Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2905
Revised: February 26, 2004
Accepted: March 4, 2004
Published online: October 1, 2004
AIM: To discuss the angiographic features of gastrointestinal stromal tumor (GIST) and to evaluate their diagnostic role.
METHODS: Twelve patients with pathologically proved GIST underwent angiography (DSA) 1 wk before operation, using Puck and digital subtraction DSA. The origin, size, morphology and angiographic appearance of the lesions were reviewed.
RESULTS: Two tumors arose from stomach, 8 from jejunum, and 2 from ileum. Seven cases were benign and 5 were malignant. Obviously thickened supplying arteries were detected in 8 tumors, and early-developed veins were found in 3. Two types of angiographic changes of GIST were observed. Four cases had twisted irregular neoplastic vessels with partially coarse and indistinct margins, which were all malignant. Eight cases had ball-like neoplastic vessels with uniform tumor staining, of which 7 were benign and 1 was malignant.
CONCLUSION: Angiography facilitates localization and diagnosis of GIST, helps define their size, range and location, and is especially valuable to patients suffering from melena with unknown reasons.
- Citation: Fang SH, Dong DJ, Zhang SZ, Jin M. Angiographic findings of gastrointestinal stromal tumor. World J Gastroenterol 2004; 10(19): 2905-2907
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2905
Gastrointestinal stromal tumor and its imaging features have been reported[1-3]. However, to our knowledge, the appearance of GIST at angiography has been seldom discussed. We analyzed retrospectively the angiographic appearance of 12 cases of GIST, which was proved by surgical pathology (immunohistochemistry), and the diagnostic value of angiography was discussed.
Twelve patients (4 women and 8 men, aged 40-70 years with a mean age of 53.3 years) with pathologically proved GIST (immunohistochemistry) who underwent angiography 1 wk before surgery were included. All the patients suffered from recurrent melena for 3 mo to 3 years, with syncope in 2 cases, a palpable abdominal mass in 1 case, and concomitant abdominal pain in another case.
After routine preparations, Seldinger’s puncture of femoral artery and super selective catherization, angiographies of superior mesenteric artery, inferior mesenteric artery and coeliac trunk were performed sequentially using 6-7 F catheters. Omnipaque (iodine content: 350 mg/mL) as contrast medium was administered with a dose of 30 mL and at a rate of 8 mL/s for superior mesenteric artery, 20 mL/s and 6 mL/s for inferior mesenteric artery, and 40 mL/s and 8 mL/s for celiac trunk. Puck changer was used in all angiographies (with the sequence 2 films/s × 3 s, 1 film/s × 4 s, 1 film/2 s × 4 s), and DSA was selected necessarily (with acquisition sequence 3.1 films/s × 5 s, 1.6 films/s × 5 s, 1 film/s × 2 s). The dose and rate of contrast medium were same in two methods. The apparatus was GE medical systems, Hiline.
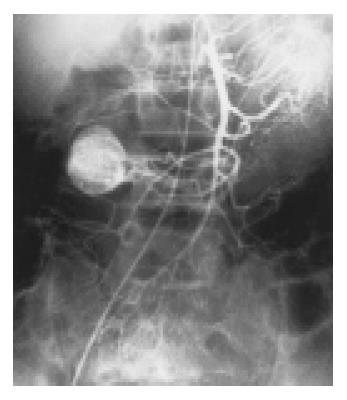
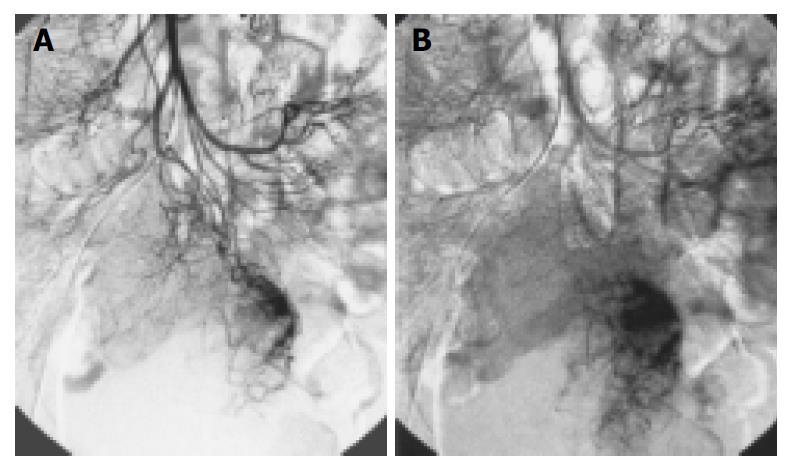
Two tumors arose from stomach, 8 from jejunum (Figure 1, Figure 2), and 2 from ileum. Most of them were originated from the jejunum with a percentage of 66.7% (8/12). The lesions had different sizes, ranged from 3 cm × 3 cm × 2 cm to 5 cm × 6 cm × 4 cm (mean 4 cm × 4.5 cm × 3 cm) in 7 benign lesions, of which 5 lesions were malignant (Figure 3). The smallest was 5.5 cm × 4.5 cm × 3 cm and the largest was 6.5 cm × 10 cm × 7 cm. All tumors were round or oval in shape, except an irregular malignant tumor and another dumbbell-shaped benign tumor growing endophytically and exophytically from the intestinal lumen at the same time.
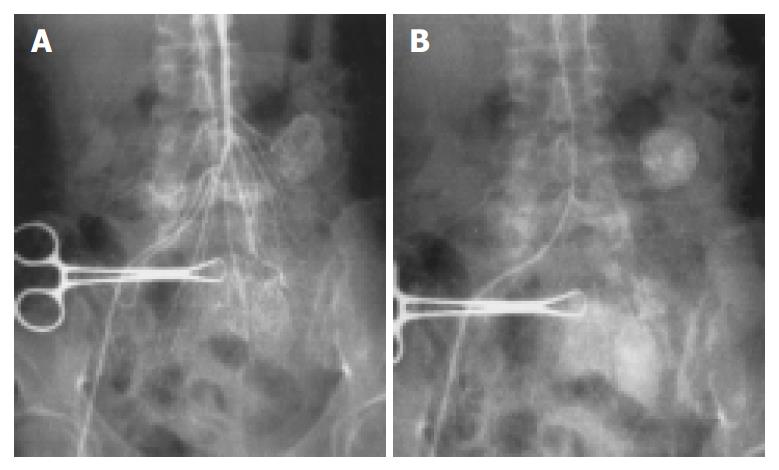
Thickened supplying arteries were observed in 8 tumors (Figure 1), of which 6 were benign and 2 were malignant. Early-developed veins were found in 3 cases (Figure 1), all of which were benign. Four cases having irregular twisted neoplastic vessels with different vessel size and partially coarse and obscure vessel margin were all malignant (Figure 3). Eight cases have ball-like neoplastic vessels with clear margins, included 7 benign and 1 malignant cases. However, the 7 benign cases all had predominantly homogeneous tumor staining with distinct margins (Figure 1, Figure 2).
Four malignant tumors had an indistinct border, and the others had a clear margin. The surrounding organs were pushed by the tumors. Intestinal intussusception was found in 1 patient with recurrent abdominal pain.
The neoplastic cells were fusiform or polygon in shape, and arranged in interlacing or swirl or fence-like. LSAB label was used in immunohistochemistry. Leiomyomas or leiomyosarcomas of other organs (uterus and soft tissue) and shwannomas of soft tissue were chosen for comparisons. High positive presentation of Vim, CD117 and CD34 was found in 12 cases of GIST. Negative presentation of CD34 was found in leiomyomas or leiomyosarcomas, and positive S-100 and negative CD34 were found in shwannomas.
Five malignant tumors contained necrotic or cystic changes with different degrees and two possessed central hemorrhage. Six patients in this study underwent CT at the same time. Masses were revealed by CT in 4 cases, only intestinal intussusception was seen in 1 patient, and diagnosis could not be established with confidence in the other case because of the relatively small size. Of the 3 patients who underwent barium examinations, one was normal, one had an intestinal filling defect and another had his intestines pushed away. No metastasis was found before surgery and no lymphadenopathy was demonstrated during operation.
GIST included the majority of primary non-epithelial tumors of gastrointestinal (GI) tract. These tumors were originated from cells located in the wall of the organ and showed a remarkable variability in their differentiation pathways. They can be divided into 4 major categories on the basis of their phenotypical features: tumors showing differentiation toward smooth muscle, tumors showing apparent differentiation toward neural elements, tumors showing dual differentiation toward smooth muscle and neural elements, tumors lacking differentiation toward either cell type even after exhaustive immunohistochemical and ultrastructural probing[4,5]. However, with the development in immunohistochemistry and ultrastructural probing, it has been agreed generally that GIST is an independent kind of tumors originated from primitive mesenchymal cells of GI tract with non-directive differentiation, some of which have incomplete differentiation parts of smooth muscles and/or nerve sheath cells[1,6-8]. Micro superstructures of these tumors are similar to those of primitive mesenchymal cells without any characteristics of differentiated cellularity. Vim, CD117 and CD34 are highly presented in immunohistochemistry. The standards were all compliant in this study.
GIST could be localized in any portion of the GI tract, but was most often found in the gastric part[9,10]. The jejunum as the most frequent place (66.7%) in this group did not reflect the fact, because it is not necessary for gastric lesion to do angiography when endoscopy, endoluminal ultrasonogrphy or barium study was available and effective.
CT and MR imaging features have been reported previ-ously[1-3,10]. To our knowledge, however, few articles included a description of the appearance of GIST on angiography. According to our study, tdwo types of angiographic changes could be concluded. First, abnormally distributed twisted neoplastic vessels with different thickness and obscure margin were one of the malignant features, and heterogeneous tumor blush with ill-defined border in capillary phase was another. Second ball like vessels with a clear margin, well defined homogeneous tumor staining in round or oval shape, and early-developed veins, suggested a benign GIST. The differentiation points have been described in many previous documents already, including that the malignant tumor was characterized by a larger size more than 5 cm, necrosis, hemorrhage, an indistinct border, and adherence to or direct invasion of the structures nearby[5,11]. The tumors in our group with first type of manifestations were all malignant, but they were found in a relatively small number of patients.
There are many diagnostic approaches for GIST, including barium meal, double-contrast barium study, ultrasonography, fibro-endoscopy, endoluminal ultrasonography, CT, MR imaging and angiography. The sensitivity of these procedures varied according to the different sites of lesions. CT has been considered as the most effective method[1,9]. Therefore, angiography is used less and less in diagnosing tumors of GI, as a result, angiography is only recommended for patients with melena when no abdominal mass is palpable and the examination of upper GI is negative. It helps indicate the supplying arteries, differentiate benign from malignant tumors, and define their size, range and origin, despite the tumors grow exophytically or endophytically. On the other hand, we do not suggest routine use of this procedure because of its traumatic property, high risk and relative complicated technique.
Edited by Wang XL and Chen WW Proofread by Xu FM
| 1. | Belloni M, De Fiori E, Mazzarol G, Curti A, Crosta C. Endoscopic ultrasound and Computed Tomography in gastric stromal tumours. Radiol Med. 2002;103:65-73. [PubMed] [Cited in This Article: ] |
| 2. | Pisanu A, Ambu R, Uccheddu A. Uncommitted gastrointestinal stromal tumour. Case report. G Chir. 2001;22:217-221. [PubMed] [Cited in This Article: ] |
| 3. | Kitabayashi K, Seki T, Kishimoto K, Saitoh H, Ueno K, Kita I, Takashima S, Kurose N, Nojima T. A spontaneously ruptured gastric stromal tumor presenting as generalized peritonitis: report of a case. Surg Today. 2001;31:350-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | LaRock RG, Ginn PE. Immunohistochemical staining characteristics of canine gastrointestinal stromal tumors. Vet Pathol. 1997;34:303-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Rosai J. Gastriointestinal tract. In: Rosai J, ed. Ackerman's sur-gical pathology. 8th ed. Mosby-year Book Inc. USA. 1996;645-648. [Cited in This Article: ] |
| 6. | Tworek JA, Appelman HD, Singleton TP, Greenson JK. Stromal tumors of the jejunum and ileum. Mod Pathol. 1997;10:200-209. [PubMed] [Cited in This Article: ] |
| 7. | Spivach A, Zanconati F, Bonifacio Gori D, Pellegrino M, Sinconi A. [Stromal tumors of the small intestine (GIST). Prognostic differences based on clinical, morphological and immunophenotypic features]. Minerva Chir. 1999;54:717-724. [PubMed] [Cited in This Article: ] |
| 8. | Mignon F, Julié C, Izzillo R, Luciani A, Guichoux F, Mesurolle B, El Hajam M, Qanadli SD, Chagnon S, Lacombe P. [Imaging features of gastric stromal tumors: radiologic-pathologic correlation. Report of 4 cases]. J Radiol. 2000;81:874-881. [PubMed] [Cited in This Article: ] |
| 9. | Wiener Y, Gold R, Zehavy S, Sandbank J, Halevy A. [Primary gastrointestinal stromal tumors]. Harefuah. 2001;140:377-380, 456, 455. [PubMed] [Cited in This Article: ] |
| 10. | Hasegawa S, Semelka RC, Noone TC, Woosley JT, Marcos HB, Kenney PJ, Siegelman ES. Gastric stromal sarcomas: correlation of MR imaging and histopathologic findings in nine patients. Radiology. 1998;208:591-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Lin SC, Huang MJ, Zeng CY, Wang TI, Liu ZL, Shiay RK. Clinical manifestations and prognostic factors in patients with gastrointestinal stromal tumors. World J Gastroenterol. 2003;9:2809-2812. [PubMed] [Cited in This Article: ] |