Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7878
Revised: March 1, 2014
Accepted: March 12, 2014
Published online: June 28, 2014
Colorectal cancer (CRC) is the third leading cause of cancer deaths worldwide and the fourth most common cancer diagnosed among men and women in the United States. Considering the risk factors of CRC, dietary therapy has become one of the most effective approaches in reducing CRC morbidity and mortality. The use of probiotics is increasing in popularity for both the prevention and treatment of a variety of diseases. As the most common types of microbes used as probiotics, lactic acid bacteria (LAB) are comprised of an ecologically diverse group of microorganisms united by formation of lactic acid as the primary metabolite of sugar metabolism. LAB have been successfully used in managing diarrhea, food allergies, and inflammatory bowel disease. LAB also demonstrated a host of properties in preventing colorectal cancer development by inhibiting initiation or progression through multiple pathways. In this review, we discuss recent insights into cellular and molecular mechanisms of LAB in CRC prevention including apoptosis, antioxidant DNA damages, immune responses, and epigenetics. The emerging experimental findings from clinical trials as well as the proposed mechanisms of gut microbiota in carcinogenesis will also be briefly discussed.
Core tip: The gastrointestinal tract inhabits trillions of bacteria that interact with the host at multiple levels to maintain its normal functions. Disruptions in this complex cross-talk ecosystem result in physiological changes associated with colorectal tumorigenesis, including cell proliferation, immune responses and apoptosis. This review summarizes the role of lactic acid bacteria as anti-tumorigenic probiotics and suggests the possibility of altering gut microbiota to prevent or halt development of colorectal cancer.
- Citation: Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol 2014; 20(24): 7878-7886
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7878.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7878
Colorectal cancer (CRC) is one of the major health challenges, representing the second cause of cancer deaths and the fourth most common cancer diagnosed among men and women in the United States[1,2]. Like other types of cancers, the resistance to programmed cell death and uncontrolled proliferation of the cells are main features of CRC influencing therapeutic efficacy. Although consequential progress in the treatment of cancers has been made during the last few decades, there are still many persistent issues chief among them the resistance to chemotherapy which requires better clinical resolution[3]. Recently, it has become increasingly evident that the large and complex bacterial population hosted by the large intestine and known as “gut microbiota” plays an critical role in colorectal carcinogenesis[4,5]. One group of them is probiotics which are “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” (WHO[6]). In fact, it is not the general probiotic bacteria that have this biotherapeutic action but those who have a particular anticancer activity well beyond the activity of the usual probiotic bacteria[7]. The most common group of this class of probiotics is lactic acid bacteria (LAB). Accumulating recent evidence shows that LAB inhibit initiation or progression of carcinogenesis through various pathways, thus paving the way for potential therapeutic methods for colorectal cancer (Table 1). In this review, we will discuss emerging findings from both experimental and clinical studies describing the latest developments of LAB’s role in CRC prevention and their potential mechanisms of action.
| Prevention | LAB strains | Functions |
| Apoptosis | Lactobacillus acidophilus | Anti-cancer cell growth and differentiation |
| Direct induction of Beclin-1 and GRP78 | ||
| L. reuteri | Proliferation (Cox-2, cyclin D1) and cell survival (Bcl-2, Bcl-xL) | |
| Enhances MAPK activities including c-Jun N-terminal kinase and p38 MAPK | ||
| L. acidophilus and L. rhamnosus | Induce Beclin-1 and GRP78, as well as indirectly through the induction of Bcl-2 and Bak | |
| L. acidophilus and L. casei | 5-fluorouracil apoptosis induction | |
| Antioxidant DNA damage | Bifidobacterium longum and L. acidophilus | Antioxidative activity, inhibiting linoleic acid peroxidation |
| Streptococcus thermophilus | Releasing ROS protective factors | |
| Immune response improvement | L. acidophilus | Stimulates DCs to produce inflammatory cytokines IL-12 and regulatory IL-10 |
| LTA-deficient L. acidophilus | Induces IL-10 in DCs, down-regulates IL-12 levels | |
| Increases densities of effector Foxp3+RORγt- Tregs | ||
| L. acidophilus, L. casei and B. longum | Enhance the total numbers of T cells, NK cells, MHC class II+ cells, and CD4-CD8+ T cells | |
| L. casei Shirota (LcS) | Induces cytokines, such as IFN-γ, interleukin-β (IL-1β) and TNF-α | |
| B. adolescentis | Increases the production of TNF-α | |
| Epigenetics | LTA-deficient L. acidophilus | Enhances the expression of tumor suppressor genes |
| New anticancerfunction | Pediococcus pentosaceus FP3 | Adhere to colon cancer cells and trigger bioproduction of SCFA |
| L. salivarius FP25 | ||
| L. salivarius FP35 | ||
| Enterococcus faecium FP51 |
As the most common types of microbes used as probiotics, LAB are comprised of an ecologically diverse group of microorganisms united by formation of lactic acid as the primary metabolite of sugar metabolism, including Lactobacillus, Streptococcus, Enterococcus, Lactococcus, Bifidobacterium and Leuconostoc[8]. Their beneficial effects were initially revealed by E. Metchnikoff (1845-1919), a Russian scientist who proposed that extended longevity of the people of Balkan could be attributed to their practice of ingesting fermented milk products[9]. Recent studies showed that LAB could be successfully used to manage diarrhea[10,11], food allergies[12], and inflammatory bowel disease (IBD)[13-15]. The potential role of LAB has been extensively reviewed elsewhere[8,16], and their beneficial effects include reinforcement of the natural defense mechanisms and protection against gastrointestinal disorders[17]. Several publications have indicated that LAB play an important role in prevention of CRC[16,18]. Although there is no general consensus on the role of LAB in CRC treatment, it is generally agreed that specific LAB strains can beneficially activate anticancer mechanisms, thereby regulating the host’s immune response[3,18].
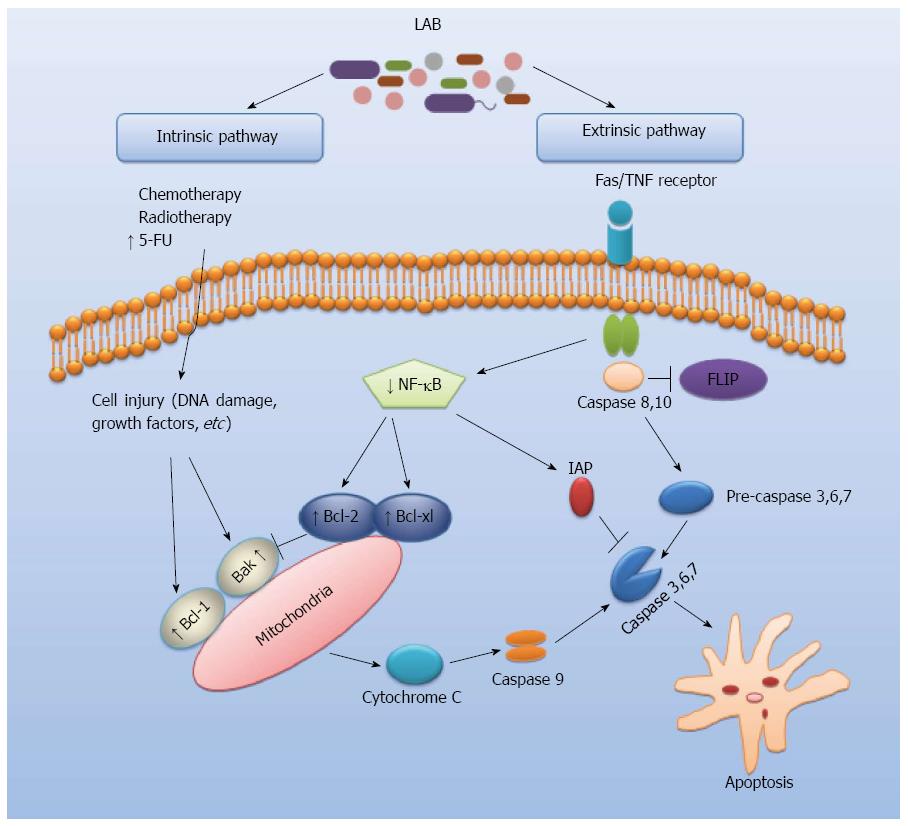
Apoptosis is a form of genetically programmed cell death, playing a key role in the regulation of cell numbers (Figure 1)[19,20]. An important pathogenetic event in many types of cancers is the reduced ability to trigger apoptosis associated with alteration of control processes of cell proliferation[21]. The regulation of cell survival and death at molecular level on the apoptotic process can have a huge chemopreventive and therapeutic potential[22]. Several studies showed that LAB can play a role in the regulation of cell apoptosis via intrinsic and extrinsic pathways which are potentially critical mechanisms in the prevention of CRC. Chen et al[23] analyzed the effect of oral administration of Lactobacillus acidophilus (L. acidophilus) on colorectal cancer in mice. Their results indicated that L. acidophilus reduced the severity of colorectal carcinogenesis and enhanced apoptosis in treated mice. It has been shown that Lactobacillus reuteri (L. reuteri) may prevent colorectal cancer via downregulating nuclear factor-kappaB (NF-κB)-dependent gene products which regulate cell proliferation (Cox-2, cyclin D1) and survival (Bcl-2, Bcl-xL)[24]. Furthermore, L. reuteri suppressed tumor necrosis factors (TNF)-induced NF-κB activation including NF-κB-dependent reporter gene expression in a dose- and time-dependent manner to slow down cancer cell growth. Such activities of L. reuteri might be involved in the extrinsic pathway of apoptosis by which LAB act to protect against CRC (Figure 1). Other studies reported that exopolysaccharides of L. acidophilus and L. rhamnosus were antitumourigenic against HT-29 colon cancer cells and that this activity was due to the activation of autophagic cell death promoted directly by the induction of Beclin-1 and GRP78, as well as indirectly through the induction of Bcl-2 and Bak[25]. Moreover, the combination of L. acidophilus and L. casei enhanced the apoptosis-induction capacity of 5-fluorouracil in colorectal carcinoma cell line LS513, suggesting that these probiotics may be used as adjuvants in anticancer chemotherapy[26]. Therefore, an improved understanding of probiotics-mediated effects on apoptosis signaling pathways is critical for development of future LAB-based CRC treatments.
The metabolic antioxidant activities of LAB may be assigned to reactive oxygen species (ROS) scavenging, enzyme inhibition, and reduction activity or inhibition of ascorbate autoxidation in the intestine by neutralizing free radicals[27]. It is assumed that ROS play a key role in IBD and CRC. Several in vitro studies showed that LAB strains possess antioxidant properties and inactivate ROS via enzymatic mechanisms such as coupled NADH oxidase/peroxidase system and catalase[28-32]. Lin et al[33] showed that a strain of Bifidobacterium longum (B. longum) and L. acidophilus display antioxidative activity, inhibiting linoleic acid peroxidation by 28%-48% which is dominant in lipid peroxidation process. The heat-killed cells of L. acidophilus 606 and the soluble polysaccharide components of this strain exhibit potent antioxidative activity[34]. Several recent studies revealed prevention of oxidative DNA damage in human derived colon (HT29) cells by LAB[35]. These results indicate that the majority of strains including Streptococcus thermophilus have a protective effect against oxidative damage by releasing ROS protective factors into the medium. Furthermore, the obligatory homofermentative lactobacilli display high antioxidant activity whereas this property is highly strain-dependent among facultative and obligate heterofermentative lactobacilli[36,37]. Taken together, these studies implicate LAB as key molecules in antioxidant activity which may prevent CRC.
The immune system plays a critical role in control of tumor promotion and progression[38]. The interaction of several elements of the immune system, such as antigen-presenting cells (APCs), different subsets of T cells, B cells, natural killer (NK) cells, and dendritic cells (DCs), is usually activated by damage, invasion or mutation[39]. Recent studies implicate LAB in immune responses critical for colorectal cancer prevention and therapeutics[40].
