Published online Sep 28, 2019. doi: 10.3748/wjg.v25.i36.5469
Peer-review started: July 10, 2019
First decision: August 5, 2019
Revised: August 17, 2019
Accepted: August 24, 2019
Article in press: August 24, 2019
Published online: September 28, 2019
Irritable bowel syndrome (IBS) is one of the most common functional gas-troenterological diseases characterized by abnormal visceral sensitivity and low-grade inflammation. The role of Clostridium butyricum (C. butyricum) in reducing intestinal low-grade inflammation via immune pathways has been well defined. However, the detailed mechanisms of the effects of C. butyricum on intestinal mucosal immunity, especially on immune cells of the lamina propria, remain unclear. Dendritic cells (DCs), which are important immune cells, secrete proinflammatory cytokines (IL-1β, IL-6, and others) and express T cell immuno-globulin and mucin domain-3 (TIM3), promoting proliferation and activation of DCs, and mediating Th1 and Th17 inflammatory responses.
To investigate the role of DCs in the development of IBS in a rat model and to understand the regulation of DCs after C. butyricum intervention.
An IBS animal model was established using C57BL/6 mice, and C. butyricum was continuously administered via the intragastric route to simulate different intestinal immune states. Intestinal visceral hypersensitivity and histopathology were assessed using the abdominal withdrawal reflex (AWR) test and hematoxylin & eosin (H&E) staining, respectively. The expression of proinflammatory cytokines (IL-1β and IL-6) and TIM3 was analyzed by Western blot analysis and real-time PCR. Flow cytometry was applied to analyze the quantity, function, and membrane molecule TIM3 of the lamina propria dendritic cells (LPDCs). The regulatory effect of C. butyricum was verified in bone marrow-derived dendritic cells by in vitro experiments.
The secretion of proinflammatory cytokines (IL-1β and IL-6) in mice with IBS was significantly increased compared with that of the control group, which suggested that the intestinal mucosa in mice with IBS was in a low-grade inflammatory state. The expression of CD11C+CD80+ and CD11c+TIM3+ in intestinal LPDCs in mice with IBS increased significantly. Meanwhile, the cytokines (IL-1β and IL-6) were significantly reduced after the intervention with probiotic C. butyricum. The amount and function of LPDCs and the TIM3 on the surface of the LPDCs were decreased with the alleviation of the intestinal inflammatory response.
The results suggest that C. butyricum regulates the amount and functional status of LPDCs in the intestinal mucosa of mice with IBS, and therefore modulates the local immune response in the intestine.
Core tip: In this study, we revealed that Clostridium butyricum can regulate the number and functional status of lamina propria dendritic cells in the intestinal mucosa of mice with irritable bowel syndrome, and therefore modulate the local immune response in the intestine.
- Citation: Zhao Q, Yang WR, Wang XH, Li GQ, Xu LQ, Cui X, Liu Y, Zuo XL. Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells. World J Gastroenterol 2019; 25(36): 5469-5482
- URL: https://www.wjgnet.com/1007-9327/full/v25/i36/5469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i36.5469
Irritable bowel syndrome (IBS) is one of the most common functional gas-troenterological diseases which affects approximately 15% of the population worldwide[1]. IBS is thought to result from the activation of the mucosal immune system and the disruption of the epithelial barrier by the intestinal microbiota[2-4]. Previous studies have reported that the intestinal flora interacts with the intestinal immune system to maintain intestinal homeostasis[5,6]. Therefore, an intervention with probiotics may be salutary for IBS treatment.
The probiotic Clostridium butyricum (C. butyricum) is a type of Gram-positive anaerobic bacterium, which has been used for the treatment of IBS in clinical settings. It has been documented that the intragastric administration of C. butyricum significantly reduces intestinal inflammation and ameliorates the intestinal microbiota[7-9]. Besides, Clostridium species have been reported to affect the accumulation of intraepithelial lymphocytes (IELs) in the colon[10] and to increase the number of Treg cells, thereby inhibiting the expression of inflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-12, IFN-r, IL-1β, and IL-6) and up-regulating the expression of inhibitory cytokines (IL-10)[11-15]. Other studies provided evidence that Clostridium species might suppress the intestinal inflammatory response via immune pathways[15-17].
The lamina propria dendritic cells (LPDCs) are important antigen-presenting cells in the intestines. They are now recognized to be essential for innate and acquired immunity[18,19]. LPDCs have a particular capacity to sample antigens from peripheral tissues and efficiently activate resting T cells and direct T cell bias (Th1, Th2, and Th17)[20]. In the steady state, LPDCs induce the differentiation of regulatory T cells to produce IL-10 and transform growth factor-beta (TGF-beta)[19,21]. Studies on LPDCs in IBS or IBD patients have revealed that LPDCs were significantly increased and the co-stimulatory molecules (CD80, CD86, and MHCII) were upregulated, the secretions of IFN-g, IL-1β, IL-6, IL-12, and TNF-α were increased, and the migration and proliferation of CD4+ T cells were enhanced. These regulations were found to be correlated with Th1 and Th17 inflammatory responses[22-24]. The immunoregulatory function of LPDCs is related to their subpopulation and the surface receptors[25]. The local microenvironment likely plays an important role in defining the phenotype and activation of dendritic cells (DCs).
Recently, detailed analyses of the effect of C. butyricum on LPDCs have been mainly focused on cytokines; e.g., C. butyricum was revealed to promot Treg cell generation in the intestine through the induction of TGF-β1 from LPDCs[16] . This strain negatively regulated the expression of IL-12/IL-23p40[26]. However, to date, there is no study that reveals the effect of C. butyricum on surface molecules of LPDCs . T cell immunoglobulin and mucin domain-3 (TIM3) is an immunoregulatory factor expressed on the surface of DCs[27,28]. It upregulates the secretion of the pro-inflammatory cytokines (TNF-α and IL-1β), promotes cell proliferation and activation, and eliminates various pathogens[29-32]. To our best known, the regulatory effect of C. butyricum on TIM3 has not been reported until now.
