Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4626
Revised: October 14, 2013
Accepted: November 2, 2013
Published online: April 28, 2014
AIM: To investigate the relationship between neuronal nitric oxide synthase (nNOS) expression and the natriuretic peptide signaling pathway in the gastric fundus of streptozotocin (STZ)-induced diabetic mice.
METHODS: Diabetic mice were induced by injection of STZ solution. Immunofluorescence labeling of HuC/D, nNOS and natriuretic peptide receptor-A, B, C (NPRs) in the gastric fundus (GF) was used to observe nNOS expression and whether NPRs exist on enteric neurons. The expression levels of nNOS and NPRs in the diabetic GF were examined by western blotting. An isometric force transducer recorded the electric field stimulation (EFS)-induced relaxation and contraction in the diabetic GF. An intracellular recording method assessed EFS-induced inhibitory junction potentials (IJP) on the GF. GF smooth muscles acquired from normal mice were incubated with different concentrations of the NPRs agonist C-type natriuretic peptide (CNP) for 24 h, after which their nNOS expressions were detected by western blotting.
RESULTS: Eight weeks after injection, 43 diabetic mice were obtained from mouse models injected with STZ. Immunofluorescence indicated that the number of NOS neurons was significantly decreased and that nNOS expression was significantly downregulated in the diabetic GF. The results of physiological and electrophysiological assays showed that the EFS-induced relaxation that mainly caused by NO was significantly reduced, while the contraction was enhanced in the diabetic GF. EFS-induced IJP showed that L-NAME sensitive IJP in the diabetic GF was significantly reduced compared with control mice. However, both NPR-A and NPR-B were detected on enteric neurons, and their expression levels were upregulated in the diabetic GF. The nNOS expression level was downregulated dose-dependently in GF smooth muscle tissues exposed to CNP.
CONCLUSION: These findings suggested that upregulation of the NPs signaling pathway may be involved in GF neuropathy caused by diabetes by decreasing nNOS expression.
Core tip: The results demonstrated that the expressions of neuronal nitric oxide synthase (nNOS) and numbers NOS neurons were significantly downregulated while natriuretic peptides (NPs) and the natriuretic peptide receptor-A, B, C (NPRs) signaling pathway were upregulated. C-type natriuretic peptide, an NPRs agonist, inhibited nNOS expression in cultured gastric fundus tissue. These findings suggested that upregulation of the NPs signaling pathway may be involved in gastric fundus neuropathy caused by diabetes, by decreasing nNOS expression. The results are interesting and may represent a molecular mechanism of diabetic gastroparesis.
- Citation: Lu HL, Huang X, Wu YS, Zhang CM, Meng XM, Liu DH, Kim YC, Xu WX. Gastric nNOS reduction accompanied by natriuretic peptides signaling pathway upregulation in diabetic mice. World J Gastroenterol 2014; 20(16): 4626-4635
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4626.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4626
Diabetic gastroparesis is a representative diabetic dysmotility, and is associated with dysphagia, heartburn, nausea, vomiting and delayed gastric emptying[1,2]. It occurs in up to 50% of patients with type 1 diabetes and in 30% of patients with type 2 diabetes[3,4]. Gastroparesis seriously affects patients’ quality of life and makes the control of blood glucose more difficult. Although diabetic gastroparesis is a significant health problem, the pathogenesis of this gastric dysfunction and its mechanisms are still not well understood. The gastric motility dysfunction may be caused by several factors, such as hyperglycemia, neuropathy, myopathy and depletion of interstitial cells of Cajal (ICC)[5,6]. The enteric nervous system (ENS), composed of excitatory and inhibitory neurons, exists throughout the entire gastrointestinal (GI) tract, which plays an important role in controlling and coordinating the GI tract motility. The ENS has a vital regulatory role in gastrointestinal motility; therefore, it has attracted more attention in recent years. It is generally considered that enteric neuropathy is one of the causative factors of diabetic gastroparesis. Numerous studies have shown that neurons expressing NOS in myenteric plexus are damaged and the number of nNOS immunoreactive positive neurons are significantly reduced[7]; both mRNA and protein expression levels of nNOS are downregulated and accompanied by attenuation of NO-induced relaxation in diabetic gastroparesis mice[8,9]. To date, the mechanisms of diabetes-induced enteric neuropathy remain unclear, and many investigators have reported that neuronal apoptosis, oxidative stress, advanced glycation end product (AGEs), changes of nerve growth factors and impaired brain-gut interactions may be involved[9]. However, whether the effects of important intracellular signaling pathways participate in diabetes-induced enteric neuropathy has not been widely examined.
Atrial natriuretic peptide was isolated from the antrium by de Bold et al[10] in 1981. From then on, brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP), dendroapsis natriuretic peptide, micrurus natriuretic peptide (MNP), and ventricular natriuretic peptide have been discovered. These natriuretic peptides are distributed all over the body and exert a variety of biological effects, such as natriuretic-diuretic, vasorelaxation, and other functions designed to decrease blood pressure and to control electrolyte homeostasis. Three types of single-transmembrane natriuretic peptide receptors (NPRs) for natriuretic peptides (NPs) have been identified[11-13]: natriuretic peptide receptor A (NPR-A), NPR-B and NPR-C. They are divided into two major categories. NPR-A and NPR-B are membrane-bound guanylyl cyclase receptors that activate guanylyl cyclase, which catalyzes the formation of cGMP from GTP[14-16]. NPR-C, primarily controls NPs concentrations via receptor-mediated internalization and degradation, and has been reported in many signaling pathways in the GI tract [17].
Nitric oxide (NO), identified as a biological signaling molecule in the 1980s, is a major nor-adrenergic, nor-cholinergic (NANC) inhibitory neurotransmitter, which mediates smooth muscle relaxation. It is synthesized by NO synthase (NOS) and its three isoforms (eNOS, nNOS and iNOS) are expressed in many tissues, including endothelium, vascular smooth muscle, specific segments of the nephron and the heart[18,19]. It has been reported that nNOS is expressed on inhibitory neurons and plays an important role in regulation of NO production in the GI tract. NO binds to soluble GC and increases cGMP levels. Many studies have reported the relationships between NPs and NOS. CNP interacts with the NPR-C receptor coupled via G proteins leading to the activation Ca2+-calmodulin dependent endothelial NOS (eNOS), and subsequent increasing in NO production would induce the reduction in cardiac myocyte contractility[20]. By contrast, it has been demonstrated that BNP can increase iNOS and eNOS expression in the rat myocardium and cultures of cardiomyocytes, respectively[21-22].
Our previous studies demonstrated that both NPR-A and NPR-B were distributed in the rat gastric smooth muscle layers and that CNP caused relaxation of the gastric circular and longitudinal smooth muscle tissues in stomachs of humans, rats and guinea pigs[23-25]. Recently, we also reported that the NPs/NPRs signaling pathway is upregulated in the gastric antrum and corpus smooth muscle layers, which may be involved in diabetes-induced loss of gastric ICC via decreasing the production of mSCF indirectly[26]. However, it is not clear whether natriuretic peptides play a role in diabetes-induced neuropathy. In this study, we investigated whether the NOS neurons are damaged and the relationships between CNP/NPRs signaling pathways and nNOS expression in the GF of STZ-induced diabetic mice.
Male imprinting control region (ICR) mice (5-wk-old) used for this study were purchased from the Experimental Animal Center of Shanghai Jiaotong University School of Medicine. One hundred mice were randomly divided into two groups: the control group and the diabetic model group. Mice in the diabetic model group were fasted overnight and intraperitoneally injected with STZ (Sigma-Aldrich, Saint Louis, MO, United States) solution. STZ was prepared freshly in 0.1 mol/L ice-cold citrate buffer (pH = 4.0) and used at a dose of 200 mg/kg body weight. Mice in the control group were intraperitoneally injected with the same volume of 0.1 mol/L citrate buffer. The animals had free access to food and water, and were maintained under standard housing conditions (room temperature 24-27 °C; humidity 60%-65%) with a 12 h light and dark cycle. After two months, blood glucose and body weight of each mouse were measured. Blood withdrawn from mouse tail vein after fasting for 8 h and the blood glucose concentration was measured with One-touch blood glucose monitoring system (Johnson and Johnson Medical Company, New Brunswick, NJ, United States). A mouse was declared diabetic when its blood glucose concentration was above 16 mmol/L.
Whole stomachs were quickly excised from the mice and placed in a Sylgard base dish with pre-oxygenated Krebs solution (containing in 118.1 mmol/L NaCl, 4.7 mmol/L KCl, 1.0 mmol/L KH2PO4, 1.0 mmol/L MgSO4, 25.0 mmol/L NaHCO3, 2.5 mmol/L CaCl2, and 11.1 mmol/L glucose), which was equilibrated with 95% oxygen and 5% CO2. The mesenteric fat was removed, and the stomach was cut along the greater curvature and pinned to the Sylgard base with the mucosa facing upward. Mucosal and submucosal layers were carefully removed under a dissecting microscope and the smooth muscle layers in the GF were used for immunohistochemistry and other experiments.
Smooth muscle tissues (10 mm × 10 mm) from the GF were fixed with ice-cold paraformaldehyde (4% w/v) for 25 min. These tissues were then washed in 0.1 mol/L phosphate buffered saline (PBS) overnight at 4 °C. To reduce non-specific antibody binding, they were preincubated in 5% bovine serum albumin (Sigma) for 1 h at room temperature before incubation with the rabbit anti-nNOS antibody (1:1000; Cell Signaling Technology, Danvers, MA, United States) and mouse anti-HuC/HuD antibody (A-21271, Abcam, Burlingame, CA, United States). To achieve greater penetration during labeling, incubation solutions with the primary antibody were mixed with Triton-X 100 (0.5%; Sigma). Tissues were incubated in the primary antibodies for 48 h at 4 °C. Following washing in 0.1 mol/L PBS overnight at 4 °C, tissues were incubated with the corresponding secondary antibody (DyLight 488 conjugated anti-rabbit IgG and DyLight 549 conjugated anti-mouse IgG, 1:400, CoWin Biotech, China) for 1 h at 25 °C. Tissues were washed in 0.1M PBS for 4 h before being mounted on a slide glass with an anti-fading agent (Molecular Probes, Mississauga, Ontario, Canada) and examined using a confocal microscope (TCS-SP2: Leica Microsystems, Wetzlar, Germany).
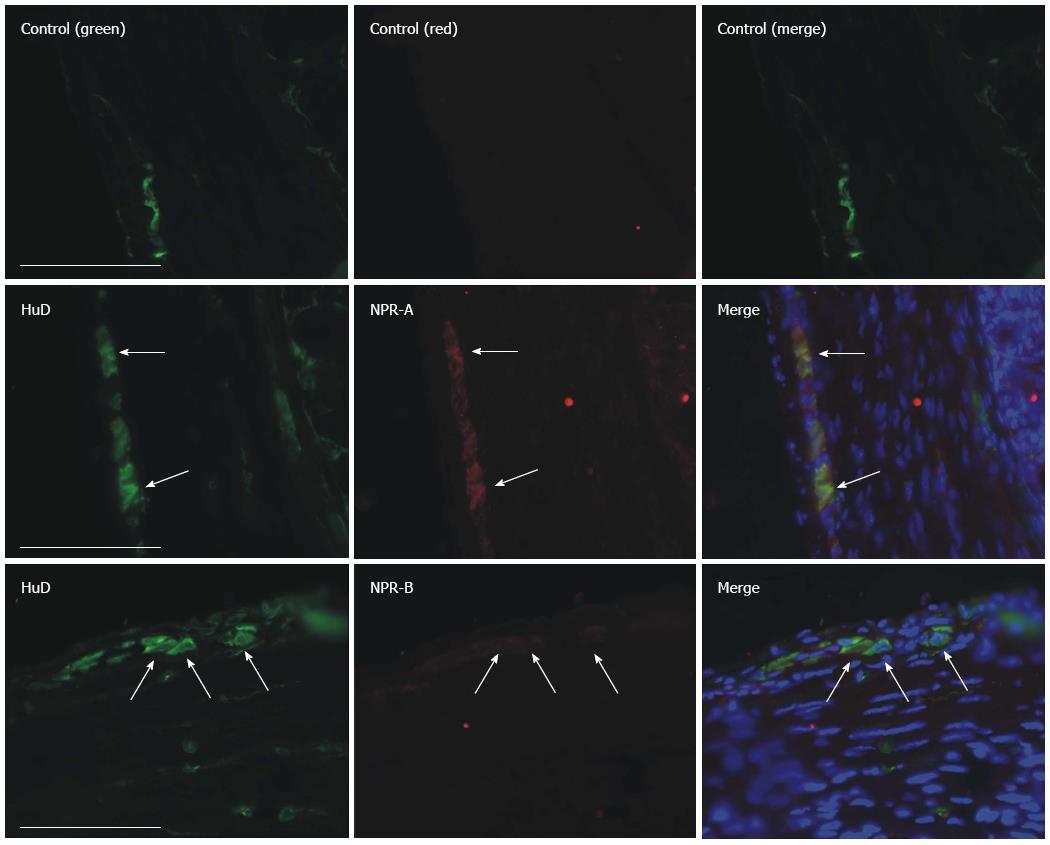
We also used frozen tissue sections to verify whether NPRs were expressed on the myenteric plexus. Small GF tissues were collected after the mice were killed by cervical dislocation. The samples were fixed with 4% paraformaldehyde overnight at 4 °C and sectioned at 5 μm thickness. The sections were then blocked with 10% goat serum in PBS for 1 h at room temperature. The blocking solution was removed and primary antibody solution added (NPR-A, ab70848, 1:100; NPR-B, ab14357, 1:200; HuC/D, A-21271, Abcam), before being incubated overnight at 4 °C. The sections were washed three times in PBS (10 min per time), followed by 1 h incubation in secondary antibody solution (DyLight 488 conjugated anti-mouse IgG and DyLight 549 conjugated anti-rabbit IgG, 1:400, CoWin Biotech, China). The negative control group was created by incubating sections without primary antibodies. Sections were then washed in 0.1 mol/L PBS for 15 min three times before being mounted on a slide glass. These slices were visualized and photographed under a fluorescence microscope (Olympus IX71, Tokyo, Japan).
Fresh GF smooth muscle strips (approximately 2 mm × 8 mm) were acquired by cutting along the circular axis from the gastric smooth muscle tissue. After a silk thread (USP 5/0) was attached to both ends of the muscle strips, the strips were mounted along the circular axis in 10 mL organ baths containing warmed (37 °C) and oxygenated (95%O2: 5%CO2) Krebs solution. An isometric force transducer (RM6240C, Chengdu Instrument Factory, China) that was connected to an amplifier recorded the isometric contraction measurements. The muscle strips were incubated at the appropriate tension for 40 min before the experiment. To observe the excitatory and inhibitory signals in the GF, electric field stimulation (EFS) was applied. Muscle strips were subjected to EFS at 1, 5, 10, 15, 20 and 25 Hz under a constant voltage was 50 V. The pulse width was 0.5 ms, and the duration of stimulation was 10 s. At every interval, 4 min were allowed for recovery of spontaneous activity. After each series of stimulations, the bath solution was exchanged. At the conclusion of each experiment, 50 mmol/L KCl was used to normalize the differences among the readings for each sample.
