Published online Aug 28, 2006. doi: 10.3748/wjg.v12.i32.5140
Revised: July 20, 2005
Accepted: July 28, 2005
Published online: August 28, 2006
AIM: To analyze the biological effects of prolonged in vitro exposure of HT-29 and LoVo colon cancer cell lines to gefitinib (Iressa™), an inhibitor of epidermal growth factor receptor (EGFR) activity, and ZD6474, an inhibitor of both KDR and EGFR activities.
METHODS: Cells were treated with each drug for up to 2 wk using either a continuous or an intermittent (4 d of drug exposure followed by 3 d of washout each week) schedule.
RESULTS: In both cell types, prolonged exposure (up to 14 d) to gefitinib or ZD6474 produced a similar inhibition of cell growth that was persistent and independent of the treatment schedule. The effects on cell growth were associated with a pronounced inhibition of p-EGFR and/or p-KDR expression. Treatment with gefitinib or ZD6474 also inhibited the expression of EGFR downstream signal molecules, p-Erk1/2 and p-Akt, although the magnitude of these effects varied between treatments and cell lines. Furthermore, expression of the drug resistance-related protein ABCG2 was shown to significantly increase after 14 d of continuous exposure to the two drugs.
CONCLUSION: We conclude that long-term exposure of colon cancer cells to gefitinib and ZD6474 does not modify their cytotoxic effects but it might have an effect on sensitivity to classical cytotoxic drugs.
- Citation: Azzariti A, Porcelli L, Xu JM, Simone GM, Paradiso A. Prolonged exposure of colon cancer cells to the epidermal growth factor receptor inhibitor gefitinib (Iressa™) and to the antiangiogenic agent ZD6474: Cytotoxic and biomolecular effects. World J Gastroenterol 2006; 12(32): 5140-5147
- URL: https://www.wjgnet.com/1007-9327/full/v12/i32/5140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i32.5140
Recently, a greater understanding of the molecular basis of cancer has fostered the development of rationally designed molecular-targeted therapies with agents that have been shown to prevent cell proliferation, differentiation and survival through the inhibition of receptor tyrosine kinases (TKs), such as epidermal growth factor receptor-1 (EGFR) and vascular endothelial growth factor receptors (VEGFRs)[1,2].
Drugs that specifically target these receptors act via inactivation of the tyrosine kinase function of EGFR receptors resulting in lack of recruitment and phosphorylation of several intracellular substrates. A major downstream signalling route involved in this process is the Ras-Raf-MAPK pathway finally leading to ERK1 and 2 inactivation[3,4]. Another important target in EGFR signalling is PI3K and the downstream AKT protein transducing signals to the cascade of survival and motility[5,6]. Recently, the relevant role of the oncosuppressor gene PTEN in uncoupling some of these signalling pathways and thus generating gefitinib resistance has also been stressed[7].
Gefitinib (Iressa) ia a well-known oral EGFR inhibitor that is able to reduce tumour growth and the formation of metastases in a range of human cancer cell lines and human tumour xenografts[8,9]. In the clinical setting, gefitinib monotherapy has demonstrated antitumour activity in patients with recurrent or refractory non-small-cell lung cancer[10,11] and it has been approved for clinical cancer treatment in several countries. ZD6474 is a novel, orally available antiangiogenic agent that selectively targets two key tumour growth pathways by inhibiting VEGFR and EGFR tyrosine kinase activities. Preclinical studies have shown ZD6474 to be a potent inhibitor of VEGF-induced endothelial cell proliferation, tumour-induced angiogenesis and tumour growth[12].
Combining gefitinib or ZD6474 with other biological or cytotoxic agents has resulted in enhanced antitumour effects in vitro and in vivo[13-17]. Several ongoing clinical trials are therefore investigating the clinical efficacy of these targeting molecules when administered (1-21 or 1-28 d) in combination with agents such as taxols, gemcitabine, cisplatin, oxaliplatin, 5-FU and irinotecan [http://www.clinicaltrials.gov]. However, we have recently demonstrated that the effect of gefitinib used in combination with some cytotoxic drugs, can be schedule-dependent and have concluded that only extensive analysis of their main pathways of action could help in the design of an optimal multi-drug therapy[13,15,16]. The great influence that schedules can have in combination treatments with TK-inhibitors and other cytotoxic drugs is also evinced by the fact that EGFR inhibitors can variously interact with proteins involved in resistance to some conventional cytotoxic drugs[18,19]. In particular, ABCG2 and mdr-P glycoprotein (P-gP), belonging to ATP-binding cassette (ABC) transporters, are involved in sensitivity to topoisomerase I inhibitors[20] and anthracyclines[21].
In vitro data analyzing the activity of TK inhibitors on cell growth , apoptosis induction or cell cycle and target modulation are mainly limited to very short term cell exposures to drugs, generally lasting no longer than 5 d[22-24]. Although such exposure times are useful to clarify the molecular mechanisms of action of this class of drugs, they do not account for some major cell mechanisms controlling the expression and function of EGFR receptors[25]. Some such mechanisms that are known to produce EGFR down-regulation are endocytosis, pH-sensitive dissociation, dephosphorilation by PTP1B, trafficking to the lysosome, etc.[26-29].
Data from in vivo studies on the effects of prolonged exposure to TK inhibitors are scant. An in vivo study on long-term exposure confirmed that TK inhibitors are able to reduce tumour growth when utilized alone or in association with other drugs[9,30] but at least one other study has demonstrated that tumour cell lines can develop resistance to gefitinib[31]. The only in vitro report analyzing the pharmacological and biological effects of prolonged exposure to an EGFR inhibitor supports the hypothesis that duration of cell exposure to such a drug is important in modulating its antitumour effect and synergism with other drugs[32].
The aim of this study was to investigate the cytotoxic and biomolecular effects of different gefitinib and ZD6474 long-term exposure modalities on colon cancer cell lines and, whether phosphorylation of their specific targets (EGFR and/or KDR), activity of downstream signalling molecules and multidrug resistance proteins were modified in an exposure time-dependent manner.
Gefitinib and ZD6474 were provided by AstraZeneca Pharmaceuticals (Macclesfield, UK). Stock solutions were prepared at 20 μmol/L in dimethyl sulphoxide (DMSO) and stored in aliquots at -20°C. Further dilutions were made in F-12/HAM or McCoy’s medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 50 000 U/L penicillin, and 80 μmol/L streptomycin.
Two colon cancer cell lines of human origin were used, LoVo and HT-29. Cells were routinely cultured in F-12/HAM (LoVo) or McCoy’s (HT-29) medium, supplemented as above, in a humidified incubator at 37°C with a 50 mL/L CO2 atmosphere. Cells were trypsinized once a week with trypsin 0.25% ethylenediaminetetraacetic acid 0.02%, and the medium was changed twice a week. Doubling times were 20 ± 1 h for HT-29 cells and 24 ± 1 h for LoVo cells.
Gefitinib or ZD6474 (1-100 μmol/L) were incubated in F-12/HAM or McCoy’s medium, supplemented as above, in a humidified incubator at 37°C with a 50 mL/L CO2 atmosphere for 1, 5 and 7 d, after which HPLC analysis was performed. The stock solutions of each drug in DMSO were used as controls. The HPLC consisted of an LC9010 system coupled with a UV detector (Varian Inc, Palo Alto, CA, USA). Purified drug was eluted from an Aspec Bond Elut-C2 column (Varian Inc) using a solution of triethylamine and acetonitrile (76:24 [v/v]) adjusted to pH 3.0 with 0.2 mol/L phosphoric acid.
Determination of the IC50 of gefitinib or ZD6474 was performed using the 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazoliumbromide (MTT) assay. On d 1, 10 000 cells/well in a volume of 200 μL were added to 96-well plates. In each plate, one column contained cells not exposed to the drug (control), and five columns contained cells exposed to increasing concentrations of the drug. Each drug concentration was repeated in six identical wells. On d 2, gefitinib or ZD6474 (0.01, 0.1, 1, 10 and 100 μmol/L) was added allowing for different times of drug exposure (18 h to 3 d). Results were expressed as dose-effect curves with a plot of the fraction of unaffected (surviving) cells versus drug concentration. The IC50 was defined as the drug concentration yielding a fraction = 0.5 of affected (not surviving) cells, compared with untreated controls.
LoVo and HT-29 cells were exposed to gefitinib (0.12 μmol/L and 1.2 μmol/L, respectively) and ZD6474 (0.6 μmol/L and 5 μmol/L, respectively). These sub-toxic concentrations induced 30% cell growth inhibition after 3 d of continuous drug exposure. Cell survival was determined by cell counts after 7 and 14 d of drug exposure, using either a continuous or intermittent (4 d with drug followed by a 3-d washout) schedule. Controls were untreated cells which, at a quite complete confluence, were counted, divided, and plated again at 70% of confluence. Results were expressed as percentage of cell survival compared with untreated controls.
LoVo and HT-29 cells were exposed to gefitinib (0.12 μmol/L and 1.2 μmol/L, respectively) and ZD6474 (0.6 μmol/L and 5 μmol/L, respectively) for 7 and 14 d, using either a continuous or intermittent (4 d with drug followed by a 3-d washout) schedule. Cells were then harvested, washed twice in ice-cold PBS (pH 7.4), fixed in 4.5 mL of 70% ethanol at -20°C and washed once again in ice-cold PBS. After resuspension of the pellet in PBS containing 1 mg/mL RNase and 0.01% NP-40, cellular DNA was stained with 50 μg/mL propidium iodide (Sigma-Aldrich, St Louis, MO, USA). Cells were stored in ice for 60 minutes before analysis. Cell cycle and apoptosis determinations were performed using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), and data were interpreted using the ModFit software provided by the manufacturer.
Expressions of EGFR, KDR, Akt, p-Akt, Erk1/2, p-Erk1/2, PTEN, ABCG2 and PgP were determined by Western blot analysis using β-actin as the standard protein. Expressions of p-EGFR and p-KDR were determined by immunoprecipitation and Western blot. Protein bands were quantified by densitometric analysis followed by analysis with Quantity One software (BioRad, Hercules, CA, USA). The primary antibodies used were anti-EGFR (clone 13) and anti-KDR (clone A-3) from BD Transduction Laboratories (San Diego, CA, USA); Anti-Akt, anti-p-Akt, anti-Erk1/2 and anti-p-Erk1/2 (clone E10) from Cell Signaling Technology (Beverly, MA, USA); Anti-ABCG2 (clone BXP-21) from Alexis Corporation (Lausen, Switzerland); Anti-PgP (clone F4) and anti-β-actin from Sigma-Aldrich; And anti-phosphotyrosine polyclonal antibody PY99 and anti-PTEN (A2B1) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The secondary antibodies used were horseradish-peroxidase-conjugated anti-mouse and anti-rabbit from Amersham Pharmacia Biotech (Upsala, Sweden).
All the in vitro experiments were performed in triplicate, and results are expressed as the mean ± SD unless otherwise indicated.
To verify gefitinib and ZD6474 stability in our experimental conditions, each drug was incubated in complete medium for 1, 5 and 7 d. The HPLC retention time and the area under the curve (AUC) for the peak of each drug were measured and compared to those of the same drugs in DMSO (standard). The HPLC retention times for the internal standards were 2.47 min for gefitinib and 1.75 min for ZD6474. Each drug showed the same retention time as its standard, irrespective of concentration, exposure time and the absence or presence of complete medium; the AUC of each peak was proportional to the drug concentration. These results confirmed the stability of gefitinib and ZD6474 in our experimental conditions. Table 1 shows the AUC and retention times of gefitinib and ZD6474 peaks obtained by HPLC after 7 d of drug exposure.
| Drug | Medium | Concentration (µmol/L) | Retentiontime (min) | AUC |
| Gefitinib | DMSO | 100 | 2.47 ± 0.03 | 1.30 ± 0.20 |
| Complete medium | 100 | 2.52 ± 0.05 | 1.27 ± 0.16 | |
| ZD6474 | DMSO | 100 | 1.75 ± 0.02 | 0.88 ± 0.08 |
| Complete medium | 100 | 1.71 ± 0.04 | 0.86 ± 0.10 |
The IC50 values for gefitinib and ZD6474 in HT-29 and LoVo cells are shown in Table 2. A time-dependent reduction in IC50 was observed for both drugs, with the IC50 after 3 d of exposure being 4-8 times lower than after 18 h of exposure.
| Exposuretime | Gefitinib (µmol/L) | ZD6474 (µmol/L) | ||
| LoVo cells | HT-29 cells | LoVo cells | HT-29 cells | |
| 18 h | 48.5 ± 2.5 | > 100 | 16 ± 5.1 | 80 ± 4.8 |
| 1 d | 29 ± 3.1 | > 100 | 13 ± 2.6 | 59 ± 3.6 |
| 2 d | 16.5 ± 1.5 | > 100 | 8.2 ± 3.8 | 19 ± 1.8 |
| 3 d | 7.3 ± 0.9 | 23.6 ± 4.1 | 3.5 ± 0.9 | 10 ± 0.4 |
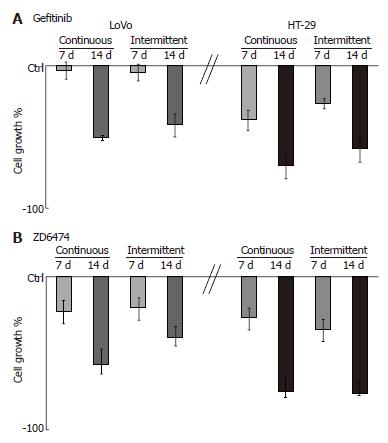
Treatment with gefitinib for 1 wk, according to a continuous or intermittent schedule, did not significantly affect LoVo cell growth, and 2 wk of treatment was necessary to show an inhibitory effect (Figure 1A). By contrast, HT-29 cell growth was inhibited by gefitinib at 1 wk and to an even greater extent at 2 wk. ZD6474 induced a progressive, exposure-dependent inhibition of cell growth in both cell lines, but HT-29 cells appeared to be more sensitive than LoVo cells (Figure 1B).
As expected, prolonged exposure of the cells to gefitinib or ZD6474 produced an exposure-dependent increase in apoptosis (Table 3). This effect was observed with both the continuous and the intermittent treatment schedules. There was also a moderate accumulation of cells in the G0/G1 phase and this effect became more evident at 2 wk with continuous exposure (Table 3).
| LoVo cells | HT-29 cells | ||||
| Drug exposure | Apoptosis(%) | G0/G1(%) | Apoptosis(%) | G0/G1(%) | |
| Control | 0 | 74.3 ± 1.3 | 0 | 69.5 ± 1.4 | |
| Gefitinib | 1 wk continuous | 10.1 ± 0.5 | 80.4 ± 1.5 | 7 ± 0.4 | 70. 7 ± 1.7 |
| 2 wk continuous | 23.2 ± 0.9 | 87.9 ± 1.3 | 20 ± 1.2 | 77.2 ± 2.0 | |
| 1 wk intermittent | 13.8 ± 0.6 | 75.4 ± 1.4 | 6.5 ± 0.3 | 67.1 ± 1.4 | |
| 2 wk intermittent | 29.7 ± 0.8 | 78.0 ± 2.1 | 18.3 ± 1.8 | 69.7 ± 1.9 | |
| Control | 0 | 74.3 ± 2.1 | 0 | 69.5 ± 1.8 | |
| ZD6474 | 1 wk continuous | 15.4 ± 0.6 | 77.6 ± 1.9 | 7 ± 0.9 | 71.7 ± 2.1 |
| 2 wk continuous | 30.3 ± 0.9 | 81.8 ± 2.3 | 15 ± 0.8 | 75.2 ± 2.4 | |
| 1 wk intermittent | 18.3 ± 1.3 | 74.6 ± 2.1 | 7.3 ± 1.1 | 70.2 ± 1.8 | |
| 2 wk intermittent | 29.6 ± 1.5 | 76.5 ± 1.5 | 16.4 ± 2.2 | 72.0 ± 1.7 | |
The ability of gefitinib and ZD6474 to modulate their specific targets and downstream effectors was analyzed by measuring the expression levels of total and phosphorylated proteins.
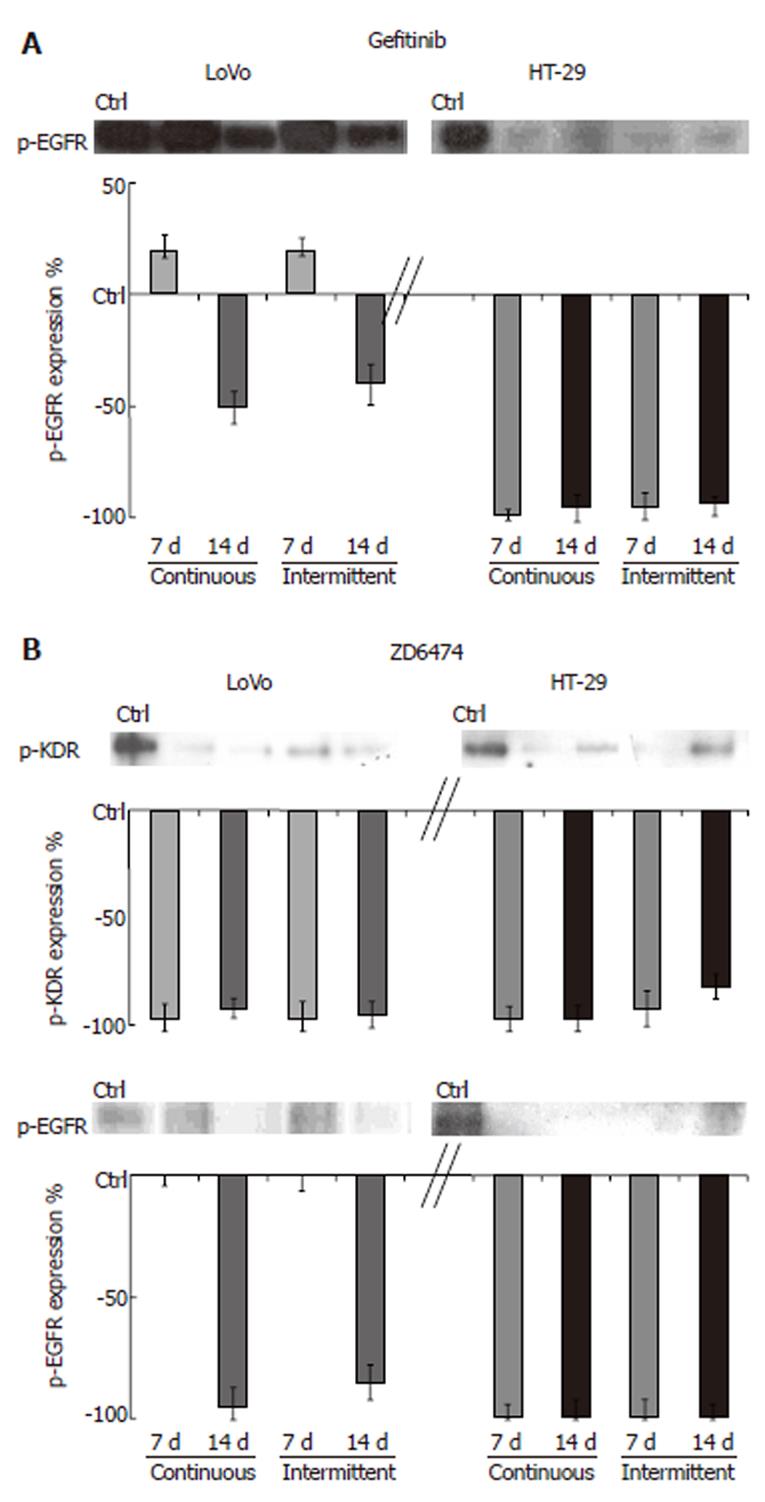
TK receptor modulation: Prolonged exposure to gefitinib and ZD6474 of both cell lines, using either a continuous or intermittent treatment schedule, did not change the total amount of EGFR or KDR protein. HT-29 cells exposed to gefitinib for 7 and 14 d showed almost no detectable p-EGFR (Figure 2). In LoVo cells, gefitinib produced partial inhibition of p-EGFR that was appreciable only after 14 d of treatment. In both cell lines, ZD6474 almost completely inhibited p-KDR (approximately 98% inhibition compared with the control; Figure 2). ZD6474 also inhibited p-EGFR, with a greater effect in HT-29 cells than in LoVo cells.
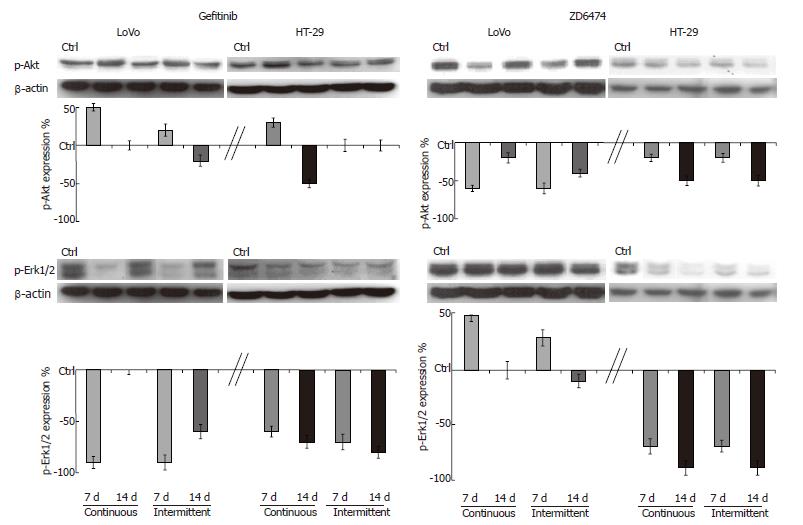
EGFR signal transduction pathway modulation: Prolonged exposure to gefitinib and ZD6474 did not change the expression of Akt and Erk1/2 in HT-29 or LoVo cells. Gefitinib produced only modest effects on p-Akt but markedly decreased p-Erk1/2, the downstream effector of the proliferation pathway (Figure 3). Compared with the 7 d exposure, the magnitude of gefitinib-mediated inhibition of p-Erk1/2 at 14 d was similar in the HT-29 cells, but had decreased in the LoVo cells.
Continuous or intermittent treatment with ZD6474 was associated with only a slight inhibition of p-Akt (Figure 3). ZD6474 induced a progressive and almost complete inhibition of p-Erk1/2 in HT-29 cells, but not in LoVo cells.
PTEN modulation: With a progressive increase in exposure time from 1 to 14 d, neither gefitinib nor ZD6474 modulated the total amount of PTEN in either cell line (data not shown).
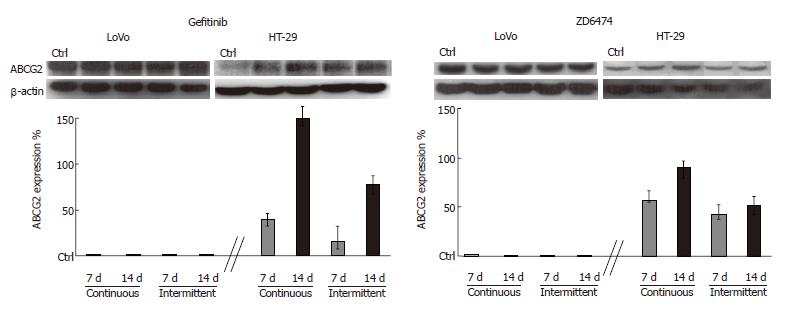
Preliminary analysis of the baseline expression levels of proteins related to drug resistance showed that ABCG2 was detectable in HT-29 and LoVo cells while P-gP was undetectable. LoVo cells also showed a higher level of ABCG2 than HT-29 cells. Interestingly, both gefitinib and ZD6474 exhibited time exposure-dependent increase in ABCG2 expression only in HT-29 cells; this effect became evident after 5 d of drug exposure (data not shown) and was progressive until 14 d (Figure 4). Stimulation of ABCG2 expression was more evident after continuous exposure to gefitinib or ZD6474 (150% and 90% increase in ABCG2 expression, respectively) than after intermittent exposure (80% and 50% increase compared with controls, respectively).
Analysis of the biomolecular effects that the TK inhibitors, gefitinib and ZD6474, can have on tumour cells after prolonged drug exposure is instrumental in leading to optimization of their use in monotherapies and in combination with other biological or conventional cytotoxic drugs[10,11,33,34]. Several cell effects have been demonstrated to depend directly on the modality and duration of cell exposure to these drugs; these effects include receptor expression, function of signal transduction, modulation of drug resistance proteins, etc. Nonetheless, and in spite of the clinical modalities of administration of these drugs, in vitro studies describing the inhibitory effect of gefitinib or ZD6474 on cell growth have considered only short drug exposures of 1-5 d[22-24].
In our study, we assessed the effects of prolonged exposure to the TK inhibitors, gefitinib and ZD6474, on cell viability and on their specific molecular targets by directly monitoring the modulation of the phosphorylated form of EGFR and of the two effectors, Akt and Erk1/2, that are important in the cell survival and proliferation pathways, respectively.
Two weeks exposure to gefitinib resulted in up to 70% cell growth inhibition and no apparent differences between the continuous and the intermittent treatment schedules. Moreover, as already reported for erbitux[32], gefitinib inhibited the phosphorylated forms of the receptor p-EGFR and of the downstream effector p-Erk1/2 involved in the proliferation pathway. The effects of gefitinib on p-Akt were less dramatic and appeared to be cell line-specific.
Comparison of these results with those obtained after short-term drug exposure[13] highlighted differences in the molecular effects produced by different drug schedules. Although a high rate of cells died after 2 wk of drug exposure in our investigation, cells tried to escape the attack by an exogenous agent by further modulating the survival and proliferation pathways. These findings suggest that a combination of Gefitinib and other PI3K/Akt pathway inhibitors, such as mTor inhibitor, may produce a more powerful synergistic effect.
ZD6474 proved to be able to inhibit the growth of our two colon cancer cell lines, thus confirming similar effects previously observed in GEO cells[23]. In both cell lines, the ZD6474-mediated inhibition of growth was associated with almost complete inhibition of p-KDR and p-EGFR, as well as a slight inhibition of p-Akt. In contrast, the modulation of p-Erk1/2 by ZD6474 was evident in HT-29 cells only.
Another aspect we considered was the effect on the expression of the tumour suppressor PTEN. Unlike Nagata, who reported PTEN modulation after 1 hour of exposure to the anti-ErbB2 antibody, trastuzumab[35], no appreciable modulation of this protein was observed in our study after short or long, continuous or alternate drug exposures. Our conclusion was that 14 d of drug exposure may be long enough for recovery of the possibly transient and short-term modulation of this gene potentially occurring in the first few hours after treatment with TK inhibitors.
Tyrosine kinase inhibitors have shown the potential to modulate cytotoxic drug resistance, through an interaction with ABCG2 and PgP[18,19]. In our study, ABCG2, which is involved in camptothecin resistance[20], was detectable in both HT-29 and LoVo cells while PgP, which is involved in anthracycline resistance, was not detectable in either cell line. The increased ABCG2 expression, following exposure to gefitinib or ZD6474, was cell line-specific. The HT-29 cells showed a progressive exposure-related increase of 100%-150%, while expression in the LoVo cells was unaffected. The increased expression of ABCG2 in HT-29 cells was schedule-dependent, and it was higher with continuous than with intermittent incubation. Our results seem to be in contrast with those obtained by Nakamura[19] but the experimental conditions used were completely different. Nakamura used a short time (15 min) to show a gefitinib-dependent increase in topotecan accumulation in transfectant cells, overexpressing ABCG2, and 4 d of gefitinib plus cytotoxic drugs exposure to led to reversal of drug resistance. In our experiments the ability of gefitinib to enhance ABCG2 expression proved to be a late effect of the drug and may account for the antagonism between Topoisomerase-I inhibitors and TK-inhibitors in HT-29 cells induced by a pre-exposure to gefitinib for 5 d[13]. Moreover, the cell-line-specific effects of gefitinib and ZD6474 on drug-related proteins may provide an explanation for the different results. Wakeling and Ciardiello obtained[30,31] when analyzing the onset of drug resistance in vivo after long-term drug intake.
In conclusion, our investigation studied, in an in vitro model of colon cancer, some crucial points related to the clinical use of these TK inhibitor drugs. Evidence has emerged that long term use (maximum 14 d) of these drugs does not lead to a loss of cell activity and intermittent or continuous exposures to these drugs do not produce significantly different toxic effects. The main signal transduction steps for TK receptor pathways were also studied and the findings demonstrated that the effects of these drugs may be highly cell-line-specific (HT-29 vs Lovo), drug-dependent (gefitinib vs ZD6474) and schedule-related (continuous vs intermittent), thus indicating that a predictive factor for these drugs cannot be easily identified or broadly applicable. The data concerning the modulation of drug resistance related proteins in tumour cells treated with long-term drug exposure are even more interesting. ABCG2 expression was shown to be induced by these drugs in a cell-line- specific and schedule-dependent manner. This evidence should suggest caution in choosing exposure times when combining these drugs with Topoisomerase-I inhibitors in particular. The findings of this study can be instrumental in the implementation of future in vitro and clinical studies on these drugs.
S- Editor Wang J L- Editor Lutze M E- Editor Bi L
| 1. | Hamid O. Emerging treatments in oncology: focus on tyrosine kinase (erbB) receptor inhibitors. J Am Pharm Assoc (2003). 2004;44:52-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Vlahovic G, Crawford J. Activation of tyrosine kinases in cancer. Oncologist. 2003;8:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Paradiso A, Cardone RA, Bellizzi A, Bagorda A, Guerra L, Tommasino M, Casavola V, Reshkin SJ. The Na+-H+ exchanger-1 induces cytoskeletal changes involving reciprocal RhoA and Rac1 signaling, resulting in motility and invasion in MDA-MB-435 cells. Breast Cancer Res. 2004;6:R616-R628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Reshkin SJ, Bellizzi A, Cardone RA, Tommasino M, Casavola V, Paradiso A. Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells. Clin Cancer Res. 2003;9:2366-2373. [PubMed] [Cited in This Article: ] |
| 5. | Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14:2185-2197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Reshkin SJ, Bellizzi A, Albarani V, Guerra L, Tommasino M, Paradiso A, Casavola V. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na(+)/H(+) exchange, motility, and invasion induced by serum deprivation. J Biol Chem. 2000;275:5361-5369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3'-kinase/Akt pathway signaling. Clin Cancer Res. 2003;9:4340-4346. [PubMed] [Cited in This Article: ] |
| 8. | Arteaga CL, Truica CI. Challenges in the development of anti-epidermal growth factor receptor therapies in breast cancer. Semin Oncol. 2004;31:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Sirotnak FM. Studies with ZD1839 in preclinical models. Semin Oncol. 2003;30:12-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21:2237-2246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2323] [Cited by in F6Publishing: 2239] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 11. | Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149-2158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2064] [Cited by in F6Publishing: 1979] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 12. | Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645-4655. [PubMed] [Cited in This Article: ] |
| 13. | Azzariti A, Xu JM, Porcelli L, Paradiso A. The schedule-dependent enhanced cytotoxic activity of 7-ethyl-10-hydroxy-camptothecin (SN-38) in combination with Gefitinib (Iressa, ZD1839). Biochem Pharmacol. 2004;68:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Tortora G, Caputo R, Damiano V, Fontanini G, Melisi D, Veneziani BM, Zunino F, Bianco AR, Ciardiello F. Oral administration of a novel taxane, an antisense oligonucleotide targeting protein kinase A, and the epidermal growth factor receptor inhibitor Iressa causes cooperative antitumor and antiangiogenic activity. Clin Cancer Res. 2001;7:4156-4163. [PubMed] [Cited in This Article: ] |
| 15. | Xu JM, Azzariti A, Colucci G, Paradiso A. The effect of gefitinib (Iressa, ZD1839) in combination with oxaliplatin is schedule-dependent in colon cancer cell lines. Cancer Chemother Pharmacol. 2003;52:442-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Xu JM, Azzariti A, Severino M, Lu B, Colucci G, Paradiso A. Characterization of sequence-dependent synergy between ZD1839 ("Iressa") and oxaliplatin. Biochem Pharmacol. 2003;66:551-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Warburton C, Dragowska WH, Gelmon K, Chia S, Yan H, Masin D, Denyssevych T, Wallis AE, Bally MB. Treatment of HER-2/neu overexpressing breast cancer xenograft models with trastuzumab (Herceptin) and gefitinib (ZD1839): drug combination effects on tumor growth, HER-2/neu and epidermal growth factor receptor expression, and viable hypoxic cell fraction. Clin Cancer Res. 2004;10:2512-2524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Ozvegy-Laczka C, Hegedus T, Várady G, Ujhelly O, Schuetz JD, Váradi A, Kéri G, Orfi L, Német K, Sarkadi B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 265] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Nakamura Y, Oka M, Soda H, Shiozawa K, Yoshikawa M, Itoh A, Ikegami Y, Tsurutani J, Nakatomi K, Kitazaki T. Gefitinib ("Iressa", ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res. 2005;65:1541-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Bates SE, Medina-Pérez WY, Kohlhagen G, Antony S, Nadjem T, Robey RW, Pommier Y. ABCG2 mediates differential resistance to SN-38 (7-ethyl-10-hydroxycamptothecin) and homocamptothecins. J Pharmacol Exp Ther. 2004;310:836-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Lehne G. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr Drug Targets. 2000;1:85-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Xu JM, Paradiso A, McLeod HL. Evaluation of epidermal growth factor receptor tyrosine kinase inhibitors combined with chemotherapy: Is there a need for a more rational design. Eur J Cancer. 2004;40:1807-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Ciardiello F, Caputo R, Damiano V, Caputo R, Troiani T, Vitagliano D, Carlomagno F, Veneziani BM, Fontanini G, Bianco AR. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546-1556. [PubMed] [Cited in This Article: ] |
| 24. | Anido J, Matar P, Albanell J, Guzmán M, Rojo F, Arribas J, Averbuch S, Baselga J. ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res. 2003;9:1274-1283. [PubMed] [Cited in This Article: ] |
| 25. | Lazar CS, Cresson CM, Lauffenburger DA, Gill GN. The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol Biol Cell. 2004;15:5470-5480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Carpentier JL, Gorden P, Anderson RG, Goldstein JL, Brown MS, Cohen S, Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982;95:73-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 141] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270:4334-4340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708-1711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 408] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 30. | Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749-5754. [PubMed] [Cited in This Article: ] |
| 31. | Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, Melisi D, De Vita F, De Placido S, Bianco AR. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 287] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 32. | Huang ZQ, Buchsbaum DJ, Raisch KP, Bonner JA, Bland KI, Vickers SM. Differential responses by pancreatic carcinoma cell lines to prolonged exposure to Erbitux (IMC-C225) anti-EGFR antibody. J Surg Res. 2003;111:274-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1323] [Cited by in F6Publishing: 1419] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 34. | Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1324] [Cited by in F6Publishing: 1285] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 35. | Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1362] [Cited by in F6Publishing: 1329] [Article Influence: 66.5] [Reference Citation Analysis (0)] |