This study aimed to investigate the role of the probiotic C. butyricum in regulating the quantity and function of LPDCs and the membrane molecule TIM3 of the LPDCs in mice with IBS and to further elucidate the effects of the probiotic C. butyricum on the local immune response in the intestinal mucosa.
Twenty-four C57BL/6 male mice (aged 6-8 wk) were randomly divided into four groups (normal control, IBS, IBS + C. butyricum intervention, and IBS + normal saline (NS) intervention (n = 6 per group)). Pentobarbitone (50 mg/kg intraperitoneal administration) was used to establish deep anesthesia in these mice[33]. A plastic catheter (outer diameter = 4 mm) was inserted into the descending colon of each mouse to a depth of 4-6 cm from the anus. 2,4,6-trinitrobenzenesulfonic acid (TNBS) (P2297, Sigma, Shanghai; 0.1 mL of 5% (w/v), diluted to 0.2 mL using 50% ethanol) was slowly instilled. In the control group, the clysis was replaced by an injection of NS. For the other groups, the mice were inverted for 10 min to prevent drug leakage after clysis. Four weeks after TNBS administration, C. butyricum (QingDao EastSea Pharmaceutical Co., Ltd., QinDao, China) was continuously administered via the intragastric route to 6 mice during 1 wk (200 μL/d; concentration of live bacteria concentration, 1 × 108 CFU/mL). NS in the same amount was administered to the mice in the control group. Hematoxylin & eosin (H&E) staining and the abdominal withdrawal reflex (AWR) test were used to evaluate the degree of colon inflammation.
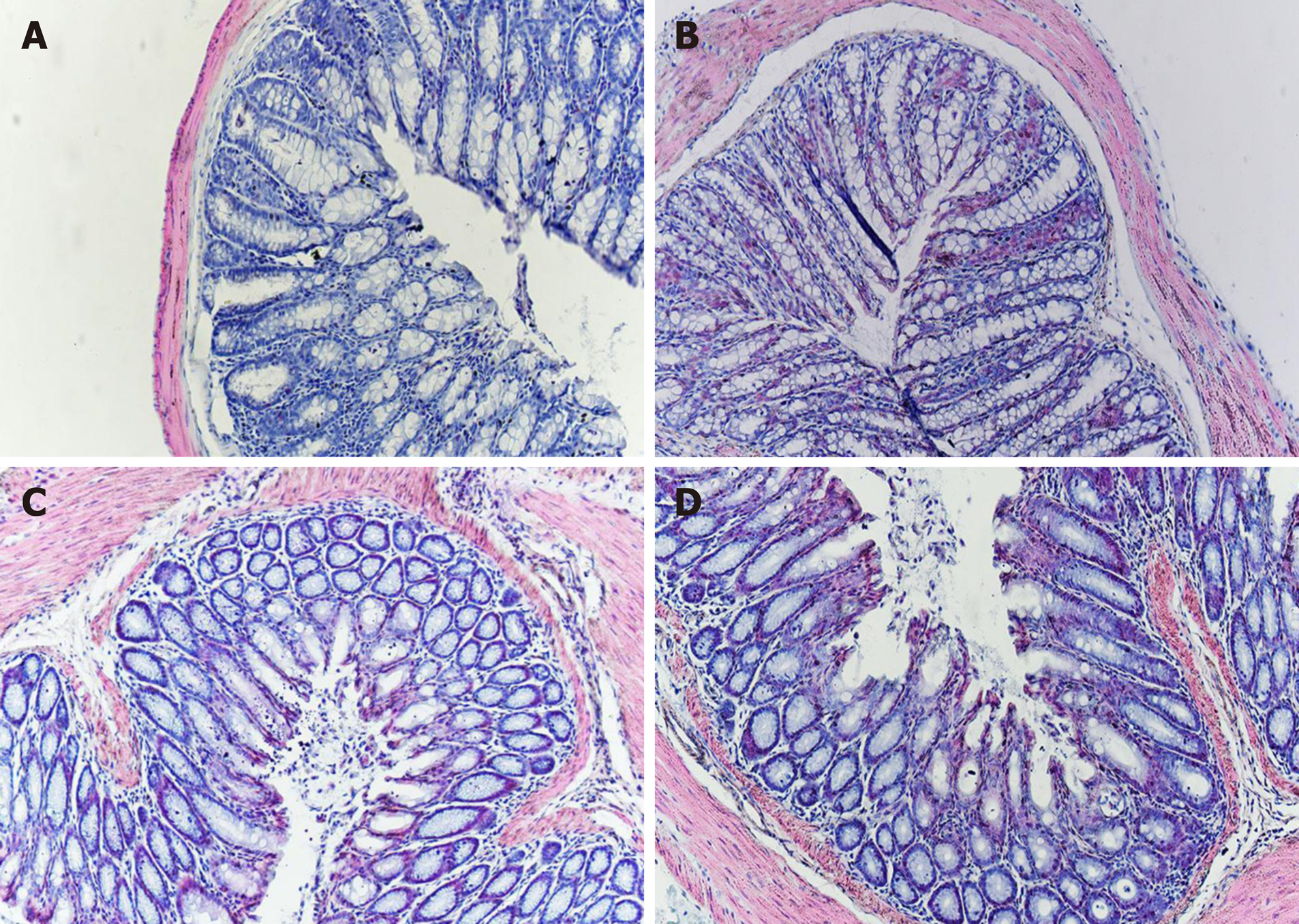
To evaluate the inflammatory grade of colon tissue in the four groups, formalin-fixed mouse colon tissue was embedded in paraffin, sectioned at 4 μm, and stained with H&E (Beijing Coribo Technology Co. Ltd., Beijing, China) for histological analysis to assess conditions such as epithelial erosion, crypt loss, and inflammatory infiltration with lymphocytes. A pathologist blindly assessed and assigned an inflammatory grade to each section.
The AWR test was perfomed at day 28 to assess visceral hyperalgesia in response to colorectal distention (CRD)[34]. A disposable silicon balloon-urethral catheter for pediatric use (6 Fr, Terumo, Tokyo, Japan) was used. Mice were briefly anesthetized with ether. The balloon was inserted into the rectum until the catheter was positioned to the anus (2 cm distal from the end of the balloon). The catheter was fixed to the base of the tail to prevent detachment. After waking up and adapting for 1 hours, CRD was performed in a stepwise fashion. An ascending-limit phasic distention (0.25, 0.35, or 0.50 mL) was applied in 30 s every 4 min. The AWR was semiquantitatively scored as previously described[35]. The AWR score was assigned as follows: 0 = no behavioral response to distension; 1 = brief head movements followed by immobility; 2 = contraction of abdominal muscles without lifting of the abdomen; 3 = lifting of the abdomen; 4 = body arching and lifting of the pelvic structure.
The mice were sacrificed by cervical dislocation, and their colon, rectum, fat, and mesentery were extracted. The colon was washed and cut into 5-mm sections, incubated in a solution containing 2% fetal calf serum (FCS) and 5 mmol/L EDTA (Sigma) for 30 min. The tissue fragments were filtered through a screen to remove the epithelial cells and were collected into a small bottle where they were washed, cut up, and incubated in RPMI-1640 containing 2% FCS, collagenase IV (4 mg/mL, Solarbio, Beijing, China), and DNase I (10 μg/mL, Solarbio) for 30 min. The suspension was stirred at 37°C, then the released cells were collected through a 300-mesh screen. The isolated cells were separated on a discontinuous 40%/75% Percoll (Sigma) gradient. The typical yield was 2-3 × 106 lymphocytes/mouse.
DCs were generated in vitro from bone marrow cells from 6- to 8-wk-old wild-type C57BL/6 mice[36] and were resuspended in 10% FCS medium (RPMI-1640: Gibco, Los Angeles, United States; GM-CSF: Biosciences, San Jose, CA, United States, [20 ng/mL]; IL-4: PeproTech, Rocky Hill, United States, [10 ng/mL]; penicillin [100 U/mL], and streptomycin [100 U/mL]). The cells were cultivated in a 6-well plate in an environment containing 5% CO2 at 37 °C. A half volume of culture medium was replaced every other day. On the 7th day, penicillin-streptomycin was removed while replacing the medium. The collected DCs were separately co-cultured with 100 μL of the C. butyricum culture supernatant and 100 µL (concentration, 1 × 108 CFU/mL) of the live bacterial suspension for 4 hours and then stimulated with 0.1 µg/mL of lipopolysaccharide (LPS; Sigma–Aldrich) for 3 hours. The DC cells were subsequently collected, and the supernatant was cultured for evaluation using flow cytometry and enzyme-linked immunosorbent assay (ELISA).
The colonic lamina propria cells were collected and suspended (1 × 106 cells/mL) in PBS with 2% FCS. The DCs were marked with anti-CD11c- FITC, anti-CD80-PEcy5.5, and anti-TIM3-APC (eBioscience, CA, United States). The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, USA), and the data were collected and analyzed with FlowJo software (Tree Star Inc., Stanford, United States).
The colon tissues were homogenized and ultrasonically treated on ice for cell lysis and protein extraction. After determining the protein content, the degeneration process was performed at 100 °C for 5 min. The proteins were separated using 10% SDS-PAGE and transferred to PVDF membranes (0.4-5 μm pore size; Millipore, United States). After blocking with 5% skimmed milk, the membranes were incubated with rabbit anti-TIM3 (1:2000), anti-IL-1β (1:2000), and anti-IL-6 (1:2000) antibodies (Abcam, Cambridge, UK) at 4 °C overnight and then incubated with secondary antibodies labeled with horseradish peroxidase (1:10000, Zhongshan Gold Bridge, Beijing, China) for 2 h at room temperature. The immunoblots were detected using an enhanced chemiluminescent substrate (Millipore) on the ChemiDoc MP system (Bio-Rad, United States). All of the detected protein bands were standardized against β-actin. The semiquantification of each band was performed using ImageJ NIH software (National Institutes of Health, Bethesda, MD, United States).
Different groups of DC culture supernatants were collected and centrifuged at 300 g for 10 min at 4 °C. The supernatants were collected and stored at -80 °C until use. According to the manufacturer’s instructions, an ELISA kit (Tianjin Anoric Biotechnology CO, Ltd.) was used to measure the mucosal cytokine levels of IL-1β and IL-6. The results are expressed as total protein (pg/mL).
Total RNA was extracted using Trizol (Invitrogen, San Diego, CA, USA). The total RNA (1 μg) was reverse transcribed into cDNA using the ReverTra Ace® qPCR RT Kit (TOYOBO, Japan) in a Mastercycler thermal cycler (Eppendorf, German). Real-time PCR was performed using the SYBR Green reagent (Takara, Japan) in a fluorescence thermocycler (LightCycler; Roche Diagnostics, Mannheim, Germany). The relative mRNA expression was determined after normalizing to β-actin levels using the 2-ΔΔCT method with the following sequence-specific primers: TIM3 forward, 5′-GC TAC GTC AAC AGC CAG CAG-3′ and reverse, 5′-CCA ATG AGG TTG CCA AGT GA-3′; IL-1β forward, 5′-GAA CCT AGC TGT CAA CGT GTG G-3′ and reverse, 5′-GCA ATG TGC TGG TGC TTC AT-3′; IL-6 forward, 5′-GCT AAG GAC CAA GAC CAT CCA-3′ and reverse, 5′-GAC CAC AGT GAG GAA TGT CCA-3′.
The statistical analyses were performed using SPSS Statistics 17.0 software. All the values are expressed as the mean ± standard error of the mean (SEM). For multiple comparisons, one-way analysis of variance (ANOVA) was applied. The comparisons with P < 0.05 were considered statistically significant.
As shown in Figure 1, histological examination showed no difference with regard to morphology (epithelium erosion, crypt loss, and inflammatory infiltration with lymphocytes) between the normal control and IBS groups in H&E staining. Furthermore, no difference in morphology was observed between the IBS + C. butyricum and IBS + NS groups.
To determine whether C. butyricum treatment could reduce intestinal visceral hypersensitivity in IBS, C. butyricum was continuously administered via the intragastric route to IBS groups during 1 week. The degree of colon pain threshold pressures was assessed by the AWR test. As shown in Figure 2, the AWR scores for the IBS group were significantly increased compared with those for the normal control group at distention volumes of 0.25, 0.35, and 0.5 mL (P < 0.05). Similarly, C. butyricum treatment significantly decreased the AWR scores at the same distention volumes (P < 0.05).
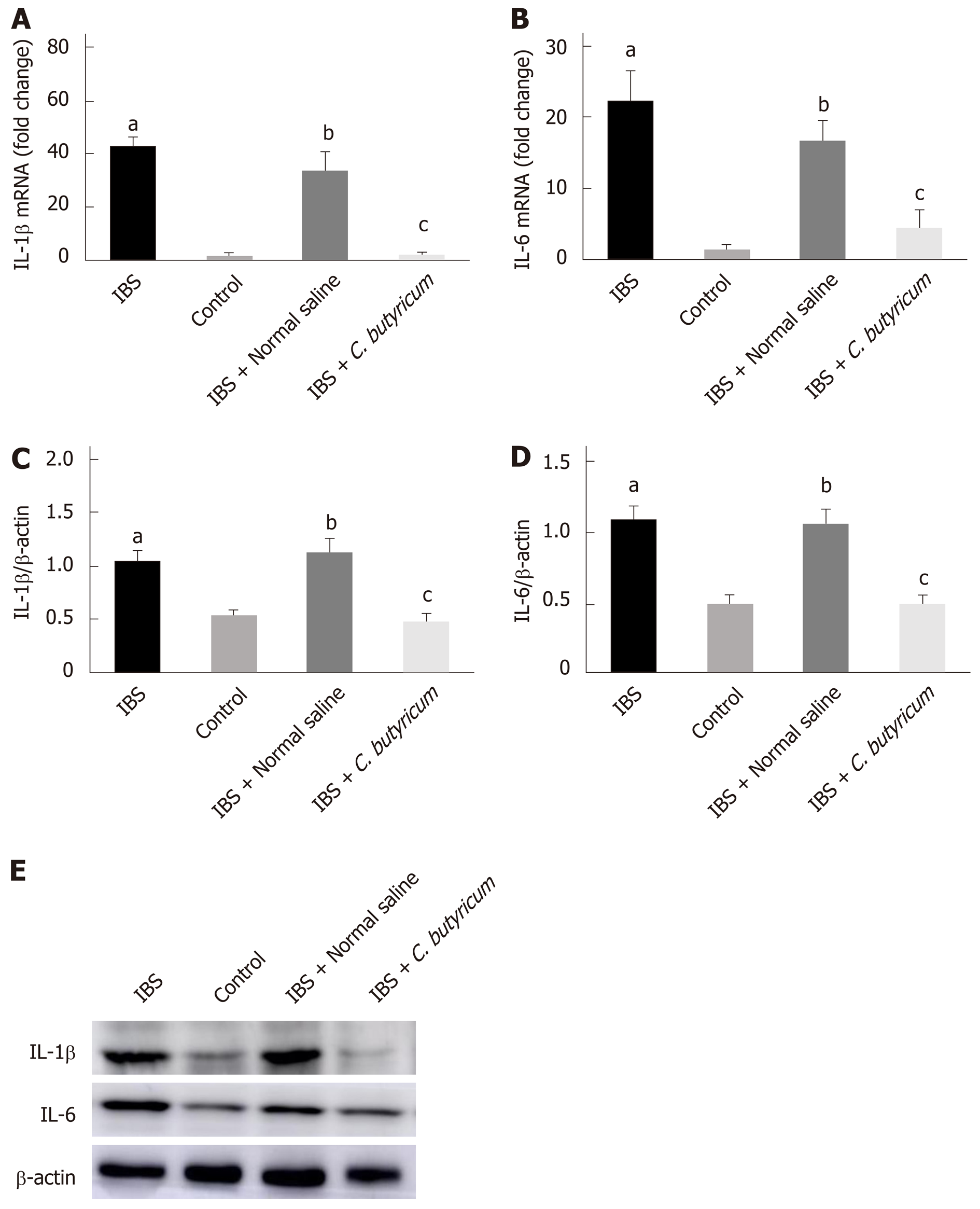
We further investigated the effects of C. butyricum on the expression of IL-1β and IL-6 proteins and their corresponding mRNAs in mice with TNBS-induced IBS. As shown in Figure 3, inflammatory cytokines (IL-1β and IL-6) were found to be significantly higher in the colon of mice with IBS compared with those in the normal control group (n = 6, P < 0.01). However, treatment with C. butyricum significantly reduced the expression of IL-1β and IL-6 proteins and the corresponding mRNAs (n = 6, P < 0.05). These results suggested that the intestinal mucosa in mice with IBS was in a low-grade inflammatory state. And C. butyricum might suppress the production of proinflammatory cytokines induced by TNBS stimulation.
In the absence of colonic mucosa histopathology, low-grade inflammation together with visceral hypersensitivity and increased inflammatory cytokines were observed in the PI-IBS animal model.
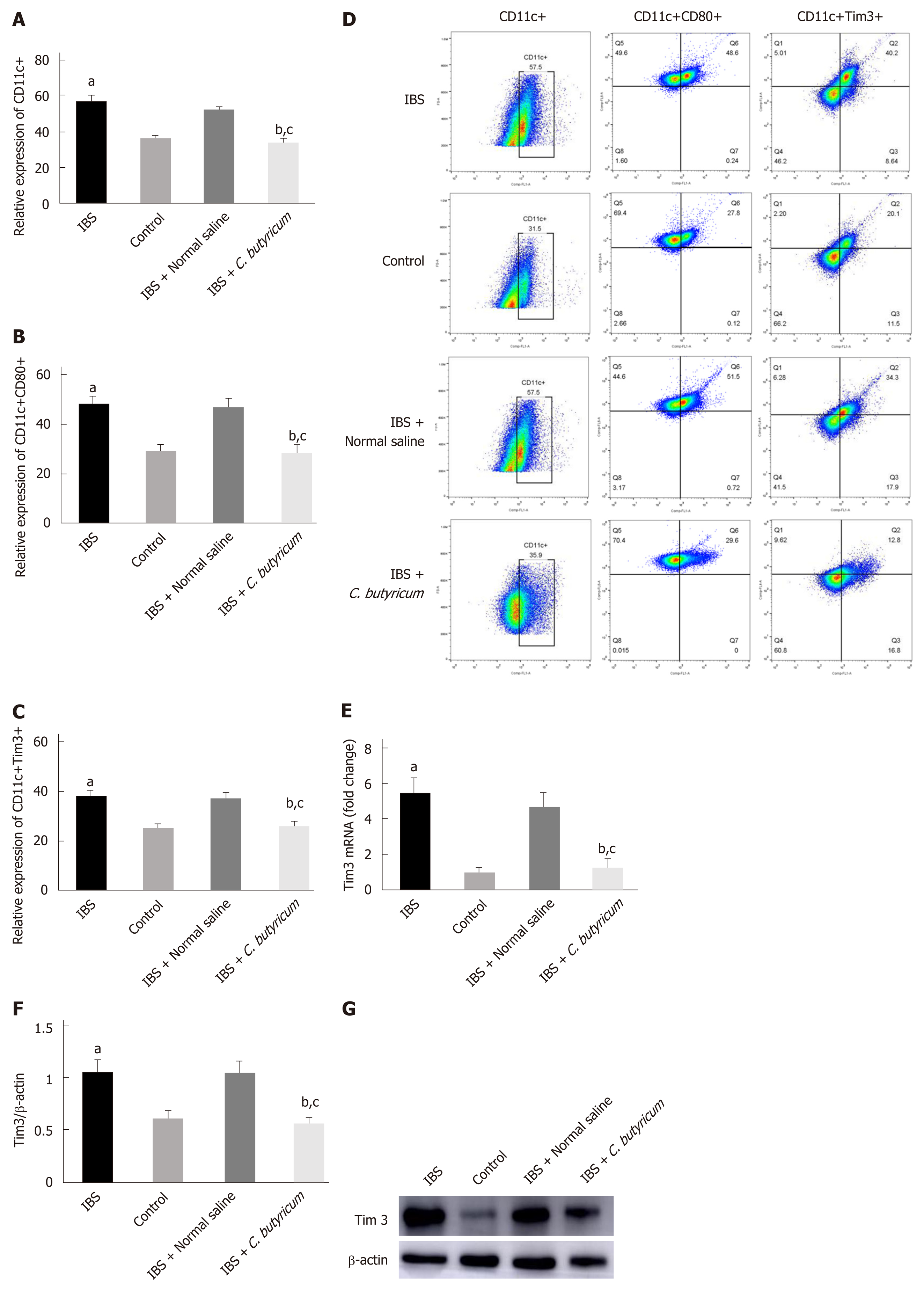
To evaluate the effects of C. butyricum on colonic LPDCs, LPDCs were extracted and analyzed using flow cytometry. Figure 4A-B shows that TNBS induced an increase in the number of CD11c+ LPDCs (IBS group: 56.90% ± 1.86% and IBS + NS group: 52.70% ± 1.13%) compared with that of the normal control group (36.43% ± 1.67%) (n = 6, P < 0.001). After treatment with C. butyricum, the number of CD11c+ LPDCs markedly decreased (34.15±1.54%), which was similar to that of the normal control group (n = 6, P > 0.5). Simultaneously, the amount of CD11c+CD80+ LPDCs increased in the IBS group (48.23% ± 2.92%) and in the IBS + NS group (47.20% ± 3.10%) compared with that of the normal control group (29.32% ± 1.19%). However, the amount of CD11c+CD80+ LPDCs was restricted by the C. butyricum treatment (28.37% ± 1.39%) (n = 6, P < 0.001).
Also, we investigated the level of an immunoregulatory molecule, TIM3, in LPDCs. Flow cytometry analysis was used to quantify the number of CD11c+TIM3+ LPDCs. Figure 4C shows that the levels of CD11c+TIM3+ LPDCs at the IBS low-grade inflammatory stage were significantly increased compared with that of the normal control group (normal control group: 25.60% ± 1.42%; IBS group: 38.52% ± 2.23%) (n = 6, P < 0.01). C. butyricum reduced the increase in the number of CD11c+TIM3+ LPDCs of the IBS group (IBS + C. butyricum group: 26.52% ± 1.78% and IBS + NS group: 37.40 ± 1.53%) (n = 6, P < 0.01) to match the number of TIM3+CD11c+ LPDCs of the normal control group (n = 6, P > 0.05). The other two methods (real-time PCR and Western blot analysis) also confirmed this conclusion at the protein and RNA levels (Figure 4E and F).
Collectively, based on our results, we concluded that C. butyricum not only reduced the number and function of LPDCs in TNBS-induced IBS mice but also down-regulated the expression of the immunoregulatory molecule TIM3 in LPDCs.
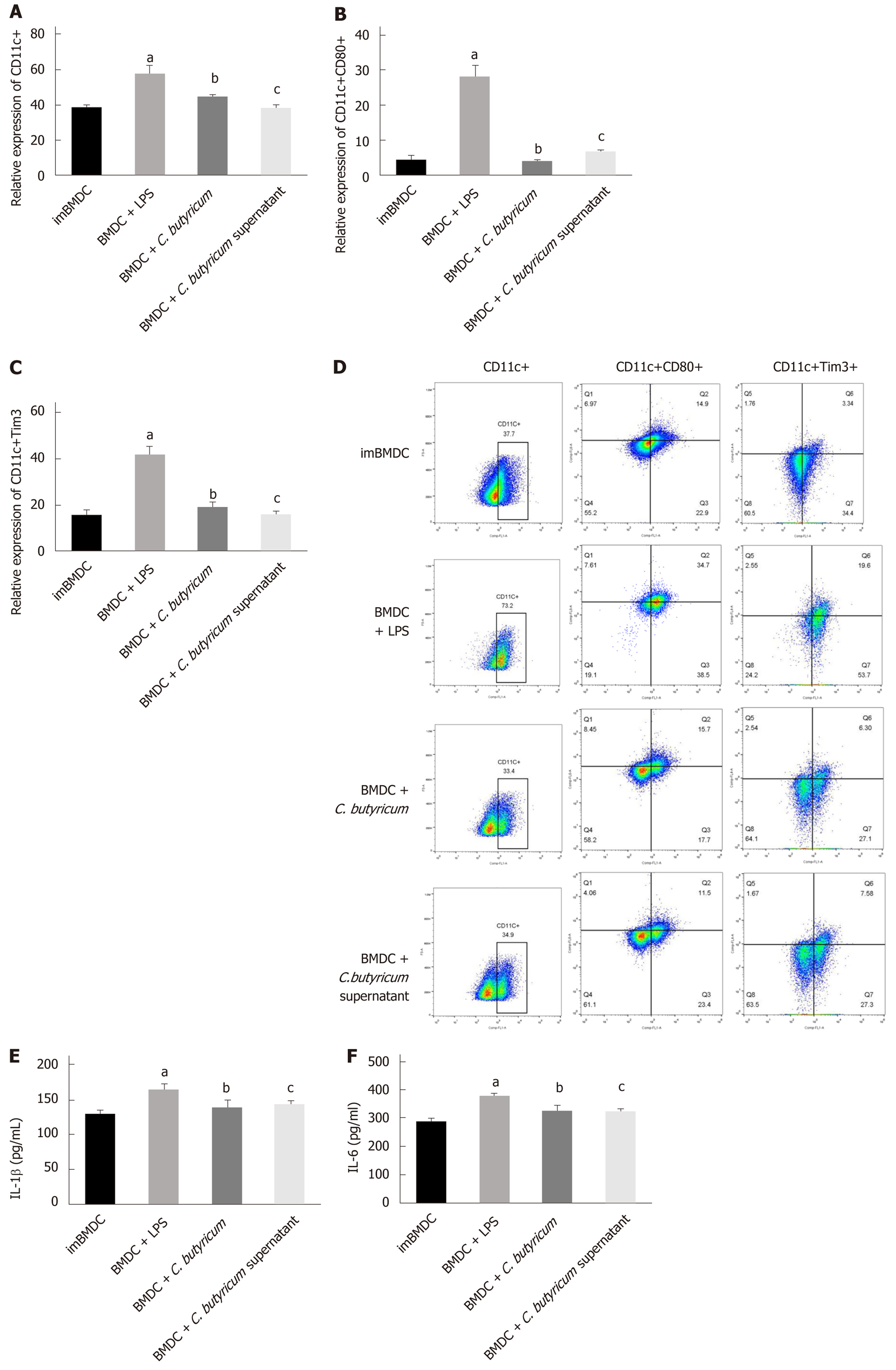
We examined the effect of C. butyricum on the BMDCs using flow cytometry analysis. Figure 5A-D shows that the C. butyricum treatment significantly decreased the number of CD11c+TIM3 + BMDCs (19.28% ± 1.57%) compared to that of the LPS group (43.33% ± 4.59%) (n = 6, P < 0.01). The number of CD11c+CD80+ DCs was also decreased in the BMDCs + C. butyricum group (n = 6, P < 0.001). The production of proinflammatory cytokines (IL-1β and IL-6) was also examined in BMDCs using ELISA. Figure 5E and F shows that C. butyricum markedly inhibited the LPS-induced secretion of IL-1β and IL-6 (n = 6, P < 0.05).
These results indicated that C. butyricum inhibited the proliferation and activation of BMDCs and reduced the secretion of pro-inflammatory cytokines, but down-regulated the expression of the TIM3 on the membrane of BMDCs.
IBS is related to various physiological alterations such as changes in the composition of gut microbiota, immune activation, gastrointestinal (GI) dysmotility, hyper-sensitivity, and the gut mucosal barrier[2,4,37,38]. Although extensive studies have recently focused on the correalations between intestinal microenvironment and immune activation, associated mechanisms have been rarely reported.
Probiotic C. butyricum produces butyric acid, which plays an important role in intestinal function by enhancing the intestinal barrier, improving the intestinal microbiota, regulating the immune system, and promoting gastrointestinal motility[39,40]. In this study, we found that in TNBS clysis-induced PI-IBS mice, the intragastric administration of C. butyricum significantly alleviated intestinal visceral hypersensitivity and reduced low-grade mucosal inflammation. Some investigators have proposed chronic low-grade mucosal inflammation as a potential etiological factor[41]. Particularly, the changes in the cytokines IL-1β and IL-6 secreted by LPDCs contribute to the development of IBS[42,43]. Our results showed that the important proinflammatory cytokines IL-1β and IL-6 were significantly increased in a murine model of IBS (n = 6, P < 0.01). However, these two inflammatory cytokines were both significantly suppressed (n = 6, P < 0.01) after the intervention with C. butyricum. Such results provide evidence for the anti-inflammatory activity of C. butyricum[7-9].
LPDCs are known to play a pivotal role in the regulation of the mucosal immune response[22]. Animal studies have highlighted the role of DCs in IBS pathogenesis based on the findings of induced visceral hypersensitivity and T cell activation[23,24]. It has been shown previously that the quantity of DCs in patients with IBS and IBD increased significantly, and the mature co-stimulatory molecules (CD80, CD86, and MHCII) were upregulated. Meanwhile, the secretion of the inflammatory cytokines was increased, and the migration and proliferation of CD4+ T cells were enhanced, resulting in the mediation of the Th1 and Th17 inflammatory responses[44-46]. In this study, we found that the expression of CD11c+ LPDCs and CD11c+CD80+ LPDCs in the colon of IBS mice was significantly higher than that of the normal control mice (n = 6, P < 0.001). These results suggested enhanced proliferation and activation of LPDCs in the local intestinal mucosa of IBS animals. Our results also demonstrated that LPDCs participated in and promoted the intestinal inflammatory immune response in PI-IBS. These findings are consistent with the results of previous studies[47].
In addition, we also found that after receiving C. butyricum intervention, the quantity of LPDCs was significantly decreased, and the co-stimulatory molecule CD80 was downregulated (n = 6, P < 0.001) in mice. These results indicate that C. butyricum alleviates the intestinal low-grade inflammation by regulating the functional status of LPDCs in mice with TNBS-induced IBS. Further investigation of the local microenvironment will be needed for the confirmation of DC phenotype and activation.
The immunoregulatory function of LPDCs is related to their subpopulation and surface receptors. TIM3 is an immune-regulatory factor. Recent studies have revealed that TIM3 expressed on macrophages and DCs upregulates the secretion of proinflammatory cytokines (TNF-α, IL-1β, IL-6 and so on) and promotes cell proliferation, activation, and phagocytosis, which result in enhanced elimination of different pathogens[29,31,32,43]. Anti-TIM3 mAb reduces the secretion of IL-12p70 in LPS-induced BMDCs and inhibits the upregulation of CD40, CD80, and CD86 expression in BMDCs[48]. The expression of TIM3 is upregulated after being stimulated to mature via the in vitro administration of IL-15 or IL-12 and IL-18 for immature natural killer cells[27,28,49]. Proinflammatory TIM3 is highly expressed in several inflammatory diseases[50,51]. TIM3/Gal9 induces the generation of the cytokines IL-1β, IL-6, and TNF-α through caspase-1, and the induced IL-1β further promotes the generation and activation of other cytokines by autocrine feedback[30,52]. To our best known, this is the first study that reports the role of TIM3 in LPDCs in the pathogenesis of IBS and the regulatory effect of C. butyricum on TIM3. In this study, the results demonstrate that the expression levels of TIM3 on LPDCs are positively correlated with intestinal low-grade inflammation according to the comparision among four different intestinal immune states. The levels of CD11c+TIM3+ LPDCs are significantly increased in the colon of mice with IBS. However, the CD11c+TIM3+ LPDCs are significantly reduced after the alleviation of the intestinal low-grade inflammation by C. butyricum intervention (n = 6, P < 0.01), but no significant difference is observed when compared to the normal control group (n = 6, P > 0.05).
Combined with previous studies, the regulatory effect of C. butyricum on DCs has been verified in the following cell tests. Compared with the BMDC + LPS group, through the stimulation of C. butyricum-treated BMDCs with LPS, the expression of CD11c+, CD11c+CD80+, and CD11c+TIM3+ LPDCs was also significantly downregulated. These results suggest that C. butyricum not only reduces the number of BMDCs and inhibits their functional status but also significantly reduces the expression of TIM3. Thus, we have reason to believe that DCs play an important regulatory role in the immune response and are regulated by the microenvironment.
In conclusion, our results indicate that intestinal LPDCs play an important role in the pathogenesis of IBS, and C. butyricum can alleviate intestinal inflammatory immune responses by regulating the amount and function of LPDCs and the expression of TIM3 on the surface of LPDCs. Our research not only provides an in-depth understanding of the local immune response mechanism in the intestinal mucosa of IBS patients, but also provides a newperspective for the application of probiotic C. butyricum in the treatment of IBS.
Irritable bowel syndrome (IBS) affects 7% to 21% of the general population. It is a chronic diseases characterized by abnormal visceral sensitivity and low-grade inflammation. The role of Clostridium butyricum (C. butyricum) in reducing intestinal low-grade inflammation via immune pathways has been well defined. However, the mechanism has not been clearly elucidated.
To test the hypothesis that the function of dendritic cells (DCs) changes in the development of IBS and to understand the regulation of DCs after C. butyricum intervention.
We aimed to investigate the mechanism of DCs in the development of IBS in a mice model and to understand the regulation of DCs after C. butyricum intervention.
An IBS animal model was established using C57BL/6 mice, and C. butyricum was continuously administered via the intragastric route to simulate different intestinal immune states. Intestinal visceral hypersensitivity and histopathology were assessed using the abdominal withdrawal reflex test and hematoxylin & eosin staining, respectively. The expression of proinflammatory cytokines (IL-1β and IL-6) and TIM3 was analyzed by Western blot analysis and real-time PCR. The flow cytometry was applied to analyze the quantity, function, and membrane molecule TIM3 of the LPDCs. The regulatory effect of C. butyricum was verified in BMDCs by in vitro experiments.
We found that the IBS mouse model has abundant expression of IL-1β, IL-6, and CD11C+CD80+ and CD11c+TIM3+ LPDCs compared with the control group. Further investigation showed that probiotic C. butyricum reduced the expression of cytokines (IL-1β and IL-6). The amount and function of LPDCs and the membrane molecule TIM3 of the LPDCs were decreased with the alleviation of the intestinal inflammatory response.
This study demonstrated that C. butyricum could induce the expression of various pro-inflammatory cytokines via regulating the amount and the functional status of LPDCs in the intestinal mucosa of mice with IBS.
This research not only provides an in-depth understanding of the local immune response mechanism in intestinal mucosa of IBS humans, but also provides a new perspective for the application of probiotic C. butyricum in the treatment of IBS.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maric I, Tanabe S S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M, Bolotin A, Friger M, Freud T, Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 2. | Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 153] [Article Influence: 25.5] [Reference Citation Analysis (2)] |
| 3. | Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 4. | Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, Filippi J, Saint-Paul MC, Tulic MK, Verhasselt V, Hébuterne X, Piche T. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3154] [Cited by in F6Publishing: 3172] [Article Influence: 211.5] [Reference Citation Analysis (0)] |
| 6. | Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol. 2013;91:204-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome--a 12 week double-blind study. Aliment Pharmacol Ther. 2014;40:51-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Hudson LE, Anderson SE, Corbett AH, Lamb TJ. Gleaning Insights from Fecal Microbiota Transplantation and Probiotic Studies for the Rational Design of Combination Microbial Therapies. Clin Microbiol Rev. 2017;30:191-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P, Yang XX, Gu X, Song LJ, Li Z, Zuo XL, Li YQ. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2018;8:2964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 10. | Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504-3511. [PubMed] [Cited in This Article: ] |
| 11. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1875] [Cited by in F6Publishing: 1929] [Article Influence: 175.4] [Reference Citation Analysis (2)] |
| 12. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2568] [Cited by in F6Publishing: 2644] [Article Influence: 188.9] [Reference Citation Analysis (0)] |
| 13. | Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1218] [Cited by in F6Publishing: 1120] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 14. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2516] [Cited by in F6Publishing: 2919] [Article Influence: 265.4] [Reference Citation Analysis (0)] |
| 15. | Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, Yoshimura A, Kanai T. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Kashiwagi I, Morita R, Schichita T, Komai K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T, Yoshimura A. Smad2 and Smad3 Inversely Regulate TGF-β Autoinduction in Clostridium butyricum-Activated Dendritic Cells. Immunity. 2015;43:65-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Hua MC, Lin TY, Lai MW, Kong MS, Chang HJ, Chen CC. Probiotic Bio-Three induces Th1 and anti-inflammatory effects in PBMC and dendritic cells. World J Gastroenterol. 2010;16:3529-3540. [PubMed] [Cited in This Article: ] |
| 18. | Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831-1840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 766] [Cited by in F6Publishing: 752] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 20. | Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005;206:132-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 552] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 22. | Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50-65. [PubMed] [Cited in This Article: ] |
| 23. | Long Y, Wang W, Wang H, Hao L, Qian W, Hou X. Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol. 2012;27:935-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Li M, Zhang L, Lu B, Chen Z, Chu L, Meng L, Fan Y. Role of dendritic cell-mediated abnormal immune response in visceral hypersensitivity. Int J Clin Exp Med. 2015;8:13243-13250. [PubMed] [Cited in This Article: ] |
| 25. | Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Semin Immunol. 2005;17:284-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Xue X, Feng T, Yao S, Wolf KJ, Liu CG, Liu X, Elson CO, Cong Y. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J Immunol. 2011;187:5879-5886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Ocaña-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I. TIM-3 Regulates Distinct Functions in Macrophages. Front Immunol. 2016;7:229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Sada-Ovalle I, Chávez-Galán L, Torre-Bouscoulet L, Nava-Gamiño L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J Immunol. 2012;189:5896-5902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207:2343-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Dai SY, Nakagawa R, Itoh A, Murakami H, Kashio Y, Abe H, Katoh S, Kontani K, Kihara M, Zhang SL, Hata T, Nakamura T, Yamauchi A, Hirashima M. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol. 2005;175:2974-2981. [PubMed] [Cited in This Article: ] |
| 32. | Zhang H, Song Y, Yang H, Liu Z, Gao L, Liang X, Ma C. Tumor cell-intrinsic Tim-3 promotes liver cancer via NF-κB/IL-6/STAT3 axis. Oncogene. 2018;37:2456-2468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750-1761. [PubMed] [Cited in This Article: ] |
| 34. | La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791-2795. [PubMed] [Cited in This Article: ] |
| 35. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] [Cited in This Article: ] |
| 36. | Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693-1702. [PubMed] [Cited in This Article: ] |
| 37. | Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51 Suppl 1:i41-i44. [PubMed] [Cited in This Article: ] |
| 38. | Raskov H, Burcharth J, Pommergaard HC, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 39. | Ternovoĭ VA, Shipitsyna VV, Iakovlev VM. [Changes in the content of various types of cholesterol and phospholipids in rat tissues during cold acclimation]. Zh Evol Biokhim Fiziol. 1989;25:15-19. [PubMed] [Cited in This Article: ] |
| 40. | Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care. 2012;15:474-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 41. | Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [PubMed] [Cited in This Article: ] |
| 43. | Gwee KA, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, McKendrick MW, Moochhala SM. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523-526. [PubMed] [Cited in This Article: ] |
| 44. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] [Cited in This Article: ] |
| 45. | Wurbel MA, McIntire MG, Dwyer P, Fiebiger E. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One. 2011;6:e16442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Drakes ML, Blanchard TG, Czinn SJ. Colon lamina propria dendritic cells induce a proinflammatory cytokine response in lamina propria T cells in the SCID mouse model of colitis. J Leukoc Biol. 2005;78:1291-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 48. | Maurya N, Gujar R, Gupta M, Yadav V, Verma S, Sen P. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein-3 via Bruton's tyrosine kinase and c-Src. J Immunol. 2014;193:3417-3425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Ndhlovu LC, Lopez-Vergès S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734-3743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 50. | Koh HS, Chang CY, Jeon SB, Yoon HJ, Ahn YH, Kim HS, Kim IH, Jeon SH, Johnson RS, Park EJ. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun. 2015;6:6340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 51. | Chen X, Song CH, Liu ZQ, Feng BS, Zheng PY, Li P, In SH, Tang SG, Yang PC. Intestinal epithelial cells express galectin-9 in patients with food allergy that plays a critical role in sustaining allergic status in mouse intestine. Allergy. 2011;66:1038-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, Behar SM. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 2013;190:4196-4204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |