showed that ezetimibe binds specifically to a single site in the brush border membrane and that this binding is lost in BBM fractions of Npc1l1- deficient mice[38]. The exact cellular localization of NPC1L1 is, however, still under debate. Iyer et al[39] showed that NPC1L1 is glycosylated and enriched in the BBM of rat enterocytes. Davies et al[40] who were the first to identify NPC1L1 as a homolog of the Niemann Pick type C (NPC) protein[40], showed in HepG2 cells that NPC1L1 is localized to a subcellular vesicular compartment but not in the plasma membrane. Using immortalized fibroblasts from wild-type and Npc1l1 knock-out mice these authors also showed that lack of NPC1L1 activity causes dysregulation of caveolin transport and localization, suggesting that the observed sterol transport defect may be an indirect result of the inability of Npc1l1-deficient cells to properly target and/or regulate cholesterol transport in the cells.
Another possible mechanism of action of ezetimibe has been proposed by Smart and colleagues[41]. These authors described the presence of a stable complex of annexin (ANX) 2 and caveolin (CAV) 1 located in enterocytes of zebrafish and mouse. Disruption of this complex by morpholino antisense oligonucleotides in zebrafish prevented normal uptake of cholesterol. Ezetimibe treatment of zebrafish, C57Bl/6 mice fed a Western type diet and LDL receptor knock-out mice disrupted the ANX2-CAV1 complex, suggesting that ANX2 and CAV1 are components of an intestinal sterol transport complex and targets for ezetimibe. Interestingly, C57BL/6 mice fed a standard diet did not show disruption of the ANX2-CAV1 complex upon ezetimibe treatment, but did show decreased cholesterol absorption[41]. Moreover, recent research using CAV1-deficient mice revealed that inhibition of cholesterol absorption by ezetimibe did not require the presence of CAV1[42]. In addition, rabbits did not appear to form the ANX2-CAV1 complexes, yet, their cholesterol absorption efficiency was still inhibited by ezetimibe[43]. Collectively, these studies make a mode of action in which ezetimibe acts by deregulating the ANX2-CAV1 complex less likely.
Other proteins critical in control of sterol absorption are the ATP-binding cassette (ABC) transporter proteins, G5 and G8. ABCG5 and ABCG8 act as functional heterodimers[44] and are localized at the canalicular membrane of hepatocytes and at the brush border membrane of enterocytes. Mutations in the human genes encoding ABCG5 or ABCG8 have been shown to cause the inherited disease sitosterolemia[30-32], which is characterized by an accumulation of plant sterols (e.g., sitosterol, campesterol) in blood and tissues due to their enhanced intestinal absorption and decreased biliary removal. Thus, ABCG5/ABCG8 limit plant sterol absorption by effective efflux back into the intestinal lumen. Since ABCG5/ABCG8 also accommodate cholesterol, as evidenced from the fact that Abcg5/g8-deficient mice show a strongly reduced biliary cholesterol secretion[45], this system also provides a means to control cholesterol absorption efficiency. Yet, Abcg5 and/or Abcg8 deficiency in mice clearly enhances phytosterol absorption[45-47], but reported effects on cholesterol absorption efficiency are minimal[45,46]. On the other hand, overexpression of ABCG5 and ABCG8 in mice as well as pharmacological induction of their expression did lead to a strongly decreased fractional cholesterol absorption[46,48,49], indicating that ABCG5 and ABCG8 play a role in control of cholesterol absorption under certain conditions.
Other transporter proteins, like the scavenger receptor BI (SR-BI) and ABCA1 have been suggested to play a role in control of cholesterol absorption. In the small intestine, SR-BI is localized both at the apical membrane and at the basolateral membrane of enterocytes, with different expression levels along the length of the small intestine[50]. It was reported that mice deficient in SR-B1, however, show only a small increase in fractional cholesterol absorption efficiency and a small decrease in fecal neutral sterol output[51]. On the other hand, intestine-specific overexpression of SR-BI in mice did lead to increased cholesterol and triglyceride absorption in short-term absorption experiments[52], indicating that SR-BI might have a role in cholesterol absorption.
Although earlier reports[53] have suggested an apical localization, it is evident that ABCA1 is localized at the basolateral membranes of chicken enterocytes[54] and human CaCo-2 cells[55]. The conflicting results yielded in studies assessing intestinal cholesterol absorption in mice lacking Abca1[56,57], suggest that the overall effect of Abca1 on absorption is very minor. However, as will be described later, this protein does have an important function in intestinal cholesterol metabolism.
After uptake, cholesterol is esterified by the enzyme ACAT 2 in the endoplasmic reticulum (ER) of enterocytes. It was reported that Acat2-deficiency in mice on a low-cholesterol chow diet did not affect cholesterol absorption efficiency, however, Acat2-deficient mice did show a clear reduction in cholesterol absorption upon feeding a high-fat/high-cholesterol diet and as a consequence, are resistant to diet-induced hypercholesterolemia[58]. Other proteins crucial for cholesterol absorption are those involved in chylomicron formation, like apolipoprotein B (apoB) and microsomal triglyceride transfer protein (MTP), and proteins involved in intracellular chylomicron trafficking such as SARA2. Mutations in the MTP gene result in abetalipoproteinemia, an inherited human disease characterized by extremely low plasma cholesterol and triglyceride levels and absence of apoB-containing particles. Patients suffer from fat and cholesterol malabsorption and neurological diseases due to malabsorption of lipid-soluble vitamins. Mutations in SARA2 cause chylomicron retention disease or Anderson disease[59], both of which are characterized by the inability to secrete chylomicrons causing severe fat malabsorption and accumulation of chylomicron-like particles in enterocytes. SARA2 belongs to the Sar1-ADP-ribosylation factor family of small GTPases and is involved in intracellular trafficking of chylomicrons through the secretory pathway[59].
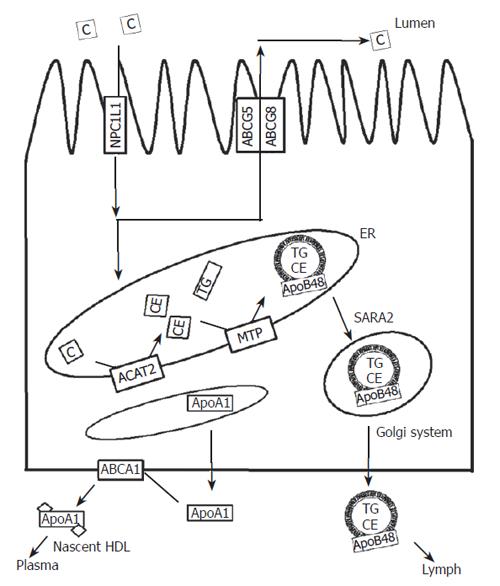
The major routes of cholesterol in enterocytes and the proteins involved are depicted schematically in Figure 1.
Figure 1 Schematic overview of the major routes of cholesterol in enterocytes.
Dietary and biliary cholesterol are taken up via the action of NPC1L1. In the ER, cholesterol is esterified and incorporated into chylomicrons, which are subsequently secreted into lymph. Non-esterified sterols can be re-secreted into the intestinal lumen via the action of ABCG5/G8 or secreted towards ApoA1 via the action of ABCA1. ABCA1, ABCG5, ABCG8: ATP-binding cassette transporter A1, G5, G8; ACAT2: Acyl-coenzyme A: Cholesterol acyl transferase 2; ApoAI, ApoB48, apolipoprotein AI, B48; C: Cholesterol; CE: Cholesterylester; ER: Endoplasmatisch reticulum; MTP: Microsomal triglyceride transfer protein; NPC1L1: Niemann Pick C 1 like 1 protein; SARA2: Sar1-ADP-ribosylation GTPase 2; TG: Triglycerides.
Regulation of cholesterol absorption
As indicated above, cholesterol can be taken up from the intestinal lumen by NPC1L1 and effluxed back into the lumen via ABCG5 and ABCG8. When both processes are active and present in the same cells, a classical futile cycle arises, enabling very sensitive regulation. Interference with this system has a great potential for reducing plasma cholesterol.
An established application hereof is provided by ezetimibe that interferes with NPC1L1 activity[29,38]. Lowering of NPC1L1 expression provides another potential means to reduce cholesterol absorption. Mechanisms involved in transcriptional control of NPC1L1 are beginning to be unraveled. The nuclear receptor peroxisome proliferator-activated receptor (PPAR) δ/β (NR1C2) has been shown to decrease cholesterol absorption, presumably by decreasing NPC1L1 expression[60]. Activation of PPARδ/β by the synthetic agonist GW610742 resulted in a 43% reduction of cholesterol absorption in mice, which coincides with unchanged intestinal expression of Abcg5 and Abcg8 but a decreased intestinal expression of Npc1l1. Treatment of human colon-derived CaCo-2 cells with ligands for PPARδ/β, but not for PPARγ or PPARα, decreased NPC1L1 expression as well[60]. Whether PPARδ/β regulates NPC1L1 directly or indirectly via transcriptional repression, is still under investigation.
The major regulatory pathways in cholesterol metabolism are controlled by the nuclear receptor liver X receptor (LXR). Two LXR isotypes have been identified in mammals, i.e., LXRα (NR1H3) which is mainly expressed in the liver, kidney, intestine, spleen and adrenals, and LXRβ (NR1H2) which is expressed ubiquitously. Natural ligands for both LXRs are oxysterols. After activation, LXR heterodimerizes with retinoid X receptor (RXR)[61,62]. Activated RXR/LXR heterodimers bind to specific LXR response elements (LXREs) in the promoter regions of their target genes and activate gene transcription. LXR target genes include many genes involved in cellular cholesterol efflux like ABCA1, ABCG1, ABCG5, and ABCG8[53,63,64], genes involved in bile acid synthesis [cholesterol-7α-hydroxylase (Cyp7a1)] in rodent models and genes involved in lipogenesis like sterol regulatory element-binding protein (SREBP) 1C, fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC). Global LXR activation by synthetic agonists therefore has a plethora of effects including elevated HDL levels, hypertriglyceridemia, hepatic steatosis, increased biliary cholesterol excretion, reduced intestinal cholesterol absorption efficiency and increased neutral sterol loss via the feces[65,66]. The decreased intestinal cholesterol absorption is primarily due to increased cholesterol efflux of cholesterol towards the intestinal lumen due to increased Abcg5 and Abcg8 expression, as fractional cholesterol absorption was reduced upon LXR activation in wild-type mice but remained unaltered in Abcg5/g8-deficient mice[49] and Abcg5-deficient mice[46] under these conditions. Other mechanisms, such as reduced intestinal Npc1l1 expression after LXR activation contribute to reduced cholesterol absorption, as recently shown in ApoE2-knock-out mice[67].
Dietary phytosterols and phytostanols and their esters have been introduced in functional foods to suppress intestinal cholesterol absorption and hence to reduce the risk for CVD[9]. Phytosterols and stanols are thought to decrease cholesterol absorption by competing with cholesterol for incorporation into mixed micelles in the intestinal lumen[68]. However, several recent studies suggested additional mechanisms involving alterations of intestinal gene expression. Igel and colleagues[69] showed for the first time that phytosterols and stanols are actually taken up by the enterocytes and subsequently re-secreted into the gut lumen, most probably through the action of Abcg5/Abcg8 transporters. This finding indicated that phytosterols and stanols, in addition to modes of action within the intestinal lumen, exert metabolic actions from inside the enterocytes. Moreover, dietary phytostanol consumption (2.5 g) once a day reduces LDL cholesterol as effective as consumption of 2.5 g phytostanols ingested in three daily portions[70], suggesting that luminal concentrations may not be the key to the control of metabolic actions. The identification of a phytosterol-derived agonist for the nuclear receptor LXR[71] has led to the proposal that phytosterols and stanols decrease cholesterol absorption via activation of intestinal LXR. In vitro studies in CaCo-2 cells indicated that phytostanols indeed are able to induce the expression of ABCA1, an established LXR target gene[72]. Recent in vivo studies, however, showed that dietary phytosterols and phytostanols decrease cholesterol absorption without activating LXR in rodent models. Field et al[73] showed that addition of 2% phytostanols to a chow diet do not affect intestinal expression of ABC sterol transporters and Npc1l1 in male golden Syrian hamsters. Likewise, Calpe-Berdien et al[74] showed very recently that decreased cholesterol absorption upon addition of 2% phytosterol to a Western type diet is not associated with transcriptional changes in Abca1, Abcg5, Abcg8 or Npc1l1 in C57BL/6J, ApoE-/- and LDLr-/- mice. Plösch and colleagues[66] have shown similar results using 0.5% phytosterol or phytostanol in a semi-synthetic diet containing 0.2% cholesterol in C57BL mice. Additionally, these authors showed that the plant sterol/stanol-induced reduction of cholesterol absorption in mice is not influenced by Abcg5-deficiency (J. Nutr., in press), indicating that intra-luminal events are most relevant for the inhibitory effect of these dietary compounds.
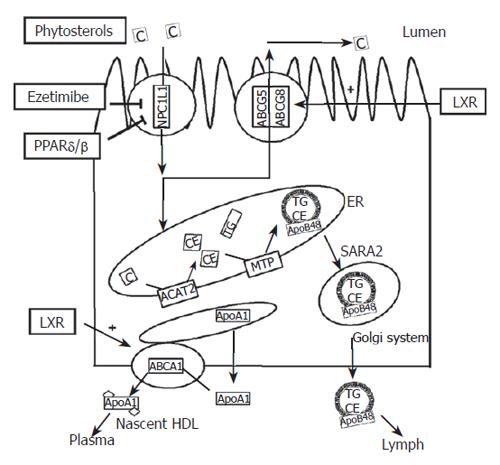
The modes of action of the different cholesterol absorption decreasing compounds are schematically depicted in Figure 2.
Figure 2 Schematic overview of the regulation of cholesterol transport in enterocytes.
Plant sterols, ezetimibe PPARδ/β agonists and LXR agonists all reduce cholesterol absorption through different mechanisms. Plant sterols interfere with micellisation of cholesterol. Ezetimibe binds to NPC1L1 and thereby interferes with the cholesterol uptake. Agonists for PPARδ/β reduce expression of NPC1L1 and thereby the amount of NPC1L1 protein. Agonists for LXR increase the expression of ABCG5 and ABCG8 and thereby enhance the efflux of cholesterol towards the intestinal lumen. LXR: Liver X Receptor; PPARδ/β: Peroxisome proliferators-activated receptor δ/β.
NOVEL ROLE OF THE INTESTINE IN REVERSE CHOLESTEROL TRANSPORT
It is clear that the intestine plays a major role in cholesterol homeostasis as a cholesterol absorbing organ. However, recent studies revealed that the intestine also acts as an excretory organ in the reverse cholesterol transport (RCT) pathway[66,75]. This pathway is classically defined as the HDL-mediated flux of cholesterol from peripheral cells to the liver, followed by its secretion into bile and disposal via the feces. RCT is extremely important in prevention of CVD as it removes excess cholesterol from macrophages present in the arterial vessel wall. The amount of cholesterol secreted into bile is substantial. As only part of it is absorbed by the intestine, it contributes significantly to cholesterol loss via the feces. However, a novel pathway that contributes to fecal cholesterol loss has recently been established.
Already in the nineteen-sixties, it was suggested that non-dietary cholesterol present in the intestinal lumen consists of a fraction secreted by the liver into the bile and a second fraction directly secreted by the intestine. Measuring dietary cholesterol, cholesterol absorption and cholesterol loss via the feces in patients with complete obstruction of common bile duct due to carcinoma of the head of the pancreas unequivocally established the presence of intestinally secreted cholesterol in the feces[76]. By intestinal perfusion studies in humans, Simmonds et al[77] have tried to quantify this route. In a triple lumen tube system, perfusion studies were carried out using micellar solutions with radio-labeled cholesterol. Decrease in specific activity was interpreted as secretion of endogenous cholesterol from the intestine and the contribution of endogenously secreted cholesterol from the intestine was estimated to be about 44% of total fecal output, but direct proof for the existence of this pathway could not be provided[77].
Since these early experiments, the focus of research has shifted more towards the liver. Biliary cholesterol and bile acid secretions are believed to represent the major pathways for removal of excess cholesterol. However, recent calculations of cholesterol fluxes in different mouse models again emphasize the relevance of intestinal cholesterol secretion. A striking example is provided by the Cyp7a1-deficient mouse[78]. Cyp7a1 is important for converting cholesterol into bile acids and catalyzing the formation of 7α-hydroxycholesterol[79]. As Cyp7a1 is rate-controlling in this pathway, it is regulated in a complex manner involving multiple nuclear receptors. Cyp7a1-deficiency in mice leads to significantly decreased fecal bile acid loss and bile acid pool size. Surprisingly, fecal neutral sterol output is increased two times in Cyp7a1-/- mice, although biliary cholesterol concentration remains unaffected[78]. As dietary intake and cholesterol absorption are known, it can be calculated from these data that direct intestinal cholesterol secretion contributes at least 30% to the increased fecal neutral sterol output.
Plösch and colleagues[66] showed that the pathway of intestinal cholesterol secretion can be induced in mice by treatment with the synthetic LXR agonist T0901317. In C57BL/6 mice, efflux of cholesterol from the intestinal epithelium into the lumen, calculated from the difference between dietary and biliary input minus fecal output, contributed up to 36% of the total fecal cholesterol loss. Pharmacological LXR activation in these mice tripled the intestinal cholesterol secretion, showing that this represents a valid, inducible pathway for removal of cholesterol in mice.
To further characterize this route, Kruit et al[75] studied the effects of LXR activation by the synthetic agonist GW3965 in wild-type and Mdr2-deficient mice. Mdr2-Pgp (or Abcb4 according to the new nomenclature) mediates the ATP-dependent translocation of phospholipids at the canalicular membrane of hepatocytes. Consequently, Mdr2-deficiency leads to the inability to secrete phospholipids into the bile. Due to the tight coupling of phospholipid and cholesterol secretion, these mice also show a severely impaired biliary cholesterol secretion[80,81]. Despite the impaired biliary cholesterol secretion, chow-fed Mdr2-/- mice showed a similar fecal neutral sterols loss as wild-type mice, suggesting that the intestine indeed contributes to the fecal neutral sterol loss. LXR activation increased fecal neutral sterol output to a similar extent in Mdr2-/- and wild-type mice, although biliary cholesterol secretion remained impaired in Mdr2-/- mice but increased in wild-type mice. These data show that the increased fecal cholesterol loss upon LXR activation is independent of biliary cholesterol secretion. Although fractional cholesterol absorption decreased to a greater extent in Mdr2-/- mice compared to wild-type mice upon LXR activation, it could be calculated that at least 57% of fecal cholesterol originates from intestinal secretion in Mdr2-/- mice[75].
The most intriguing question, namely the origin of intestine-derived cholesterol has remained unanswered so far. Part of the cholesterol could, in theory, originate from enhanced sloughing of intestinal cells or reflect a consequence of increased intestinal de novo cholesterol synthesis. Indeed, increased intestinal cholesterol synthesis has been found in Cyp7a1-/- mice[78]. Upon LXR activation, however, intestinal HMGCoA reductase gene expression remained unchanged[66,75], indicative for unchanged cholesterol synthesis, while fecal sterol loss increased 3 times. Staining for the proliferation marker Ki-67 has revealed no signs of increased intestinal cell proliferation upon LXR activation, making the possibility of enhanced cell shedding less likely[75]. Using intravenously injected radiolabeled cholesterol as a marker, Kruit and colleagues[75] additionally showed that fecal loss of plasma-derived cholesterol is 1.7-fold higher upon LXR activation in Mdr2-/- mice, suggesting that the intestine plays an important role independently of biliary cholesterol in cholesterol transport from plasma to the feces.
Further research should be done to identify the putative proteins involved in this pathway. The sterol efflux proteins, ABCG5/ABCG8, seem to be good candidates, as increased fecal neutral sterol output upon LXR activation requires the presence of Abcg5 and Abcg8[49] and transgenic mice overexpressing human ABCG5 and ABCG8 (hG5G8Tg) showed significantly-increased fecal neutral sterol loss[48]. However, deficiency of Abcg5 and/or Abcg8 leads to only mild[45,49] or no[46] decrease in fecal neutral sterol loss and the increased fecal neutral sterol excretion loss in the hG5G8Tg mice was inhibited in hG5G8Tg mice lacking Mdr2 (Mdr2-/-hG5G8Tg mice), suggesting that biliary cholesterol secretion is responsible for the increased fecal sterol loss in hG5G8Tg mice[82]. However, hG5G8Tg mice showed a high expression of human ABCG5 and ABCG8 in the liver but their expression in the intestine was far less pronounced[48]. Thus, the question whether intestinal ABCG5 and ABCG8 are important for intestinal cholesterol efflux under normal conditions still remains unanswered.
Virtually nothing is known about transporter systems involved in uptake of plasma cholesterol by enterocytes prior to its excretion into the intestinal lumen. LXR activation can upregulate a number of cholesterol transporters, of which only SR-BI is known to be involved in cholesterol uptake, at least in the liver. Chow-fed SR-B1-/- mice show only a small decrease in fecal neutral sterol loss, suggesting a relatively small contribution of intestinal SR-B1 to the control of fecal cholesterol excretion. However, basolaterally localized SR-B1 in enterocytes could theoretically play a role in cholesterol. When free cholesterol in enterocytes decreases due to activation of ABCG5 and ABCG8, uptake of the sterol from the plasma compartment may become energetically favorable.
INTESTINAL CONTRIBUTION TO HDL BIOGENESIS
The intestine along with the liver, has been known for many years to synthesize and secrete apolipoprotein A-I (ApoA-I), the principal apolipoprotein of HDL. Already in 1977, Glickman and Green[83] have described the synthesis of ApoA-I by the intestine of rats. One year later, Wu and Windmueller[84] estimated that intestinally synthesized ApoA-I contributes up to 56% of total plasma ApoA-I in rats. A potential role for the intestine in HDL particle assembly was initially suggested from experiments in hepatectomized dogs and studies describing the presence of HDL in mesenteric lymph[86-89]. More recently, in vitro studies using the human colon carcinoma cell line CaCo-2 showed that basolateral efflux of cholesterol occurs in high density ApoB-free, ApoA-I containing lipoproteins[90,91].
In addition to ApoA-I, ATP-binding cassette (ABC) transporter 1 (ABCA1) is of crucial importance for HDL formation. Three different groups have independently reported mutations of the ABCA1 gene as the cause of Tangier disease[92-94]. Tangier disease is characterized by almost complete absence of plasma HDL, abnormal accumulation of cholesteryl esters in reticuloendothelial cells of many tissues and early incidence of atherosclerosis. No abnormalities in the ApoA-I protein[95] or in protein synthesis have been found. These findings and the subsequent generation of Abca1-/- mice which also lack plasma HDL[57], underscore that ABCA1 is crucial for HDL formation.
ABCA1 performs the rate-controlling step in HDL formation by mediating the efflux of cholesterol and phospholipids to nascent ApoA-I. ABCA1 is widely expressed throughout the body[96], however not all tissues are important for the regulation of plasma HDL. Bone marrow transplantation studies in which bone marrow of wild-type and Abca1-/- mice was transplanted into Abca1-/- or wild-type mice, respectively, revealed that macrophage expression of Abca1 contributes only minimally to plasma HDL[97]. Macrophage ABCA1 is, however, important for the development of atherosclerosis because deficiency of Abca1 in bone marrow-derived cells increased the susceptibility to atherosclerosis in sensitive strains of mice[98,99]. Conversely, overexpression of ABCA1 in bone marrow-derived cells inhibited the progression of atherosclerotic lesions in such mice[100].
As both the liver and intestine synthesize ApoA-I and express significant levels of ABCA1, they are prone to contribute to plasma HDL levels. Indeed, mice overexpressing human ABCA1 in the liver and macrophages showed increased plasma HDL levels. Since macrophage ABCA1 can only minimally increase plasma HDL[97], this indicates that plasma HDL is controlled by hepatic ABCA1. A similar conclusion can be drawn from studies employing adenoviral Abca1 transfer to mouse liver in vivo[101,102]. Basso et al showed that treatment of C57BL/6 mice with adenovirus containing rABCA1-GFP resulted in a 2-fold increase in plasma HDL levels. Wellington et al[102] treated mice with increasing doses of ABCA1-containing adenoviruses, resulting in a dose-dependent increase in hepatic ABCA1 protein expression. HDL cholesterol was increased in mice injected with low doses of adABCA1, but surprisingly higher doses did not further raise plasma HDL levels[102]. Liver-specific Abca1 knockdown by 50% in mice using siRNA resulted in a 40% decrease of plasma HDL cholesterol levels, indicating that hepatic Abca1 expression correlates with plasma HDL levels in mice[103].
The creation of liver-specific Abca1 knock-out (Abca1-L/-L) mice definitively showed that the liver is the major contributor to plasma HDL as liver-specific deficiency of Abca1 results in a decrease of plasma HDL cholesterol levels by 80%. Further analysis revealed that in vivo catabolism of HDL ApoA-I isolated from wild-type mice was 2-fold higher in Abca1-L/-L mice due to a 2-fold higher rate of catabolism of ApoA-I in the kidneys[104]. These data unequivocally demonstrate that hepatic Abca1 is responsible for the maintenance of the circulating plasma HDL by direct lipidation of lipid-poor ApoA-1 containing particles. These data also show that, although the liver is the major organ responsible for HDL levels, additional extra-hepatic sites also contribute to HDL biogenesis.
To address the contribution of intestinal Abca1 to plasma HDL, intestine-specific Abca1 knockout (Abca1-i/-i) mice were created using the Cre/Lox system with the Cre transgene under the control of the villin promoter[105]. Brunham et al showed that intestinal Abca1 deficiency resulted in a 30% decrease in plasma HDL cholesterol levels, indicating that intestinal Abca1 is critically involved in HDL biogenesis. Combined deletion of both hepatic and intestinal Abca1 resulted in a 90% decrease of plasma HDL, which was similar to the level found in the whole-body Abca1-/- mice, proving that the liver and intestine are really the two major sites for HDL biogenesis. Absence of intestinal Abca1 resulted in decreased transport of dietary cholesterol into plasma HDL, but total intestinal cholesterol absorption was not affected. Surprisingly, lymphatic HDL content was hardly affected in Abca1-i/-i mice. In contrast, HDL was virtually absent in lymph of Abca1-L/-L mice, indicating that lymph HDL originates from the plasma compartment rather than directly from the intestine[105]. This finding has solved a long-lasting debate on the origin of lymphatic HDL[83,86-88,106,107]. It would be interesting to see whether lack of intestinal Abca1 influences the development of atherosclerosis.
Modulation of plasma HDL by intestine- specific LXR activation
As discussed above, LXR is a major regulator of cholesterol metabolism and LXR agonists are considered promising candidates for novel treatment strategies against atherosclerosis. Indeed, treatment of ApoE-/- and LDLr-/- mice, both are sensitive to atherosclerosis development, with synthetic LXR agonists inhibited the development of atherosclerosis[108,109]. However, general LXR activation also leads to increased lipogenesis, hypertriglyceridemia and hepatic steatosis in rodents[65] and is therefore not recommended for its use in humans. Specific LXR activation in the intestine may be beneficial in this respect, as it can theoretically lead to decreased cholesterol absorption, increased intestinal cholesterol excretion and plasma HDL levels. The preliminary data from our laboratory, using an intestine-specific LXR agonist in Wistar rats, showed that intestine-specific LXR activation indeed has the desired effect in this model without adverse effects on triglyceride metabolism.
CONCLUSION
During the past 5 years, a number of developments have greatly contributed to appreciation of the important role of the intestine in maintenance of cholesterol homeostasis (Figure 3). The most important developments include the identification of transporter proteins involved in uptake and secretion of cholesterol by enterocytes, the establishment of the direct cholesterol excretion pathway of the intestine, and the definition of the role of the intestine in HDL biogenesis.
Figure 3 Schematic overview of the involvement of the intestine in cholesterol homeostasis.
The intestine is critically involved in the control of plasma cholesterol due to its role in intestinal cholesterol absorption (1), direct cholesterol excretion into the intestinal lumen (2), and HDL biogenesis (3). CM: Chylomicron; HDL: High density lipoprotein; LDL: Low density lipoprotein; VLDL: Very low density lipoprotein.
A wealth of data indicate that the intestine should be considered a promising target for development of anti-atherosclerotic drugs that, in addition to interference with cholesterol absorption, may directly modulate cholesterol excretion and plasma HDL cholesterol levels.
S- Editor Liu Y L- Editor Wang XL E- Editor Bai SH