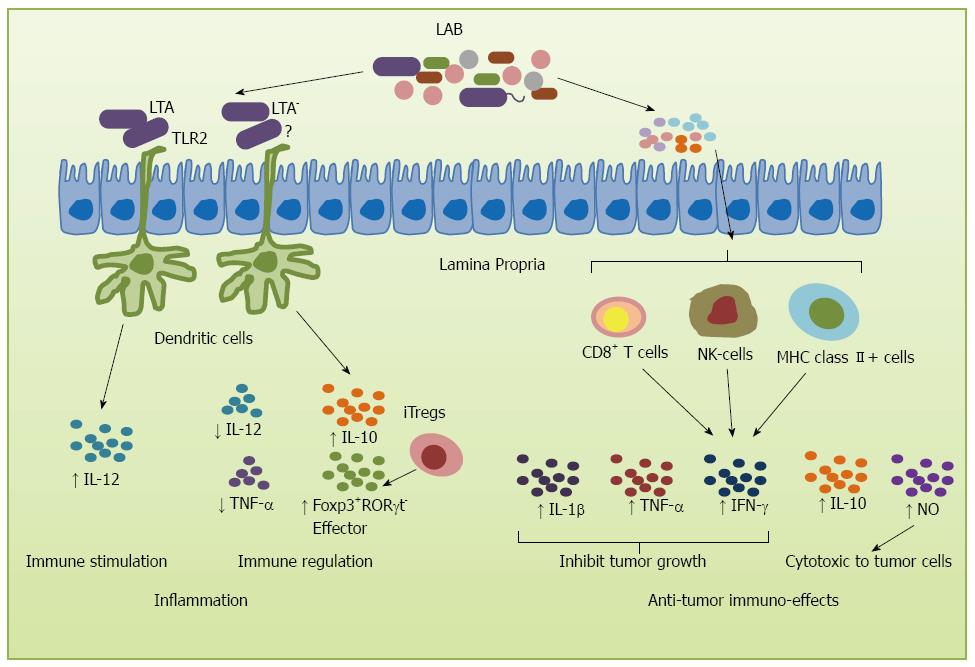
During tumor development, the first line of immune defense is formed by innate immune cells that confer protection against acute inflammation[41]. de Visser et al[42] showed that intestinal inflammation parallels the development of CRC. Probiotics play an important role in this process, with several studies showing their role in increasing the production of IL-10, an anti-inflammatory cytokine[43-45]. In fact, recent studies showed that L. acidophilus stimulated innate cells to produce inflammatory and regulatory cytokines by interacting surface layer proteins with other cell surface components such as lipoteichoic acid (LTA) which is a zwitterionic glycolipid found in the cell wall of several Gram-positive bacterial strains[46-50]. LTA can stimulate DCs through Toll-like receptor 2, resulting in cytokine release[51,52]. Some specific Lactobacillus species can stimulate DCs to produce IL-12 and regulatory, inflammatory cytokine IL-10[46,47]. However, disruption of LTA synthesis resulted in a L. acidophilus derivative that acts on intestinal immune cells to augment production of IL-10 in DCs, down-regulate IL-12 levels, and significantly mitigate dextran sulfate sodium- and CD4+CD45RBhigh T cell-mediated colitis in mice[53]. These alterations of cell surface components of L. acidophilus provide a potential strategy for the treatment of inflammatory intestinal disorders and cancer therapy. Reinforcing the role of LTA, studies using LTA-deficient L. acidophilus (NCK2025) strain led to normalization of innate and adaptive pathogenic immune responses and caused regression of established colonic polyps. Not only IL-12 and TNF-α were down-regulated by NCK2025, but also IL-10 in DCs was significantly enhanced and CD4+ T-cells were activated. The mice acquired significant protection from colitis with increased densities of effector Foxp3+RORγt- Tregs in response to oral administration of L. acidophilus NCK2025[54] (Figure 2).
Under pathological states, such as colon cancer, probiotics may inhibit disease via modulation of the mucosal and systemic immune response and by reduction of the inflammatory response to host microbiota[41]. Another possible anti-tumor mechanism is the activation of immunity by immune cells to fight with the tumor cells, delay the onset of tumor or increase the survival rate. Galdeano et al[55] analyzed the profile of cytokines induced by some LAB strains and observed that the most remarkable effect for all the probiotic strains tested is the increase in TNF-α, interferon-γ (IFN-γ) and the regulatory cytokine IL-10. LAB such as L. acidophilus, Lactobacillus casei (L. casei) and B. longum have been shown to possess immunomodulatory and antitumor effects by suppressing the proliferation of tumor cells and prolonging survival[56]. The increase in survival was correlated with an increase in cellular immunity as reflected by the enhancement in the total numbers of T cells, NK cells and MHC class II+ cells, and CD4-CD8+ T cells in flow cytometry analysis. Several strains of LAB have been shown to exert powerful anti-tumor effects. For example, L. casei Shirota (LcS) has been shown to exert strong anti-metastatic effects on transplantable tumor cells and to suppress chemically-induced carcinogenesis[57]. Intrapleural administration of LcS into tumor-bearing mice induced production of several cytokines, such as IFN-γ, interleukin-β (IL-1 β) and TNF-α, inhibiting tumor growth and increasing survival[58,59]. Furthermore, oral feeding of LcS significantly enhanced NK cell cytotoxicity which delayed tumor onset or suppressed tumor incidence[60]. Likewise, a butanol extract of another LAB strain, B. adolescentis, significantly increased the production of TNF-α and NO, which regulate immune modulation and are cytotoxic to tumor cells[61] (Figure 2). Taken together, these studies provide convincing evidence demonstrating the important role of LAB and their byproducts in the protection against carcinogenesis processes.
The term “epigenetics” is used to describe those mechanisms which are able to modify the expression levels of selected genes without necessarily altering their DNA sequences, including DNA methylation, histone tail modifications, chromatin remodeling, as well as mechanisms mediated by non-coding RNA molecules. Epigenetic modifications are often induced by environmental factors[62]. It is now clear that epigenetic phenomena occur together with gene mutation and contribute to the progression of normal colonic mucosa to CRC[63]. Recently, in the field of cancer biology, increasing attention has been given to the role of epigenetic alterations in the etiology of cancer. A particularly active area of research involves histone deacetylase inhibitors (HDACi), a well known class of epigenetic drugs, used not only for cancer therapy but for cancer chemoprevention through strong anti-proliferative effects on tumor cells[64-66]. Probiotic metabolites such as butyrate, a short-chain fatty acid (SCFA), are therefore an important class of therapeutic compounds. Waldecker et al[67] reported that butyrate was one of the most potent HDACi in human colon cancer cell lines, suggesting an integral role of butyrate as an anti-inflammatory derivative of microbial fermentation in the colon. In studies using fecal fermentation, supernatants were found to be rich in butyrate and exhibited strong HDAC inhibitory properties in several colon cancer cell lines[68]. The ability of butyrate to de-repress epigenetically silenced genes in cancer cells, such as cell cycle inhibitor p21 and the pro-apoptotic protein Bcl-2 homologous antagonist/killer, and to activate these genes in normal cells, has important implications for cancer prevention and therapy[69]. It has been hypothesized that increased colonic concentration of butyrate can be an important mediator against CRC[70,71]. Recently, Lightfoot et al[72,73] tested possible epigenetic modifications induced by LTA-deficient L. acidophilus and found that oral NCK 2025 enhances the expression of tumor suppressor genes. This indicates that differential epigenetic regulation of CRC-related genes by NCK2025 represents a potential therapy against CRC.
In addition to the anticancer properties of LAB discussed above, recent findings showed that LAB also exerts antiproliferation activities of colon cancer cells via synergistic actions between adherence to cancer cells and SCFA bioproduction. To this end, Thirabunyanon et al[74] investigated probiotic action of LAB in the prevention and biotherapy of colon cancer. Four probiotic bacteria Pediococcus pentosaceus FP3, Lactobacillus salivarius (L. salivarius) FP25, L. salivarius FP35, and Enterococcus faecium FP51 showed antiproliferation properties at the rates of 17%-35%. The proposed mechanism of the proliferative inhibition was assigned to the synergic induction by directly adhering to colon cancer cells and triggering bioproduction of SCFA, mainly butyric and propionic acids. Using cell-free supernatants from LAB L. casei- and L. rhamnosus-treated cells, Escamilla et al[75] showed a decrease in colon cancer cell invasion in vitro by inducing matrix metalloproteinase-9 activity and zona occludens-1. These properties of L. casei and L. rhamnosus GG may prove useful in designing strategies for CRC prevention or treatment.
Clinical trials examining the effect of LAB or probiotics on cancer are presently ongoing. The SYNCAN project funded by the European Union involving a 12-wk randomized, double blind trial of a food supplement containing Lactobacillus GG, Bifidobacterium Bb-12 in adenoma patients aims at testing colon cancer risk biomarkers[76]. It is hoped that the results of this study will provide much-needed information on the cancer protective effects of these bacterial strains. In randomized clinical trials, probiotics have been shown to decrease postoperative infectious complications in patients with CRC[77]. Similarly, functional outcome and health-related quality of life were significantly improved in patients who underwent surgical resection of CRC following L. acidophilus and Bacillus natto treatment[78]. Although there are no clinical studies in which LAB or probiotics are shown to reduce recurrence of CRC, L. casei has been shown to decrease significantly the recurrence of other cancers such as superficial bladder cancer[79]. Thus, based on current evidence, the effects of LAB in CRC are encouraging, although clinical as well as mechanistic studies are needed to identify the bacterial products and their interaction with the host in prevention or treatment of CRC. It is worth mentioning that some progress has been made in identification of “bacterial biomarkers” for cancer detection. In this regard, Brim et al[80] analyzed the SLC5A8 gene, which encodes a transporter of butyrate, in 50 colon adenomas from patients and found that 82% of these patients displayed a high level of methylation, pointing to its potential use as a marker for early detection.
It is clear that LAB are of paramount importance in the prevention of CRC. Insights into the cellular and molecular mechanisms which include apoptosis, antioxidant, immune responses, and epigenetics opened the door for the development of novel therapeutic approaches. Although a wide range of studies have shown remarkable potential of LAB strains in interfering with colorectal carcinogenesis, conclusive clinical evidence supporting the role of probiotics in CRC treatment is still lacking. More epigenetic studies on LAB are required to demonstrate their effects in cancer prevention. Although several mechanisms of actions of LAB in carcinogenesis have been described in in vitro and animal model studies, we are still far from pinpointing the exact cellular signaling responsible for their effects. Nevertheless, the demonstrated functions of LAB in repairing defective apoptotic processes or controlling cell proliferation in cancer have made them an attractive tool for helping treat CRC. For example, based on the mechanism of apoptosis, one could modify LAB such as L. reuteri, L. acidophilus and L. rhamnosus to increase their anticancer effects in vivo. Likewise, the use of individual LAB like L. acidophilus or combination of different LAB strains to strengthen the ability of the immune system against cancer development can prove useful in prevention and treatment. However, it is clear that further investigations are strongly required to uncover the usefulness of probiotics in CRC treatment in clinical settings.
P- Reviewers: DePaolo RW, Francino MP, Joseph Lau WY, Kuda T, Santoro GA S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Tenesa A, Dunlop MG. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet. 2009;10:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 2. | Garagnani P, Pirazzini C, Franceschi C. Colorectal cancer microenvironment: among nutrition, gut microbiota, inflammation and epigenetics. Curr Pharm Des. 2013;19:765-778. [PubMed] [Cited in This Article: ] |
| 3. | Daniluk U. Probiotics, the new approach for cancer prevention and/or potentialization of anti-cancer treatment? J Clin Exp Oncol. 2012;1:1000e105. [Cited in This Article: ] |
| 4. | Rowland IR. The role of the gastrointestinal microbiota in colorectal cancer. Curr Pharm Des. 2009;15:1524-1527. [PubMed] [Cited in This Article: ] |
| 5. | Zhu Q, Gao R, Wu W, Qin H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013;34:1285-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Food and Agriculture Organization/World Health Organization. Report of Joint FAO/WHO (Food and agriculture organization/World health organization) Working group on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada: Guidelines for the evaluation of probiotics in food 2002; 1-11. [Cited in This Article: ] |
| 7. | Fotiadis CI, Stoidis CN, Spyropoulos BG, Zografos ED. Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J Gastroenterol. 2008;14:6453-6457. [PubMed] [Cited in This Article: ] |
| 8. | Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol. 2011;37:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Hove H, Nørgaard H, Mortensen PB. Lactic acid bacteria and the human gastrointestinal tract. Eur J Clin Nutr. 1999;53:339-350. [PubMed] [Cited in This Article: ] |
| 10. | Chouraqui JP, Van Egroo LD, Fichot MC. Acidified milk formula supplemented with bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004;38:288-292. [PubMed] [Cited in This Article: ] |
| 11. | Gaón D, García H, Winter L, Rodríguez N, Quintás R, González SN, Oliver G. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (B Aires. ). 2003;63:293-298. [PubMed] [Cited in This Article: ] |
| 12. | Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, Savilahti E. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow’s milk allergy. J Allergy Clin Immunol. 2004;114:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am J Clin Nutr. 2003;78:675-683. [PubMed] [Cited in This Article: ] |
| 14. | Azcárate-Peril MA, Sikes M, Bruno-Bárcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401-G424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | del Carmen S, de LeBlanc AM, Miyoshi A, Rocha CS, Azevedo V, LeBlanc JG. Potential application of probiotics in the prevention and treatment of inflammatory bowel diseases. Ulcers. 2011;2011:1-13. [Cited in This Article: ] |
| 16. | Rafter JJ. The role of lactic acid bacteria in colon cancer prevention. Scand J Gastroenterol. 1995;30:497-502. [PubMed] [Cited in This Article: ] |
| 17. | Bengmark S, Gil A. Bioecological and nutritional control of disease: prebiotics, probiotics and synbiotics. Nutr Hosp. 2006;21 Suppl 2:72-84, 73-86. [PubMed] [Cited in This Article: ] |
| 18. | Hirayama K, Rafter J. The role of lactic acid bacteria in colon cancer prevention: mechanistic considerations. Antonie Van Leeuwenhoek. 1999;76:391-394. [PubMed] [Cited in This Article: ] |
| 19. | de Vries EG, Gietema JA, de Jong S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin Cancer Res. 2006;12:2390-2393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Bucur O, Ray S, Bucur MC, Almasan A. APO2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in prostate cancer therapy. Front Biosci. 2006;11:1549-1568. [PubMed] [Cited in This Article: ] |
| 21. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7556] [Cited by in F6Publishing: 8609] [Article Influence: 506.4] [Reference Citation Analysis (0)] |
| 22. | Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 832] [Cited by in F6Publishing: 811] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 23. | Chen CC, Lin WC, Kong MS, Shi HN, Walker WA, Lin CY, Huang CT, Lin YC, Jung SM, Lin TY. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br J Nutr. 2012;107:1623-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell Microbiol. 2008;10:1442-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Kim Y, Oh S, Yun HS, Oh S, Kim SH. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett Appl Microbiol. 2010;51:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer. 2010;62:371-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol. 2013;97:809-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 288] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 28. | Amanatidou A, Bennik MH, Gorris LG, Smid EJ. Superoxide dismutase plays an important role in the survival of Lactobacillus sake upon exposure to elevated oxygen. Arch Microbiol. 2001;176:79-88. [PubMed] [Cited in This Article: ] |
| 29. | Bruno-Bárcena JM, Andrus JM, Libby SL, Klaenhammer TR, Hassan HM. Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity. Appl Environ Microbiol. 2004;70:4702-4710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr. 2003;90:449-456. [PubMed] [Cited in This Article: ] |
| 31. | Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol. 2002;72:215-224. [PubMed] [Cited in This Article: ] |
| 32. | Lee J, Hwang KT, Heo MS, Lee JH, Park KY. Resistance of Lactobacillus plantarum KCTC 3099 from Kimchi to oxidative stress. J Med Food. 2005;8:299-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. 2000;45:1617-1622. [PubMed] [Cited in This Article: ] |
| 34. | Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol. 2006;42:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Koller VJ, Marian B, Stidl R, Nersesyan A, Winter H, Simić T, Sontag G, Knasmüller S. Impact of lactic acid bacteria on oxidative DNA damage in human derived colon cells. Food Chem Toxicol. 2008;46:1221-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Annuk H, Shchepetova J, Kullisaar T, Songisepp E, Zilmer M, Mikelsaar M. Characterization of intestinal lactobacilli as putative probiotic candidates. J Appl Microbiol. 2003;94:403-412. [PubMed] [Cited in This Article: ] |
| 37. | Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, Kumar P, Poddar D, Aggarwal PK, Henry CJ. Cancer-preventing attributes of probiotics: an update. Int J Food Sci Nutr. 2010;61:473-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Uccello M, Malaguarnera G, Basile F, D’agata V, Malaguarnera M, Bertino G, Vacante M, Drago F, Biondi A. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012;12 Suppl 1:S35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Jounai K, Ikado K, Sugimura T, Ano Y, Braun J, Fujiwara D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS One. 2012;7:e32588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Gabrilovich D, Pisarev V. Tumor escape from immune response: mechanisms and targets of activity. Curr Drug Targets. 2003;4:525-536. [PubMed] [Cited in This Article: ] |
| 41. | Loredana Baffoni FG, Gioia DD, Biavati B. Role of intestinal microbiota in colon cancer prevention. Ann Microbiol. 2012;62:15-30. [Cited in This Article: ] |
| 42. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1633] [Cited by in F6Publishing: 1589] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 43. | Pessi T, Sütas Y, Hurme M, Isolauri E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy. 2000;30:1804-1808. [PubMed] [Cited in This Article: ] |
| 44. | Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237-3246. [PubMed] [Cited in This Article: ] |
| 45. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [PubMed] [Cited in This Article: ] |
| 46. | Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880-2885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 47. | Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474-19479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 48. | Mohamadzadeh M, Duong T, Sandwick SJ, Hoover T, Klaenhammer TR. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci USA. 2009;106:4331-4336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 49. | Goh YJ, Azcárate-Peril MA, O’Flaherty S, Durmaz E, Valence F, Jardin J, Lortal S, Klaenhammer TR. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2009;75:3093-3105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | Goh YJ, Klaenhammer TR. Genomic features of Lactobacillus species. Front Biosci (Landmark Ed). 2009;14:1362-1386. [PubMed] [Cited in This Article: ] |
| 51. | Mayer ML, Phillips CM, Townsend RA, Halperin SA, Lee SF. Differential activation of dendritic cells by Toll-like receptor agonists isolated from the Gram-positive vaccine vector Streptococcus gordonii. Scand J Immunol. 2009;69:351-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Mayer ML, Phillips CM, Stadnyk AW, Halperin SA, Lee SF. Synergistic BM-DC activation and immune induction by the oral vaccine vector Streptococcus gordonii and exogenous tumor necrosis factor. Mol Immunol. 2009;46:1883-1891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4623-4630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 54. | Khazaie K, Zadeh M, Khan MW, Bere P, Gounari F, Dennis K, Blatner NR, Owen JL, Klaenhammer TR, Mohamadzadeh M. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. 2012;109:10462-10467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigón G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007;14:485-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 56. | Lee JW, Shin JG, Kim EH, Kang HE, Yim IB, Kim JY, Joo HG, Woo HJ. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. J Vet Sci. 2004;5:41-48. [PubMed] [Cited in This Article: ] |
| 57. | Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22:599-605. [PubMed] [Cited in This Article: ] |
| 58. | Matsuzaki T, Yokokura T, Mutai M. Antitumor effect of intrapleural administration of Lactobacillus casei in mice. Cancer Immunol Immunother. 1988;26:209-214. [PubMed] [Cited in This Article: ] |
| 59. | Matsuzaki T. Immunomodulation by treatment with Lactobacillus casei strain Shirota. Int J Food Microbiol. 1998;41:133-140. [PubMed] [Cited in This Article: ] |
| 60. | Takagi A, Ikemura H, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Relationship between the in vitro response of dendritic cells to Lactobacillus and prevention of tumorigenesis in the mouse. J Gastroenterol. 2008;43:661-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Lee do K, Jang S, Kim MJ, Kim JH, Chung MJ, Kim KJ, Ha NJ. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer. 2008;8:310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Coppedè F. Epigenetic biomarkers of colorectal cancer: Focus on DNA methylation. Cancer Lett. 2014;342:238-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Migliore L, Migheli F, Spisni R, Coppedè F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J Biomed Biotechnol. 2011;2011:792362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 64. | Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics. 2012;4:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 65. | Acharya MR, Sparreboom A, Venitz J, Figg WD. Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol. 2005;68:917-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 66. | Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 67. | Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 68. | Waldecker M, Kautenburger T, Daumann H, Veeriah S, Will F, Dietrich H, Pool-Zobel BL, Schrenk D. Histone-deacetylase inhibition and butyrate formation: Fecal slurry incubations with apple pectin and apple juice extracts. Nutrition. 2008;24:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007;17:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 70. | Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496-1501. [PubMed] [Cited in This Article: ] |
| 71. | Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 72. | Lightfoot YL, Mohamadzadeh M. Tailoring gut immune responses with lipoteichoic acid-deficient Lactobacillus acidophilus. Front Immunol. 2013;4:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Lightfoot YL, Yang T, Sahay B, Mohamadzadeh M. Targeting aberrant colon cancer-specific DNA methylation with lipoteichoic acid-deficient Lactobacillus acidophilus. Gut Microbes. 2013;4:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Thirabunyanon M, Hongwittayakorn P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl Biochem Biotechnol. 2013;169:511-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer. 2012;64:871-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Escamilla J; SYNCAN. Synbiotics and cancer prevention in humans. Available from: http://www.syncan.be/. [Cited in This Article: ] |
| 77. | Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, Jiang Y, Zhang H, Yang Z, Wang Y. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study. Aliment Pharmacol Ther. 2011;33:50-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 78. | Ohigashi S, Hoshino Y, Ohde S, Onodera H. Functional outcome, quality of life, and efficacy of probiotics in postoperative patients with colorectal cancer. Surg Today. 2011;41:1200-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, Kitamura T, Kawabe K, Kotake T, Kuroda M. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002;68:273-280. [PubMed] [Cited in This Article: ] |
| 80. | Brim H, Kumar K, Nazarian J, Hathout Y, Jafarian A, Lee E, Green W, Smoot D, Park J, Nouraie M. SLC5A8 gene, a transporter of butyrate: a gut flora metabolite, is frequently methylated in African American colon adenomas. PLoS One. 2011;6:e20216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |