Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1791
Revised: January 10, 2003
Accepted: February 16, 2003
Published online: August 15, 2003
AIM: To explore the role of bile liquid crystal in the process of gallbladder stone formation and to provide bases for preventing and treating cholelithiasis.
METHODS: 46 guinea pigs, half males and half females, were randomly divided into control group and stone-causing group. Normal feed and stoneleading feed were used respectively to raise guinea pigs in the control group and stone-causing group. The guinea pigs were killed in three batches during the raising period. Under polarizing microscope, the pattern changes of bile liquid crystal in the gallbladder biles of the guinea pigs in the control group and stone-causing group were dynamicly observed respectively in single-blind trial.
RESULTS: It was found that there were few crystals in the guinea pigs’ biles of the control group, and their Malta cross was small and scattered, and existed in single form. With the increase of the feeding days, bile liquid crystals grew and Malta cross became bigger with their distribution densified, denser somewhere, but always existed in single form. While those of the stone-causing group had more bile liquid crystals, Malta cross was big and merged in strings. With the increase of the feeding days, bile liquid crystals grew in amount and strings of Malta cross increased and became bigger. The crosses in strings were arranged more and more regularly and they gradually changed into stone crystals.
CONCLUSION: Formation of gallbladder stone is a process of nucleation from different substances, and the causing-stone gallbladder bile is a constantly supersaturated solution, and bile liquid crystal is a nucleation factor in the formation of gallbladder stones. The process of nucleation includes gathering, merging and phase-changing of bile liquid crystals. The process of gathering, merging of bile liquid crystal is the key to nucleation.
- Citation: Yang HM, Wu J, Li JY, Gu L, Zhou MF. Role of nucleation of bile liquid crystal in gallstone formation. World J Gastroenterol 2003; 9(8): 1791-1794
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1791
Bile liquid crystal is a type of liquid crystal in the living beings’ bile. Since American scholar Olszewski et al[1] found the substance of liquid crystal in the bile liquid of human body in 1973, some scholars at home and abroad have explored the matter of liquid crystal in the living beings’ bile[2-4], but the relation between bile liquid crystal and formation of gallbladder stone has not been clarified so far. In order to explore the role of bile crystal in the process of gallbladder-stone formation and to provide certain bases for the prevention and treatment of gallbladder stone, we made dynamic observations and studied on the nucleation of bile liquid crystal in gallbladder stone through animal tests in guinea pigs.
46 guinea pigs (provided by the animal branch of the Scientific Research Department in Kunming Medical College, with qualified animal testing certificate issued by Yunnan Province), each weighing about 300 grams, half males and half females, were randomly divided into control group (n = 23) and stone-causing group (n = 23).
Animal raising The control group was given normal feed which was provided by the animal branch of the Scientific Research Department in Kunming Medical College, while the stone-causing group was given stoneleading feed. The two groups were separately raised in flocks, with free access to water containing 1% vitamin C.
Stone-causing feed The components of the stone-causing feed were as follows: starch 25%, bran 25%, glucose 12%, lard 1.5%, cholesterol 1.5%, normal feed 35%[5]. They were mixed evenly and made granule with feed machine.
Sample preparation Guinea pigs were killed in three batches. The first batch was killed after raised for 10 days, with 5 pigs in each group, totally 10 pigs in the 2 groups. The 2nd batch was killed after raised for 25 days, with 5 pigs in each group, totally 10 pigs in the 2 groups. The 3rd batch was killed after raised for 60 days, with 13 pigs in each group, totally 26 pigs in the 2 groups. The pigs were whacked at head to stupor with a blood vessel forceps, immediately cut open belly and their cystic duct was blocked with a blood vessel forceps to take out gallbladder and all bile. The bile was centrifugalized for 30 minutes with a China-made TGL-16B bench centrifuge, 4000 r/min, and observed under polarizing microscope.
Polarizing microscope observation A China-made W-104 micro-sample advancer (precision of 0.2 μL) was used to stratify the centrifuged bile and drip 6.0 μL of it on a carrier flake, then it was covered with the cover-glass and the bile naturally spread out to form slices. Under an advanced polarizing microscope (BHSP model of Olympus, Japan), the pattern changes of the bile liquid crystal in the process of gallbladder stone formation were observed.
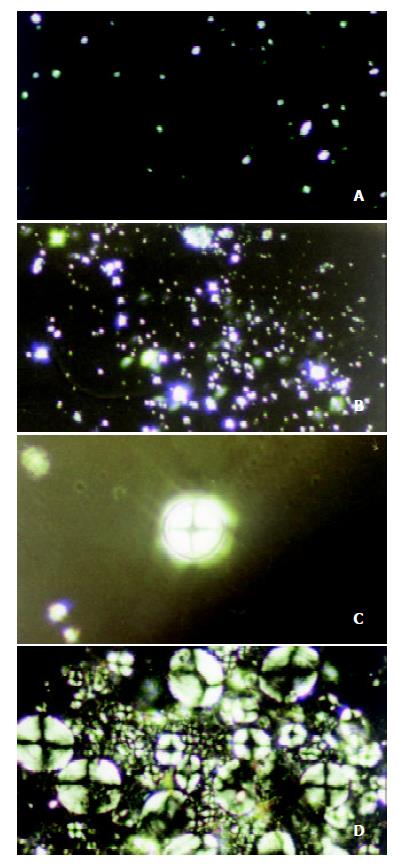
Under the perpendicular polarized microscope, the morphological changes of bile liquid crystal in the gallbladder bile of guinea pigs in the control group were observed, and it was found that there were few matters of liquid crystal in gallbladder bile, and that the interference pattern, named Malta cross, was small and scattered, in single form. With the increase of the feeding days, bile liquid crystals grew larger and Malta cross became bigger with their distribution densified unevenly, but always existed in single form, as shown in Figure 1 (a), (b), (c) and (d). No regularly shaped stone crystal was found in the biles.
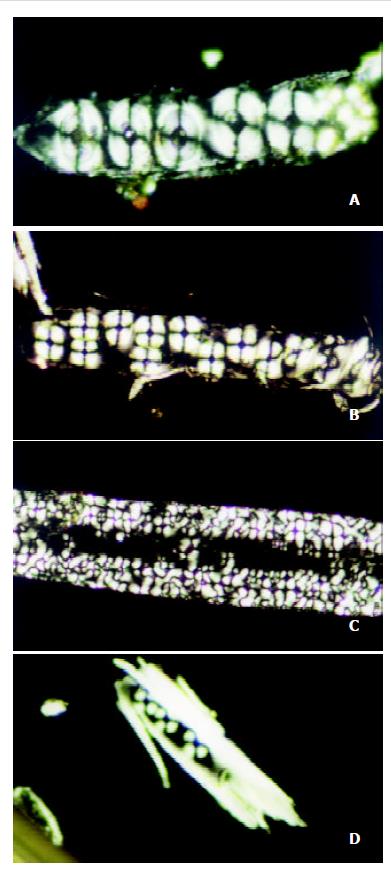
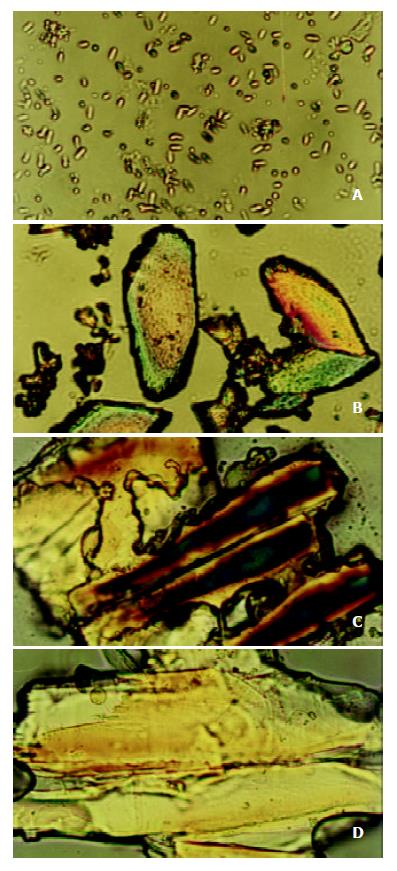
Under the polarizing microscope, the morphological changes of bile liquid crystal in guinea pigs’ gallbladders in stone-causing group were observed, and it was found that there were more matters of liquid crystal in gallbladder biles, and that the interference pattern, Malta cross was big and merged into strings. With the increase of the feeding days, bile liquid crystals grew in number and strings of Malta crosses increased and became bigger. The crosses in strings were arranged more regularly so that they gradually phase-changed into stone crystals, as shown in Figure 2 (a), (b), (c) and (d). It exhibited variously shaped stone crystals in the biles, as shown in Figure 3 (a), (b), (c) and (d).
The cause of gallbladder stone is very complicated, various factors are involved[5-9]. The basic factor is now considered to be the change of compositions and physical-chemistry characteristics of biles, which results in cholesterol in the state of super-saturation in the bile. And it is very likely to form crystals, which is then deposited to form gallbladder stones. It also causes imbalance between nucleation-leading factors and antinucleation factors and abnormal function of the gallbladder and so on[10-19]. From the viewpoint of crystallogeny, in a system of solution, the precondition for solute to be separated out and to form crystal is that the solute in the system must be in the state of super-saturation, which is an unstable state in thermodynamics. Only in this state, can the solute be crystallized and separated out. The formation of crystals has two steps of nucleation and crystallization, it firstly forms a crystalline nucleus containing hundreds of molecules, then crystals grow. In a system of solution, spontaneous nucleation of solute often requires a very high degree of saturation, the supersaturated solution in this state is called unstably supersaturated solution. When a certain type of special nucleating factors is added, supersaturated solution of a relatively low degree also can produce nucleation, which is called heterogeneous nucleation, and the supersaturated solution in this state is called substably supersaturated solution[20,21]. Homogeneous nucleation of bile cholesterol requires a very high degree of saturation (estimated at about 300%), which is not possible to occur in human body, and even less possible in guinea pigs’ body. So the formation of gallbladder-stone should be a process of heterogeneous nucleation, stone-causing gallbladder bile should be a substably supersaturated solution, and bile liquid crystal should be a type of nucleating factors for gallbladder stones.
As we observed under the polarizing microscope, there were many substances of liquid crystal in the gallbladder bile of the guinea pigs in the stone-causing group, which were big and string of Malta crosses. With the increase of the feeding days, bile liquid crystals grew in number, more and more strings of Malta cross appeared and steadily became bigger and bigger, and their arrangement became more and more regular, and gradually phase-changed into stone crystals. These have proved that the formation of gallbladder stone is indeed a process of heterogeneous nucleation, and stone-causing gallbladder bile is a type of substably supersaturated solution, and that bile liquid crystal is a type of nucleating factors in gallbladder-stone. Its process of nucleation is such that bile liquid crystals get together, merge and phase change. In another word, single Malta crosses assemble into strings, then merge as slices, and finally phase-change into crystals. The assemblage and merger of bile liquid crystals are the key for nucleation. Although there were also substances of liquid crystal existing in gallbladder biles in the control group and bile crystals also increased with feeding time, they neither assembled nor merged, and Malta crosses always existed in single forms which were unable to nucleate to form stone. If some way can be found to prevent or retard the assemblage and merger of bile liquid crystals, i. e., formation of the nucleus, it will be of a great significance in theory and practice for prevention and treatment of cholelithiasis.
Cholesterol can not dissolve in water, but it can dissolve in bile. The concentration of cholesterol in bile is about 10-20 mmol/L, this concentration is about 106 times[22] denser than that of the solubility of cholesterol in water. Studies concerned pointed out that cholesterol in bile maintained its state of dissolution in forms of micelle and vesicle. Micelle is the polymer of cholesterol, phosphatide and cholate, while vesicle is the compound of cholesterol and phosphatide[23-31]. These two form a system of thermodynamic balance in bile so that they are interlinked and interconverted, and play the role of modulation for dissolution and separation of cholesterol. Under the effect of nucleation-leading factors and antinucleation factors in bile, vesicles in bile assemble, merge and separate out crystalline nuclei of cholesterol which then gradually grow up to form stones[32]. As we observed under the polarizing microscope, single Malta cross in bile liquid crystal of guinea pigs in stone-causing group, gradually assembled into strings, then merged into slices, and finally phase-changed into stone crystals. The present study supports the above viewpoints from the studies of morphology. Whether bile crystal is such a kind of vesicles or vesicles aggregate and what factors cause or stop bile liquid crystal assembling and merging, still need to be further studied.
Edited by Xu XQ and Zhu LH
| 1. | Olszewski MF, Holzbach RT, Saupe A, Brown GH. Liquid crystals in human bile. Nature. 1973;242:336-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Yang HM, Wu J, Li JY, Zhou JL, He LJ, Xu XF. Optic properties of bile liquid crystals in human body. World J Gastroenterol. 2000;6:248-251. [PubMed] [Cited in This Article: ] |
| 3. | Yang H, Wu J, Cu L, Zhou J, He L, Cao S. The optical properties of bile liguid crystals in organisms. Chin J Biomedi Engin. 1999;8:67-68. [Cited in This Article: ] |
| 4. | Savina LV, Kokueva OV, Golovanova ES, Kadygrob GV. [Crystallo-optic structures of bile in chronic acalculous cholecystitis]. Eksp Klin Gastroenterol. 2002;59-62, 128. [PubMed] [Cited in This Article: ] |
| 5. | Han TQ, Jiang ZY, Zhang SD. Research progress in formation of gallstone. Zhongguo Shiyong Waike Zazhi. 2001;21:123-125. [Cited in This Article: ] |
| 6. | Zhang SD, Han TQ. Prospects for studies of gallstone. Waike Lilun Yu Shijian. 1999;4:5-6. [Cited in This Article: ] |
| 7. | Nishioka T, Tazuma S, Yamashita G, Kajiyama G. Partial replacement of bile salts causes marked changes of cholesterol crystallization in supersaturated model bile systems. Biochem J. 1999;340:445-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Wang DQ, Cohen DE, Lammert F, Carey MC. No pathophysiologic relationship of soluble biliary proteins to cholesterol crystallization in human bile. J Lipid Res. 1999;40:415-425. [PubMed] [Cited in This Article: ] |
| 9. | Rubin M, Pakula R, Konikoff FM. Microstructural analysis of bile: relevance to cholesterol gallstone pathogenesis. Histol Histopathol. 2000;15:761-770. [PubMed] [Cited in This Article: ] |
| 10. | Wu ZD. Surgery. 5th ed. Beijing: People's Health Publishing House. 2002;617-619. [Cited in This Article: ] |
| 11. | Yu JP, Sheng ZX, Luo HS. Practical Digestive Diseases. 1st ed. Beijing: Science Press. 1999;1228-1232. [Cited in This Article: ] |
| 12. | Yang GH. Pathology. 5th ed. Beijing: People's Health Publishing House. 2001;217-218. [Cited in This Article: ] |
| 13. | Zhao JC, Shu Y, Cheng NS, Xiao LJ, Zhu H. Changes of choles-terol metabolism in cholesterol gallstone formation in the rabbit. Zhongguo Putong Waike Zazhi. 2000;9:124-128. [Cited in This Article: ] |
| 14. | Li QR, Gong YH, Zhou JP, Gao Q, Pang BZ. Formation of gall-stones by feeding on high cholesterol diet. Zhonghua Linchuang Ganzangbingxue Zazhi. 2002;18:320-321. [Cited in This Article: ] |
| 15. | Zhang JH, Yang KZ, Han BL. The kinetic mechanism of gall-stone formation. Zhongguo Putong Waike Zazhi. 2001;16:424-426. [Cited in This Article: ] |
| 16. | Ye SA, Tang WH. Research progress in gallbladder's effect in the process of gallstone formation. Guowai Yixue Waike Fence. 2001;28:221-223. [Cited in This Article: ] |
| 17. | Liu CL, Higuchi WI. Cholesterol crystallite nucleation in supersaturated model biles from a thermodynamic standpoint. Biochim Biophys Acta. 2002;1588:15-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Jirsa M, Groen AK. Role of biliary proteins and non-protein factors in kinetics of cholesterol crystallisation and gallstone growth. Front Biosci. 2001;6:E154-E167. [PubMed] [Cited in This Article: ] |
| 19. | Qin YL, Tang WH. Research progress in glycoprotein in gallstone. Guowai Yixue Waike Fence. 1999;26:344-347. [Cited in This Article: ] |
| 20. | Zhang KC, Zhang LH. Crystal Growth Science and Technology. 2nd Edition. Beijing: Science Press. 1997;178-182. [Cited in This Article: ] |
| 21. | Cheng JZ. Modern Crystal Chemistry-Theories and Technique. 1st Edition. Beijing: Higher Education Press. 2001;126-131. [Cited in This Article: ] |
| 22. | Zhang QY. Diseases of the Biliary System. 1st Edition. Nanjing: Jiangsu Science and Technology Press. 1997;167. [Cited in This Article: ] |
| 23. | Luk AS, Kaler EW, Lee SP. Structural mechanisms of bile salt-induced growth of small unilamellar cholesterol-lecithin vesicles. Biochemistry. 1997;36:5633-5644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Prigun NP, Korolevich AN. [Changes in human biliary vesicle sizes in pathological states]. Biofizika. 2002;47:1095-1100. [PubMed] [Cited in This Article: ] |
| 25. | Portincasa P, Venneman NG, Moschetta A, van den Berg A, Palasciano G, vanBerge-Henegouwen GP, van Erpecum KJ. Quantitation of cholesterol crystallization from supersaturated model bile. J Lipid Res. 2002;43:604-610. [PubMed] [Cited in This Article: ] |
| 26. | Venneman NG, Huisman SJ, Moschetta A, vanBerge-Henegouwen GP, van Erpecum KJ. Effects of hydrophobic and hydrophilic bile salt mixtures on cholesterol crystallization in model biles. Biochim Biophys Acta. 2002;1583:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Palasciano G, van Erpecum KJ. Cholesterol crystallization in model biles: effects of bile salt and phospholipid species composition. J Lipid Res. 2001;42:1273-1281. [PubMed] [Cited in This Article: ] |
| 28. | Moschetta A, Frederik PM, Portincasa P, vanBerge-Henegouwen GP, van Erpecum KJ. Incorporation of cholesterol in sphingomyelin- phosphatidylcholine vesicles has profound effects on detergent-induced phase transitions. J Lipid Res. 2002;43:1046-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Renooij WL, Groen AK, van Erpecum KJ. Hydrophilic bile salts enhance differential distribution of sphingomyelin and phosphatidylcholine between micellar and vesicular phases: potential implications for their effects in vivo. J Hepatol. 2001;34:492-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Eckhardt ER, Moschetta A, Renooij W, Goerdayal SS, van Berge-Henegouwen GP, van Erpecum KJ. Asymmetric distribution of phosphatidylcholine and sphingomyelin between micellar and vesicular phases. Potential implications for canalicular bile formation. J Lipid Res. 1999;40:2022-2033. [PubMed] [Cited in This Article: ] |
| 31. | Sakomoto M, Tazuma S, Chayama K. Less hydrophobic phosphatidylcholine species simplify biliary vesicle morphology, but induce bile metastability with a broad spectrum of crystal forms. Biochem J. 2002;362:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Gantz DL, Wang DQ, Carey MC, Small DM. Cryoelectron microscopy of a nucleating model bile in vitreous ice: formation of primordial vesicles. Biophys J. 1999;76:1436-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |