INTRODUCTION
Henoch-Schönlein purpura (HSP) is a systemic vasculitic disorder of the small vessels of the skin, joints, GI tract, and kidney. The clinical manifestations usually include arthralgia, a purpuric skin rash, nephritis, abdominal pain, and GI bleeding. It commonly affects young children, with the peak age of onset between 4 and 7 years. The pathologic changes are mediated by IgA deposition[1], complement activation, granulocyte recruitment, and destruction of endothelial cells. The American College of Rheumatology published diagnostic criteria for HSP in 1990[2] including (1) age less than or equal to 20 years at disease onset, (2) the presence of palpable purpura, (3) GI bleeding, and (4) a biopsy showing granulocytes in the walls of small arterioles or venules. The diagnosis is made when three or more of those criteria are fulfilled.
CASE REPORT
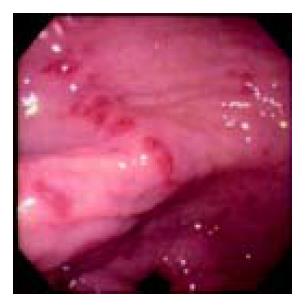
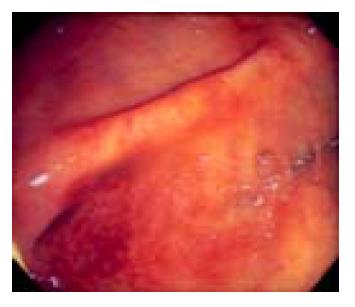
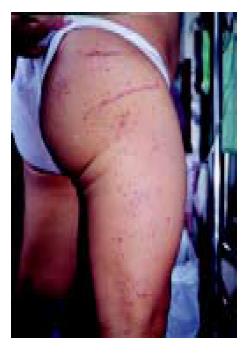
A 60-year-old man presented with three days of colicky abdominal pain with bloody diarrhea, arthralgia in the knees, and ankle edema. His medical history was unremarkable except for a self-limited upper respiratory infection one week previously. Physical examination revealed diffuse lower abdominal rebound tenderness. There was no fever and no skin rash on admission. The initial leukocyte count was 17830 /mm3, and the C-reactive protein was 12 mg/dL. A plain abdominal film showed generalized ileus and no evidence of free air. Stool cultures were negative for enteric pathogens, and no ova or parasites were found. Upper endoscopy revealed multiple discrete coin-like petechiae and hemorrhagic erosions in the gastric antrum and lower body (Figure 1). Colonoscopy revealed patches of hyperemic and ecchymotic lesions in the cecum (Figure 2), ileocecal valve, and sigmoid colon. The patient was managed with fasting and intravenous fluids. The day after admission, he developed numerous erythematous, purpuric lesions, predominantly on the lower extremities and buttocks. The lesions rapidly became palpable (Figure 3). A platelet count, prothrombin time and partial thromboplastin time were normal. Based on this constellation of findings, HSP was suspected. Tissue from a skin biopsy of the lesions was compatible with leukocytoclastic vasculitis with deposition of IgA, confirming the diagnosis of HSP. The urinalysis and renal function were normal. Parenteral steroid therapy (5 mg/(kg·d) of hydrocortisone) was instituted initially, resulting in relief of the abdominal pain and arthralgia and gradual resolution of the skin lesions within 5 d. The treatment was switched to oral prednisolone 20 mg/d with gradual tapering over 4 wk. The patient has remained well over 2 years of follow up.
Figure 1 Upper endoscopic view of multiple discrete coin-like petechiae in the lower gastric body.
Figure 2 Colonscopic view of hyperemic and ecchymotic lesions in the cecum.
Figure 3 Numerous erythematous papules and palpable purpura on the lower extremities and buttocks.
DISCUSSION
The above-mentioned patient fulfilled three of four criteria for the diagnosis of HSP. The only exception was his age. The diagnostic sensitivity and specificity of three out of the four criteria are 89.4% and 88.1%, respectively[2]. Although the cause of HSP is unknown, immunizations, certain food allergies, insect bites, infection[3], and some medications[4] may play a role in the development of the disease. It may be that our patient’s recent cold predisposed him to HSP. The final diagnosis of HSP was confirmed by histologic examination of a skin biopsy specimen demonstrating a vasculitis with leukocyte infiltration and deposition of IgA.
The diagnosis of HSP can be difficult, especially when abdominal symptoms precede cutaneous lesions. A skin rash is nearly always present, although not necessarily in the earliest stages. Lanzkowsky reported that 14% of patients had abdominal symptoms preceding the skin rash[5]. As in our case, the skin rash is typically on the lower extremities and buttocks, less commonly on the face and trunk. It starts as erythematous papules that quickly become palpable purpura. Normal platelets counts, prothrombin time, and partial thromboplastin time exclude a platelet or hemorrhagic disorder as the cause of purpura. Other laboratory tests are usually not conclusive.
Abdominal symptoms reportedly occur in 50-85% of HSP patients, possibly secondary to edema and hemorrhage within the bowel wall and mesentery. The abdominal pain is always colicky and poorly localized. On physical examination the abdomen may be rigid or distended, occasionally mimicking an acute abdomen and resulting in unnecessary exploratory laparotomy. Signs suggestive of intussusception including an abdominal mass may be present in children. GI bleeding occurs in one-third of cases and may be overt, with hematemesis, melena, or bloody stools[6,7]. While bloody diarrhea is common in HSP, it may also occur in eosinophilic gastroenteritis[8], systemic lupus erythematosus, parasitic infection, and drug-induced vasculitis. Distinguishing between these different vasculitic disorders depends on clinical, serological, hematologic, and histologic findings. Arthralgia is usually symmetrical, with the ankles being the most commonly affected joints. Joint involvement is considered as an indication for systemic steroids. Proteinuria and hema-turia indicate renal involvement and generally occur within the first 3 mo after the onset of the disease.
The hallmarks of HSP on computed tomography are multiple focal areas of bowel thickening with skip lesions, mesenteric edema, vascular engorgement, and non-specific lymphadenopathy[9]. However, similar thickening may also be seen in eosinophilic gastroenteritis[8], lymphoma, Crohn’s disease, and granulomatous disease. It is thus not a specific sign for HSP. Ultrasound may reveal generalized thickening of the intestinal wall, ascites, and sometimes intussusception.
GI involvement in HSP is seen predominantly in the small bowel[10,11] but may also affect the esophagus, stomach, terminal ileum[12,13] and colon[14]. Our patient had diffuse, poorly localized abdominal pain and bloody diarrhea, prompting us to visualize both the upper and lower GI tract. The endoscopic findings in our patient included discrete coin-like petechiae, hemorrhagic erosions, and skip hyperemic and ecchymotic lesions. These were seen in the gastric antrum, cecum, ileocecal valve, and sigmoid colon. It is important to recognize these characteristic endoscopic findings when a previously healthy patient presents with the sudden onset of an acute abdomen. When encountered, they should suggest the possibility of HSP. Although we did not biopsy the lesions in the GI tract, we expected to find histologic abnormalities similar to those found on skin biopsy. However, endoscopic biopsies often miss the subm-ucosal vessels and only reveal nonspecific inflammation.
HSP is primarily a medical disease and requires only supportive treatment once other acute surgical conditions have been excluded. Simple analgesics or non-steroidal anti-inflammatory drugs are used as first-line therapy for relief of arthralgia. Parenteral steroids have been advocated for more severe abdominal or joint pains, or painful angioedema, but they should be used with caution if there is active GI bleeding. All patients with HSP should have their urine analyzed on several occasions during the initial stages of the disease. Proteinuria and hematuria indicate possible renal involvement which, if it progresses to renal insufficiency, has a poor long-term outcome[15,16]. Our patient’s urinalysis was normal, and his renal function has been remained normal over two years of follow up.
In conclusion, the majority of patients with HSP recover fully. The prognosis is best in the absence of renal involvement. The diagnosis of HSP may be difficult, especially when abdominal symptoms precede the characteristic palpable purpura. Typical endoscopic findings may alert gastroent-erologists to consider this disease early and thus avoid unnecessary laparotomy.