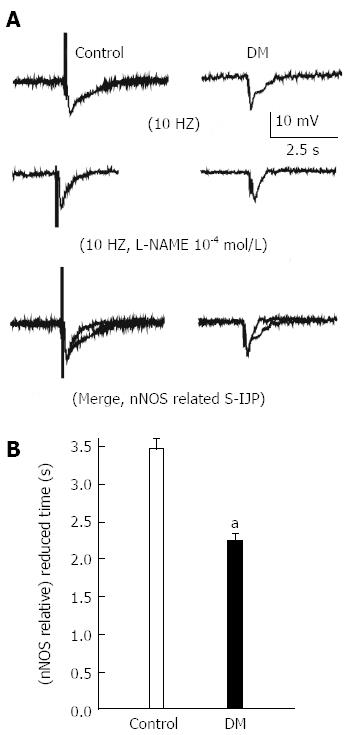
Muscle strips (approximately 5 mm × 10 mm) dissected from the GF were isolated and pinned onto the base of a Sylgard-coated chamber, circular muscle side up, and continuously perfused with warmed (37 °C) and oxygenated Krebs solution. Strips were allowed to equilibrate for approximately 2 h before the recording commenced. Experiments were carried out in the presence of nicardipine (1 μmol/L) to minimize the movement of muscles. Glass microelectrodes filled with 3 mol/L KCl (30-60 MΩ of resistance) were inserted into the cells. Membrane potentials were recorded using a standard electrometer (Duo 773, WPI Inc., Sarasota, FL, United States). EFS was applied in this experiment under a constant voltage of 50 V. The pulse width was 0.5 ms, the duration of stimulation was 20 ms and the slow inhibitory junction potentials (IJP) of circular smooth muscle in normal and diabetic GF were recorded.
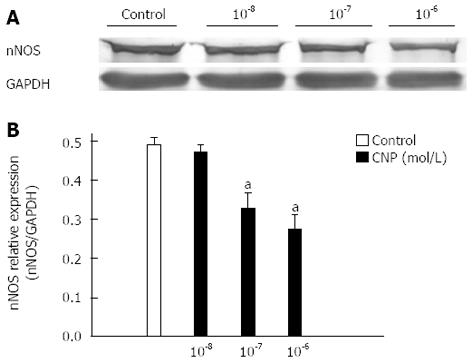
Smooth muscle tissues in the GF were obtained from normal mice as mentioned above. They were washed using sterile PBS three times for five minutes each. To study the relationship between natriuretic peptides (NPs) and nNOS expression in the GF, these tissues were exposed to DMEM containing 0.5% FCS and different concentrations of CNP (10-8, 10-7, 10-6 mol/L) for 24 h. These tissues were then cleaved into protein sample solutions and detected by western blotting.
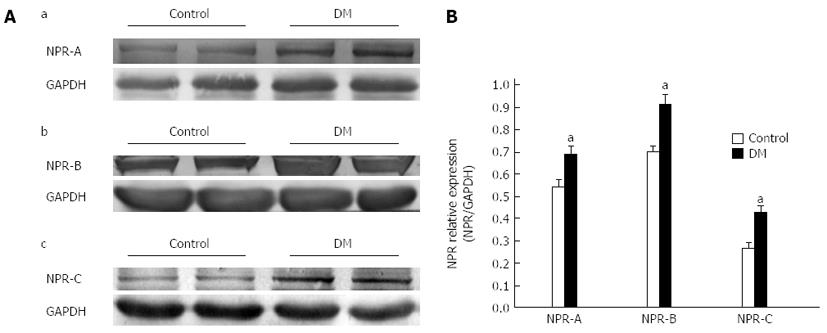
Protein samples were extracted from the smooth muscle tissues in the GF as recommended by the manufacturer of RIPA buffer (Beyotime chemical Co., Jiangsu, China). They were mixed with 2 × loading buffer and in a 100 °C water bath for 10 min before a protein assay (Bio-Rad, Hercules, California, United States) was used to determine the protein content. Equivalent amounts of protein (normally 40 μg per lane) and pre-stained markers were separated by 10% SDS-PAGE and electro transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ, United States). Membranes were then blocked in Tris buffered saline-Tween 20 (TBS-T) with 5% (w/v) non-fat dry milk for 2 h at room temperature. The membranes were incubated overnight at 4 °C with rabbit anti-nNOS polyclonal antibody (1:1000; Cell Signaling Technology, Boston, MA, United States), rabbit anti-NPR-A antibody (1:400; sc-25485, Santa Cruz Biotechnology, Dallas, Texas, United States), rabbit anti-NPR-B antibody (1:300; sc-25486, Santa Cruz Biotechnology, United States), rabbit anti-NPR-C antibody (1:400; sc-25487, Santa Cruz Biotechnology) or rabbit anti-GAPDH monoclonal antibody (1:1000; Cell Signaling Technology,). After washing three times (five minutes each) with TBS-T, the membranes were incubated with the alkaline phosphatase (AP)-conjugated goat anti-rabbit IgG (1:1000; CoWin Biotech, Beijing, China) for 1 h at room temperature. Following removal of the secondary antibody, membranes were washed three times and BCIP/NBT Phosphatase Substrate System (KPL Inc., Gaithersburg, MD, United States) was used to detect the signals on the blots. The image from each western blotting was quantitatively analyzed by using Quantity One software (Bio-Rad) and normalized by the GAPDH signal.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Science and Technology Commission of PRC (STCC Publication No. 2, revised 1988). The Committee on the Ethics of Animal Experiments of Shanghai Jiaotong University School of Medicine approved the protocol (permit number: Hu 686-2009).
The data were expressed as the mean ± SE. Analysis of differences between multiple groups of data was performed with one-way ANOVA, followed by a post hoc Bonferroni test. For comparison between two data sets, a paired or unpaired Student’s t-test was used. Differences were considered to be significant at a P value less than 0.05.
Two months after injection of STZ, the majority mice exhibited hyperglycemia. Plasma glucose concentrations of 43 of the STZ-treated mice were above 16 mmol/L and were thus defined as diabetic. Their mean blood glucose concentration was 23.6 ± 1.9 mmol/L (n = 43), which was significantly higher than the control group (5.9 ± 0.6 mmol/L, n = 43, P < 0.01). Their average body weight was 20.5 ± 0.6 mg, which was significantly lower than the control group (31.7 ± 0.6 mg, n = 43, P < 0.01).
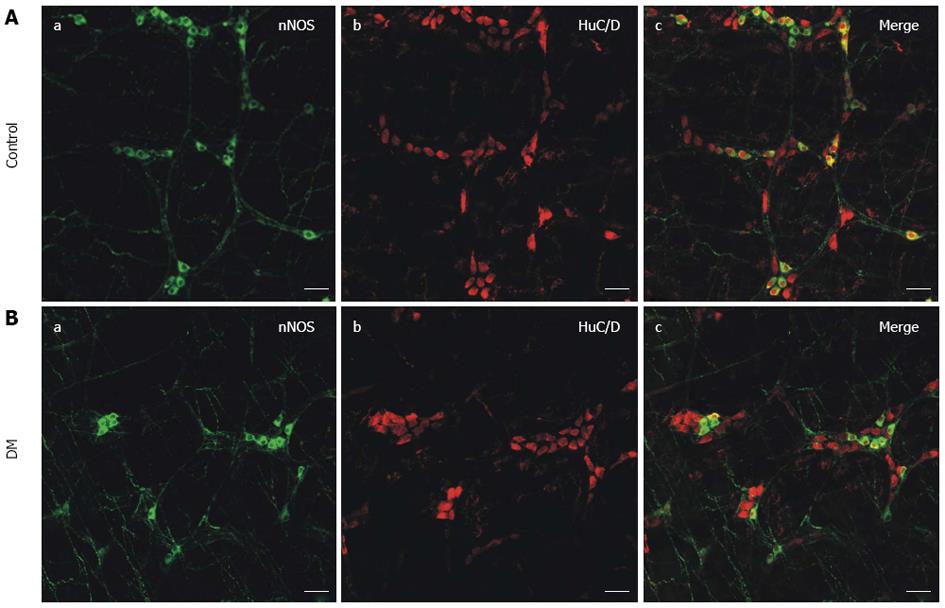
To determine whether diabetes-induced neuropathy had occurred, myenteric neurons were observed in the control and STZ-induced diabetic mice. According to other reports, specific anti-nNOS antibodies and anti-HuC/D antibodies were used to detect NOS neurons and all myenteric neurons in gastric fundus smooth muscle, respectively[27-29]. Green fluorescence showed nNOS and red fluorescence showed HuC/D. Fewer nNOS immunopositive cells were detected in the diabetic mice (Figure 1B) compared with the control mice (Figure 1A) and the NOS neurons were significantly damaged in the STZ-induced diabetic mice (Figure 1B), as observed from the typical merged images (Figure 1).
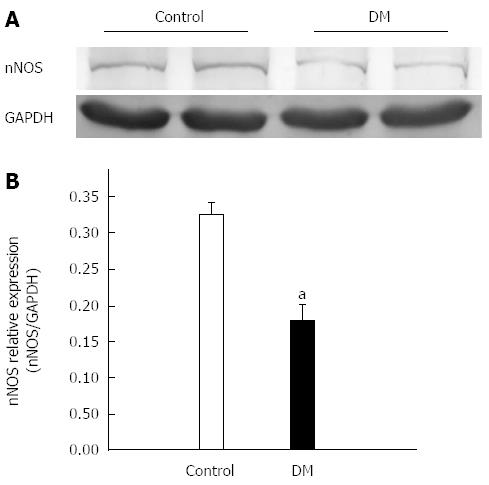
The protein expression level of nNOS in gastric fundus smooth muscle tissue was further analyzed by western blotting. The results showed that the nNOS expression level in STZ-induced diabetes was 0.17 ± 0.03, which was significantly lower than that of control mice (0.33 ± 0.02, Figure 2, n = 7, P < 0.05).
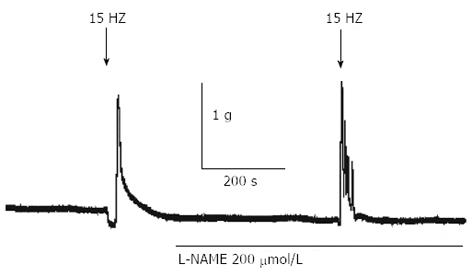
To further confirm whether NOS neurons were damaged and to observe the functional changes in the GF caused by diabetes, physiological and electrophysiological methods were applied. EFS (50 V, 0.5 ms pulse width, 10 s duration, 15 Hz) induced a relaxation and following contraction of gastric fundus smooth muscle strips in normal mice. However, the EFS-induced relaxation disappeared in the presence of 200 μmol/L L-NAME, an NOS inhibitor (Figure 3, n = 5). The results showed that EFS-induced relaxation in the GF was caused by NO synthesized by NOS neurons.
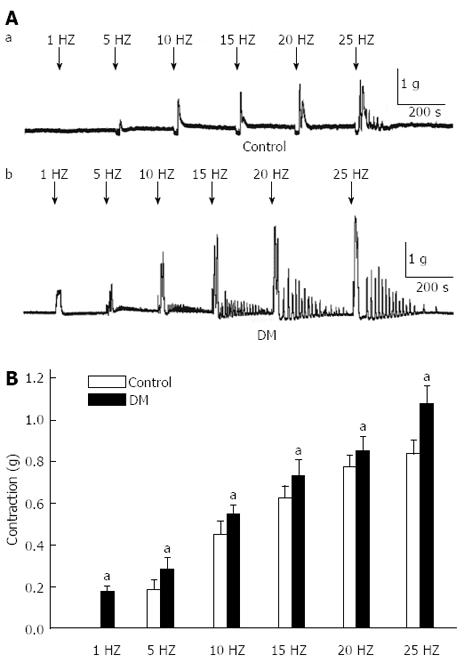
Different frequencies of EFS (1, 5, 10, 15, 20, 25 Hz) were applied in the GF, respectively (Figure 4A). In the diabetic mice, the EFS-induced relaxation was almost completely inhibited while the excitability contraction amplitude was significantly enhanced compared with control mice (0.18 ± 0.02 , 0.28 ± 0.04, 0.55 ± 0.03, 0.73 ± 0.05, 0.86 ± 0.05 and 1.1 ± 0.07 g in diabetic mice, and 0, 0.18 ± 0.04, 0.45 ± 0.03, 0.63 ± 0.03, 0.77 ± 0.03 and 0.83 ± 0.04 g in controls, respectively, Figure 4B, n = 8, P < 0.05).
An intracellular recording technique was used to determine the EFS-induced IJP on gastric fundus smooth muscle tissues (Figure 5A and B). The duration of the L-NAME sensitive, NO-mediated IJP in diabetes was 2.3 s ± 0.07, which was significantly reduced compared with the control (3.4 s ± 0.08, Figure 5C, n = 9, P < 0.05).
To examine whether there were significant changes in NPRs expression in the diabetic GF, total homogenate of GF tissues was used and analyze by western blotting. Figure 6A shows that NPRs were detected in the GF. The expression levels of NPR-A, NPR-B, NPR-C in diabetic and control mice were 0.68 ± 0.03, 0.94 ± 0.03 and 0.43 ± 0.03, and 0.54 ± 0.03, 0.7 ± 0.02 and 0.20 ± 0.02, respectively. The expression levels of NPRs were all upregulated in STZ-induced diabetic mice (Figure 6B, n = 8, P < 0.05).
NPRs were overexpressed in diabetic GF smooth muscle; therefore, the role of NPs in diabetes-induced neuropathy should be investigated. Firstly, we tried to evaluate whether NPRs were expressed on myenteric neurons. Cryosection staining results revealed that both NPR-A and NPR-B were detected on myenteric neurons (red fluorescence, Figure 7). To further investigate the relationship between upregulation of NPRs and nNOS expression, GF smooth muscles were exposed to different concentrations of CNP, a NPRs agonist and the nNOS expression levels were detected. The nNOS expression levels were 0.49 ± 0.02 in the control and 0.47 ± 0.02, 0.35 ± 0.04, 0.28 ± 0.03 in the presence of 10-8, 10-7, 10-6 mol/L CNP, respectively. CNP significantly reduced nNOS expression in cultured GF tissues in a concentration-dependent manner (Figure 8B, n = 7, P < 0.05).
Gastroparesis is a syndrome characterized by delayed gastric emptying in the absence of mechanical obstruction of stomach. It is a well-recognized chronic complication of long-standing diabetes and affects patients’ digestion and absorption functions seriously. Although diabetic gastroparesis (DGP) is a significant health problem, the pathogenesis of gastric dysfunction is still not well understood. The mutual cooperation and coordination between ENS, ICC, and smooth muscle play an important role in maintaining normal gastrointestinal motility. Several studies have reported that DGP may be caused by many factors, such as the depletion of ICC, diabetes-induced neuropathy and damage to NOS neurons[30-33]. High-density NOS neurons are primarily involved in gastric receptive relaxation and pyloric sphincter relaxation, which is extremely important for normal gastric emptying[34-37]. The first part of this study was focused on whether the NOS neurons were destroyed in STZ-induced diabetic mice. Our results indicated that the relative fluorescence intensity of nNOS was much weaker in diabetic GF smooth muscle compared with that of the control (Figure 1). The nNOS expression detected by western blotting revealed a consistent decrease in the protein level (Figure 2). The results suggested that the number of NOS neurons was significantly decreased and further detection showed nNOS expression levels to be significantly downregulated in diabetic mice compared with the control.
There is no spontaneous rhythmic contraction in the GF, therefore, electric field stimulation (EFS) was used to induce relaxation and contraction. Firstly, we tried to study whether NO is involved in EFS-induced response in GF smooth muscle tissues (Figure 3). Different frequencies of EFS were applied on the GF of diabetic and control mice. The results showed that the EFS-induced relaxation was significantly reduced while contraction was enhanced in the diabetic GF (Figure 4). Junction potentials (JPs) occur spontaneously and can be evoked by EFS. Output from the enteric nervous system to the gastric smooth muscle can be detected as neuromuscular excitatory and inhibitory junction potentials (EJPs and IJPs)[38]. The IJP has both rapid and slow components. The rapid component of the IJP is mediated by P2Y1 receptors and is widely considered to be transmitted by ATP[39,40]. The slow component of the IJP is nitrogen, and can be blocked by NOS inhibitors[41]. In this experiment, IJPs were evoked by EFS on GF smooth muscles and the slow component (NO component) duration was significantly reduced in diabetic mice compared with the control (Figure 5). This result might imply that diabetes causes serious neuropathy, especially NOS neurons damage, resulting in reduced NO production, further inducing abnormal excitability contraction and reduced sIJP duration time.
The NPs system is a local endocrine system in the gastrointestinal tract. It plays an important role in regulation of motility, secretion and absorption. Our previous studies showed that NPs can induce smooth muscle relaxation and the NPs signaling pathway participates in diabetes-induced ICC damage[23-26]. Many studies have reported the relationships between NPs and NOS[20-22]. In this study, we tried to evaluate whether NPs are involved in NOS neuron damage. Firstly, the protein expression levels of NPRs in diabetic GF smooth muscle were detected by western blotting. The results showed that the expression levels of three types of natriuretic peptide receptor (NPR-A, B, C) in diabetes were much higher than in control mice (Figure 6). Secondly, we observed the distribution of NPRs on GF enteric nerve system in frozen sections by immunohistochemistry. The results showed that there were many NPR-A, B proteins expressed on myenteric neurons (Figure 7). To investigate the relationship between upregulated NPRs and nNOS expression, GF smooth muscles were incubated with different concentrations of CNP and then the nNOS expression level was detected. The results showed that CNP decreased nNOS expression in a concentration-dependent manner (Figure 8). We can conclude that NPs may be involved in diabetes-induced neuropathy via decreasing nNOS expression.
In summary, we found that the number of NOS neurons was reduced and nNOS expression was downregulated, while the NPRs expressions were upregulated in GF smooth muscle of STZ-induced diabetic mice. Diabetes-induced NOS neuron damage resulted in poor production of NO, which eventually caused abnormal excitability contraction and damaged relaxation in diabetic GF. Diabetes-induced upregulation of the NPs signaling pathway may be involved in NOS neurons injury.
Diabetic gastroparesis is a common complication of diabetic dysmotility. It is generally considered that enteric neuropathy is one of the causes of diabetic gastroparesis. Numerous studies have shown that neurons that synthesize the nitric oxide synthase (NOS) in the myenteric plexus were damaged and the number of nNOS immunoreactive neurons were significantly reduced; however, the mechanism of diabetes-induced enteric neuropathy remain unclear.
The natriuretic peptides (NPs) system is a local endocrine system in the gastrointestinal tract. It plays an important role in regulation of motility, secretion and absorption. Previous studies have reported that NPs can induce smooth muscle relaxation and the NPs signaling pathway participates in diabetes-induced interstitial cells of Cajal damage. In this study, the authors demonstrated that upregulation of the NPs signaling pathway might be involved in gastric fundus neuropathy caused by diabetes via decreasing nNOS expression.
Recent reports have highlighted the importance of damage to NOS neurons accompanied by upregulation of NPs/NPRs/cGMP signaling pathway in the diabetic gastric fundus. C-type natriuretic peptide (CNP), a NPRs agonist, inhibited nNOS expression in cultured gastric fundus tissue. This is the first study to report the relationship between NPs/NPRs signaling pathway and NOS neuron damage in STZ-induced diabetic gastric fundus tissues.
By understanding the mechanism by which NOS neurons are damaged in diabetic gastroparesis, this study may represent a future strategy for therapeutic intervention in the treatment of patients with diabetic gastroparesis.
The NPs are a family of three polypeptide hormones termed atrial natriuretic peptide, brain natriuretic peptide, and CNP. In gastrointestinal tract NPs are involved in gastrointestinal motility, absorption and secretion.
The authors examined numbers of NOS neurons, and the expressions of nNOS and natriuretic peptide receptor-A, B, C (NPRs) in diabetic gastric fundus. The results demonstrated that the numbers of NOS neurons and the expression of nNOS were significantly downregulated while the NPs/NPRs signaling pathway was upregulated. CNP, a NPRs agonist, inhibited nNOS expression in cultured gastric fundus tissue. The results are interesting and may represent a molecular mechanism of diabetic gastroparesis.
P- Reviewer: Feng CG S- Editor: Zhai HH L- Editor: Stewart GJ E- Editor: Liu XM
| 1. | Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989-1996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 398] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Samsom M, Vermeijden JR, Smout AJ, Van Doorn E, Roelofs J, Van Dam PS, Martens EP, Eelkman-Rooda SJ, Van Berge-Henegouwen GP. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116-3122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47-56. [PubMed] [Cited in This Article: ] |
| 4. | Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16-S22. [PubMed] [Cited in This Article: ] |
| 6. | Camilleri M. The stomach in diabetes: from villain to ally. Clin Gastroenterol Hepatol. 2009;7:285-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wang XY, Huizinga JD, Diamond J, Liu LW. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095-1e92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, Ferris CD. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [PubMed] [Cited in This Article: ] |
| 10. | de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Reprinted from Life Sci. 28: 89-94, 1981. J Am Soc Nephrol. 2001;12:403-409; discussion 403-408; 408-409. [PubMed] [Cited in This Article: ] |
| 11. | Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 729] [Cited by in F6Publishing: 695] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 437] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989;341:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 483] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 762] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991;252:120-123. [PubMed] [Cited in This Article: ] |
| 16. | Rose RA, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol. 2008;586:353-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Piech A, Dessy C, Havaux X, Feron O, Balligand JL. Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovasc Res. 2003;57:456-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Cheng PY, Chen JJ, Yen MH. The expression of heme oxygenase-1 and inducible nitric oxide synthase in aorta during the development of hypertension in spontaneously hypertensive rats. Am J Hypertens. 2004;17:1127-1134. [PubMed] [Cited in This Article: ] |
| 19. | Costa MA, Elesgaray R, Caniffi C, Fellet A, Arranz C. Role of cardiovascular nitric oxide system in C-type natriuretic peptide effects. Biochem Biophys Res Commun. 2007;359:180-186. [PubMed] [Cited in This Article: ] |
| 20. | Wang T, Yan M, Li J, Zheng X. The role of iNOS-derived NO in the antihypertrophic actions of B-type natriuretic peptide in neonatal rat cardiomyocytes. Mol Cell Biochem. 2007;302:169-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Ren B, Shen Y, Shao H, Qian J, Wu H, Jing H. Brain natriuretic peptide limits myocardial infarct size dependent of nitric oxide synthase in rats. Clin Chim Acta. 2007;377:83-87. [PubMed] [Cited in This Article: ] |
| 22. | Guo HS, Jin Z, Jin ZY, Li ZH, Cui YF, Wang ZY, Xu WX. Comparative study in the effect of C-type natriuretic peptide on gastric motility in various animals. World J Gastroenterol. 2003;9:547-552. [PubMed] [Cited in This Article: ] |
| 23. | Guo HS, Cui X, Cui YG, Kim SZ, Cho KW, Li ZL, Xu WX. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscle of rat. Acta Pharmacol Sin. 2003;24:1021-1026. [PubMed] [Cited in This Article: ] |
| 24. | Guo HS, Cai ZX, Zheng HF, Li XL, Cui YF, Wang ZY, Xu WX, Lee SJ, Kim YC. Role of calcium-activated potassium currents in CNP-induced relaxation of gastric antral circular smooth muscle in guinea pigs. World J Gastroenterol. 2003;9:2054-2059. [PubMed] [Cited in This Article: ] |
| 25. | Wu YS, Lu HL, Huang X, Liu DH, Meng XM, Guo X, Kim YC, Xu WX. Diabetes-induced loss of gastric ICC accompanied by up-regulation of natriuretic peptide signaling pathways in STZ-induced diabetic mice. Peptides. 2013;40:104-111. [PubMed] [Cited in This Article: ] |
| 26. | Qiu ZX, Mei B, Wu YS, Huang X, Wang ZY, Han YF, Lu HL, Kim YC, Xu WX. Atrial natriuretic peptide signal pathway upregulated in stomach of streptozotocin-induced diabetic mice. World J Gastroenterol. 2010;16:48-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 27. | Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998-9009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Freytag C, Seeger J, Siegemund T, Grosche J, Grosche A, Freeman DE, Schusser GF, Härtig W. Immunohistochemical characterization and quantitative analysis of neurons in the myenteric plexus of the equine intestine. Brain Res. 2008;1244:53-64. [PubMed] [Cited in This Article: ] |
| 29. | Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl). 2004;209:19-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. [PubMed] [Cited in This Article: ] |
| 31. | Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535-1544. [PubMed] [Cited in This Article: ] |
| 33. | Gangula PR, Mukhopadhyay S, Ravella K, Cai S, Channon KM, Garfield RE, Pasricha PJ. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692-G699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973;390:1-38. [PubMed] [Cited in This Article: ] |
| 35. | Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 401] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Plourde V, Quintero E, Suto G, Coimbra C, Taché Y. Delayed gastric emptying induced by inhibitors of nitric oxide synthase in rats. Eur J Pharmacol. 1994;256:125-129. [PubMed] [Cited in This Article: ] |
| 37. | Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817-1824. [PubMed] [Cited in This Article: ] |
| 38. | Furness J, Moore G, Waud E. The identification of victims of mass disasters, and also the identification of individual remains. A method of coding teeth. Br Dent J. 1969;127:501-504. [PubMed] [Cited in This Article: ] |
| 39. | Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA. 2007;104:16359-16364. [PubMed] [Cited in This Article: ] |
| 40. | King BF, Townsend-Nicholson A. Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther. 2008;324:1055-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Watson MJ, Lang RJ, Bywater RA, Taylor GS. Characterization of the membrane conductance changes underlying the apamin-resistant NANC inhibitory junction potential in the guinea-pig proximal and distal colon. J Auton Nerv Syst. 1996;60:31-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |